Abstract
Background
Inositol Hexaphosphate (IP6) is a naturally occurring polyphosphorylated carbohydrate that is found in food sources high in fiber content. We hypothesized that IP6 would inhibit the cell growth rate of bladder cancer in vitro.
Methods
T24 and TCCSUP bladder cancer cell lines were treated with titrating doses of IP6 (0.3, 0.6 and 0.9 mM/well). Cell viability and vascular endothelial growth factor levels were measured.
Results
Significant reductions (p < 0.001) in cellular growth were noted in both cell lines at all doses and time points tested, with the exception of 0.3 mM IP6 at 24 hours in the T24 cell line. The percent inhibition of vascular endothelial growth factor was significantly higher than that observed in the TCCSUP cell line at 48 and 72 hours with 0.3 mM IP6 (p < 0.001). The T24 cells exhibited the same level of inhibition at 24 and 48 hours with 0.6 mM dose of IP6 and at 72 hours with the 0.3 mM dose (p < 0.001).
Conclusions
In vitro treatment of bladder cancer with the common dietary polyphosphorylated carbohydrate IP6 significantly decreased cellular growth by anti-angiogenic mechanisms. We feel that this data warrants further investigation and consideration for initiation of clinical trials to evaluate the safety and clinical utility of this agent.
Key Words: Cellular anti-proliferation, Bladder cancer, Angiogenesis, Inositol hexaphosphate, Vascular endothelial growth factor
Introduction
There will be 73,510 newly diagnosed cases of bladder cancer in 2012, with 55,600 being men and 17,910 being women. Approximately 1 in 5 of those who develop bladder cancer will die due to the disease (relative mortality 20.2%) [1]. Bladder cancer has become the second most prevalent cancer after cancer of the prostate in middle-aged to elderly male individuals. Many patients do not die of their disease, but have multiple recurrences [2]. This lends to the fact that the 5-year cost to Medicare attributed to bladder cancer in the United States is over one billion dollars [3].
Carcinogen exposure is the leading cause of bladder cancer and cigarette smoking accounts for approximately 66% of the male and 30% of the female cancers with a 2 to 4-fold increase in risk when compared to non-smokers [4, 5, 6, 7]. Leading presenting symptoms include hematuria (occurring up to 85%) and irritative voiding symptoms such as dysuria and frequency [8]. Approximately 60–80% of newly diagnosed bladder cancers are superficial, either Ta, T1, or carcinoma in situ [9]. With local resection and no adjuvant intravesical therapy, progression rates for tumor grades I, II, and III and stage Ta and T1 to muscle-invasive cancer is 2, 11, and 45%, respectively [10].
Intravesical chemotherapy with thiotepa [11], mitomycin C [12], or doxorubicin [13] had historically been the standard treatment for those patients suffering with bladder cancer. However, these chemotherapies have been unable to provide significant long-term benefit over that of transurethral resection alone [14]. Bacillus Calmette-Guerin (BCG) exhibits significant protection over that of surgical resection alone [15, 16] and has proven to be superior to that of chemotherapy with Doxorubicin in preventing tumor recurrence [17]. However, BCG is not without treatment related side effects and many patients will fail to respond to treatment. Therefore, alternative and potentially more effective and less toxic forms of therapy are needed.
Inositol hexaphosphate (IP6), a naturally occurring polyphosphorylated carbohydrate, is found in foods high in fiber content such as legumes and cereals [18], and is easily absorbed from the gastrointestinal tract making it safe in a regular diet. IP6 has been shown to inhibit both the in vivo and in vitro growth of numerous tumor and cell lines [19]. Present in most mammalian cells, IP6 plays an important role in regulating cell function, proliferation and differentiation [20]. The mechanisms by which IP6 exerts its anti-proliferative effects are through regulation of cell cycle, apoptosis and angiogenesis. Angiogenesis is the process in which new blood vessels are formed for tumor growth to occur [21]. One of the more common and specific methods used to detect angiogenic activity is through the measurement of vascular endothelial growth factor (VEGF) [22].
We hypothesized IP6 would inhibit the growth rate of bladder cancer in vitro and prove to be a potentially effective form of intravesical therapy for bladder cancer.
