Abstract
We studied the bee specialist fungus Ascosphaera in wild solitary bees to investigate the diversity of the genus in nature and the ecology of these fungi with their bee hosts. A new morphologically distinctive species was discovered which also has a unique nrITS sequence. This new species, here named Ascosphaera callicarpa, is common on the larval feces of the solitary bee Chelostoma florisomne which nests in the Phragmites reeds of thatched roofs in Europe. Because collections of Ascosphaera from wild bees are scarce and because little is known about the ecology and distribution of the majority of the species in the genus, a key to the species thus far reported for Europe is included.
Introduction
Ascosphaera is a genus of 28 species of bee specialist fungi with a worldwide distribution in the temperate to tropical regions. The genus is remarkable for its host and habitat specificity with all species completing their entire life cycle within the nests of bees (Apoidea: Anthophila). Ascosphaera was first discovered in the early 20th century in Europe after A. apis, the type species, was identified as the causative agent of a brood disease affecting honeybees [1], [2]. This brood disease, known as chalkbrood, was later observed in a solitary bee in London [3]. Ascosphaera is widely known as the chalkbrood fungus, although at least half of the species lead a saprotrophic rather than pathogenic lifestyle [4], [5]. Saprotrophic Ascosphaera species flourish on diverse substrates within the bee nest, for example on pollen provisions, on materials used by the bees to construct the nest and on larval feces [6], [7]. Little is known about these saprotrophs which appear to live innocuously inside the brood cells of the bees. Consequently, the potential for research on the ecological and functional role of these fungi within the bee nest remains wide open.
Ascosphaera is placed in Ascosphaeraceae (Pezizomycotina: Eurotiomycetidae), a small family of ascomycetes primarily characterized by a unique fruiting body type called a spore cyst. Spore cysts are unicellular, cyst-like fruiting bodies that form from the expansion of a single cell called a nutriocyte [8]. The wall of a spore cyst is a double-layered membrane. Asci are free-floating and evanescent. Because of their anomalous fruiting bodies, the taxonomic affinities of Ascosphaera and its relatives remained uncertain until ontological studies led C.F. Spiltoir and L.S. Olive [8] to confidently place them among the Ascomycota within Eurotiomycetidae [as Plectascales]. This position was later confirmed by additional morphological study [9] and DNA sequenced-based phylogenies [10], [11].
A distinguishing feature of Ascosphaera is the presence of spore balls [8]. A spore ball is a compact aggregation of spores formed by groups of asci that are united by a single membrane [12]. The membrane surrounding a spore ball disintegrates and only remnants of it are sometimes observed in mature spore cysts [5]. Spore balls may contain as few as two to as many as several hundred ascospores [5], [13]. The average number of ascospores per spore ball and the persistence of spore balls at maturity are meaningful taxonomic characters.
Pathogenic Ascosphaera species afflict only the larval stage of bees. Typically diseased larvae die in the larval stage; however, in rare occurrences, larvae have been observed to enter pupation before being overcome by the fungus (Wynns pers. obs.). Pathogenic species of Ascosphaera appear to be highly specialized fungi with ascospores typically germinating only when within the midgut of their host. Spore germination is followed by rapid hyphal growth, with the fungus consuming the larva from the inside out [14]. Two widespread pathogenic species, Ascosphaera aggregata and A. apis, are of economic interest because of their potential to negatively impact populations of commercial pollinators, namely Apis mellifera L. and Megachile rotundata (Fabricius) [15], [16].
Although Ascosphaera lives in association with both solitary and social bees the majority of species (25 out of 28) were originally described from solitary bees. Within the nests of solitary bees Ascosphaera grows on pollen provisions where an egg has failed to develop, on larval feces, on the surface of cocoons, within larvae, and on the diverse materials used by different bee species for brood cell construction [4], [5], [6]. Unlike their social relatives (e.g., honey bees), solitary bees lack adult-larva interaction, there is no nursing of the brood and no cooperative behavior (including social immunity) [17]. A consequence of no adult-larva contact and no nursing is that the brood is mass provisioned rather than progressively provisioned like their social counterparts; this means that once an egg hatches the larva has all the food it will need to complete development into an adult [17]. Following their flight and nesting period solitary bees overwinter in their individual brood cells with no activity until emergence the following spring or early summer. In this way solitary bee nests provide a relatively stable, undisturbed micro-environment that appears suitable for the growth of these specialised fungi.
The only monographic work on Ascosphaera [5] focused on collections from an important commercial pollinator in Canada, the alfalfa leafcutting bee Megachile rotundata. While limited in scope, this monograph, which included the first key to the genus, remains the most useful and comprehensive reference for the identification of Ascosphaera species. Given the importance of wild pollinators and their increasing role in buffering the loss of honeybee pollination services [18] a more complete monograph with an updated key to these bee-specialist fungi is much needed.
Seven of the 28 described species of Ascosphaera are currently known from Europe. Here we describe a new species from Denmark occurring in the nests of the wild solitary bee Chelostoma florisomne L. To stimulate interest and to facilitate the identification of Ascosphaera species so far known from Europe, we provide a key and descriptions for these species. Cumulative host reports and species distributions are also included with the hope that this information will result in additional records for these under-collected fungi.
