Abstract
Background:
Tumor immunology research has led to the identification of a number of tumor-associated self antigens, suggesting that most tumors trigger an immunogenic response, as is the case in osteosarcoma, where the detection of natural serum IgM antibodies might achieve the diagnosis of osteosarcoma. Natural IgM antibodies to tumor-associated proteins may expand the number of available tumor biomarkers for osteosarcoma and may be used together in a serum profile to enhance test sensitivity and specificity. Natural IgM antibodies can be consistently detected in the peripheral blood sera months to years before the tumor is diagnosed clinically. The study of the level of a potential biomarker many months (or years) prior to diagnosis is fundamentally important. Integrated circulating and imaging markers in clinical practice treating osteosarcoma have potential applications for controlling tumor angiogenesis.
Objectives:
To study the expression of natural IgM antibodies to the tumor antigens of angiogenesis in the peripheral blood sera of osteosarcoma patients and healthy individuals, and to develop serum-based predictive biomarkers.
Methods:
Peripheral venous blood samples were collected from 117 osteosarcoma patients and 117 patients with other tumors. All diagnosis was histologically confirmed. Staging of patients was performed according to the Enneking Surgical Staging System. The control group consisted of 117 age- and sex- matched healthy individuals. In this study, novel immunoconjugates were designed, synthesized and then used to develop a rapid, specific and sensitive enzyme-linked immunosorbent assay (ELISA) method to detect angiogenin (ANG)–IgM directly in the peripheral blood sera of humans.
Results:
Serum ANG–IgM levels are significantly higher in osteosarcoma patients than in healthy individuals (P < 0.005). Serum ANG–IgM levels varied widely, but were highly dependent on the concentration of IgM (r = 0.85; P < 0.0005). We found ANG–IgM in the sera of 85% of newly diagnosed osteosarcoma patients and ANG–IgM levels were significantly higher in osteosarcoma patients compared to any other tumors (P < 0.001).
Conclusions:
These results demonstrated that the combined biomarker ANG–IgM has greater sensitivity and specificity in early diagnosis of osteosarcoma patients than the traditional biomarkers (ANG and vascular endothelial growth factor). Circulating ANG–IgM immune complexes can potentially serve as a biomarker for increased risk of osteosarcoma, because relatively high serum levels were also detected in otherwise healthy individuals with a first degree family history of osteosarcoma and in patients with a diagnosis of benign conditions. Immunological aspects of angiogenesis for managing osteosarcoma will have a practical value in early diagnosis, prognosis and monitoring response to antiangiogenic therapy.
Keywords: osteosarcoma, tumor angiogenesis, angiogenin, immunoglobulin M immune complexes, multivalent IgM, cancer yearly diagnosis, natural immunity, serum biomarkers, ELISA, serology
Introduction
Osteosarcoma is a primary malignant tumour of the skeleton characterized by the direct formation of immature bone or osteoid tissue by the tumor cells1 (Fig. 1). More rarely osteosarcoma may arise in the soft tissue.2 World Health Organization (WHO) histologic classification of bone tumors divides osteosarcoma into central and surface tumors, and recognizes a number of subtypes within each group. Classic osteosarcoma represents approximately 15% of all biopsy-analysed primary bone tumors. Among primary malignant bone tumors, osteosarcoma ranks second in frequency after multiple myelomas. The incidence of classic osteosarcoma is 3 cases/million population/year. It represents 0.2% of all malignant tumors. Further reduction in the mortality will require successful strategies for the early detection and screening of osteosarcoma.
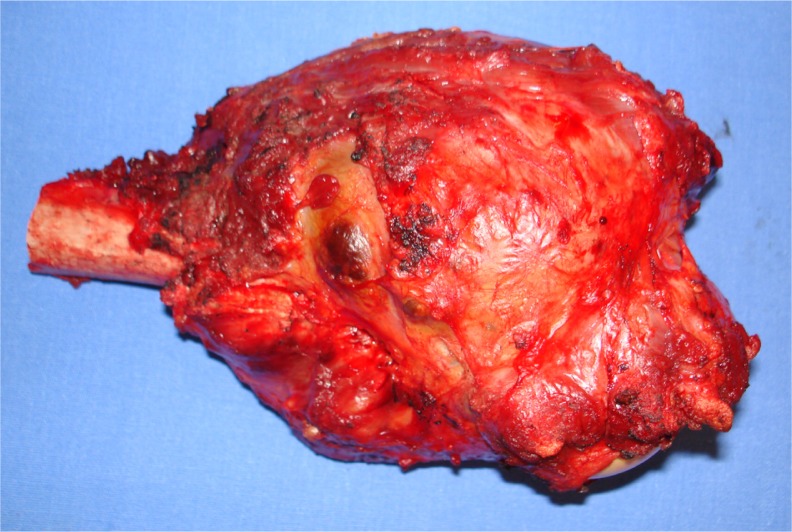
Figure 1.
Photograph of osteosarcoma after surgical removal.
Continuing advances in tumor angiogenesis have opened up new potential methods to help determine the prognosis and prediction of response in many solid tumor types, including osteosarcoma. Traditionally, the stage of the disease at clinical presentation and patient characteristics (ie, performance status, symptom severity, age and tumor size) were the major determinants of disease prognosis and treatment strategy. While these traditional factors continue to play important roles in treatment choice and outcomes, significant strides have been made in the evaluation and correlation of circulating and imaging biomarkers as potential tools in diagnosis, prognosis, treatment strategy and treatment evaluation. This article will provide a brief overview of the role of the biomarkers involved in tumor angiogenesis and of their value in clinical research, and will examine how these circulating and imaging biomarkers may eventually influence routine clinical practice. The methodology of evaluation is more controversial than the markers for early detection of osteosarcoma.
The results of imaging tests can be viewed as markers for early detection. Because they involve a subjective component, the method of evaluation differs from that of circulating markers. Advances in imaging are transforming our understanding of tumor angiogenesis of osteosarcoma3–5 (Fig. 2). Vascular imaging makes it possible to quantify the number and spacing of blood vessels, measure blood flow and vascular permeability, and analyze cellular and molecular abnormalities in blood vessel walls.6,7 An examples of an imaging marker for the early detection of osteosarcoma is low-dose computed tomography angiography (CTA). Imaging angiogenesis in osteosarcoma is useful for clarificying the structural and functional abnormalities of angiogenic blood vessels.
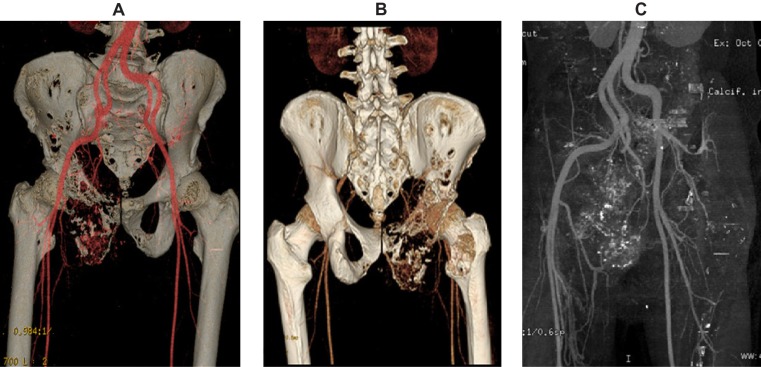
Figure 2.
Example of osteosarcoma reconstruction by volume-rendered 3D CTA image in anatomic orientation. A) This volume-rendered 3D CTA image, in anteroposterior projection, demonstrates the vascularity of the pelvic region, especially near the lytic lesion of the right pelvic bones. B) This volume-rendered 3D CTA image, in posteroanterior projection, demonstrates the vascularity of the pelvic region, especially near the lytic lesion of the right pelvic bones. C) Reconstruction of osteosarcoma by 3D CTA with bone sustraction showing the arterial vascularity of pelvic region.