Materials and Methods
Tissue Culture Media
RPMI 1640 tissue culture media (Invitrogen, Grand Island, NY) was employed for both cell passage and experimental procedures. RPMI was supplemented with 10% Fetal Bovine Serum (ATCC, Manassas, VA), 1% Penicillin Streptomycin (Invitrogen, Grand Island, NY) and 1% Sodium Pyruvate (Sigma Chemical Company, St. Louis, MO).
IP6
IP6 (dodecasodium salt hydrate) derived from rice was purchased from Sigma Chemical Company (St. Louis, MO). IP6 was solubilized in RPMI 1640 tissue culture media (as described above) to the desired concentrations of 0.3, 0.6, and 0.9 mM/well. After solubilization, the pH of IP6 was checked and determined to be 9.5. The pH was then adjusted to a neutral pH of 7.0 using 1N Hydrochloric acid, IP6 was then filtered using a 0.2 micron filter to assure sterility. Tissue culture media alone served as the control for all experimental procedures.
Cell Culture and Reagents
Two bladder cancer cell lines, TCCSUP (Grade IV), and T24 (Grade III) (ATCC, Manassas, VA) were maintained as monolayers in their preferred media (as described above) at 37°C in 5% CO2. Cell were trypsinized and then plated in sterile 96-well plates at 1 × 105 cells/ml. Cells were then returned to the incubator for 24 hours to allow adherence prior to exposure to IP6. Cells were treated with IP6 at the desired concentrations 0.3, 0.6 and 0.9 mM/well and returned to the incubator for an additional 24, 48, and 72 hours. Prior to MTT assay, supernatants were collected and stored for later assay and quantification of VEGF levels (pg/ml).
MTT Assay
The MTT colorimetric assay was performed to detect tumor cell viability after 24, 48 and 72 hours of incubation. MTT, a tetrazolium dye (3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; thiazolyl blue, Sigma chemical Co., St. Louis, MO) was added to each well as described previously [23]. Plates were incubated in the presence of MTT dye for 4 hours. Mitochondrial dehydrogenase activity reduced the yellow MTT dye to a purple formazan, which was then solubilized with acidified isopropanol and absorbance was read at 570 nm.
VEGF ELISA
Human VEGF Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) was used to detect VEGF (pg/ml) after 24, 48 and 72 hours of incubation. Cell culture supernatants, standards, or control samples were added in 200 μl increments to a precoated microplate containing a monoclonal antibody specific for VEGF. VEGF present in the samples will bind to the antibody. The microplate was then washed to release any unbound substances and an enzyme-linked polyclonal antibody specific for VEGF was added to each well. After incubation, a wash buffer was used to remove any unbound antibody-enzyme reagent and a substrate solution was added for color development. Once the color development was complete, the plate was read at 450 nm.
Statistical Analysis
Determination of statistical significance was performed by analysis of variance (ANOVA) [24]. Post hoc comparison of individual concentration means with the control was completed using the Tukey-Kramer Multiple Comparison test [25]. All data are reported as means ± SD.
Results
MTT Cell Viability Assay
TCCSUP All doses of IP6 tested significantly reduced the proliferation of cells after 24 hours of exposure (table 1). An 11.7 ± 7.0% reduction in proliferation was observed with 0.3 mM IP6 (p = 0.004), followed by 73.8 ± 6.4%with 0.6 mM IP6 (p < 0.001) and 82.3 ± 3.6% with 0.9 mM IP6 (p < 0.001). After 48 hours, 0.9 mM IP6 exhibited the greatest degree of anti-proliferation with a reduction in cell growth of 91.5 ± 1.0% (p < 0.001), closely followed by 0.6 mM (85.4 ± 2.6%, p < 0.001). A 32.2 ± 4.4% reduction was seen with 0.3 mM IP6 (p < 0.001). After 72 hours, 0.9 mM IP6 (92.9 ± 0.8%, p < 0.001) and 0.6 mM IP6 (92.3 ± 0.6%, p < 0.001) exhibited nearly equivalent anti-proliferative effects in the TCCSUP cell line (table 1), while a 26.2 ± 4.6% (p < 0.001) reduction was observed with 0.3 mM IP6.