Materials and Methods
Morphological study
Descriptions of spore cysts and ascospores were made from observations of spore cysts mounted in water on a glass slide. Measurements and light photomicrographs were made on an Olympus AX70 Provis light microscope and Olympus SZX16 dissecting microscope. Herbarium acronyms follow those of Index Herbariorum [19].
Culture and isolation
Attempts to isolate and culture the fungus were made by placing spore cysts and hyphae on three different solid agar media: malt agar with 20% dextrose (MY20), V8® agar with 2% yeast extract (V8YE), and malt extract agar (MEA). To induce spore germination spore suspensions were prepared from spore cysts placed in a modified V8 spore germination broth [20] and exposed to CO2 as described in Wynns et al. [13].
Molecular study
Genomic DNA was obtained by plucking 5–10 spore cysts and grinding them inside a 1.5 ml Eppendorf tube. DNA was isolated using the Qiagen DNeasy Plant Mini Kit (Hilden, Germany) using the standard protocol and eluted in two separate 50–100 µl fractions to avoid over-dilution.
We sequenced the entire nuclear ribosomal ITS region (ITS1-5.8S-ITS2) for A. callicarpa sp. nov. Genomic DNA was amplified using ITS1F and ITS4 primers [21]. PCR reactions were prepared for a final 50 µl volume containing 29.8 µl of sterile deionized water, 5 µl of Taq polymerase reaction buffer (Sigma®), 1.0 µl 10 mM dNTPs, 3.0 µl 25 mM MgCl2, 0.2 µl Taq DNA polymerase (Sigma®), 5.0 µl each 10 µM primer and 1 µl of genomic DNA template. PCR was performed on a Biometra® thermocycler (Whatman) under the following conditions: step 1) 1 min at 95 C, 2) 45 sec at 95 C, 3) 40 sec at 52 C, 4) 1 min 30 sec at 72 C, 5) return to step 2 30 times, 6) final step of 10 min at 72 C. Samples were kept at 4 C until electrophoresis was performed on a 1% agarose TAE gel and visualized with EZvision One® (Amresco). PCR reactions were cleaned using Qiaquick® PCR purification kit (Qiagen) and sent to Eurofins MWG Operon AG (Ebersberg, Germany) for sequencing. The nucleotide sequence was assembled using BioEdit [22] and subjected to a BLASTn search in GenBank.
Nomenclature
The electronic version of this article in Portable Document Format (PDF) in a work with an ISSN or ISBN will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants, and hence the new names contained in the electronic publication of a PLOS ONE article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies. In addition, new names contained in this work have been submitted to MycoBank from where they will be made available to the Global Names Index. The unique MycoBank number can be resolved and the associated information viewed through any standard web browser by appending the MycoBank number contained in this publication to the prefix http://www.mycobank.org/MB/. The online version of this work is archived and available from the following digital repositories: PubMed Central, LOCKSS.
Results and Discussion
Culture and isolation
Despite repeated attempts, we were unable to obtain in vitro mycelial growth or induce ascospore germination of Ascosphaera callicarpa.
Molecular study
An ITS sequence was obtained for A. callicarpa (GenBank accession: JX070046). A BLASTn search of the ITS sequence revealed a highest sequence-similarity to other Ascosphaera species.
Key to European species of Ascosphaera
1. Wall of spore cyst smooth; ascospores cylindrical with rounded ends, (3.1–) 4.0×1.6(−2.0) µm; saprotrophA. callicarpa A.A. Wynns (Fig. 1)
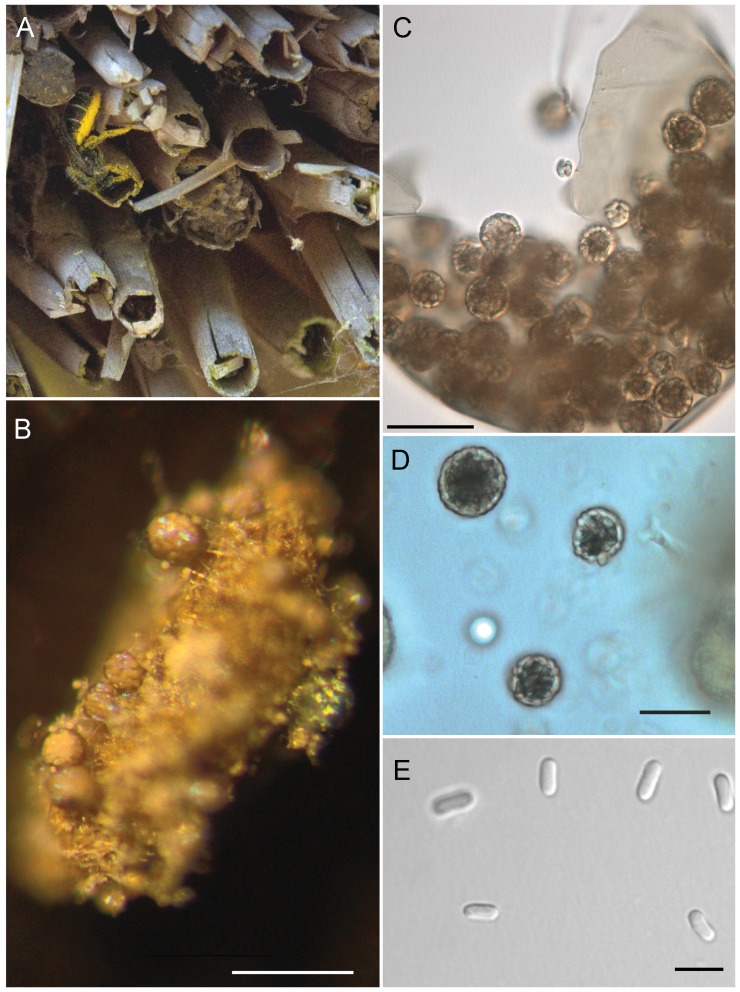
Figure 1. Ascosphaera callicarpa.
