Abstract
Utilizing ENU mutagenesis, we identified a mutant mouse with elevated platelets. Genetic mapping localized the mutation to an interval on chromosome 19 that encodes the Jak2 tyrosine kinase. We identified a A3056T mutation resulting in a premature stop codon within exon 19 of Jak2 (Jak2 K915X), resulting in a protein truncation and functionally inactive enzyme. This novel platelet phenotype was also observed in mice bearing a hemizygous targeted disruption of the Jak2 locus (Jak2 +/-). Timed pregnancy experiments revealed that Jak2 K915X/K915X and Jak2 -/- displayed embryonic lethality; however, Jak2 K915X/K915X embryos were viable an additional two days compared to Jak2 -/- embryos. Our data suggest that perturbing JAK2 activation may have unexpected consequences in elevation of platelet number and correspondingly, important implications for treatment of hematological disorders with constitutive Jak2 activity.
Introduction
Cytokines play an integral role in hematopoiesis by providing growth signals to progenitor and committed cells that promote mitogenesis, survival and in some cases differentiation. Once bound to their cognate receptors, cytokines mediate downstream signaling through activation of components of the Jak-Stat signaling pathway. Thrombopoietin (Tpo) is the principal cytokine regulator of megakaryopoiesis, through binding to its cognate receptor Mpl. Tpo activates the Jak2 and Tyk2 tyrosine kinases [1] as well as the Stat3 and Stat5 transcription factors [2,3,4]. The importance of Tpo, its receptor and proximal signaling pathways in platelet function is illustrated by the discovery of gain-of-function mutations in Tpo [5], Mpl [6,7] and Jak2 [8,9,10,11] that all result in Essential Thrombocythemia (ET). Similarly, loss-of-function mutations in Mpl have been documented in Congenital Amegakaryocytic Thrombocytopenia [12,13]. Jak2 is critical for murine embryogenesis as mice lacking Jak2 expression die of anemia at E12.5 [14,15].
While screening ENU mutagenized mice for dominant hematopoietic defects, we identified a mouse with thrombocythemia and determined that the mutation resulted in a truncated allele of Jak2 that lacked catalytic activity. Analysis of this mutation has uncovered a novel function of Jak2 in the megakaryocyte/platelet lineage.
Materials and Methods
Mice and ENU mutagenesis
C57Bl6/J (B6) and 129S1/SvImJ (129) mice were purchased from The Jackson Laboratory. Jak2 +/- mice (on the B6 genetic background) were provided by Dr. James Ihle, Memphis, TN. All mice were maintained in specific-pathogen free facilities at the Toronto Centre for Phenogenomics or Ontario Cancer Institute. Animal protocols were approved by the OCI Animal Care Committee (Permit Number 1517). All efforts were made to reduce animal suffering.
To induce random mutations, one intraperitoneal injection of 150mg/kg ENU was administered to male 129 mice (mutagenized strain) [16]. The F1 generation (129;B6) was produced by out-crossing ENU-mutagenized males to B6 (mapping strain) females – pups from this breeding were designated generation 1 (G1). G1 mice were screened to detect dominant traits deviating from normal homeostatic venous blood parameters by at least two standard deviations from ‘normal’ G1 parameters. Affected mice with elevated platelets were sequentially back-crossed to B6 mice for genetic mapping. The Jak2 K915X allele was maintained on a B6 background by intercrossing heterozygous or wild type (WT) mice. Timed matings were performed on G9 animals and peripheral blood analysis was completed on G10 mice.
Hematologic analysis, genetic mapping and sequencing
Peripheral blood from 6-8 week old mice was collected by saphenous venipuncture. Complete blood counts (CBC) were performed using a Coulter Ac-T Differential Hematology Analyzer. Jak2 +/+ mice were littermate controls of Jak2 +/- animals. Jak2 Control mice are littermate controls of Jak2 K915X G10 breedings. Bone marrow sections were prepared from femurs of 12-week old mice. Femurs were fixed in 10% formaldehyde and then sectioned (4 µm) and stained with Hematoxylin and Eosin (H&E) at the CMHD pathology core (http://www.cmhd.ca/enu_mutagenesis/pathology.html). Affected mice were sequentially bred to B6 to confirm heritability and to genetically map the mutation using microsatellite base genome scan and single-nucleotide polymorphism markers differentiating 129 and B6 alleles [16]. Once the mutation was mapped to a 6.7Mb region of chromosome 19, candidate gene analysis was used to select genes for exon sequencing [14,15].
Genotyping
Multiplex PCR was used to genotype Jak2 K915X and Jak2 +/- mice using genomic DNA prepared from tail or biopsy tissue [14]. All Jak2K915X genotyping was performed at The Centre for Applied Genomics using a custom TaqMan SNP genotyping assay. The custom assay was used to discriminate between the wild type allele (A 3056nt) and the Jak2K915X allele (T 3056nt).
Clonogenic assays
CFU-C, CFU-E and CFU-Mk assays were performed as previously described [17,18].