Notes: CTA demonstrates a high sensivity and specificity, and positive predictive value for evaluating osteosarcoma. CTA imaging shows the adjacent blood vessels and clearer details of the extent of bone destruction. Being able to see the relationship of these structures to the tumor is very important in planning the appropriate surgery for removal of osteosarcoma tumors.
A sensitive assay to identify serological markers that can accurately determine the onset of osteosarcoma—especially if the technique is of low risk to the patient, such as taking a blood sample—is ideal for the early detection of cancer. Immunoassays are of general interest for all proteomic and diagnostic approaches in which several parameters have to be determined simultaneously from a limited amount of sample material. The Human Proteome Organization states that blood still represents an ideal clinical source of biomarkers because of its minimally invasive and standardized acquisition methods, and because of its known role in reflecting systemic changes associated with disease. Improved analytical methods are required to accommodate the analysis of large numbers of samples for biological and epidemiological monitoring.8,9 Enzyme-linked immunosorbent assays (ELISAs), which have been used widely since the 1970s for clinical analyses10,11 and, more recently, for environmental analyses,12,13 have been developed for several biomarkers of exposure found in human blood. ELISA is a sensitive, high-throughput technique that quantitatively measures the amount of analyte present in a physiological fluid such as serum. The question remains whether ELISAs should be introduced as routine. We examined this clinically important question by studying a large number of the sera from patients with established osteosarcoma using a simple ELISA assay system.
Tumor angiogenesis is one of the most important hallmarks of cancer, which enables its development, progression and metastasis.14 Osteosarcoma is a heterogeneous disease with tumors that express a variety of aberrant proteins of angiogenesis.15–17 Current blood tests that identify circulating tumor antigens associated with angiogenesis are elevated most commonly in patients with metastatic disease and appear to reflect tumor bulk. They are too insensitive to be used for screening and early diagnosis of primary osteosarcoma.18 Angiogenesis analysis of peripheral blood serum shows the presence of biomarkers that characterize cancer. Proangiogenic factors are found in the serum osteosarcoma patients, ie, vascular endothelial growth factor (VEGF), placental growth factor (PLGF), basic fibroblast growth factor (bFGF) and angiogenin (ANG).7,18–20 Elevated levels of serum ANG, VEGF, PLGF and bFGF are also valuable diagnostic parameters.7,21,22 However, because of the difficulties in general standardization, this parameter may be difficult to interpret in younger patients.23–27
ANG is the unique factor for all mammals.28,29 Human ANG is a single chain, non-glycosylated polypeptide of 14.4 kDa.30,31 ANG shows 33% homology to ribonuclease A. The homology of human and bovine ANG is 65% at the protein level. The ANG gene has been called RNASE5 (ribonuclease A family 5). ANG is homologous with pancreatic ribonuclease (RNASE1) and yeast RNASE1. The understanding that the growth of tumors is dependent on angiogenesis has led to the development of new approaches to treatment and new agents directed at tumor vasculature.7,32
The expression of ANG is currently regarded as the major proangiogenic factor for most types of human cancer. ANG displays multiple physiological and pathological functions by targeting both vascular and non-vascular systems.6,33,34 In contrast to the detection of serum tumor angiogenic antigens, the detection of natural serum immunoglobin M (IgM) antibodies to tumor-associated antigen may provide reliable markers for osteosarcoma diagnosis and prognosis.20,35–38 Natural IgM antibodies to tumor-associated antigens circulate in the blood much earlier than serum antigen.39–41 Natural IgM antibodies produced against such tumor-associated antigens of angiogenesis may provide an in vivo amplification of an early carcinogenic signal and therefore may allow earlier detection of cancer than current methods allow.42 Natural IgM antibodies to tumor antigens have been reported in patients with early-stage cancer, and a panel of serum antibodies can detect cancer many years prior to radiograph detection.4,35,43–45 Early tumor detection is a key to ensure effective treatment. The immune system thus may play a role in preventing osteosarcoma by destroying cancer cells soon after they arise or by destroying viruses that lead to cancer or both. It stands to reason that maintaining a healthy immune system will help prevent cancer.46,47
Natural antibodies of the IgM isotype are predominantly present in healthy individuals. Natural IgM has multiple roles in the immune system. They are key to the homeostasis of the immune system, particularly relating to B lymphocytes and autoimmunity. All the tumor-specific antibodies belong nearly exclusively to the IgM class. It makes sense that anti-tumor immunity seems to be a part of natural immunity, and immune memory is not needed and therefore not induced. The detection of natural IgM antibodies against tumor-specific antigens of angiogenesis, such as ANG in osteosarcoma, has raised the possibility of an auto-immune aspect to this disease.43,48,49
Investigators in the European Organization for Research and Treatment of Cancer have been studying marker–IgM immune complexes, which play a role in diagnosis and prognosis in cancer. We have recently discovered the occurrence of cancer markers associated with IgM in liver and colorectal cancer, and we have demonstrated that marker–IgM immune complexes are a novel class of tumor markers with a greater diagnostic potential compared to the corresponding free biomarker (Fig. 3). When detected as IgM complexes, both alpha-fetoprotein (AFP; the conventional serological marker for the detection of hepatocellular carcinoma (HCC)) and squamous cell carcinoma antigen (SCCA; a novel HCC biomarker),50 improved our diagnosis of liver cancer.50–52 Further evidence of the diagnostic relevance of IgM immune complexes in cancer was also provided by carcinoembryonic antigen (CEA), which is the serological gold standard for the diagnosis of colorectal cancer (CRC).53 The assessment of CEA–IgM levels in CRC patients allowed the identification of a much higher number of patients compared to free CEA, and co-determination of both biomarkers increased sensitivity without compromising assay specificity.53
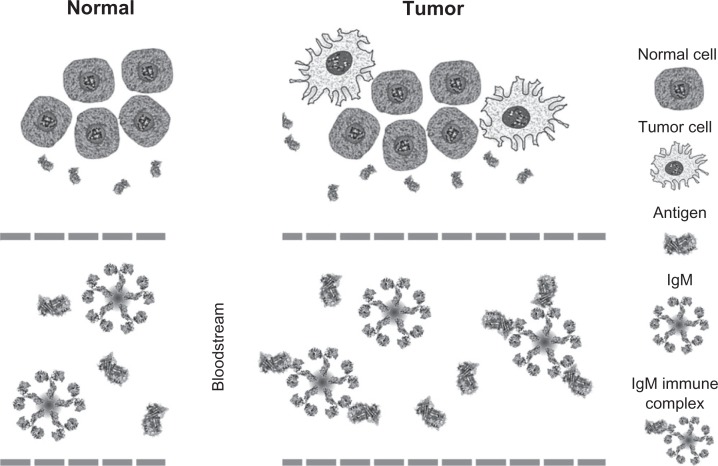
Figure 3.
Schematic illustration of the immune response to cancer and development of IgM immune complexes in the bloodstream.