A) habitat. Phragmites reeds and female Chelostoma florisomne returning with pollen for her brood. B) fecal pellet of C. florisomne larva covered with spore cysts; pale spore balls are visible through the transparent spore cyst wall. C) close-up of spore cyst showing spore balls and smooth, unornamented spore cyst wall. D) spore balls. E) bacilliform ascospores. B, photographed from A.A. Wynns 5168; C–E from A.A. Wynns 5166. Scale bars: B = 200 µm, C = 50 µm, C = 10 µm, D = 15 µm, E = 10 µm.
1. Wall of spore cyst with minute or conspicuous dark spots; ascospores not cylindrical; saprotroph or pathogen2
2. Ascospores broadly sub-falcate, with a tendency to be trigonal when view on-end, 1.9–3.5×0.6–0.9 µm; saprotrophA. tenax Skou (Figs. 2A–B)
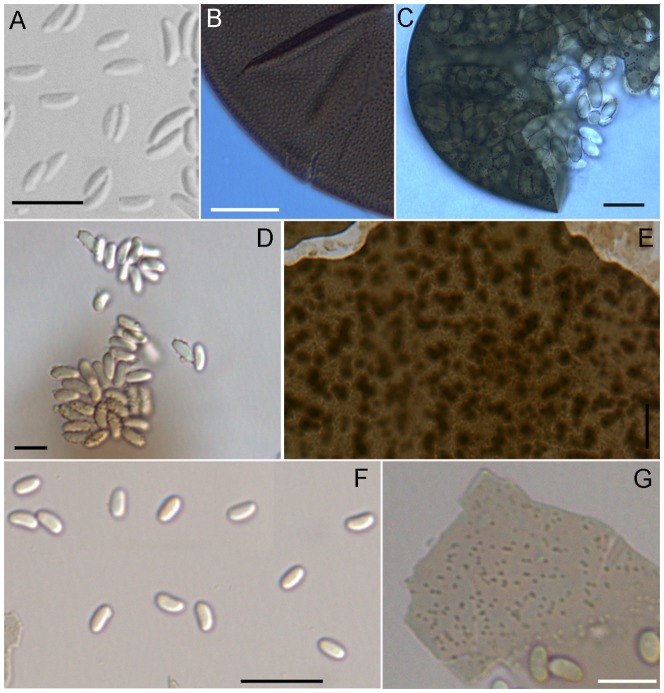
Figure 2. Light microphotographs of Ascosphaera tenax, A. atra, A. major, and A. apis.
Ascosphaera tenax A) ascospores. B) punctate spore cyst wall.; A. atra C) broken spore cyst with ascospores.; A. major D) ascospores with attached granules. E) close-up of spore cyst wall.; A. apis F) ascospores. G) detail of pale spore cyst wall with minute spots. A–B photographed from holotype; C from ARSEF 693; D from A.A. Wynns 5170; E from A.A. Wynns 5175; F from A.A. Wynns 5174. Scale bars A = 5 µm, B–C = 10 µm, D = 5 µm, E–F = 10 µm.
2. Ascospores not sub-falcate or trigonal in cross section3
3. Ascospores always >2 µm wide, 4–7.9×2.3–6.5 µm, ellipsoid to broadly ellipsoid; spore balls not persistent; saprotrophA. atra Skou & K. Hackett (Fig. 2C)
3. Ascospores otherwise; spore balls persistent; pathogen or saprotroph4
4. Spore cysts not exceeding 125 µm diameter5
4. Spore cysts mostly exceeding 125 µm diameter6
5. Ascospores 3.0–5.0×1.3–1.8 µm; at least some ascospores and spore balls with attached granules; spore cyst wall brown with small spots visible at low magnification; saprotrophA. fimicola Skou (Fig. 3)
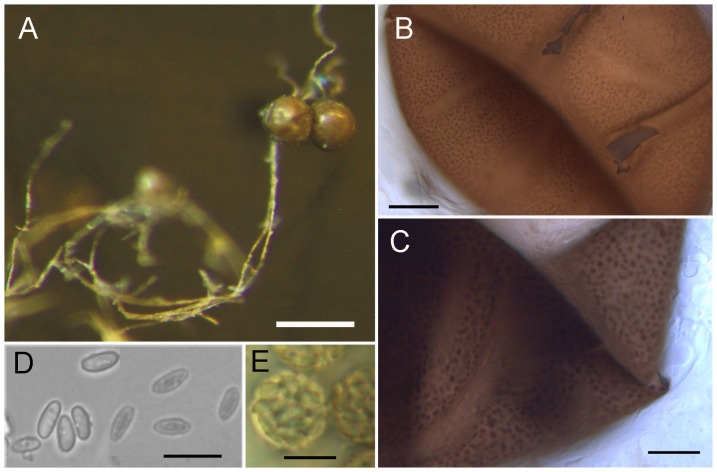
Figure 3. Light microphotographs of Ascosphaera fimicola.