5-fluorouracil and Phenylhydrazine Priming
Six to eight-week old mice were injected with 5-fluorouracil (5FU) or Phenylhydrazine (PHZ), as previously described [18,19]. Briefly, 5FU was administered at 120 µg/kg and blood was collected at Days 0, 6, 8 and 13. PHZ was delivered by intraperitoneal injection at 100 µg/kg and peripheral blood was harvested at Days 0, 1, 7 and 9. Complete blood counts were performed with a HEMAVET 950 (Drew Scientific Inc.).
Transfection and Cell Culture
293T cells (ATCC) were transfected with HA-tagged Jak2 or HA-Jak2 K915X. Alternatively, Jak2 constructs were generated that expressed the 3’ UTR or had the 3’ UTR removed. Thirty-six hr after transfection, cells were washed, lysed as described [20]. Lysate fractions were resolved via SDS-PAGE and transferred to PVDF membranes for Western blotting experiments.
Western blotting
Membranes were blocked in optimal blocking agent (either 2.5% bovine serum albumin or 5% skim milk powder in 50 mM TrisHCl (pH 8.0), 150 mM NaCl, 0.1% Tween 20 (TBST)) for 1 hr. Primary antibody incubations were performed for 1 hr, followed by 30 min washing in TBST. Secondary incubations were performed with HRP-Sheep anti-mouse IgG (GE Healthcare Life Sciences, Mississauga, ON) or HRP-Protein A (GE Healthcare Life Sciences, Mississauga, ON) for 30 min. After washing, membranes were developed by ECL.
Antibodies
Anti-Jak2 and β-tubulin antibodies were purchased from Cell Signaling Technology (Beverley, MA) and Millipore (Billerica, MA). The HA antibody was from Covance (Laval, QC). Phosphorylation-specific pSer-523 [21] and pTyr-570 [22] Jak2 antibodies have been previously characterized.
Microscopy
Megakaryopoiesis was assessed by microscopic examination of bone marrow of femurs on histology sections. Megakaryocytes are identified by their characteristic morphology of large size, lobulated nuclei and abundant cytoplasm and quantified by counting the number of megakaryocytes per microscopic field under a 40x objective.
Results
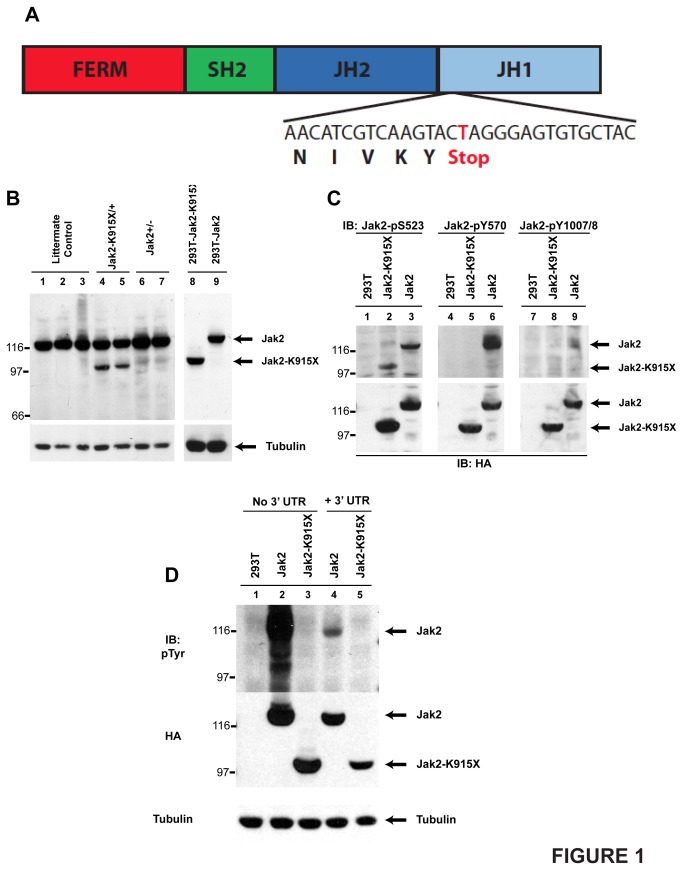
We identified a G1 mouse, strain 7254, with elevated platelets. Back-crossing on to the B6 strain and SNP-based mapping resulted in the identification of a 6.7 Mb heritable region on chromosome 19 as the interval encoding the responsible mutation. We noted that Jak2 was within this interval and hypothesized that the mutation underlying the 7254 phenotype may be in Jak2. We performed genomic DNA sequencing which identified an A3056T mutation in exon 19 of the Jak2 locus. This mutation leads to a K915X premature stop codon in the functional JH1 kinase domain of Jak2 (Figure 1A).
Figure 1. Strain 7254 is defined by a K915X mutation and generates a non-functional truncated Jak2 protein.
