Abstract
Introduction
To examine the role of inflammation in bladder cancer, we assessed the relationship between a systemic inflammation prognostic score (modified Glasgow Prognostic Score, mGPS), the tumor inflammatory cell infiltrate as measured by the Klintrup-Makinen score and tumor necrosis with cancer specific survival in patients with bladder cancer.
Materials and Methods
The cohort consisted of 68 bladder cancer patients, 47 with localised disease and 21 with muscle invasive disease. The mGPS response was constructed by measuring C-reactive protein and albumin concentrations and the Klintrup-Makinen score was evaluated histologically for the local inflammatory response. Pathological parameters such as grade, T stage and tumor necrosis were also assessed.
Results
Median follow was 47 months and 24 patients died of their disease. On univariate analysis, T stage (p < 0.001), grade (p < 0.001) and mGPS (p = 0.002) were significant predictors of cancer specific survival. On multivariate analysis, T stage (hazard ratio 5.98, 95% confidence interval 3.18–11.24, p < 0.001) and mGPS (hazard ratio 1.78, 95% confidence interval 1.09–2.9, p = 0.02) were significant independent predictors of cancer specific survival.
Conclusion
A preoperative systemic inflammatory response is an independent predictor of poor cancer specific survival in patients with bladder cancer.
Key Words: Bladder cancer, C-reactive protein, Inflammation, Glasgow prognostic score, Klintrup-Makinen score
Introduction
Worldwide there are approximately 360,000 new cases of bladder cancer diagnosed each year. In the UK alone, bladder cancer is the fifth most common solid non-cutaneous malignancy with approximately 10,000 new cases and 5,000 deaths annually. Overall survival is variable as only half of patients diagnosed survive 5 years [1].
There has been a long standing interest in identifying those patients most at risk of disease progression and ultimately dying from their disease. Ideally, a factor or combination of factors that could clearly stratify patients into those who do not progress and those that progress and are at higher risk of dying from their cancer would be highly beneficial. Currently, the TNM stage and tumor grade are the most widely used tools to predict survival but could be improved upon.
It is now established that disease progression in cancer patients is not solely determined by the tumor characteristics but also by the host response. There is increasing evidence that both local and systemic inflammatory responses play an important role in the progression of various solid tumors [2, 3].
Recent evidence suggests that intensity of local inflammatory infiltrate within the tumor bed predicts prognosis: a pronounced lymphocytic infiltration in colorectal cancer is associated with improved survival [4, 5, 6]. Also, quantifying the degree of infiltration by lymphocyte subsets such as CD8+ and CD4+ T cells provides prognostic information in a various tumor types [6, 7, 8] including bladder cancer [9]. The process of assessing lymphocyte infiltration is time consuming and has not been adopted into routine clinical practice. It is therefore of interest that Klintrup et al. [10] have reported a simplified method of assessing the inflammatory cell infiltrate at the tumor margin shown on routine hematoxylin and eosin stained sections, that tumor inflammatory infiltrate, including all white cell types, can be graded high or low grade, with a high grade infiltrate being associated with improved survival in colorectal cancer.
In addition, increasing evidence supports a role of the systemic inflammatory response, indicated by elevated levels of C-reactive protein (CRP) being an independent predictor of survival in patients with a variety of common solid tumors including gastrointestinal, lung, renal, prostate and bladder cancers [11, 12, 13, 14, 15, 16]. A retrospective analysis of patients undergoing radical cystectomy has demonstrated that CRP in combination with other pathological parameters offers additional prognostic information in these patients but some of these parameters are collated post procedure [17]. The modified Glasgow Prognostic Score (mGPS), incorporates CRP and albumin serum levels preoperatively [18]. mGPS score provides additional prognostic information in patients with various solid malignancies including lung, gastroesophageal, renal and colorectal cancers [19, 20, 21, 22].
The prognostic role of tumor necrosis is well established in malignancies such as renal, breast, lung and colorectal cancers [23, 24, 25, 26]. However, there are few reports on the prognostic role of necrosis in bladder cancer.
The aim of this study was to assess relationships between a systemic inflammation prognostic score (mGPS), the local tumor inflammatory cell infiltrates, tumor necrosis and cancer specific survival in patients with bladder cancer.