A) two opaque, iridescent spore cysts still attached to hyphae. B–C) close-up of spore cyst showing maculate wall, D) ellipsoid ascospores with a few small granules attached to their surface. E) spore ball. A, photographed from A.A. Wynns 5167; B–C, E from A.A. Wynns 5130; D from J.P. Skou s.n. (paratype). Scale bars: A = 500 µm, B = 20 µm, C = 10 µm, D = 5, µm E = 15 µm.
5. Ascospores 2.1–3.9×1.1–1.7 µm; ascospores and spore balls always without granules; spore cyst wall pale greenish to yellowish brown, with nearly smooth walls, minute spots visible at high magnification; obligate parasite, cause of chalkbrood disease of honeybeesA. apis (Maasen ex Claussen) L.S. Olive & Spiltoir (Figs. 2F–G)
6. Ascomata often ≥400 µm diameter, forming a dense layer beneath the cuticle of bee larvaewith chalkbrood disease; ascospores 3.4–5.9×1.3–2.6 µm, ellipsoid, sub-cylindrical or allantoid; obligate pathogenA. aggregata Skou (Figs. 4A–B, E–F)
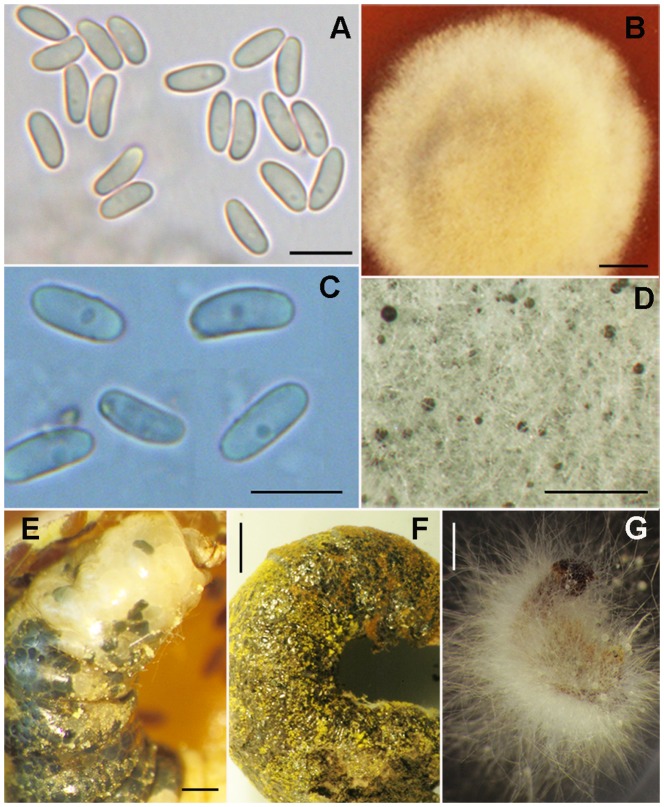
Figure 4. Light microphotographs of Ascosphaera aggregata and A. proliperda.
Ascosphaera aggregata A) ascopores. B) culture on V8 agar medium showing pale buff mycelium.; A. proliperda C) ascospores. D) culture on SDA showing white mycelium and scattered black spore cysts. E–F) bee larvae with chalkbrood caused by A. aggregata. E) fresh Chelostoma florisomne cadaver showing mature (black) and immature (white) spore cysts below the cuticle. F) dry Osmia bicornis larva swollen from fungal growth and spore cyst production below the cuticle. G) aerial hyphae and spore cysts on bee larva with chalkbrood caused by A. proliperda. A, photographed from A.A. Wynns 5144; B from A.A. Wynns 5162; C from A.A. Wynns 5055. Scale bars: A = 10 µm, B = 2 cm, C = 5 µm, D = 825 µm, E = 2 mm, F = 1 mm, G = 2 mm.
6. Spore cysts mostly less than and not exceeding 400 µm in diameter, developing on aerial hyphae above the cuticle of larvae with chalkbrood disease or growing saprotrophically on the cocoon, feces or leaf lining of a brood cell7
7. Ascospores (2.4–) 2.8–4.0 (−5.0)×1.0–1.8 (−2.0) µmA. major (Prökschl & Zobl) Skou (Figs. 2D–E)
7. Ascospores 3.5–6.5×1.7–3.5 µmA. proliperda Skou (Figs. 4C–D,G)
Taxonomy
Ascosphaera aggregata Skou, Friesia 11: 64, 1975.
Type: DENMARK.ThurØ, on larvae of Osmia rufa L., J.P. Skou s.n. (holotype, c!).
Description
Mating system unknown but possibly homothallic [4]. Pathogenic. Infected larvae swollen, black, and filled with a solid core of pale buff mycelium. Ascomata black to dark brown spore cysts produced below surface of larval cuticle in a crowded continuous layer [23] or scattered and appearing as small individual boils [24], 280–750×130–290 µm, spherical or conical and faceted from being tightly packed beneath the larval cuticle; wall light reddish brown to black, minutely punctate. Spore balls pale brown to yellowish brown with small brown granules attached to surface, 9–25 µm diameter, mostly persistent. Ascospores ellipsoid to sub-cylindrical or allantoid, 3.4–5.9×1.3–2.6 µm. Culture on V8YE with moderate growth after 14 days, low, pale buff with a darker, brownish centrum with age, occasionally producing nutriocytes on aerial hyphae (A.A.Wynns pers. obs.) and immature spore cysts below agar surface [4].