(A) The Jak2 domain structure is indicated. The DNA sequence from 7254 splenocytes and corresponding protein sequence are also shown. (B) Splenocytes were harvested from phenylhydrazine primed Jak2 K915X and Jak2 +/- mice and their wild type littermates. HA-tagged Jak2 and Jak2K915X were also expressed in 293T cells. A Western blot was performed with a peptide-specific JAK2 antibody. (C) 293T cells were transfected with cDNAs encoding HA-Jak2 or HA-Jak2 K915X. Western blotting was performed with phosphorylation-specific antibodies that recognize pSer-523, pTyr-570 and pTyr-1007/1008 in Jak2. The membranes were stripped and reprobed with an anti-HA antibody. (D) HA-tagged versions of Jak2 and Jak2K915X with or without the JAK2 3’ UTR were expressed in 293T cells. Western blots were performed with 4G10 anti-phosphotyrosine and HA antibodies. Immunoblotting with anti β-tubulin was performed to demonstrate equal loading.
Western blotting of splenocytes isolated from Jak2 K915X , Jak2 +/- and Jak2 +/+ mice revealed a novel, truncated 95 kDa protein in Jak2 K915X mice (Figure 1B, lanes 4 and 5), that co-migrated with the expressed Jak2K915X protein in 293T cells (lanes 8 and 9).
Recent evidence has suggested that the Jak2 JH2 domain possesses weak intrinsic kinase activity [23,24]. Considering that the JAK2 K915X mutation resides in the Jak2 JH1 domain, we tested whether JAK2 K915X protein product is catalytically active. Both wild type Jak2 and Jak2 K915X are phosphorylated at Ser-523 [21] (Figure 1C, lanes 2 and 3). However, only wild type Jak2 is phosphorylated at Tyr-570 [22] (lane 6) and Tyr-1007/1008 (lane 9).
The possibility of nonsense-mediated decay occurring was eliminated by expression of Jak2 cDNAs that lacked or contained the Jak2 3’ untranslated region. The presence of the Jak2 3’ UTR resulted in comparable protein expression (Figure 1D, lanes 4 and 5). While Jak2 and Jak2K915X protein was reduced compared to the cDNA lacking the 3’ UTR (lanes 2 and 3), both forms of Jak2 were readily detected when the 3’ UTR was present.
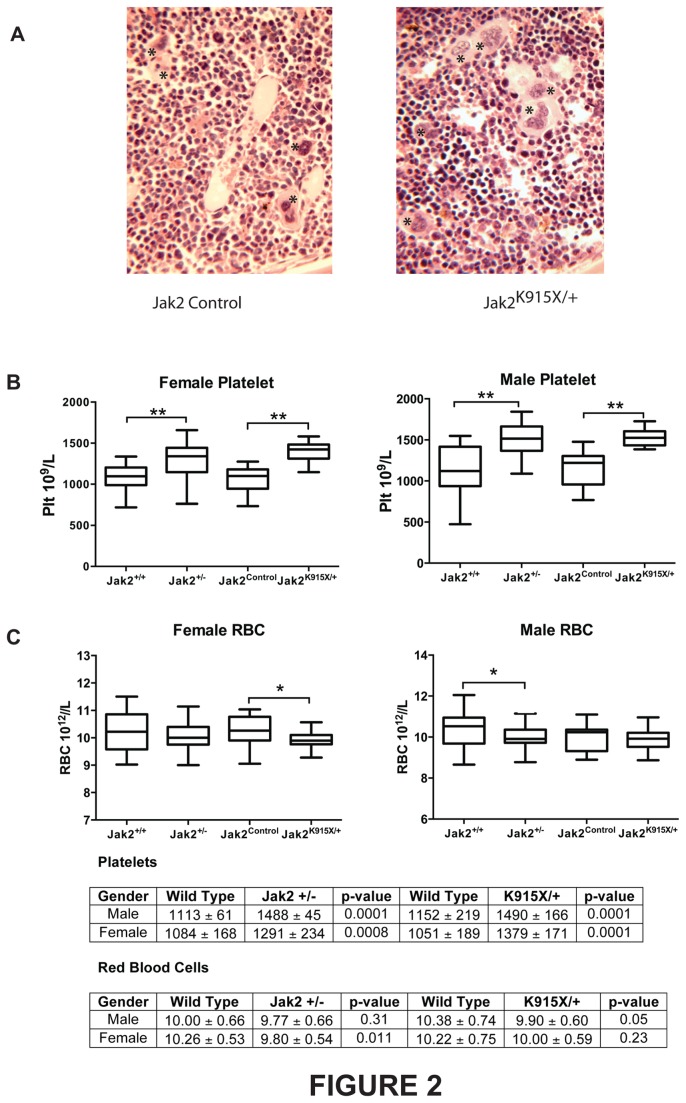
To determine whether the Jak2 K915X allele phenocopied a Jak2 null allele or represented a neomorphic allele, we compared mice bearing homo- and heterozygous mutations in these loci. Increased megakaryocytes were observed upon enumeration of bone marrow sections isolated from Jak2 K915X/+ mice (Jak2+/+ = 7.5 ± 1.8; Jak2K915X/+ = 9.3 ± 2.4; p = 0.016). Representative sections from wild type and Jak2 K915X/+ mice illustrate increased megakaryocytes in mutant mice (Figure 2A). Elevated platelets were found in male and female mice in Jak2 K915X/+ mice at 8 weeks of age (Figure 2B). Interestingly, although this has not previously been reported, Jak2 +/- mice showed an identical phenotype. In contrast to the platelet phenotype, red blood cell numbers (Figure 2C) and other hematological parameters (data not shown) were comparable in both Jak2 K915X/+ and Jak2 +/- mice, with the exception of decreased RBC in Jak2 +/- male and Jak2 K915X/+ female mice at 8 weeks, compared to Jak2 +/+ and Jak2 Control littermates.