To our knowledge, no investigations have been reported on the occurrence of ANG–IgM immune complexes in the serum of patients with osteosarcoma. It has been established that natural IgM antibodies are useful serological markers in the diagnosis of systemic autoimmune diseases54,55,66 and that their diagnostic value is related to their immunologic specificity. Since natural IgM antibodies are prevalent in human sera and are part of the normal immune response,26,56 one important problem that is inherent to IgM-based methods for identifying tumor-related antigens is demonstrating their tumor relevance.27 Natural IgM antibodies to angiogenic proteins encoded by oncogenes and tumor suppressor have been thought to represent prime candidates for biomarkers for early diagnosis of osteosarcoma. Numerous angiogenic antigens found in the sera of osteosarcoma patients have been proposed as diagnostic or prognostic markers of the disease.57–62 More recently, high throughput technologies have made major contributions to the study of self-antigen–antibody systems as serologic biomarkers in osteosarcoma.10,11
In this study, we have used a novel ELISA to evaluate the presence of circulating ANG–IgM immune complexes in the peripheral blood sera of patients with osteosarcoma and healthy individuals, and also to evaluate the usefulness of ANG–IgM detection for an early diagnosis of osteosarcoma.
Materials and Methods
Patients
The study included 117 patients with newly diagnosed osteosarcoma in the Department of Bone Tumors of the National Institute of Rehabilitation, Mexico City, Mexico between January 2007 and September 2009; their mean age was 23.8 years (range: 4–74 years), and 76 men and 41 women took part. Tumor staging was based on radiography, computed tomography, operative findings and pathology reports in accordance with the Enneking Surgical Staging System. All patients had histopathologic confirmation of osteosarcoma according the WHO classification. The median follow-up time was 43 months (range: 24–72 months). Individual patient records were traced where possible.
The comparative group consisted of 117 patients with other tumors (osteocondroma, fibrous displasia, encondroma, condroblastoma, Ewing’s tumor, giant cell bone tumor, desmoplastic fibroma, chondromyxoid fibroma, simple bone cyst, aneurysmal bone cyst, Langerhans’ cell histiocytosis) matched for age (±5 years), gender and ethnicity in the Department of Bone Tumors of National Institute of Rehabilitation, Mexico City, Mexico.
Control subjects
The general reference (normal) control samples consisted of 117 healthy individuals (75 men and 42 women with a mean age of 25.2 years; range: 5–73 years) in the Blood Transfusion Service of the National Institute of Rehabilitation. The absence of disease was confirmed by physical examination, clinical history and routine laboratory tests.
Blood sampling
Seven milliliters of the peripheral venous blood was drawn into a serum separator tube (Vacutainer Systems, code 607213, Becton-Dickinson, USA). Blood was allowed to clot for 1 h at room temperature (RT). Sera was obtained after centrifugation at 3000 r.p.m. for 10 min at 4 °C. All serum samples were stored in 300 μL aliquots at −80 °C until analysis. Peripheral venous blood samples were collected from cancer patients before or after surgery, and during antiangiogenic therapy.
Reagents
All reagents were of analytical grade and were obtained from Sigma–Aldrich Ltd, Poole, UK, unless otherwise indicated.
Gel-filtration
Serum samples from 117 patients with biopsy-proven osteosarcoma were grouped and subjected to gelfiltration analysis. One hundred microliters of pooled sera were analyzed as previously described. The presence of ANG–IgM and ANG in the fractions collected from the gel-filtration column every 30s were tested by ELISA.
ANG–IgM assay
In this study, novel immunoconjugates were designed, synthesized and then used to develop a rapid, specific and sensitive ELISA method to detect ANG–IgM directly in the peripheral blood sera of humans.
Human ANG was coupled with high molecular weight matrix (HMWM; polyphenylacrilate) according to “in-house” protocols provided by Tissue Engineering, Cell Therapy and Regenerative Medicine Unit (National Institute of Rehabilitation). Briefly, 4 mg of HMWM was coupled with 4 mg of ANG in 0.1 M buffer, pH 4.5, containing 2 mg of 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) in a final volume of 1.6 mL. The mixture was incubated for 2 h at RT. The conjugated ANG–HMWM was then dialyzed against phosphate buffered saline (PBS), pH 7.4, at 4 °C. Polystyrene microtiter ELISA plates with 96 wells (Maxi-sorb, NUNC, Rochester, NY, USA) were incubated overnight at 4 °C with ANG–HMWM (1 μg/mL) in 0.1 M carbonate/bicarbonate buffer, pH 9.6. The final volume of this (as well as of all other steps) was 100 μL per well, unless stated otherwise. After washing the plates twice with PBS, residual binding sites were blocked (1 h at RT) with 200 μL per well of PBS containing 2% w/v human serum albumin (HAS). Human sera were appropriately diluted in assay buffer (veronal buffer containing 0.1% w/v HSA, 2 mM CaCl2 and 0.1% w/v Tween-20; pH 7.4), and incubated for 1 h at RT. After this and the subsequent incubation steps, the plates were washed with PBS containing 0.1% w/v Tween-20 (PBST). IgM bound to ANG–HWMM was quantified with horseradish peroxidase-labeled anti-human IgM (IgM-HPR) diluted in assay buffer. Finally, horseradish peroxidase activity was visualized by incubation with 100 μg/mL 3.3′,5.5′-tetra-methylbenzidine (TMB) in 0.11 M sodium acetate, pH 5.5, containing 0.003% v/v H2O2. The reaction was stopped after 10 min by addition of 2 M H2SO4, and the absorbance at 450 nm was measured in a microtiter plate reader (Bio-Kinetics Reader; Bio-Tek Instruments, Winooski, VT, USA). Tests were performed in duplicate. All measurement (patients and control subjects) were made on the same day and under the same experimental conditions.
Dilutions of a pool of normal sera obtained from 117 healthy volunteers were used to generate a standard curve in each microtiter plate. This standard was arbitrarily proposed to contain ANG–IgM. Results with serum samples were related to this standard and expressed as ANG–IgM. The specificity of the binding of ANG–IgM to ANG–HWMM was determined by competition immunoassay. The standard curve was pre-incubated with increasing amounts of the competitors (VEGF, bFGF and PLGF). After 1 h incubation, the standard, with or without competitors, was added to the ANG–HWMM-coated plates and tested as described above.
ANG detection
The ANG levels of gel-filtration fractions were determined as follows: 96-well ELISA plates were coated with 1 mg of goat anti-human ANG antibody in 100 mL of PBS (pH 7.2) per well at 4 °C over-night and then blocked for 2 h with 3% bovine serum albumin (BSA) in PBS. After blocking, 100 mL of ANG gel-filtration fractions containing 1% HSA and 0.05% Tween 20 were incubated for 1 h at RT. The wells were then washed with PBS containing 0.05% Tween 20 and incubated with 100 mL of rabbit anti-human ANG diluted in PBST at a final concentration of 10 mg/mL. ANG was revealed using peroxidase-conjugated goat anti-rabbit IgG (Sigma–Aldrich) and developed with 2,20-azino-bis(3-ethylbenzothi-azoline-6-sulfonic acid) (ABTS) (Sigma–Aldrich) and hydrogen peroxide as the substrate. The results of ANG determination in the gel-filtration fractions were reported in optical density (OD).
Purification of human ANG–IgM
Serum samples donated by normal human volunteers were collected through the Blood Transfusion Service of National Institute of Rehabilitation. Human IgM was purified in an IgM affinity column (HiTrap IgM Purification HP, Amersham Pharmacia Biotech, NJ, USA) and AKTAFPLC (Amersham Pharmacia Biotech). Procedures were carried out according to the manufacturer’s instructions. Briefly, serum samples were first prepared with ammonium sulfate until the final concentration was 0.8 M. The prepared sera were applied to the affinity column which had been pre-calibrated. IgM affinity binding buffer (20 nM sodium phosphate and 0.8 M (NH4)2SO4, pH 7.5) was then applied to wash out unbound factors; bound IgM was eluted by 20 nM sodium phosphate, pH 7.5. The flow rate of the overall purification procedure was 1 mL/min.