Ecology and distribution
Ascosphaera aggregata is an obligate pathogen with a preference for bees belonging to the family Megachilidae. This species has a broad distribution, with reports from both North America and Europe. Like A. apis, the distribution of A. aggregata is probably closely tied to the exchange and transport of bees (e.g. M. rotundata) for the pollination of commercial crops. In Europe A. aggregata is known from Denmark [23], Germany [present study], Spain [23], [25] and Sweden [present study]. Attempts to isolate and grow A. aggregata in culture often results in the co-isolation of another pathogenic species, A. proliperda. Although A. proliperda and A. aggregata can be difficult to separate based on microscopic morphological features their growth in culture is strikingly different (Figs. 4B,D; see also A. proliperda for further discussion on its co-occurrence with A. aggregata).
Additional specimens examined
CANADA. Alberta: Brooks, on larvae of M. rotundata, 1988, J Jakobsen s.n. (c). DENMARK. Thurø: Svendborg Kommune, on Osmia rufa ( = O. bicornis L.), 1974, J.P. Skou s.n., paratype (c); On O. rufa, 1974, J.P. Skou s.n., holotype, (c); Zealand: Frederikssund Kommune, Slangerup, organic apple orchard, on larva of O. rufa, 2010, A.A. Wynns 5152; Roskilde Kommune, Roskilde, on larva of O. rufa, 2010, A.A. Wynns 5144; Taastrup Kommune, Taastrup, Højbakkegård, on larvae of O. rufa, 2010, A.A. Wynns 5145 (c). USA. Nevada: On Megachile pacifica (Panzer) ( = M. rotundata), 1975, K. Hackett s.n. paratype (c). SPAIN: On M. rotundata, 1972, J.P. Skou s.n., paratype (c); on M. rotundata, 1973, J.P. Skou s.n. paratype (c).
Ascosphaera apis (Maasen ex Claussen) L.S. Olive & Spiltoir in Spiltoir & Olive, Mycologia 47: 242, 1955.
≡ Pericystis apis Maasen ex Claussen, Mitt. biol. BundAnst. Ld-u. Forstw.10: 470. 1921.
Description
Mating system heterothallic. Pathogenic. Infected larvae shrunken, pale buff, covered by a weft of hyphae, with or without the production of ascomata. Ascomata greenish (immature) to black (mature) spore cysts produced on aerial hyphae above the larval cuticle, 40–119 µm in diameter; wall pale greenish to yellowish-brown, nearly smooth with minute punctae at high magnification. Spore balls hyaline to pale yellowish, without granules, 7–20 µm in diameter, mostly persistent. Ascospores ellipsoid to sub-allantoid, 2.1–3.9×1.1–1.7 µm. Culture on SDA with rapid growth after 2–6 days, white with abundant production of spore cysts when both mating strains are present.
See Skou [7], Bissett [5], and Aronstein & Murray [26] for additional descriptions.
Ecology and distribution
Ascosphaera apis is an opportunistic pathogen of honeybees. Experimental trials showed A. apis is able to induce chalkbrood in the solitary bee M. rotundata [14]; however, A. apis is not known to live in association with solitary bees in nature. Reports of chalkbrood caused by A. apis in solitary bees before 1972 are most likely attributable to pathogenic species described after this time; e.g., A. major, A. aggregata or A. proliperda (the later two species are pathogens specific to solitary bees). Originally described from Germany, A. apis, is now known from all continents where honeybees are kept.
Additional specimens examined. USA. Texas: Weslaco, 26 Jun 2003, K.D. Murray s.n., ARSEF 7405 (+), 7406 (−).
Ascosphaera atra Skou & K. Hackett, Friesia 11: 279, 1979.
Type: U.S.A. Nevada, isolated from larva of Megachile pacifica with ragged-brood disease, 36836 (c), CBS 524.75 (holotype, c!).
Description
Mating system homothallic. Ascomata black, globose spore cysts, 30–140 µm; wall dark brown, punctate, punctae appearing as uniform dark circles often of variable size. Spore balls hyaline to pale yellowish brown, 8–17 µm diameter, evanescent. Ascospores ellipsoid to broadly ellipsoid, 4–7.9×2.3–6.5 µm, with or without small granules attached to the surface of the spore wall. Culture on SDA with moderate growth after 7 days, white to greyish-buff with abundant production of black spore cysts on aerial hyphae and on hyphae growing beneath the surface of the agar.
Ecology and distribution
Ascosphaera atra is a fast-growing saprotroph associated primarily with solitary bees. This species is typically found growing on pollen provisions. Less common substrates from which A. atra has been isolated include the surface of a diseased M. rotundata larva with chalkbrood caused by A. aggregata [27], from pollen within the gut of an otherwise healthy M. rotundata larva [7] and from the honey of A. mellifera [4]. Ascosphaera atra is the only species of the genus that has been found growing on plant material (grass silage) outside of the bee habitat [6]. Pathogenicity studies [14], [27] demonstrated that A. atra is not a pathogen of solitary bees; however, Vojvodic et al. [28] concluded that it is a weak pathogen of honeybees. More work is needed to determine if A. atra is comparable to some of its bee- pathogen congeners e.g. A. aggregata and A. apis. The perceived pathogenicity of this species in honey bee larvae may be more closely tied to its rapid growth on suitable substrates. Ascosphaera atra is the most extensively studied saprotrophic species of Ascosphaera. This is reflected in the multiple reports from N. America [4], [14], Europe [[6], present study], New Zealand and Australia [4].