Figure 2. Jak2 K915X/+ mice have elevated megakaryocytes and platelets.
(A) Bone marrow sections were prepared from 12 week Jak2 +/+ and Jak2 K915X/+ mice and stained with H and E. Representative sections are illustrated at 20x magnification. Megakaryocytes are indicated by an asterisk. (B) Platelets from male and female wild type, Jak2 +/- and Jak2 K915X mice at 8 wk of age were monitored. (C) Red blood cells were evaluated from male and female mice at 8 wk of age from WT, Jak2 +/- and Jak2 K915X/+ mice. Jak2 +/+ or JAK2 Control mice were littermate controls of Jak2 +/- or Jak2 K915X/+ breedings, respectively. Statistically significant differences between groups are denoted as *, p< 0.05 and **, p<0.0001. Each group has n=20-30.
Clonogenic assays were performed on bone marrow and spleen cells from Jak2 K915X/+ and Jak2 +/- mice. Interestingly, no statistically significant differences were observed in CFU-Megakaryocyte assays from bone marrow isolated from both strains of mice (Figure S1). There were no significant differences in hematopoietic progenitor number or morphology between the genotypes (Figures S1 and S2).
Jak2 K915X/+ and Jak2 +/- mice were challenged with 120 µg/g 5-fluorouracil or 100 µg/g phenylhydrazine. Recovery curves were similar for all genotypes in response to hematopoietic stress induced by 5-fluorouracil (Figure S3) or phenylhydrazine (Figure S4).
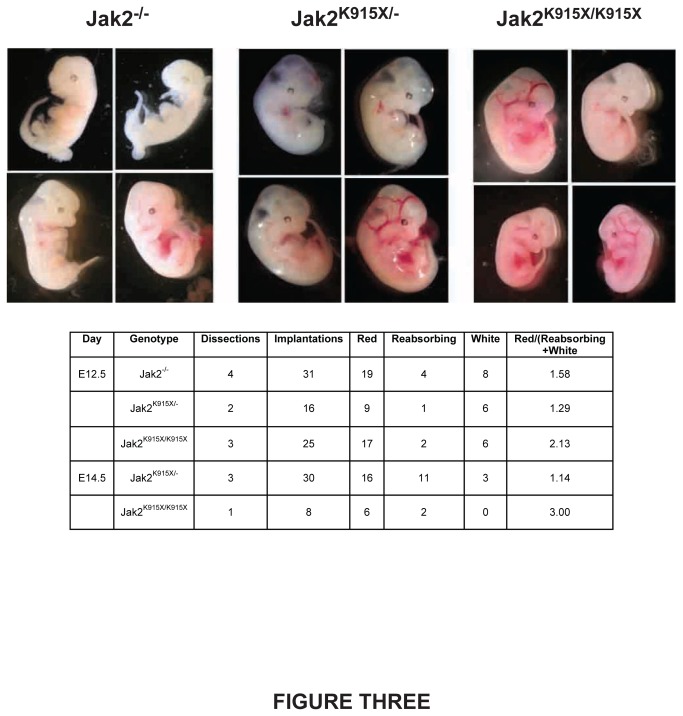
Jak2 -/- embryos die at E12.5 due to a block in fetal erythropoiesis. Timed matings were conducted to generate Jak2 K915X/K915X , Jak2 K915/- and Jak2 -/- embryos and determine whether embryonic lethality is similar between the Jak2 alleles (Figure 3). Embryos were dissected at E12.5 and E14.5 and embryos were segregated into healthy red, anemic white or terminal re-absorbing categories. Reabsorbing and white embryos were assumed to be incapable of producing viable pups. No viable Jak2 -/- embryos were observed at E14.5 from three dissections comprising 26 implantations. However, viable Jak2 K915X/- or Jak2 K915X/K915X embryos were present at E14.5. No viable Jak2 K915X/K915X embryos were identified later than E14.5.
Figure 3. The K915X mutation in Jak2 enhances viability of mouse embryos.
Embryos from timed matings were sacrificed at E12.5-E14.5. Representative embryos from E12.5 are shown. A summary of the results is provided in tabular format.
Discussion
The initial characterization of the Jak2 knockout mouse revealed that Jak2 played a critical role in erythropoiesis and thrombopoiesis, with embryonic lethality observed at E12.5 [14,15]. Since the EPO and EPO-R null mice die at E13.5, the slightly earlier death observed in Jak2 -/- embryos was attributed to the recruitment of Jak2 to other cytokine receptors including the TPO-R. Beyond this initial evaluation of the homozygote mice, little characterization of Jak2 +/- mice has been performed.