Affinity of ANG–IgM
Immunoabsorbent columns were prepared with the antigens of interest coupled with cyanogen bromide-activated Sepharose (Pharmacia Biotech).49 Two milligrams of protein were used for coupling with 1.5 mL CNBr-activated Sepharose. One gram of IVIg in 100 mL of PBS was loaded on the immunoadsorbent columm and run twice on the column at a speed of 1 mL/min at RT, followed by washing with PBS until the absorbance of the flow-through at 280 nm reached baseline values. Bound antibodies were eluted using a glycine–HCl (0.1 M) buffer, pH 2.8, and 2 M NaCl followed by PBS and then diethanolamine (0.1 M) buffer, pH 11, and 2 M NaCl. The eluates obtained at different pH levels were brought to pH 7.0 and pooled. Two milliliters of the flow-through fractions were allowed to run through the sorbents for two more cycles and then used as effluent fractions. Eluates and effluents were dialyzed against PBS.
Quantification of total IgM
Total IgM concentration was determined by nephelometry (Behring Nephelometer Analyzer, Marburg, Germany), according to standard procedures.
Total ANG and VEGF measurement
The concentrations of total ANG and VEGF were measured by using commercially available ELISA kits (Quantikine Human Angiogenic Factor Immunoassay, R&D Systems, Minneapolis, MS, USA) according to the manufacturer’s protocol.
Histology
At the National Institute of Rehabilitation, malignant pathologic study is a routine part of evaluating osteosarcoma. Each fresh biopsy specimen was fixed in formalin, embedded in paraffin and subjected to histological evaluation. Formalin-fixed and paraffin-embedded sections were subjected to histological evaluation by light microscopy including staining by hematoxylin-and-eosin (H&E) staining ccording to standard conditions. Serial sections were examined with a Zeiss Axiophoto microscope (Cal Zeiss, Inc. Jena, Germany). Each section was reviewed by two pathologists specializing in bone tumors.
Vascular imaging of osteosarcoma by CTA
CTA was performed in all of the osteosarcoma patients.3 Iopamidol 370 was administered via injection at a rate of 7 mL/s into the celiac artery, or 4 mL/s via the right, the left or the proper hepatic artery, depending on tumor location. Tumor vascularity was assessed by enhancement during the arterial phase of CTA. In brief, tumors that were markedly enhanced by CTA were assessed as very hypervascular lesions, those minimally to mildly enhanced by CTA were assessed as hypervascular, and those slightly enhanced or not enhanced by CTA were assessed as hypovascular.
Ethical approval
All patients and healthy controls provided informed, written consent and the study was approved by the Ethics Committee of National Institute of Rehabilitation, Mexico City, Mexico.
Statistical analysis
Statistically significant differences between the groups were determined by the median test and the differences were shown by using box–whisker plots. All analyzes were performed using Analyse-it® software (Analyse-it Software, Leeds, UK).
Results
Patient characteristics
The clinicopathological characteristics of 117 patients with osteosarcoma are listed in Table 1.
Table 1.
Clinicopathological characteristics of osteosarcoma patients.
| Number of patients | |
|---|---|
| Sex | |
| Women | 41 |
| Men | 76 |
| Age | |
| Mean (years) | 23.8 |
| SD (years) | 16.6 |
| Range (years) | 4–74 |
| Tumor site | |
| Humerus and radius | 12 |
| Pelvis | 10 |
| Femur | 66 |
| Tibia | 21 |
| Fibula | 6 |
| Tarsal bones | 2 |
| Extent of tumor | |
| Intraosseous | 6 |
| +cortical breakthrough | 16 |
| +soft tissue extension | 95 |
| Type of osteosarcoma | |
| Classical high grade | 98 |
| Other types | 19 |
| Largest tumor diameter | |
| Mean (cm) | 10.1 |
| SD (cm) | 3.9 |
| Range (cm) | 3–20 |
| Diagnostic delay | |
| Mean (months) | 5.3 |
| SD (months) | 3.9 |
| Range (months) | 3–20 |
| Type of surgical treatment | |
| None | 5 |
| Tumor resection | 26 |
| Amputation | 86 |
| Histological radicality of sugery | |
| Intralesional | 4 |
| Marginal resection | 5 |
| Wide resection (radical) | 49 |
| Amputation (radical) | 54 |
| Recurrent disease | |
| No recurrence | 51 |
| +recurrence | 56 |
| Persistent disease | 10 |
| Survival status | |
| Survivors | 58 |
| Death caused by osteosarcoma | 58 |
| Death from unrelated disease | 1 |
Quantification of serum ANG–IgM with ELISA
To quantify ANG–IgM in humans, an ELISA was developed using ANG coupled with HMWM (polyphenilacrilat) as the capture antigen on the plates (Fig. 4). The coating antigen was validated by assessing VEGF binding, which has a similar specificity to ANG–IgM. In general, IgM is notorious for non-specific antigens sticking to its solid phases. Hence, we included a number of controls to rule out the possibility that the observed binding of IgM to ANG–HMWM was specific. Binding of IgM to HMWM- or non-coated plates upon incubation with dilutions of normal human sera (NHS) was negligible. However, significant binding of IgM to VEGF–HMWM was observed, although this binding was less than that to ANG–HMWM-coated plates. We did competition experiments to further substantiate the specificity of the ELISA for ANG-IgM (Table 2).
Figure 4.
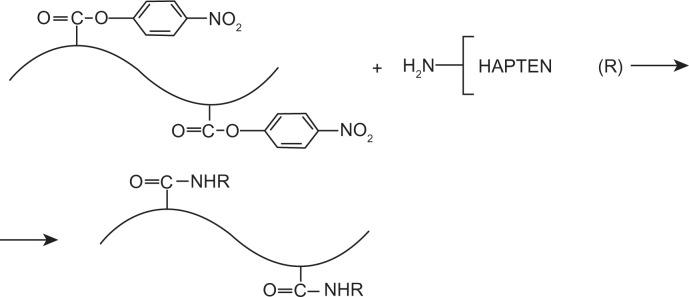
Scheme of synthesis and structure of hapten–HMWM (ie, angiogenin–polyphenilacrilat) conjugates used as coating antigens for ELISA.
Table 2.
Reproducibility, sensitivity and specificity of detection of natural IgM antibodies in osteosarcoma (OS) patients.
| % |
Self antigen
|
|||
|---|---|---|---|---|
| ANG | VEGF | bFGF | PDFG | |
| Reproducibility Sensitivity |
97 | 95 | 89 | 87 |
| OS stage IA | 29 | 21 | 11 | 9 |
| OS stage III | 18 | 9 | 5 | 4 |
| Specificity | 96 | 96 | 91 | 89 |
Binding of IgM to ANG–HMWM-coated plates was almost completely inhibited in the presence of increasing concentrations of ANG during the sample incubation. In addition, four sera absorbed into ANG–Sepharose yielded negative results in the ELISA with ANG–HMWM-coated plates, whereas the same sera absorbed onto glycine–Sepharose exhibited unaffected IgM binding to ANG–HMWM-coated plates. Together, these experiments demonstrated the specificity of the ELISA with ANG–HMWM-coated plates for ANG–IgM.
We next tested the effect of the method of blood collection, and that of freezing and thawing of samples, on levels of ANG–IgM as measured using ELISA (Table 3). IgM antibodies reacting to ANG were present in the sera of patients with osteosarcoma as well as in the sera of normal individuals. Serum ANG–IgM levels were significantly higher in all patients (100%) with osteosarcoma than in healthy individuals (P < 0.005). Serum ANG–IgM levels were significantly higher in the group of osteosarcoma patients as compared with other tumors (P < 0.001).