Table 1.
TCCSUP cell line: evaluation of the dose and time dependent in vitro anti-proliferative effects of IP6 as measured by MTT assay
| Incubation time | Mean growth inhibition, % | Number | SD | p (vs. control) |
|---|---|---|---|---|
| 24 hours | ||||
| Control | 0 | 6 | 0.1 | |
| 0.3 mM IP6 | 11.7 | 6 | 7.0 | 0.004 |
| 0.6 mM IP6 | 73.8 | 6 | 6.4 | 0.001 |
| 0.9 mM IP6 | 82.3 | 6 | 3.6 | <0.001 |
| 48 hours | ANOVA | <0.001 | ||
| Control | 0 | 6 | 0.1 | |
| 0.3 mM IP6 | 32.2 | 6 | 4.4 | <0.001 |
| 0.6 mM IP6 | 85.4 | 6 | 2.6 | <0.001 |
| 0.9 mM IP6 | 91.5 | 6 | 1.0 | <0.001 |
| 72 hours | ANOVA | <0.001 | ||
| Control | 0 | 6 | 0.1 | |
| 0.3 mM IP6 | 26.2 | 6 | 4.6 | <0.001 |
| 0.6 mM IP6 | 92.3 | 6 | 0.6 | <0.001 |
| 0.9 mM IP6 | 92.9 | 6 | 0.8 | <0.001 |
| ANOVA | <0.001 | |||
T24 Both 0.6 mM IP6 (26.1 ± 4.7%) and 0.9 mM IP6 (54.0 ± 7.2%) significantly inhibited cellular proliferation after 24 hours of incubation (table 2, p < 0.001). After 48 hours, all 3 IP6 doses tested exhibited significant reductions in cellular proliferation (table 2). The greatest reduction in cellular proliferation was observed with 0.9 mM IP6 (88.0 ± 4.3%, p < 0.001), followed by 0.6 mM IP6 (59.1 ± 1.6%, p < 0.001), and 0.3 mM IP6 (20.1 ± 4.3%, p < 0.001). As was observed at 48 hours, all 3 doses of IP6 exhibited significant reductions in cell proliferation after 72 hours of incubation (table 2, p < 0.001). Again 0.9 mM IP6 exhibited the greatest reduction in cellular proliferation (90.3 ± 1.3%, p < 0.001), followed by 0.6 mM IP6 (85.7 ± 2.1%, p < 0.001), and 0.3 mM IP6 (12.6 ± 5.0%, p < 0.001).
Table 2.
T24 cell line: evaluation of the dose and time dependent in vitro anti-proliferative effects of IP6 as measured by MTT assay
| Incubation time | Mean growth inhibition, % | Number | SD | p (vs. control) |
|---|---|---|---|---|
| 24 hours | ||||
| Control | 0 | 6 | 0.1 | |
| 0.3 mM IP6 | 6.4 | 6 | 3.5 | NS |
| 0.6 mM IP6 | 26.1 | 6 | 4.7 | <0.001 |
| 0.9 mM IP6 | 54.0 | 6 | 7.2 | <0.001 |
| 48 hours | ANOVA | <0.001 | ||
| Control | 0 | 6 | 0.1 | |
| 0.3 mM IP6 | 20.1 | 6 | 4.3 | <0.001 |
| 0.6 mM IP6 | 59.1 | 6 | 1.6 | <0.001 |
| 0.9 mM IP6 | 88.0 | 6 | 4.3 | <0.001 |
| 72 hours | ANOVA | <0.001 | ||
| Control | 0 | 6 | 0.1 | |
| 0.3 mM IP6 | 12.6 | 6 | 5.0 | <0.001 |
| 0.6 mM IP6 | 85.7 | 6 | 2.1 | <0.001 |
| 0.9 mM IP6 | 90.3 | 6 | 1.3 | <0.001 |
| ANOVA | <0.001 | |||
NS = Not significant.