Utilizing random mutagenesis, we have demonstrated a critical, yet subtle, role for Jak2 in the regulation of megakaryopoiesis. Loss of one functional allele of Jak2, either through truncation in Jak2 K915X/+ or deletion in Jak2 +/-, leads to elevated platelet production. Mutation of JAK2 is observed in several hematological disorders including ET [8,9,10,11], Polycythemia Vera [8,9,10,11], Primary Myelofibrosis [8,9,10,11] and Acute Lymphoid Leukemia (ALL) [25,26] as well as chromosomal translocations involving the fusion partners TEL [27,28,29], BCR [30], PCM1 [31,32,33], PAX5 [34], SEC 31A [35] and SSBP2 [36]. The JAK2 signaling network also participates in disease mediated by MPL mutations in ET and CRLF2 mutations in T cell ALL [37,38,39,40].
The JAK2 K915X protein product does not appear to possess catalytic activity when phosphorylation of Y570 in the JH2 domain is used to monitor activity. In contrast, phosphorylation of S523 is observed in JAK2 K915X. Mutation of S523 increased catalytic activity of wild type Jak2, suggesting that S523 is a negative regulator of kinase activity [21,41]. Earlier studies suggested that phosphorylation of this residue is mediated by a proline-directed and Mek1-dependent kinase, potentially Erk [21]. Regarding the phenotype observed in Jak2 K915X/+ mice, both EPO [42] and TPO [43] activate Erk kinase activity and phosphorylation of Jak2 K915X could potentiate increased survival observed in timed pregnancy experiments.
Genomic resequencing efforts have identified sporadic nonsense mutations in JAK2. For example, W777X [44] and Q1112X mutations were identified in lung cancer, E890X [45] was observed in colon cancer and E1097X mutation was uncovered in a case of kidney cancer. None of these mutations were recurrent and all but the W777X mutation was confirmed. However, no further characterization of the protein products has been performed and it is unclear how these loss-of-function JAK2 mutations interact with the other genetic abnormalities observed in these patients.
Clinical trials using Jak2 inhibitors including INCB018424 [46,47], CYT387 [48] and SAR302503 (TG101380) [49] have been completed or are underway to treat primary myelofibrosis. Some patients initially responsive to JAK2 inhibition have become insensitive to JAK2 inhibitors. Whether this is due to intrinsic resistance, mutation of JAK2 [50,51,52] and its effectors, persistence due to heterodimerization with other JAK kinases [53] or other mechanisms remains to be investigated. While patients report higher quality-of-life scores, JAK2 inhibitors have not reduced allele burden, potentially due to their ability to target a spectrum of tyrosine kinases [47,54]. No studies have reported a paradoxical thrombocytosis in response to JAK2 inhibition to date. Our research suggests that altering JAK2 activation may lead to unexpected clinical outcomes.
Supporting Information
Erythroid and Megakaryocyte progenitors are unaltered in Jak2K915X/-and Jak2+/- adult mice and do not show cytokine independent growth. (A) CFU-MK frequency in the bone marrow grown in the presence or absence of TPO. (B) Bone marrow CFU-E frequency grown with or without EPO. (C) Splenic CFU-E frequency grown in the presence or absence of EPO. All CFU-E and CFU-Mk were derived from Jak2 K915X/-and Jak2+/- and littermate controls at 12-14 wks of age. Data are presented as ± SEM; n=5-8.
(TIF)
Functional loss of Jak2 in Jak2K915X/-and Jak2+/- does not disrupt CFU-C frequency in the bone barrow or spleen. (A) Total CFU-C frequency in the bone marrow. (B) The frequency of CFU-C in the spleen. (C) CFU-C differential count of bone marrow derived colonies included: CFU-G (granulocyte), CFU-M (monocyte), CFU-GEMM (granulocyte, erythrocyte, monocyte and megakaryocyte), CFU-GM (granulocyte and monocyte) and BFU-E (erythroid). (D) Splenic CFU-C differential. All CFU-C were derived from Jak2 K915X/-and Jak2 +/- and littermate controls at 12-14wks of age. Data are presented as ± SEM; n=5-8.
(TIF)
5FU hematopoietic challenge of Jak2K915X/- and Jak2+/- results in similar recovery. The recovery curves for 5FU induced hematopoietic stress in Jak2 K915X/- (A, C, E) and Jak2 +/- (B, D, F). The recovery curves for red blood cells (A and B), platelets (C and D) and white blood cells (E and F). Data are presented as ± S.D. and n=9-11.
(TIF)
PHZ challenge of erythropoiesis in Jak2K915X/- and Jak2+/-. Red blood cell recovery curves of PHZ challenged of Jak2 +/- (A) and Jak2 K915X/- (B). The data are presented as ± S.D.; n=8-9.
(TIF)
Acknowledgments
We would like to acknowledge Dr. James Ihle for provision of Jak2 deficient mice.