Table 3.
Serum ANG–IgM levels detected in patients with early diagnosed cancer compared with healthy individuals: ELISA test.
| Study group | N | Serum ANG–IgM levels, ODx1000 | P-value |
|---|---|---|---|
| Osteosarcoma | 117 | 825 ± 265 | 0.005 |
| Other tumors | 117 | 525 ± 118 | 0.001 |
| Healthy individuals | 117 | 499 ± 163 |
Multivariate Analysis of angiogenic serum factors in osteosarcoma patients
The sera of patients with biopsy-proven osteosarcoma (Fig. 5) were analyzed with gel filtration. This is the first evidence of circulating ANG–IgM complexes. Serum samples from 117 patients with primary and advanced osteosarcoma were analyzed for the presence of ANG–IgM immune complexes (Table 4). The serum levels of ANG–IgM were significantly higher in the group of advanced osteosarcoma patients than in those with primary osteosarcoma. Serum ANG–IgM is a promising approach for early detection and diagnosis of osteosarcoma. Once refined, the assay must be applied to a prospective patient population to demonstrate its applicability. Our results shows the indexes of diagnostic accuracy obtained by using ANG–IgM assays in order to differentiate patients with primary bone osteosarcoma from patients with metastasis. ANG–IgM detected by the novel ELISA methods are candidate biomarkers that could be useful for the early diagnosis of osteosarcoma and are likely to be present in the early stages of tumorigenesis.
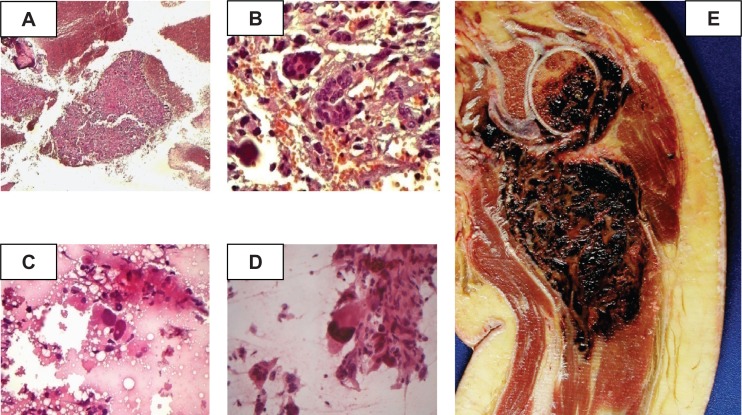
Figure 5.
Representative histologic images of specimens of telangiectatic osteosarcoma by computer-assisted imaging analysis. A, B) Photomicrograph of fine needle aspiration biopsy of a humeral lesion (×10). Photograph B shows that the humeral lesion is composed of multinucleated giant cells (×40). C, D) Photomicrograph of a cell with irregular and hypercromatic nuclei that are obviously malignant. Another view (D) shows a cell with large, irregular and hypercromatic nuclei. E) Photomacrograph of the product of disarticulation of a telangiectatic osteosarcoma. The photograph shows a litic, destructive and hemorrhagic humeral lesion.
Table 4.
ELISA characterization of collected ANG–IgM fractions.
| Study group | Serum ANG–IgM level, ODx1000 | P-value |
|---|---|---|
| Primary osteosarcoma | 675 ± 105 | 0.005 |
| Advanced ostesarcoma | 917 ± 191 | 0.006 |
| Healthy individuals | 499 ± 163 |
Serum ANG–IgM and tumor angiogenesis progression assessed by CTA in osteosarcoma patients
The present study demonstrated increased ANG–IgM expression in the peripheral blood sera of osteosarcoma patients and a positive correlation between its expression levels and tumor vascularity as evaluated by CTA. Osteosarcoma patients were categorized into three groups based on tumor vascularity as assessed by CTA. Mean serum ANG–IgM levels were 621 ± 121, ODx1000 in the hypovascular group, 755 ± 118, ODx1000 in the hypervascular group, and 921 ± 195 in the very hypervascular group, the difference in the serum ANG–IgM among the three groups is statistically significant (P < 0.001; Table 5).
Table 5.
Correlation between circulating and imaging biomarkers of tumor angiogenesis in patients with osteosarcoma.
| Serum ANG–IgM levels, ODx1000 | Type of vascularity | P-value |
|---|---|---|
| 621 ± 121 | Hypo
|
0.001 |
| 755 ± 118 | Hyper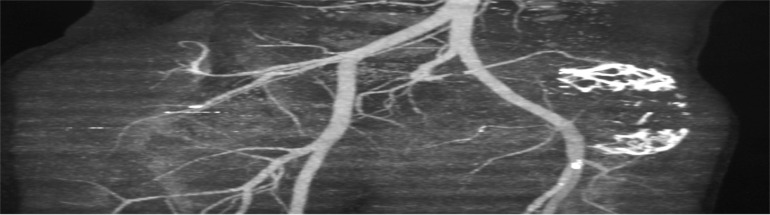
|
0.002 |
| 921 ± 195 | Very hyper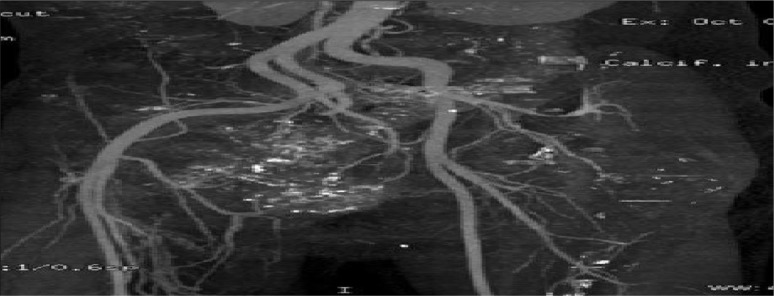
|
0.001 |
| 499 ± 136 | Healthy individuals |
Detection of early antiangiogenic response in patients with osteosarcoma using ANG–IgM ELISA
The intrinsic redundancy of the signaling mechanisms associated with angiogenesis will lead to partial or complete resistance of the tumor vessels to antiangiogenic therapy. Antiangiogenic therapy needs to be administered for several months to a year or more.21,63 Interest in circulating markers techniques that can provide early indicators of effectiveness at serum level has therefore increased. The response of osteosarcoma to treatment can be detected by ELISA, and this can be used to monitor changes.
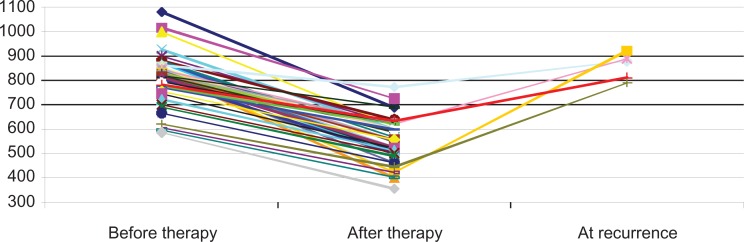
Fifty patients received antiangiogenic therapy for the treatment of osteosarcoma. Serum was taken from these patients before and after antiangiogenic therapy, and samples were frozen for analysis. After antiangiogenic therapy, all patients achieved a response, with serum ANG–IgM levels being significantly lower compared to untreated patients (mean (m) ± standard deviation (SD), OD x100: 623 ± 155 versus 835 ± 195; P < 0.05), but still higher than in healthy controls (m ± SD: 499 ± 163 versus 623 ± 155; P < 0.01) (Fig. 6). Higher serum ANG–IgM levels were significantly associated with poor treatment response (P < 0.001). Serum ANG–IgM concentration decreased after successful treatment and increased in five cases of recurrent osteosarcoma, indicating that measuring serum ANG–IgM concentrations may be useful in monitoring treatment efficacy. The fact that the patients with the concentrations higher than 650, ODx1000 had a worse prognosis supports the previous notion that the higher the vascularity, the worse the prognosis.