Human VEGF Quantikine ELISA
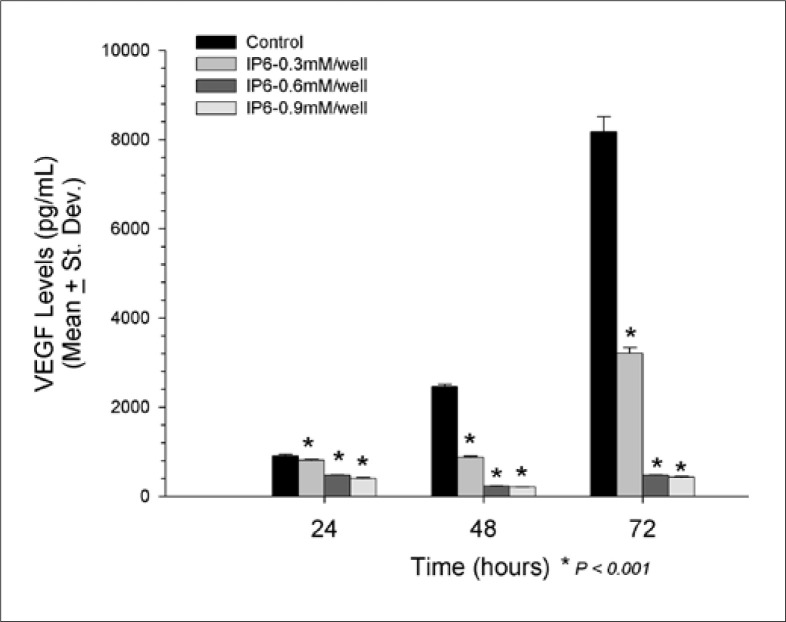
TCCSUP Significant reduction in VEGF was observed in all 3 IP6 doses tested and all time points assayed (fig. 1). At 24 hours, 0.9 mM IP6 exerted the greatest reduction in VEGF (397.6 ± 18.5 pg/ml, p < 0.001), followed by 0.6 mM IP6 (476.2 ± 11.9 pg/ml, p < 0.001), and 0.3 mM IP6 (808.5 ± 15.5 pg/ml, p < 0.001) when compared to control (908.4 ± 33.7 pg/ml). These reductions continued at 48 hours as well, with 0.9 mM IP6 showing the greatest reduction in VEGF (204.1 ± 8.8 pg/ml, p < 0.001), followed by 0.6 mM IP6 (228.9 ± 8.1 pg/ml, p < 0.001), and 0.3 mM IP6 (877.1 ± 27.5 pg/ml, p < 0.001) while the control levels of VEGF was 2459.4 ± 57.6 pg/ml. After 72 hours, the mean level of VEGF was 8171.0 ± 338.1 pg/ml in the controls, with significant reductions observed by the addition of 0.9 mM IP6 (431.4 ± 18.0 pg/ml, p < 0.001), 0.6 mM IP6 (478.4 ± 8.4 pg/ml, p < 0.001) and 0.3mM IP6 (3203.1 ± 131.9 pg/ml, p < 0.001). After 48 hours, the level of VEGF was significantly reduced when compared to the 24 hours results in the cells treated with both 0.6 mM IP6 (228.9 ± 8.1 vs. 476.2 ± 11.9 pg/ml, p < 0.001) and 0.9 mM IP6 (204.0 ± 8.8 vs. 397.6 ± 13.5 pg/ml, p < 0.001). No other time-dependent reductions in VEGF were noted in the TCCSUP cells.
Fig. 1.
Significant reduction in VEGF was observed in all 3 IP6 doses tested and all time points assayed in TCCSUP cell line.