Materials and Methods
Patients with bladder cancer diagnosed in the North Glasgow NHS Trust were included. Patients were staged pathologically according to the TNM classification and graded according to the World Health Organisation/International Society of Urological Pathology criteria [27]. The Research Ethics Committee of North Glasgow NHS Trust approved the study.
Diagnostic hematoxylin and eosin sections from pathology archives were reviewed. A minimum of 3 slides from the deepest area of tumor invasion were reviewed and were scored according to the Klintrup-Makinen criteria (K/M) [10]. This method is based on scoring inflammation at the deepest point of invasion identified from the 3 slides. A 4-point scale was used. A score of 0 was given for no increase of the inflammatory cells at the invasive margin; a score of 1 denoted a mild and patchy increase of inflammatory cells. Score 2 was assigned when inflammatory cells formed a band-like infiltrate at the invasive margin. A score of 3 was given when a prominent inflammatory reaction formed a cup-like zone at the margin. Scores of 0 and 1 were combined (low grade inflammation) and scores of 2 and 3 combined (high grade inflammation). Scoring was supervised by a pathologist (J.J.G.).
Preoperative systemic inflammatory response was assessed using the mGPS [18]. Patients with both elevated CRP (> 10mg/l) and hypoalbuminemia (< 35g/l) scored 2. Patients in whom both were normal scored 0. Patients with elevated CRP alone were scored as 1 while those with hypoalbuminemia alone were scored as 0.
The presence or absence of tumor necrosis was evaluated on histological sections under the supervision of a pathologist (J.J.G.).
Statistical analysis was undertaken using SPSS. Disease specific survival rates were generated using the Kaplan-Meir method. The log rank test was utilised to compare significant differences between subset groups using univariate analysis. Multivariate analysis was carried out based on the results of the univariate analysis. Multivariate Cox regression analysis was performed to identify factors independently associated with disease specific death. A stepwise backward procedure was utilised to ascertain which of the variables had a significant independent relationship with survival.
Results
Sixty-eight patients were studied, 47 with localised disease and 21 with muscle invasive disease. Median age at diagnosis was 72 years (range 43–93 years). Median follow-up was 47 months (range 1.2–201 months). Forty patients had recurrence and of these, 28 patients had evidence of progression and 24 patients died of their disease.
χ2 demonstrated that the mGPS was positively correlated with tumor stage, grade and progression (p = 0.033, 0.007 and 0.033, respectively) (table 1). There was also a positive correlation between necrosis and local inflammatory infiltrate (p = 0.027) (table 1). In a subset analysis looking at localised and muscle invasive cancer disease separately, the only correlation was a negative one with mGPS and tumor necrosis in those with localised disease (p = 0.025). There was no significant difference in mGPS, tumor necrosis and the local inflammatory response when comparing those with muscle invasive and localised disease.
Table 1.