Figure 6.
Dynamic changes of serum ANG–IgM levels in patients with osteosarcoma during antiangiogenic therapy.
We have shown that ANG–IgM could be used as a specific biomarker for monitoring the efficacy of antiangiogenic therapy in patients with osteosarcoma. The association of laboratory investigations with clinical trials will be instrumental for the validation of this biomarker of angiogenesis (ANG–IgM), and for improving the design, monitoring and evaluation of antiangiogenic treatments.
Affinity of osteosarcoma-associated human ANG–IgM antibodies
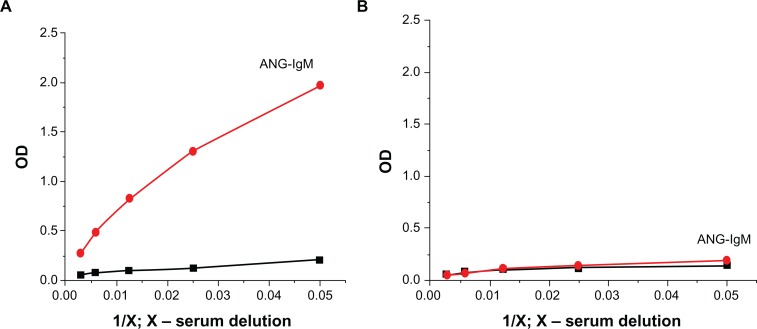
In a traditional approach, identifying the tumor biomarker is the first step. After that, it is necessary to isolate natural antibodies against this biomarker. Affinity chromatography yielded ANG-specific IgM from the sera of healthy individuals (Fig. 7). Purified ANG–IgM displayed the expected characteristics and was fully functionally active. Low affinity ANG–IgM was the predominant isotype of natural antibodies present in healthy individuals. The affinity constants ranged between 10−6 and 10−7 M. The affinity constants of ANG–IgM purified from osteosarcoma patients ranged between 10−9 and 10−11 M. Serum ANG–IgM was detectable in 85% of osteosarcoma patients, with 100% specificity for osteosarcoma. ANG–IgM showed no cross-reactivity to other structurally similar inhibitors. Identification of novel broadly cross-reactive osteosarcoma-neutralizing ANG–IgM in the sera has major implications for the development of treatment, angiogenesis, and tools to study the mechanisms of this type of cancer.
Figure 7.
Affinity chromatography produced ANG-specific IgM from the sera of healthy individuals. A) concentration of ANG–IgM before chromatography. B) concentration of ANG–IgM after chromatography.
Discussion
Investigations into tumor immunology has led to the identification of a number of tumor-associated antigens, suggesting that most tumors trigger an immunogenic response, as is the case in osteosarcoma,64 where the detection of serum IgG antibodies might achieve the diagnosis of prostate cancer.64,65 Cancer immunosurveillance predicts that the immune system can recognize the precursors of cancer (immunoediting) by native or adaptive immune effectors and, in most cases, can destroy the cancer cells before they become clinically apparent.18,56
However, the immune response is often too inefficient to prevent the development of cancer, either because tumor cells that can evade the immune response survive and invad, or because tumor antigen-specific immunotolerance is induced.56 Tumor antigens associated with immunoglobulins, mainly IgG, can form circulating immune complexes, and this has been reported for few biomarkers, such as carcinoembryonic antigen (CEA) and TA90 for colon cancer,67,68 and MUC-1 or p53 for breast cancer.69 We have recently reported that alfa-fetoprotein (AFP) and squamous cell carcinoma antigen (SCCA) (for liver cancer) and CEA ( for colorectal cancer) could be detected in the serum of patients with cancer, forming complexes with IgM, opening a new gateway for cancer detection.50–52
Multivalent IgMs are typically the main component of innate immunity, with the ability to bind a wide range of tumor antigens.70 It is well established that natural IgM plays an important role in the first line of defense against infectious agents, in regulating the proliferation of immune cells and in immunosurveillance against transformed malignant cells.71 It may be speculated that the observed enhancement of diagnostic indexes of ANG–IgM immune complexes for cancer diagnosis could be linked to the ability of natural IgM antibodies to act as “early markers” for the recognition and binding of abnormal proteins synthesized by the transformed cells.18 Although osteosarcoma patients were not submitted to radical surgery to remove the tumor for biopsy, it can be assumed that they did not harbor any cancer because all patients underwent an adequate biopsy sampling and none of them had a diagnosis of osteosarcoma within a year after the original biopsy.
In this study, novel immunoconjugates were designed, synthesized and then used to develop a rapid, specific and sensitive indirect ELISA method to detect ANG–IgM directly in the peripheral blood sera of humans. The hapten ANG was first designed and used to covalently couple to HMWM. Based on the “substructural coating antigen” concept, an optimized indirect ELISA method was established that exhibited good specificity and high sensitivity for detecting ANG–IgM.
The analysis of osteosarcoma patients’ sera confirmed the presence of specific ANG–IgM, whereas all control sera from healthy subjects were negative (100% specificity). ANG–IgM test discriminated the benign condition of the disease with greater resolution. The ANG–IgM test also improved diagnostic sensitivity for the identification of ostosarcoma patients compared to the ANG assay. The data presented here are the first evidence of the occurrence of ANG–IgM in patients affected by osteosarcoma. The ANG–IgM assay also improved sensitivity and specificity indexes with respect to the ANG test for differentiating patients with advanced osteosarcoma.
We describe a specific and reproducible ELISA for ANG–IgM. Levels of this IgM differ by up to 100-fold among healthy persons. ANG–IgM levels were significantly elevated in the patients with osteosarcoma with ANG in the range of 350–558 ng/mL compared to the patients with benign metastasis, achieving a diagnosis of cancer in the “grey zone” of osteosarcoma, where the outcome of biopsies is highly equivocal and unpredictable.72 The gain achieved in cancer detection by using the combination of ANG and IgM suggests that the ANG–IgM complex could be a complementary serological biomarker of osteosarcoma. Immunological aspects of angiogenesis for the management of osteosarcoma will have a practical value in early diagnosis, prognosis and monitoring response to antiangiogenic therapy.
In conclusion, we describe a specific and reproducible ELISA for ANG–IgM. We demonstrate a good correlation between the levels of circulating ANG–IgM and osteosarcoma vascularity. Future studies on ANG–IgM should delineate its role in human diseases such as osteosarcoma. Immune markers of angiogenesis may serve an important part in predicting a particular patient’s clinical course. In addition, angiogenesis markers may help to indicate which patients with osteosarcoma may benefit most from antiangiogenic therapies and can be used to monitor patients receiving these therapies.
Acknowledgments
The authors are grateful to Dr. Luis Guillermo Ibarra Ibarra (National Institute of Rehabilitation, Mexico City, Mexico) for his generous support and invaluable advice. This work was support by a grant from FONSEC SSA/IMSS/ISSSTE–CONACyT (number SALUD-2010-01-138883 to Y.A.S.).