Additional specimens examined. Ascosphaera atra. AUSTRALIA. Peel: Waroona, A. mellifera honey, Nov 1994, D.L. Anderson 198, ARSEF 5147. CANADA. Alberta: Beaverlodge, Peace River region, from pollen in M. rotundata cells, Jan 1985, D. Farney s.n., DAOM 188981. USA. Oregon: Ontario. M. rotundata, Jun 1979, J.D. Vandenberg 6, ARSEF 693.
Ascosphaera callicarpa A.A. Wynns, sp. nov. [urn:lsid:indexfungorum.org:names:518624]
Type. DENMARK. Zealand: Lejre Kommune, Sagnlandet (“Land of Legends”) Lejre, Landbohusene, on fecal pellets of Chelostoma florisomne nesting in the Phragmites reeds of thatched roof of shed behind 19th century cottage, 55°37′11″N; 11°22′13″, 2010, A.A.Wynns 5165 (holotype, c).
Description
Mating system unknown. Ascomata pale brown, semi-transparent and somewhat iridescent spore cysts (Fig. 1B), globose to subglobose 64–101 µm in diameter; wall smooth (Fig. 1C). Spore balls 10–16 µm in diameter, center grayish–brown to colorless, ascospores arranged spirally or not (Fig. 1C, D). Ascospores bacilliform, (3.1–)4.0×1.6(−2.0) µm, colorless or slightly brownish (Fig. 1D–E); no attached granules. Mycelium sparse, white. No growth in culture on MY20, V8YE or MEA; no spore germination in V8 spore germination broth, either with or without the addition of carbon dioxide.
Ecology and distribution
Common in the nest reeds of the solitary bee C. florisomne where it grows on the fecal pellets of this bee. Although not definitely known, the distribution of A. callicarpa is probably closely tied to that of C. florisomne. This fungus was not found in association with other bees, e.g. Osmia and Megachile, although these bees were observed nesting in the same Phragmites reeds as C. florisomne. Ascosphaera callicarpa appears to be solely saprotrophic; it was not found in association with diseased bees or where a larva had failed to develop. Ascosphaera callicarpa is so far known only from the island of Zealand, Denmark.
Etymology
The epithet callicarpa means with beautiful fruits, here referring to the spore cysts.
Preliminary conservation status
Ascosphaera callicarpa should be sought in other aggregations of C. florisomne in thatched roofs throughout Europe in order to assess its conservation status. As a possible obligate associate of the bee C. florisomne, the conservation of this fungus should be considered dependent on the conservation of its host.
Additional specimens examined
DENMARK. Zealand: Lejre Kommune, Sagnlandet Lejre, Landbohusene, shed behind 19th century houses. All specimens on fecal pellets of C. florisomne nesting in the Phragmites reeds of the thatched roof, 2008, A.A.Wynns 5011, 5012, 5013, 5014, 5015, 5018, 5025, 5026, 5027, 5072, 5074, 5136, all specimens in c; 2011, A.A.Wynns 5166, 5168 (c); Sorø Kommune, Kristiansminde, University of Copenhagen field station, east facing side of classroom building, growing on the fecal pellets of C. florisomne nesting in Phragmites reeds of the thatched roof, 2012, A.A. Wynns 5169, 5170 (c).
Morphological comparison of A. callicarpa with A. fimicola
Ascosphaera callicarpa most closely resembles A. fimicola Skou which also grows on the fecal pellets of bees. This new species is distinguishable from A. fimicola by a pale brown, highly transparent fragile spore cyst (Fig. 1B–C) with a wall (Fig. 1C) that is not sculptured or maculate as in A. fimicola (Fig. 3B–C). The spore cysts of A. fimicola (Fig. 3A) are dark brown to pale brown, also somewhat iridescent, and if transparent, not as strikingly so as in A. callicarpa (Fig. 1B). The spores of A. fimicola are ellipsoid-fusiform (Fig. 3D) and often have small brown granules attached to their surface while the spores of A. callicarpa are bacilliform (Fig. 1E) without surface granules. Ascosphaera callicarpa grows on digested Ranunculus pollen voided by C. florisomne. It is not clear if A. callicarpa grows on pollen collected from other plants since C. florisomne is strictly oligolectic on Ranunculus species [29].
Ascosphaera fimicola Skou, Friesia 11: 68, 1975.
Type: DENMARK, ThurØ, on fecal pellets from larvae of Osmia rufa, J.P. Skou s. n., (holotype, c!).
Description
Mating system not known. Ascomata light to dark brown, somewhat iridescent, glistening spore cysts, (25–) 64–125 µm in diameter; wall brown, punctate, punctae minute and of uniform in size. Spore balls yellowish, with small granules on the surface, (5–)10–15(−20) µm in diameter, mostly persistent. Ascospores ellipsoid to sub-allantoid, 3.0–5.0×1.3–1.8 µm with or without small granules attached to the spore wall. Mycelium on natural substrate noticeable, stringy, white and opaque. No growth in culture.