Funding Statement
NA was supported by the Dina Gordon Malkin Ontario Graduate Scholarship in Science and Technology and a Heart and Stroke/Richard Lewar Centre of Ecellence Scholarship. EB was supported by a NSERC Canada Graduate Scholarship award. This sutdy was funded by a group operatiing grant form the Canadian Institutes of Health Research (FRN 74611) to DLB, RP, CW, MDM, KMM and WLS and a grant from the Heart and Stroke Foundation of Ontario (NA-6363) to WLS. WLS is supported by a Canada Research Chair. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tortolani PJ, Johnston JA, Bacon CM, McVicar DW, Shimosaka A et al. (1995) Thrombopoietin induces tyrosine phosphorylation and activation of the Janus kinase, JAK2. Blood 85: 3444-3451. PubMed: 7780132. [PubMed] [Google Scholar]
- 2. Bacon CM, Tortolani PJ, Shimosaka A, Rees RC, Longo DL et al. (1995) Thrombopoietin (TPO) induces tyrosine phosphorylation and activation of STAT5 and STAT3. FEBS Lett 370: 63-68. doi:10.1016/0014-5793(95)00796-C. PubMed: 7544303. [DOI] [PubMed] [Google Scholar]
- 3. Morita H, Tahara T, Matsumoto A, Kato T, Miyazaki H et al. (1996) Functional analysis of the cytoplasmic domain of the human Mpl receptor for tyrosine-phosphorylation of the signaling molecules, proliferation and differentiation. FEBS Lett 395: 228-234. doi:10.1016/0014-5793(96)01047-2. PubMed: 8898102. [DOI] [PubMed] [Google Scholar]
- 4. Pallard C, Gouilleux F, Bénit L, Cocault L, Souyri M et al. (1995) Thrombopoietin activates a STAT5-like factor in hematopoietic cells. EMBO J 14: 2847-2856. PubMed: 7796811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wiestner A, Schlemper RJ, van der Maas AP, Skoda RC (1998) An activating splice donor mutation in the thrombopoietin gene causes hereditary thrombocythaemia. Nat Genet 18: 49-52. doi:10.1038/ng0198-49. PubMed: 9425899. [DOI] [PubMed] [Google Scholar]
- 6. Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA et al. (2006) MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 108: 3472-3476. doi:10.1182/blood-2006-04-018879. PubMed: 16868251. [DOI] [PubMed] [Google Scholar]
- 7. Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL et al. (2006) MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLOS Med 3: e270. doi:10.1371/journal.pmed.0030270. PubMed: 16834459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N et al. (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365: 1054-1061. doi:10.1016/S0140-6736(05)71142-9. PubMed: 15781101. [DOI] [PubMed] [Google Scholar]
- 9. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R et al. (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352: 1779-1790. doi:10.1056/NEJMoa051113. PubMed: 15858187. [DOI] [PubMed] [Google Scholar]
- 10. Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M et al. (2005) The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood 106: 3377-3379. doi:10.1182/blood-2005-05-1898. PubMed: 16081687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James C, Ugo V, Le Couédic, Staerk J, Delhommeau F et al. (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434: 1144-1148. doi:10.1038/nature03546. PubMed: 15793561. [DOI] [PubMed] [Google Scholar]
- 12. Ballmaier M, Germeshausen M, Schulze H, Cherkaoui K, Lang S et al. (2001) c-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood 97: 139-146. doi:10.1182/blood.V97.1.139. PubMed: 11133753. [DOI] [PubMed] [Google Scholar]
- 13. Ihara K, Ishii E, Eguchi M, Takada H, Suminoe A et al. (1999) Identification of mutations in the c-mpl gene in congenital amegakaryocytic thrombocytopenia. Proc Natl Acad Sci U S A 96: 3132-3136. doi:10.1073/pnas.96.6.3132. PubMed: 10077649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC et al. (1998) Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93: 385-395. doi:10.1016/S0092-8674(00)81167-8. PubMed: 9590173. [DOI] [PubMed] [Google Scholar]
- 15. Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U et al. (1998) Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93: 397-409. doi:10.1016/S0092-8674(00)81168-X. PubMed: 9590174. [DOI] [PubMed] [Google Scholar]
- 16. Hughes MR, Anderson N, Maltby S, Wong J, Berberovic Z et al. (2011) A novel ENU-generated truncation mutation lacking the spectrin-binding and C-terminal regulatory domains of Ank1 models severe hemolytic hereditary spherocytosis. Exp Hematol 39: 305-320, e301-302 doi:10.1016/j.exphem.2010.12.009. PubMed: 21193012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ito CY, Li CY, Bernstein A, Dick JE, Stanford WL (2003) Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood 101: 517-523. doi:10.1182/blood-2002-06-1918. PubMed: 12393491. [DOI] [PubMed] [Google Scholar]
- 18. Anderson NM, Berberovic Z, Berndl E, Bailey ML, Flenniken AM et al. (2012) Cytopenia induction by 5-fluorouracil identifies thrombopoietic mutants in sensitized ENU mutagenesis screens. Exp Hematol 40: 48-60. doi:10.1016/j.exphem.2011.09.007. PubMed: 21924221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lenox LE, Perry JM, Paulson RF (2005) BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood 105: 2741-2748. doi:10.1182/blood-2004-02-0703. PubMed: 15591122. [DOI] [PubMed] [Google Scholar]