Abbreviations
- ABTS
2,20-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid);
- ANG
angiogenin;
- ANG–IgM
natural IgM antibodies against angiogenin;
- bFGF
basic fibroblast growth factor;
- BSA
bovine serum albumin;
- CTA
computed tomography angiography;
- EDC
1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride;
- ELISA
enzyme-linked immunosorbent assay;
- HMWM
high molecular weight matrix;
- HSA
human serum albumin;
- IgM
immunoglobulin M;
- IgM–HRP
immunoglobulin M–horseradish peroxidase conjugate;
- NHS
normal human sera;
- OD
optical density;
- PBS
phosphate-buffered saline;
- PBST
phosphate-buffered saline with Tween 20;
- PDGF
platelet-derived growth factor;
- TMB
3,3′,5,5′-tetramethylbenzidine;
- VEGF
vascular endothelial growth factor.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Bielack SS, Bernstein ML. Cancer in Children: Clinical management. 5th ed. Oxford University Press; 2005. Osteosarcoma; p. 280. [Google Scholar]
- 2.Gasparini M, Rouesse J, van OA, et al. Phase II study of cisplatin in advanced osteogenic sarcoma. European Organization for Research on Treatment of Cancer, Soft Tissue and Bone Sarcoma Group. Cancer Treat Rep. 1985;69:211–3. [PubMed] [Google Scholar]
- 3.Honda H. Hepatocellular carcinoma: correlation of CT, angiographic, and histopathologic findings. Radiology. 1993;189:857–62. doi: 10.1148/radiology.189.3.8234716. [DOI] [PubMed] [Google Scholar]
- 4.Marti C, Jackson A, Jayson G. Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin Cancer Res. 2007;13(12):3449–59. doi: 10.1158/1078-0432.CCR-07-0238. [DOI] [PubMed] [Google Scholar]
- 5.Pratt CB, Howarth C, Ransom JL, et al. High-dose methotrexate used alone and in combination for measurable primary or metastatic osteosarcoma. Cancer Treat Rep. 1980;64:11–20. [PubMed] [Google Scholar]
- 6.Cai W. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des. 2008;14(28):2943–73. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 7.Ping-Chung L, Chao Y, Shou-Dong L. Prognostic significance of vascular endothelial growth factor, basis fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgery. Ann Surg Oncol. 2003;10(4):355–62. doi: 10.1245/aso.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Smeland S, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488–94. doi: 10.1016/s0959-8049(02)00747-5. [DOI] [PubMed] [Google Scholar]
- 9.Strander H, Bauer HC, Brosjo O, et al. Long-term adjuvant interferon treatment of human osteosarcoma. A pilot study. Acta Oncol. 1995;34:877–80. doi: 10.3109/02841869509127199. [DOI] [PubMed] [Google Scholar]
- 10.Lequin RM. Enzyme-linked immunosorbent assay (ELISA) Clin Chem. 2005;51:2415–8. doi: 10.1373/clinchem.2005.051532. [DOI] [PubMed] [Google Scholar]
- 11.van Weemen B. The rise of ELISA. Clinical Chemistry. 2005;51(12):2225–6. doi: 10.1373/clinchem.2005.059626. [DOI] [PubMed] [Google Scholar]
- 12.Brosjo O. Osteosarcoma and interferon. Studies of human xenografts in the nude mouse. Acta Orthop Scand Suppl. 1989;229:1–36. [PubMed] [Google Scholar]
- 13.Einhorn S, Strander H. Is interferon tissue specific? Effect of human leukocyte and fibroblast interferons on the growth of lymphoblastoid and osteosarcoma cell lines. J Gen Virol. 1977;35:573–7. doi: 10.1099/0022-1317-35-3-573. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J.Angiogenesis research: from laboratory to clinic Forum Genova 1999July–Dec9359–62. [PubMed] [Google Scholar]
- 15.de Kraker J, Voute PA. Ifosfamide, mesna and vincristine in paediatric oncology. Cancer Treat Rev. 1983;10(Suppl A):165–6. doi: 10.1016/s0305-7372(83)80024-3. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J. Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost. 2001;86(1):23–33. [PubMed] [Google Scholar]
- 17.Folkman J. Tumor angiogenesis. Cancer Med. 1993:153–70. [Google Scholar]
- 18.Volkova YA, Kushlinskii NE, Babkina IV, Solovev YN, Trapeznikov NN. Vascular endothelium growth factor and angiogenin in the serum of patients with osteosarcoma and Ewing’s tumor. Bull Exp Biol Med. 2005;20(3):927–37. doi: 10.1007/BF02682107. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 20.Poon RT. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19(4):1207–25. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 21.Folkman J. Diagnostic and therapeutic applicationes of angiogenesis research. C R Acad Sci III. 1993;316(9):909–18. [PubMed] [Google Scholar]
- 22.Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Eng J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 23.Aljubran AH. Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Ann Oncol. 2009;20(6):1136–41. doi: 10.1093/annonc/mdn731. [DOI] [PubMed] [Google Scholar]
- 24.Álvarez LA. Tumores Óseos Cartilaginosos en Niños. Reporte Epidemiológico de 20 años en Nuestro Hospital (Cartilaginous bone tumors in children: a 20-year epidemiological report from our hospital) Acta Orto Mex. 2004;18(5):191–5. [Google Scholar]
- 25.Rabin K. Personalized care of pediatric cancer patients. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:173–85. 185–8. doi: 10.1159/000146259. discussion. [DOI] [PubMed] [Google Scholar]
- 26.Rosen G. Preoperative (neoadjuvant) chemotherapy for osteogenic sarcoma: a ten year experience. Orthopedics. 1985;8:659–64. doi: 10.3928/0147-7447-19850501-19. [DOI] [PubMed] [Google Scholar]
- 27.Saznurkowska K, Lenckowski R, Popadiuk S, Korzon M. Assessment of angiogenesis in children’s osteosarcoma. Med Wieku Rozwoj. 2006;10(3):737–44. [PubMed] [Google Scholar]
- 28.Badet J. Angiogenin. Vascular Biology and Pathology: an Encyclopedic Reference. 2000:16–29. [Google Scholar]
- 29.Riordan JF. Angiogenin. Methods Enzymol. 2001;341:263–73. doi: 10.1016/s0076-6879(01)41157-8. [DOI] [PubMed] [Google Scholar]
- 30.Folkman J. Clinical applications of research on angiogenesis. N Eng J Med. 1995;333(26):1750–7. [Google Scholar]
- 31.Fritzler MJ. Challenges to the use of autoantibodies as predictors of disease onset, diagnosis and outcomes. Autoimmun Rev. 2008 Sep;7(8):616–20. doi: 10.1016/j.autrev.2008.06.007. Epub 2008 Jul 9. [DOI] [PubMed] [Google Scholar]
- 32.Raica M. Angiogenesis in pre-malignant conditions. Eur J Cancer. 2009 Apr 28;45(11):1924–34. doi: 10.1016/j.ejca.2009.04.007. Epub 2009 May 4. 2009 Jul. [DOI] [PubMed] [Google Scholar]
- 33.Folkman J. Tumor–vascular interactions and tumor dormancy. APMIS. 2008;116:569–85. doi: 10.1111/j.1600-0463.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 34.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 35.Jaffe N, Levy-Soussan P, Berneman A, Avrameas S. Differences in the natural autoantibody patterns of patients with schizophrenia and normal individuals. J Psych Neuroscience. 1996;21(2):89–95. [PMC free article] [PubMed] [Google Scholar]
- 36.Lekens S. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61(3):253–70. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- 37.Mejia-Arangue JM. Edad de aparición de los diferentes tumores malignos en la infancia. (Age at appearance of different malign tumors in infancy) Rev Med IMSS. 2005;43(1):25–37. [PubMed] [Google Scholar]