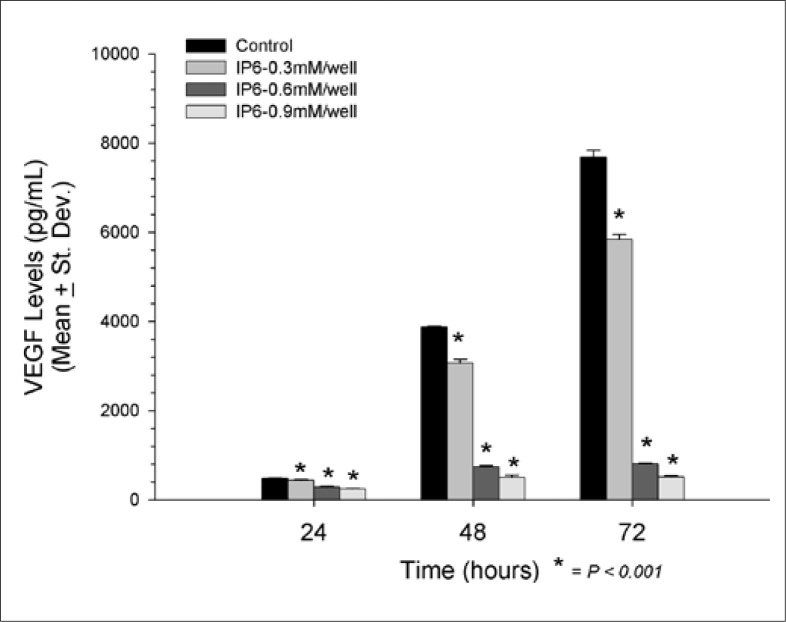
T24 After 24 hours, VEGF levels were significantly reduced in all doses of IP6 used (fig. 2). The greatest reduction in VEGF was observed by 0.9 mM IP6 (247.8 ± 12.2 pg/ml, p < 0.001) compared to control (481.2 ± 16.2 pg/ml), followed by 0.6 mM IP6 (287.1 ± 16.3 pg/ml, p < 0.001) and 0.3 mM IP6 (435.4 ± 22.2 pg/ml, p < 0.001). The dose-dependent reduction in VEGF production continued after 48 hours with 0.9 mM IP6 showing the greatest inhibitory effect (507.1 ± 45.6 pg/ml, p < 0.001), followed by 0.6 mM IP6 (738.6 ± 31.2 pg/ml, p < 0.001) and 0.3 mM IP6 (3065.7 ± 88.2 pg/ml, p < 0.001). The control group yielded a VEGF level of 3875.6 ± 13.0 pg/ml after 48 hours. The protective effects of IP6 in regards to VEGF production continued at 72 hours, with 0.9 mM IP6 exhibiting the most dramatic reduction in VEGF levels (514.5 ± 24.7 pg/ml, p < 0.001), again followed by 0.6 mM IP6 (805.5 ± 23.8 pg/ml, p < 0.001), and 0.3 mM IP6 (5834.4 ± 120.5 pg/ml, p < 0.001). All three of these levels were significantly reduced when compared to the control groups at 72 hours (7681.5 ± 152.4 pg/ml).
Fig. 2.
VEGF changes in all 3 IP6 doses tested and all time points assayed in T24 cell line.
Discussion
Although many patients will respond well to conventional intravesical therapies, a significant number will experience tumor recurrence and/or treatment limiting toxicities. Intravesical therapy with BCG gained popularity in the 1980's based on reports by Morales et al. [15] and Lamm et al. [16] showing that BCG provided significant protection from tumor recurrence, progression and mortality. A Southwest Oncology Group study randomized 262 eligible patients to receive either chemotherapy with Doxorubicin or immunotherapy with BCG. BCG was shown to provide significantly better protection from tumor recurrence with 70% of those patients receiving BCG achieving a complete response compared to 34% in the doxorubicin arm (p < 0.001) [17]. Although the use of intravesical immunotherapy with BCG has been shown to be the most effective form of treatment to date for those patients with bladder cancer, it is not without treatment related toxicity and many patients will fail to respond to initial treatment. Therefore, alternative and potentially more effective and less toxic forms of therapy are needed.
IP6 is a naturally occurring carbohydrate that is found in most cereals, grains, legumes, seeds and other foods that are high in fiber such as wheat bran and flaxseed [26]. Anti-proliferative effects of IP6 have been expressed in various tumors such as breast [27, 28], colon [29], liver [30], prostate [31, 32, 33], pancreas [34], melanoma [35] and Barrett's adenocarcinoma [36]. The effects of IP6 have been reported to be cancer cell specific and not cytotoxic nor cytostatic against normal cells. When leukemic progenitor cells were treated with IP6, there was no reported effect on normal progenitor CD34+ cells derived from bone marrow [37].