- 20. Javadi M, Hofstätter E, Stickle N, Beattie BK, Jaster R et al. (2012) The SH2B1 adaptor protein associates with a proximal region of the erythropoietin receptor. J Biol Chem 287: 26223-26234. doi:10.1074/jbc.M112.382721. PubMed: 22669948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mazurkiewicz-Munoz AM, Argetsinger LS, Kouadio JL, Stensballe A, Jensen ON et al. (2006) Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor. Mol Cell Biol 26: 4052-4062. doi:10.1128/MCB.01591-05. PubMed: 16705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Argetsinger LS, Kouadio JL, Steen H, Stensballe A, Jensen ON et al. (2004) Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol Cell Biol 24: 4955-4967. doi:10.1128/MCB.24.11.4955-4967.2004. PubMed: 15143187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bandaranayake RM, Ungureanu D, Shan Y, Shaw DE, Silvennoinen O et al. (2012) Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol 19: 754-759. doi:10.1038/nsmb.2348. PubMed: 22820988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ungureanu D, Wu J, Pekkala T, Niranjan Y, Young C et al. (2011) The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol 18: 971-976. doi:10.1038/nsmb.2099. PubMed: 21841788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bercovich D, Ganmore I, Scott LM, Wainreb G, Birger Y et al. (2008) Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down’s syndrome. Lancet 372: 1484-1492. doi:10.1016/S0140-6736(08)61341-0. PubMed: 18805579. [DOI] [PubMed] [Google Scholar]
- 26. Kearney L, Gonzalez De Castro D, Yeung J, Procter J, Horsley SW et al. (2009) Specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood 113: 646-648. doi:10.1182/blood-2008-08-170928. PubMed: 18927438. [DOI] [PubMed] [Google Scholar]
- 27. Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT et al. (1997) A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278: 1309-1312. doi:10.1126/science.278.5341.1309. PubMed: 9360930. [DOI] [PubMed] [Google Scholar]
- 28. Peeters P, Raynaud SD, Cools J, Wlodarska I, Grosgeorge J et al. (1997) Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood 90: 2535-2540. [PubMed] [Google Scholar]
- 29. Ho JM, Beattie BK, Squire JA, Frank DA, Barber DL (1999) Fusion of the ets transcription factor TEL to Jak2 results in constitutive Jak-Stat signaling. Blood 93: 4354-4364. PubMed: 10361134. [PubMed] [Google Scholar]
- 30. Griesinger F, Hennig H, Hillmer F, Podleschny M, Steffens R et al. (2005) A BCR-JAK2 fusion gene as the result of a t(9;22)(p24 q11.2) translocation in a patient with a clinically typical chronic myeloid leukemia. Genes, chromosomes & cancer 44: 329-333. [DOI] [PubMed] [Google Scholar]
- 31. Reiter A, Walz C, Watmore A, Schoch C, Blau I et al. (2005) The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer research 65: 2662-2667.. [DOI] [PubMed] [Google Scholar]
- 32. Murati A, Gelsi-Boyer V, Adelaide J, Perot C, Talmant P et al. (2005) PCM1-JAK2 fusion in myeloproliferative disorders and acute erythroid leukemia with t(8;9) translocation. Leuk Off J Leukemia Society Of America Leukemia Res Fund UK 19: 1692-1696. doi:10.1038/sj.leu.2403879. [DOI] [PubMed] [Google Scholar]
- 33. Bousquet M, Quelen C, De Mas V, Duchayne E, Roquefeuil B et al. (2005) The t(8;9)(p22;p24) translocation in atypical chronic myeloid leukaemia yields a new PCM1-JAK2 fusion gene. Oncogene 24: 7248-7252.. [DOI] [PubMed] [Google Scholar]
- 34. Nebral K, Denk D, Attarbaschi A, König M, Mann G et al. (2009) Incidence and diversity of PAX5 fusion genes in childhood acute lymphoblastic leukemia. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK 23: 134-143. doi:10.1038/leu.2008.306. PubMed: 19020546. [DOI] [PubMed] [Google Scholar]
- 35. Van Roosbroeck K, Cox L, Tousseyn T, Lahortiga I, Gielen O et al. (2011) JAK2 rearrangements, including the novel SEC31A-JAK2 fusion, are recurrent in classical Hodgkin lymphoma. Blood 117: 4056-4064. [DOI] [PubMed] [Google Scholar]
- 36. Poitras JL, Dal Cin P, Aster JC, Deangelo DJ, Morton CC (2008) Novel SSBP2-JAK2 fusion gene resulting from a t(5;9)(q14.1;p24.1) in pre-B acute lymphocytic leukemia. Genes Chromosomes Cancer 47: 884-889. doi:10.1002/gcc.20585. PubMed: 18618714. [DOI] [PubMed] [Google Scholar]
- 37. Hertzberg L, Vendramini E, Ganmore I, Cazzaniga G, Schmitz M et al. (2010) Down syndrome acute lymphoblastic leukemia, a highly heterogeneous disease in which aberrant expression of CRLF2 is associated with mutated JAK2: a report from the International BFM Study Group. Blood 115: 1006-1017. doi:10.1182/blood-2009-08-235408. PubMed: 19965641. [DOI] [PubMed] [Google Scholar]
- 38. Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin MG, Liu W et al. (2009) Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet 41: 1243-1246. doi:10.1038/ng.469. PubMed: 19838194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russell LJ, Capasso M, Vater I, Akasaka T, Bernard OA et al. (2009) Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood 114: 2688-2698. doi:10.1182/blood-2009-03-208397. PubMed: 19641190. [DOI] [PubMed] [Google Scholar]