Interrelationships between clinicopathological characteristics of patients with bladder cancer
| Numbers | Sex | Grade | T stage | Tumor necrosis | Local inflammatory cell infiltrate | mGPS | Recurrence | Progression | |
|---|---|---|---|---|---|---|---|---|---|
| Age (>65/<65) | 25/43 | 0.037 | 0.223 | 0.037 | 0.962 | 0.82 | 0.775 | 0.17 | 0.095 |
| Sex (male/female) | 46/22 | 0.546 | 0.593 | 0.539 | 0.147 | 0.653 | 0.975 | 0.975 | |
| Grade (1/2/3) | 15/28/25 | <0.001 | 0.445 | 0.207 | 0.007 | 0.021 | <0.001 | ||
| T stage (Ta/T1/T2/T3/T4) | 34/13/20/1/0 | 0.376 | 0.311 | 0.033 | <0.001 | <0.001 | |||
| Tumour Necrosis (absence/presence) | 22/46 | 0.027 | 0.212 | 0.31 | 0.11 | ||||
| Local inflammatory cell infiltrate (low/high) | 59/9 | 0.444 | 0.61 | 0.098 | |||||
| mGPS (0/1/2) | 44/13/11 | 0.436 | 0.033 | ||||||
| Recurrence (no/yes) | 28/40 | <0.001 | |||||||
| Progression (no/yes) | 40/28 |
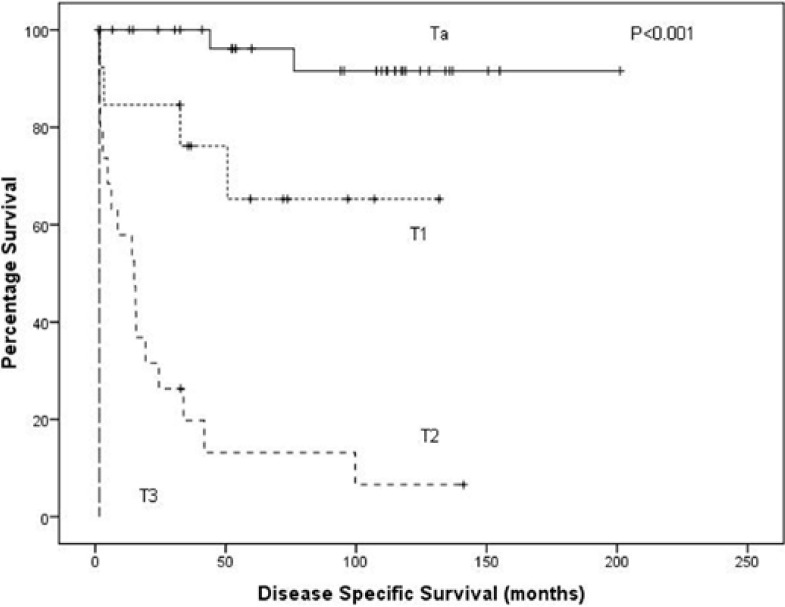
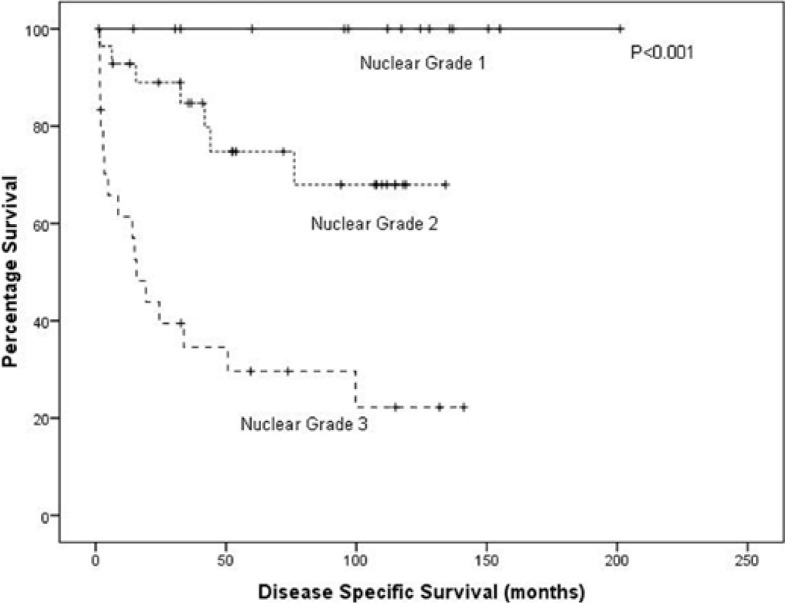
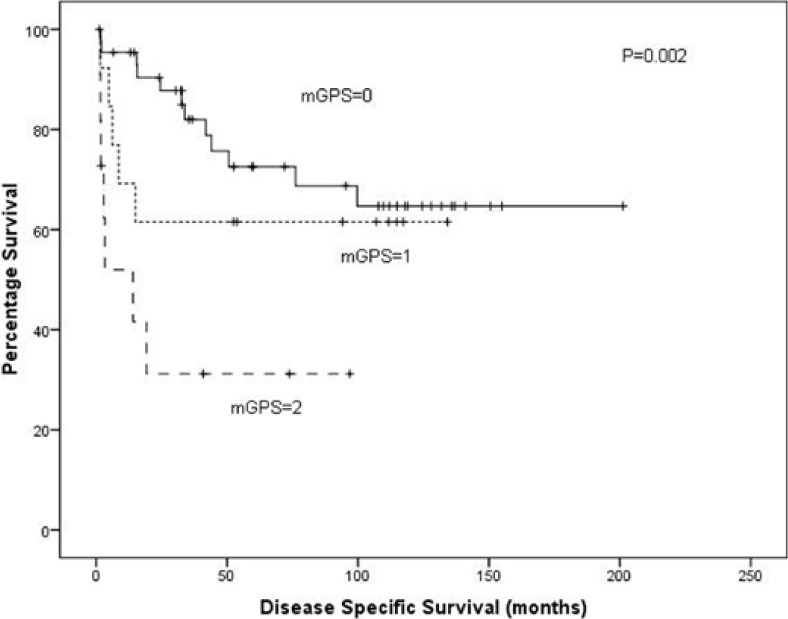
On univariate analysis, T stage (p < 0.001, fig. 1), grade (p < 0.001, fig. 2) and mGPS (p = 0.002, fig. 3) were significant predictors of cancer specific survival whilst necrosis and the local inflammatory response did not show significance (p = 0.286 and 0.253, respectively) (table 2). On multivariate analysis of the significant individual covariates T stage (hazard ratio 5.98, 95% confidence interval 3.18–11.24, p < 0.001) and mGPS (hazard ratio 1.78, 95% confidence interval 1.09–2.90, p = 0.02) were significant independent predictors of cancer specific survival.
Fig. 1.
A Kaplan-Meier plotted for the T stage against disease specific survival (log rank, p < 0.001).
Fig. 2.
A Kaplan-Meier plotted for the nuclear grade against disease specific survival (log rank, p < 0.001).
Fig. 3.
A Kaplan-Meier plotted for the mGPS score against disease specific survival (log rank p = 0.002).
Table 2.
The relationship between clinicopathological characteristics, tumou necrosis, local inflammatory response, systemic inflammatory response and cancer specific survival in patients undergoing treatment for bladder cancer
| Numbers | Univariate analysis, p | Multivariate analysis, p | Multivariate analysis, HR | |