In our current study, the in vitro growth of T24 and TCCSUP bladder cancer cell lines treated with titrating doses of IP6 (0.3, 0.6 and 0.9 mM/well) was significantly reduced. Cell viability was measured by MTT after 24, 48 and 72 hours. Significant reductions (p < 0.001) in cellular growth were noted in both cell lines at all doses and time points tested, with the exception of 0.3 mM/well IP6 at 24 hours in the T24 cell line.
Regulating cell cycle, apoptosis and angiogenesis are among the reported mechanisms by which IP6 exerts its anti-proliferative effects. Through angiogenesis, tumor cells leave the primary site and enter the blood stream where new blood vessels form and tumor cells metastasize. The most common tumor angiogenic factors are the fibroblast growth factor and the VEGF [22, 38]. As cells undergo angiogenesis, both fibroblast growth factor and VEGF levels increase during the proliferative process. A reduction in these levels as a direct result of treatment, as in this case IP6, indicates that agent is acting as a modulator of angiogenesis [22].
Vucenik et al. [22] reported inhibitory effects of IP6 on angiogenic activity in the HepG2 cells. These researchers pretreated HepG2 cells with 0.5, 1.0 and 2.0 mM IP6 for 24 hours. At specific time points, aliquots of cell supernatants were removed to determine VEGF protein levels as measured by ELISA. These results showed that IP6 reduced VEGF levels by about 50% in a concentration-dependent manner. The HepG2 cells were re-cultured in serum-free media in the absence of IP6 for an additional 24 hours. A continued reduction in VEGF levels was observed by 79% with 1.0 mM IP6 and 86% with 2.0 mM IP6.
Our laboratory has previously reported that IP6 inhibited VEGF levels in two human pancreatic cancer cell lines [34]. The results showed that VEGF levels were significantly reduced to 343 ± 23 pg/ml when compared to control (1084 ± 44 pg/ml) in the MIA PaCa-2 cells and decreased from control of 1465 ± 319 to 936 ± 35 pg/ml with 0.8 mM/well IP6 in the PANC-1 cells.
In the present study, VEGF (pg/ml) was significantly reduced (p < 0.001) in both cell lines at all time points and doses tested. However, to determine if the reduction of VEGF was due to the relative decrease in cell growth as observed by the MTT assay, VEGF levels (pg/ml) were converted to percent (%) change versus control. The percent inhibition of VEGF was significantly higher than that observed by MTT (p < 0.001) in the TCCSUP cell line at both 48 and 72 hours with 0.3 mM IP6. T24 cells exhibited the same level of inhibition at 24 and 48 hours with 0.6 mM/well dose of IP6 and at 72 hours with the 0.3 mM/well dose (p < 0.001). The observed reductions in VEGF production would indicate that IP6 reduces cellular proliferation and growth at least in part by anti-angiogenic mechanisms.
Conclusion
IP6 has demonstrated significant and reproducible reductions in the growth of bladder cancer in vitro. The significant reductions in VEGF would suggest that IP6 reduces cellular growth at least in part by anti-angiogenic mechanisms. The results presented herein warrants further investigation, both in vitro and in vivo, leading to the initiation of Phase II clinical trials to evaluate the safety and clinical utility of this agent.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Pelucchi C, Bosetti C, Negri E, Malvezzi M, La Vecchia C. Mechanisms of disease: the epidemiology of bladder cancer. Nat Clin Pract Urol. 2006;3:327–340. doi: 10.1038/ncpuro0510. [DOI] [PubMed] [Google Scholar]