Ecology and distribution
Ascosphaera fimicola grows saprotrophically on the larval feces and cocoons of the solitary bee Osmia bicornis (syn. O. rufa) and was recently collected on the larval feces of Cacoxenus indagator (Diptera: Drosophilidae) a cleptoparasite of this bee. Despite extensive collecting, A. fimicola was not found on the larval feces of the solitary bee C. florisomne. The composition of the pollen provisions of these bees may play a role in the absence or presence of A. fimicola in their nests. Chelostoma florisomne feeds exclusively on pollen from the plant genus Ranunculus (Ranunculaceae) [29] while O. bicornis often collects pollen from the plant family Rosaceae [30]. The last report of A. fimicola prior to our study was in 1975 [23]. We found that this species is more common than the previous few collections indicate. The known distribution of A. fimicola is restricted to Denmark but, like other species in the genus, this narrow distribution is most likely an artifact of under-collecting because of a more focused interest in the pathogens rather than the saprotrophs.
Additional specimens examined
DENMARK. Zealand: Taastrup Kommune, Højbakkegård Allé 3, on feces of Cacoxenus indagator in O. rufa brood cell, 2008, A.A. Wynns 5123 (c); on feces and cocoon of O. rufa, 2010, A.A. Wynns 5147, 5167 (c). Frederikssund Kommune, Slangerup, residence and farm of Verner Andersen, on pollen and feces in nest cell of O. rufa, 2010, A.A. Wynns 5149, 5151 (c). langeland: Langeland Kommune, Rudkøbing, Skovsgaard, Kågårdsvej 12, on cocoon of O. rufa and C. indagator, 2008, A.A. Wynns 5130 (c); on cocoon and feces of O. rufa, 2008, A.A. Wynns 5131 (c). THURØ: Svendborg Kommune, on fecal pellets of O. rufa, 1972, J.P. Skou s.n., paratype (c).
Ascosphaera major (Prökschl & Zobl) Skou, Friesia 10:15, 1972.
type: DENMARK. Zealand: Glostrup, isolated from chalkbrood cells of Megachile centuncularis, cbs 686.71 (neotype, cbs h-9050, non vidi).
≡Pericystis apis Maasen ex Claussen var. major Prökschl & Zobl in Prökschl, Arch. Microbiol. 18: 200. 1953.
≡Ascosphaera apis (Maasen ex Claussen) L.S. Olive & Spiltoir var. major (Prökschl & Zobl) L.S. Olive & Spiltoir in Spiltoir & Olive, Mycologia 47: 243. 1955.
Description
Mating system heterothallic. Ascomata dark brown to black, spore cysts, 60–150(−380) µm in diameter; wall greenish brown, with indistinct puntcae or small granules attached to the inner surface, occasionally with larger crystalliferous brown precipitations with age. Spore balls hyaline to greyish-brown, (6–)14–18(−24) µm in diameter, usually with granules attached to the surface. Ascospores suballantoid or bacilliform. (2.4–)2.8–4.0(−5.0)×1.0–1.8(−2.0) µm, at least some with small granules attached to the spore wall. Mycelium white to greyish-white. Culture on V8YE with moderate growth after 10 days, with abundant production of spore cysts when both strains are present.
Ecology and distribution
Ascosphaera major causes chalkbrood in Apis mellifera [31], [32] and Megachile centuncularis [7]. It is more often found growing saprotrophically on larval feces within the brood cells of M. centuncularis [7]. In the present study A. major was found growing on the larval feces and leaf material lining the brood cell of a species of Megachile and on the larval feces and pollen provisions of O. bicornis. The frequency of A. major as a cause of chalkbrood in honeybees is not known. Outwardly A. apis and A. major induce the same disease symptoms; therefore, the etiology of chalkbrood in honeybees should be carefully verified by morphological study of the fungus to distinguish infections by A. apis or A. major or to identify co-infection with both species. Ascosphaera major is known from N. America [33], [34] and Europe. In Europe this species is reported from Switzerland [32], Austria [31] and Denmark [7], [9].
Additional specimens examined
DENMARK. Zealand: Frederikssund Kommune, Slangerup, organic apple orchard belonging to Verner Andersen, growing on pollen and feces of O. bicornis, 2010, A.A. Wynns 5150 (c); Lejre Kommune, Lejre Forsøgscenter, growing on cocoon and between walls of leaf lining of healthy Megachile sp., 2008, A.A. Wynns 5038 (c); Roskilde Kommune, Roskilde, Gøderupvej 5, on leaf-lining of brood cell belonging to Megachile sp., 2010, A.A. Wynns 5173, on larval feces of Megachile sp. without disease, 2010, A.A. Wynns 5175.
Ascosphaera proliperda Skou, Friesia 10: 15. 1972.
type: DENMARK. Zealand: Frederiksberg Kommune, Frederiksberg, in Megachile centuncularis larvae collected from the greenhouse of the Royal Veterinary and Agricultural University, J.P. Skou s.n., Jun 1967, CBS 687.71 (holotype, cbs h-6723, non vidi).