|---|---|---|---|---|
| Age (<65/>65) | 25/43 | 0.024 | ||
| Sex (male/female) | 46/22 | 0.756 | ||
| Grade (1/2/3) | 15/28/25 | <0.001 | ||
| T stage (Ta/T1/T2/T3/T4) | 34/13/20/1/0 | <0.001 | <0.001 | 5.98 (3.18–11.24) |
| Tumor Necrosis (absence/presence) | 22/46 | 0.286 | ||
| Local inflammatory cell infiltrate (low/high) | 59/9 | 0.253 | ||
| mGPS (0/1/2) | 44/13/11 | 0.002 | 0.02 | 1.78 (1.09–2.90) |
Discussion
Results from the present study demonstrate, for the first time to our knowledge that an elevated mGPS independently correlates to a poor cancer specific survival in those with bladder cancer. We have also demonstrated that an elevated mGPS is directly associated with tumor stage, grade and progression.
Previous studies have demonstrated that tumor necrosis [23, 24, 25, 26] and the local inflammatory response [10, 11] play a prognostic role in various malignancies. It has also been reported that quantifying the degree of infiltration by lymphocyte subsets provides prognostic information in bladder cancer [9]. It was therefore of interest that a simplified assessment of the local inflammatory cell infiltrate [10] which is not as time consuming as quantifying lymphocyte subsets has been shown to prognostic in colorectal cancer [10, 11]. In this study, there was a positive correlation with necrosis and the local inflammatory infiltrate but neither of these pathological parameters showed any significance to cancer specific survival, this could reflect the number of patients in this cohort.
The mGPS utilises objective, well established serum based variables to predict cancer specific survival in patients undergoing treatment for bladder cancer. The variables used are common ones and offer the benefit of being objective and obtainable. These results support the evaluation and introduction of the inflammation based (mGPS) scoring system as an independent predictor of poor cancer specific survival in bladder cancer. Limitations of this study include patient numbers. Given the different prognosis between non-muscle invasive disease and muscle invasive disease, ideally these should be analysed separately, but this is not possible given the low numbers in each group.