- 40. Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K et al. (2010) Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 107: 252-257. doi:10.1073/pnas.0911726107. PubMed: 20018760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ishida-Takahashi R, Rosario F, Gong Y, Kopp K, Stancheva Z et al. (2006) Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling. Mol Cell Biol 26: 4063-4073. doi:10.1128/MCB.01589-05. PubMed: 16705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menon MP, Karur V, Bogacheva O, Bogachev O, Cuetara B et al. (2006) Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J Clin Invest 116: 683-694. doi:10.1172/JCI25227. PubMed: 16511603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rouyez MC, Boucheron C, Gisselbrecht S, Dusanter-Fourt I, Porteu F (1997) Control of thrombopoietin-induced megakaryocytic differentiation by the mitogen-activated protein kinase pathway. Mol Cell Biol 17: 4991-5000. PubMed: 9271377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ et al. (2012) Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 150: 1107-1120. doi:10.1016/j.cell.2012.08.029. PubMed: 22980975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Network. CGA (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487: 330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R et al. (2012) JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366: 787-798. doi:10.1056/NEJMoa1110556. PubMed: 22375970. [DOI] [PubMed] [Google Scholar]
- 47. Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V et al. (2012) A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 366: 799-807. doi:10.1056/NEJMoa1110557. PubMed: 22375971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tyner JW, Bumm TG, Deininger J, Wood L, Aichberger KJ et al. (2010) CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood 115: 5232-5240. doi:10.1182/blood-2009-05-223727. PubMed: 20385788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M et al. (2011) Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29: 789-796. doi:10.1200/JCO.2011.36.9280. PubMed: 21220608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deshpande A, Reddy MM, Schade GO, Ray A, Chowdary TK et al. (2012) Kinase domain mutations confer resistance to novel inhibitors targeting JAK2V617F in myeloproliferative neoplasms. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK 26: 708-715. doi:10.1038/leu.2011.255. PubMed: 21926964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marit MR, Chohan M, Matthew N, Huang K, Kuntz DA et al. (2012) Random mutagenesis reveals residues of JAK2 critical in evading inhibition by a tyrosine kinase inhibitor. PLOS ONE 7: e43437. doi:10.1371/journal.pone.0043437. PubMed: 22916261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weigert O, Lane AA, Bird L, Kopp N, Chapuy B et al. (2012) Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med 209: 259-273. doi:10.1084/jem.20111694. PubMed: 22271575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M et al. (2012) Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature 489: 155-159. doi:10.1038/nature11303. PubMed: 22820254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verstovsek S, Kantarjian HM, Estrov Z, Cortes JE, Thomas DA et al. (2012) Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood 120: 1202-1209. doi:10.1182/blood-2012-02-414631. PubMed: 22718840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Erythroid and Megakaryocyte progenitors are unaltered in Jak2K915X/-and Jak2+/- adult mice and do not show cytokine independent growth. (A) CFU-MK frequency in the bone marrow grown in the presence or absence of TPO. (B) Bone marrow CFU-E frequency grown with or without EPO. (C) Splenic CFU-E frequency grown in the presence or absence of EPO. All CFU-E and CFU-Mk were derived from Jak2 K915X/-and Jak2+/- and littermate controls at 12-14 wks of age. Data are presented as ± SEM; n=5-8.
(TIF)
Functional loss of Jak2 in Jak2K915X/-and Jak2+/- does not disrupt CFU-C frequency in the bone barrow or spleen. (A) Total CFU-C frequency in the bone marrow. (B) The frequency of CFU-C in the spleen. (C) CFU-C differential count of bone marrow derived colonies included: CFU-G (granulocyte), CFU-M (monocyte), CFU-GEMM (granulocyte, erythrocyte, monocyte and megakaryocyte), CFU-GM (granulocyte and monocyte) and BFU-E (erythroid). (D) Splenic CFU-C differential. All CFU-C were derived from Jak2 K915X/-and Jak2 +/- and littermate controls at 12-14wks of age. Data are presented as ± SEM; n=5-8.
(TIF)
5FU hematopoietic challenge of Jak2K915X/- and Jak2+/- results in similar recovery. The recovery curves for 5FU induced hematopoietic stress in Jak2 K915X/- (A, C, E) and Jak2 +/- (B, D, F). The recovery curves for red blood cells (A and B), platelets (C and D) and white blood cells (E and F). Data are presented as ± S.D. and n=9-11.
(TIF)
PHZ challenge of erythropoiesis in Jak2K915X/- and Jak2+/-. Red blood cell recovery curves of PHZ challenged of Jak2 +/- (A) and Jak2 K915X/- (B). The data are presented as ± S.D.; n=8-9.
(TIF)