- 3.Yabroff K, Lamont E, Mariotto A, Warren JL, Topor M, Meekins A, Brown ML. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 4.Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, Lopez-Abente G, Tzonou A, Chang-Claude J, Bolm-Audorff U, Jöckel KH, Donato F, Serra C, Wahrendorf J, Hours M, T'Mannetje A, Kogevinas M, Boffetta P. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000;86:289–294. doi: 10.1002/(sici)1097-0215(20000415)86:2<289::aid-ijc21>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Brennan P, Bogillot O, Greiser E, Chang-Claude J, Wahrendorf J, Cordier S, Jöckel KH, Lopez-Abente G, Tzonou A, Vineis P, Donato F, Hours M, Serra C, Bolm-Audorff U, Schill W, Kogevinas M, Boffetta P. The contribution of cigarette smoking to bladder cancer in women (pooled European data) Cancer Causes Control. 2001;12:411–417. doi: 10.1023/a:1011214222810. [DOI] [PubMed] [Google Scholar]
- 6.Burch JD, Rohan TE, Howe GR, Risch HA, Hill GB, Steele R, Miller AB. Risk of bladder cancer by source and type of tobacco exposure: a case-control study. Int J Cancer. 1989;44:622–628. doi: 10.1002/ijc.2910440411. [DOI] [PubMed] [Google Scholar]
- 7.Baris D, Karagas MR, Verrill C, Johnson A, Andrew AS, Marsit CJ, Schwenn M, Colt JS, Cherala S, Samanic C, Waddell R, Cantor KP, Schned A, Rothman N, Lubin J, Fraumeni JF, Jr, Hoover RN, Kelsey KT, Silverman DT. A case-control study of smoking and bladder cancer risk: emergent patterns over time. J Natl Cancer Inst. 2009;101:1553–1561. doi: 10.1093/jnci/djp361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakui M, Shiigai T. Urinary tract cancer screening through analysis of urinary red blood cell volume distribution. Int J Urol. 2000;7:248–253. doi: 10.1046/j.1442-2042.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- 9.Shelley MD, Mason MD, Kynaston H. Intravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta-analyses. Cancer Treat Rev. 2010;36:195–205. doi: 10.1016/j.ctrv.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Heney NM, Ahmed S, Flanagan MJ, Frable W, Corder MP, Hafermann MD, Hawkins IR. Superficial bladder cancer: progression and recurrence. J Urol. 1983;130:1083–1086. doi: 10.1016/s0022-5347(17)51695-x. [DOI] [PubMed] [Google Scholar]
- 11.Byar D, Blackard C. Comparisons of placebo, pyridoxine and topical thiotepa in preventing recurrence of stage I bladder cancer. Urology. 1977;10:556–561. doi: 10.1016/0090-4295(77)90101-7. [DOI] [PubMed] [Google Scholar]
- 12.Huland H, Otto U. Mitomycin instillation to prevent recurrence of superficial bladder carcinoma. Results of a controlled, prospective study in 58 Patients. Eur Urol. 1983;9:84–86. doi: 10.1159/000474054. [DOI] [PubMed] [Google Scholar]
- 13.Niijima T, Koiso K, Akaza H. Randomized clinical trial on chemoprophylaxis of recurrence in cases of superficial bladder cancer. Cancer Chemother Pharmacol. 1983;11(Suppl):S79–82. doi: 10.1007/BF00256725. [DOI] [PubMed] [Google Scholar]
- 14.Lamm DL, Riggs DR, Traynelis CL, Nseyo UO. Apparent failure of current intravesical chemotherapy prophylaxis to influence the long-term course of superficial transitional cell carcinoma of the bladder. J Urol. 1995;153:1444–1450. [PubMed] [Google Scholar]
- 15.Morales A, Eidinger D, Bruce AW. Intracavity Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 16.Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette-Guerin immunotherapy of superficial bladder cancer. J Urol. 1980;124:38–40. doi: 10.1016/s0022-5347(17)55282-9. [DOI] [PubMed] [Google Scholar]
- 17.Lamm DL, Blumenstein BA, Crawford ED, Montie JE, Scardino P, Grossman HB, Stanisic TH, Smith JA, Jr, Sullivan J, Sarosdy MF. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325:1205–1209. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- 18.Fox CH, Eberl M. Phytic acid (IP6), novel broad spectrum anti-neoplastic agent: a systematic review. Complement Ther Med. 2002;10:229–234. doi: 10.1016/s0965-2299(02)00092-4. [DOI] [PubMed] [Google Scholar]