Further work is required to see if the mGPS can be used as a guide for adjuvant treatment in bladder cancer.
Acknowledgments
This work was supported by Think Pink Endowment Fund.
References
- 1.www.cancerresearchuk.org 5-6-0011.
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 4.Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987;1(8545):1303–1306. doi: 10.1016/s0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487–495. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 8.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 9.Hilmy M, Campbell R, Bartlett JM, McNicol AM, Underwood MA, McMillan DC. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic infiltration and COX-2 expression and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer. 2006;95:1234–1238. doi: 10.1038/sj.bjc.6603415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klintrup K, Makinen JM, Kauppila S, Vare PO, Melkko J, Tuominen H, Tuppurainen K, Makela J, Karttunen TJ, Makinen MJ. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645–2654. doi: 10.1016/j.ejca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg. 2009;249:788–793. doi: 10.1097/SLA.0b013e3181a3e738. [DOI] [PubMed] [Google Scholar]
- 12.Hilmy M, Bartlett JM, Underwood MA, McMillan DC. The relationship between the systemic inflammatory response and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer. 2005;92:625–627. doi: 10.1038/sj.bjc.6602406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamb GW, McArdle PA, Ramsey S, Mc-Nichol AM, Edwards J, Aitchison M, McMillan DC. The relationship between the local and systemic inflammatory responses and survival in patients undergoing resection for localized renal cancer. BJU Int. 2008;102:756–761. doi: 10.1111/j.1464-410X.2008.07666.x. [DOI] [PubMed] [Google Scholar]
- 14.McArdle PA, Qayyum T, McMillan DC. Systemic inflammatory response and survival in patients with localised prostate cancer: 10-year follow-up. Urol Int. 2010;84:430–435. doi: 10.1159/000313364. [DOI] [PubMed] [Google Scholar]
- 15.O'Gorman P, McMillan DC, McArdle CS. Prognostic factors in advanced gastrointestinal cancer patients with weight loss. Nutr Cancer. 2000;37:36–40. doi: 10.1207/S15327914NC3701_4. [DOI] [PubMed] [Google Scholar]
- 16.Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87:264–267. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gakis G, Todenhofer T, Renninger M, Schilling D, Sievert KD, Schwentner C, Stenzl A. Development of a new outcome prediction model in carcinoma invading the bladder based on preoperative serum C-reactive protein and standard pathological risk factors: the TNR-C score. BJU Int. 2011;108:1800–1805. doi: 10.1111/j.1464-410X.2011.10234.x. [DOI] [PubMed] [Google Scholar]
- 18.McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67:257–262. doi: 10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- 19.Crumley AB, McMillan DC, McKernan M, McDonald AC, Stuart RC. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer. 2006;94:637–641. doi: 10.1038/sj.bjc.6602998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 22.Qayyum T, McArdle PA, Lamb GW, Going JJ, Orange C, Seywright M, Horgan PG, Oades G, Aitchison MA, Edwards J. Prospective study of the role of inflammation in renal cancer. Urol Int. 2012;88:277–281. doi: 10.1159/000334971. [DOI] [PubMed] [Google Scholar]
- 23.Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79:991–995. doi: 10.1038/sj.bjc.6690158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollheimer MJ, Kornprat P, Lindtner RA, Harbaum L, Schlemmer A, Rehak P, Langner C. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol. 2010;41:1749–1757. doi: 10.1016/j.humpath.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta S, Lohse CM, Leibovich BC, Frank I, Thompson RH, Webster WS, Zincke H, Blute ML, Cheville JC, Kwon ED. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;104:511–520. doi: 10.1002/cncr.21206. [DOI] [PubMed] [Google Scholar]
- 26.Swinson DE, Jones JL, Richardson D, Cox G, Edwards JG, O'Byrne KJ. Tumour necrosis is an independent prognostic marker in non-small cell lung cancer: correlation with biological variables. Lung Cancer. 2002;37:235–240. doi: 10.1016/s0169-5002(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 27.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]