- 38.Vollmers HP. Natural IgM antibodies, the ignored weapons in tumour immunity. Histol Histopathol. 2004;19(3):897–905. doi: 10.14670/HH-19.897. [DOI] [PubMed] [Google Scholar]
- 39.Adamson A, Brostrom LA. Circulating immune complexes in human neuroblastoma: direct assay and role in blocking specific cellular immunity. Int J Cancer. 1974;13(6):824–38. doi: 10.1002/ijc.2910130610. [DOI] [PubMed] [Google Scholar]
- 40.Muller HM, Zitt M, Goebel G. Circulating nucleic acids in plasma or serum (CNAPS) as prognostic and predictive markers in patients with solid neoplasias. Disease Markers. 2005;21:105–20. doi: 10.1155/2005/218759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vollmers HP. Natural IgM antibodies and cancer. J Autoimmun. 2007;29:295–302. doi: 10.1016/j.jaut.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol. 2000;37:1141–9. doi: 10.1016/s0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 43.Scofield H. Autoantibodies as predictors of disease. Lancet. 2004;363:1544–6. doi: 10.1016/S0140-6736(04)16154-0. Lancet. Review. Scofield RH is alone. 2004 May 8;363(9420):1544–6. [DOI] [PubMed] [Google Scholar]
- 44.Vollmers HP. Natural IgM antibodies: from parias to parvenus. Histol Histopathol. 2006;21(12):1355–66. doi: 10.14670/HH-21.1355. [DOI] [PubMed] [Google Scholar]
- 45.Vollmers HP, Brandlein S. The “early birds”: natural IgM antibodies and immune surveillance. Histol Histopathol. 2000;130(7):691–3. doi: 10.14670/HH-20.927. [DOI] [PubMed] [Google Scholar]
- 46.Leslie D. Autoantibodies as predictors of disease. J Clin Invest. 2001;89:2092–101. doi: 10.1172/JCI14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avrameas S. Natural Autoantibodies: the Other Side of the Immune System. Res Immunol. 1995;146:235. doi: 10.1016/0923-2494(96)80259-8. [DOI] [PubMed] [Google Scholar]
- 48.Manson JJ. Natural serum IgM maintains immunological homeostasis and prevents autoimmunity. Springer Semin Immunopathology. 2005;26:425–32. doi: 10.1007/s00281-004-0187-x. [DOI] [PubMed] [Google Scholar]
- 49.Ro-Mi K, Ji-Yeon K, Mi-Hong L, Tae JK. Detection of antibodies against glucose 6-phosphate isomerase in synovial fluid of rheumatoid arthritis using surface plasmon resonance (BIAcore) Exp Mol Medicine. 2003;4(35):310–6. doi: 10.1038/emm.2003.42. [DOI] [PubMed] [Google Scholar]
- 50.Pontisso P, Quarta S, Caberlotto C, et al. Progressive increase of SCCA–IgM immune complexes in cirrhotic patients is associated with development of hepatocellular carcinoma. Int J Cancer. 2006;119:735–40. doi: 10.1002/ijc.21908. [DOI] [PubMed] [Google Scholar]
- 51.Beneduce L, Castaldi F, Marino M, et al. Squamous cell carcinoma antigen–IgM complexes as novel biomarkers for hepatocellular carcinoma. Cancer. 2005;103:2558–65. doi: 10.1002/cncr.21106. [DOI] [PubMed] [Google Scholar]
- 52.Beneduce L, Castaldi F, Marino M, et al. Improvement of liver cancer detection with simultaneous assessment of circulating levels of free alpha-fetoprotein (AFP) and AFP–IgM complexes. Int J Biol Markers. 2004;19:155–9. doi: 10.1177/172460080401900211. [DOI] [PubMed] [Google Scholar]
- 53.Castaldi F, Marino M, Beneduce L, et al. Detection of circulating CEA–IgM complexes in early stage (stage 1) colorectal cancer. Int J Biol Markers. 2005;20:204–8. doi: 10.1177/172460080502000402. [DOI] [PubMed] [Google Scholar]
- 54.Mevio E. Use of serum markers in the diagnosis and management of laryngeal cancer. Clin Otolaryngol. 1991;16:90–2. doi: 10.1111/j.1365-2273.1991.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 55.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate specific antigen in a community based population of healthy men: establishment of age specific reference ranges. JAMA. 1993;270:860–4. [PubMed] [Google Scholar]
- 56.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;10:715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 57.Bielack SS, Kempf-Biealck B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities of trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 58.Bieling P, Rehan N, Winkler P, et al. Tumour size and prognosis in aggressively treated osteosarcoma. J Clin Oncol. 1996;14:848–58. doi: 10.1200/JCO.1996.14.3.848. [DOI] [PubMed] [Google Scholar]
- 59.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 60.Friedman MA, Carter SK. The therapy of osteogenic sarcoma: current status and thoughts for the future. J Surg Oncol. 1972;4(5):482–510. doi: 10.1002/jso.2930040512. [DOI] [PubMed] [Google Scholar]
- 61.Ochs JJ, Freeman AI, Douglass HO, et al. cis-Dichlorodiammineplatinum (II) in advanced osteogenic sarcoma. Cancer Treat Rep. 1978;62:239–45. [PubMed] [Google Scholar]
- 62.Randall LM. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group Study. Gynecol Oncol. 2009;112(3):583–9. doi: 10.1016/j.ygyno.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lei Y. Therapeutic angiogenesis. Devising new strategies based on past experiences. Basic Res Cardiol. 2004;99(2):121–32. doi: 10.1007/s00395-004-0447-x. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Yu J, Sreekumar A, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353:1224–35. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 65.Costrzhevskaja EG. Circulating IgG-containing immune complexes in malignant growth. Eksp Onkol. 1984;8(3):10–7. [PubMed] [Google Scholar]
- 66.Nesterova M, Johnson N, Cheadle C, Cho-Chung YS. Autoantibody biomarker opens a new gateway for cancer diagnosis. Biochim Biophys Acta. 2006 Apr;1762(4):398–403. doi: 10.1016/j.bbadis.2005.12.010. Epub 2006 Jan 30. [DOI] [PubMed] [Google Scholar]
- 67.Fuchs C, Krapf F, Kern P, Hoferichter S, Jager W, Kalden JR. CEA containing immune complexes in sera of patients with colorectal and breast cancer–analysis of complexed immunoglobulin classes. Cancer Immunol Immunother. 1988;26:180–4. doi: 10.1007/BF00205613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Habal N, Gupta RK, Bilchick AJ, Johnson T, Morton DL. TA90-IC, a new marker for advanced colon cancer. Ann Surg Oncol. 2000;7:352–6. doi: 10.1007/s10434-000-0352-y. [DOI] [PubMed] [Google Scholar]
- 69.Peyrat JP, Bonneterre J, Lubin R, Vanlemmens L, Fournier J, Soussi T. Prognostic significance of circulating p53 antibodies in patients undergoing surgery for loco-regional breast cancer. Lancet. 1995;345:621–2. doi: 10.1016/s0140-6736(95)90523-5. [DOI] [PubMed] [Google Scholar]
- 70.Lacroix-Desmazes S, Kaveri SV, Mouthon L, et al. Self-reactive antibodies (natural autoantibodies) in healthy individuals. J Immunol Methods. 1998;216:117–37. doi: 10.1016/s0022-1759(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 71.Varambally S, Bar-Dayan Y, Bayry J, et al. Natural human polyreactive IgM induces apoptosis of lymphoid cell lines and human peripheral blood mononuclear cells. Int Immunol. 2004;16:517–24. doi: 10.1093/intimm/dxh053. [DOI] [PubMed] [Google Scholar]
- 72.Whelan J, Weeden S, Uscinska B, et al. Localized extremity osteosarcoma: Mature survival data from two European Osteosarcoma Intergroup randomised clinical trials. Proc of the Ame Soc of Clin Onco. 2000;19:1281a. [Google Scholar]