- 19.Vucenik I, Shamsuddin AM. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J Nutr. 2003;133(11 Suppl 1):3778S–3784S. doi: 10.1093/jn/133.11.3778S. [DOI] [PubMed] [Google Scholar]
- 20.Vucenik I, Shamsuddin AM. Protection against cancer by dietary IP6 and inositol. Nutr Cancer. 2006;55:109–125. doi: 10.1207/s15327914nc5502_1. [DOI] [PubMed] [Google Scholar]
- 21.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 22.Vucenik I, Passaniti A, Vitolo MI, Tantivejkul K, Eggleton P, Shamsuddin AM. Antiangiogenic activity of inositol hexaphosphate (IP6) Carcinogenesis. 2004;25:2115–2123. doi: 10.1093/carcin/bgh232. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol. 1998;25:1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 25.Dixon WJ, Massey FJ. Introduction to statistical analysis. ed 4. New York: McGraw-Hill; 1983. [Google Scholar]
- 26.Matejuk A, Shamsuddin A. IP6 in cancer therapy: past, present and future. Curr Cancer Ther Rev. 2010;6:1–12. [Google Scholar]
- 27.Shamsuddin AM, Yang GY, Vucenik I. Novel anti-cancer functions of IP6: growth inhibition and differentiation of human mammary cancer cell lines in vitro. Anticancer Res. 1996;16:3287–3292. [PubMed] [Google Scholar]
- 28.Shamsuddin AM, Vucenik I. Mammary tumor inhibition by IP6: a review. Anticancer Res. 1999;19:3671–3674. [PubMed] [Google Scholar]
- 29.Jenab M, Thompson LU. Phytic acid in wheat bran affects colon morphology, cell differentiation and apoptosis. Carcinogenesis. 2000;21:1547–1552. [PubMed] [Google Scholar]
- 30.Vucenik I, Zhang ZS, Shamsuddin AM. IP6 treatment of liver cancer. II. Intra-tumoral injection of IP6 regresses pre-existing human liver cancer xenotransplanted in nude mice. Anticancer Res. 1998;18:4091–4096. [PubMed] [Google Scholar]
- 31.Shamsuddin AM, Yang GY. Inositol hexaphosphate inhibits growth and induces differentiation of PC-3 human prostate cancer cells. Carcinogenesis. 1995;16:1975–1979. doi: 10.1093/carcin/16.8.1975. [DOI] [PubMed] [Google Scholar]
- 32.Singh RP, Agarwal C, Agarwal R. Inositol hexaphosphate inhibits growth, and induces G1 arrest and apoptotic death of prostate carcinoma DU145 cells: modulation of CDKICDK-cyclin and pRb-related protein-E2F complexes. Carcinogenesis. 2003;24:555–563. doi: 10.1093/carcin/24.3.555. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal C, Dhanalakshmi S, Singh RP, Agarwal R. Inositol hexaphosphate inhibits growth and induces G1 arrest and apoptotic death of androgen-dependent human prostate carcinoma LNCaP cells. Neoplasia. 2004;6:646–659. doi: 10.1593/neo.04232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMillan B, Riggs DR, Jackson BJ, Cunningham C, McFadden DW. Dietary influence on pancreatic cancer growth by catechin and inositol hexaphosphate. J Surg Res. 2007;141:115–119. doi: 10.1016/j.jss.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 35.Rizvi I, Riggs DR, Jackson BJ, Ng A, Cunningham C, McFadden DW. Inositol hexaphosphate (IP6) inhibits cellular proliferation in melanoma. J Surg Res. 2006;133:3–6. doi: 10.1016/j.jss.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 36.McFadden DW, Riggs DR, Jackson BJ, Cunningham C. Corn-derived carbohydrate inositol hexaphosphate inhibits Barrett's adenocarcinoma growth by pro-apoptotic mechanisms. Oncol Rep. 2008;19:563–566. [PubMed] [Google Scholar]
- 37.Deliliers GL, Servida F, Fracchiolla NS, Ricci C, Borsotti C, Colombo G, Soligo D. Effect of inositol hexaphosphate (IP(6)) on human normal and leukaemic haematopoietic cells. Br J Haematol. 2002;117:577–587. doi: 10.1046/j.1365-2141.2002.03453.x. [DOI] [PubMed] [Google Scholar]
- 38.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]