Description
Mating system homothallic [5]. Pathogenic. Infected larvae shrunken, covered by erect or low compact aerial hyphae bearing ascomata. Ascomata black spore cysts produced on tips of aerial hyphae above the larval cuticle, 60–250(−400) µm in diameter; wall dark-brown, appearing mottled from the confluence of very fine granules on the inner surface. Spore balls pale brown to sub-hyaline, 9–17(−25) µm, often with small brown granules on the surface. Ascospores sub-cylindrical or sub-allantoid, 3.5–6.5×1.7–3.5 µm, hyaline to sub-hyaline, with or without minute granules attached to the surface. Culture on MY20 with rapid growth after 7 days, white with abundant production of spore cysts.
Ecology and distribution
Ascosphaera proliperda causes chalkbrood in Megachile centuncularis [9], M. rotundata [35] and O. bicornis [present study]. Ascosphaera proliperda has repeatedly been isolated from surface sterilized chalkbrood cadavers of M. rotundata and O. bicornis infected with A. aggregata [15], [35]. Interestingly, these cadavers exhibit typical symptoms for A. aggregata infection i.e. the cuticle of the host is intact with spore cysts just below the cuticle rather than above the cuticle as is typical for A. proliperda. The co-occurrence of A. proliperda and A. aggregata in chalkbrood larvae adds to the difficulty of isolating A. aggregata since the former species is much faster growing. Unless isolation or PCR diagnostics (see [36]) are attempted, A. proliperda can be easily overlooked in chalkbrood cadavers where A. aggregata is the predominant fungus. Ascosphaera proliperda is known from Europe [9] and N. America [35]. This species may prove to be more widespread if possible co-infections with A. aggregata in chalkbrood larvae are taken into consideration.
Additional specimens examined
GERMANY. Isolated from the surface of a sterilized A. aggregata chalkbrood cadaver of O. bicornis, 2010, leg. T. Conrad, A.A. Wynns 5055 (c).
Ascosphaera tenax Skou & S.N. Holm, Mycotaxon 35: 212, 1989.
Type: DENMARK, Nekselø: Kalundborg Kommune, inside cocoons of Megachile willughbiella, 1985, J.P. Skou s.n., (holotype, c!).
Description
Mating system unknown. Ascomata lustrous black, less often dark brown, spore cysts, (33–)40–90(−105) µm diameter; wall dark brown,1.5 µm thick, tough and leathery, smooth or minutely punctate. Spore balls hyaline, (7.7–)9–14(−15.4) µm diameter. Ascospores sub-falcate, with a tendency to be trigonal when viewed on-end, 1.9–3.5×0.6–0.9 µm.
Ecology and distribution
Ascosphaera tenax grows saprotrophically on pollen provisions, larval feces and the inner side of cocoons of Megachile willughbiella and M. rotundata. Spore cysts are common beneath the inside of the leaf cap of Megachile cells. The last collections of A. tenax date from 1988, when the species was found growing in nearly half (18 out of 44) M. willughbiella cocoons examined [24]. Ascosphaera tenax is known only from Denmark on the islands of Nekselø and Zealand. More focused collecting is needed to determine its real geographical range.
Conclusion
Our study is the first to provide a regional key to Ascosphaera. With the addition of A. callicarpa sp. nov., eight Ascosphaera species are now known from Europe (Table 1). Our collections of A. fimicola (see discussion under A. fimicola) from a dipteran cleptoparasite of Osmia bicornis add to the mounting evidence that, although undoubtedly a bee specialist, Ascosphaera is not restricted to bees; further evidence includes an isolated report of the saprotroph A. atra growing on grass [6] and molecular based identification of Ascosphaera DNA from Eristalis (Diptera: Syrphidae) and Vespula (Hymenoptera: Vespidae) species [37]. As previously suggested by Wynns [42], Ascosphaera should be sought outside the bee habitat in association with other pollenivorous insects and where high-sugar substrates are available. Reports of Ascosphaera in non-apoidean insects are quite possibly relevant for the control of chalkbrood in commercial bee pollinators since these insects may act as pathogen reservoirs or vectors of Ascosphaera. More frequent collections of Ascosphaera are needed to begin to grasp the diversity and ecology of these fungi in nature and to elucidate their potentially significant role within the bee habitat. Additional regional keys, such as the one provided here, may ease identification for the non-specialist and bring attention to the lesser-known species of both saprotrophs and pathogens.
Table 1. Distribution, host and substrate reports of Ascosphaera species in Europe.
| Ascosphaera species | Distribution | Hosts | Substrate |
| aggregata | cosmopolitan | Coelioxys echinata | larvae [25] |
| Megachile pugnata | larvae [38] | ||
| M. relativa | larvae [39] | ||
| M. rotundata | larvae [5], [23] | ||
| Osmia bicornis | larvae [23] | ||
| apis | cosmopolitan | Apis cerana | larvae [40] |
| A. mellifera | larvae [2], [26] | ||
| Xylocopa californica | larvae [41] | ||
| atra | N. America, Europe, Oceania | Chalicodoma aethiops | pollen provisions [4] |
| M. rotundata | pollen provisions [5], surface of chalkbrood larva [6], pollen inside the gut of a healthy larva [27] | ||
| Megachile sp. | leaf-lining of brood cell* | ||
| — | grass silage [6] | ||
| callicarpa | Denmark | Chelostoma florisomne * | larval feces* |
| fimicola | Denmark | Cacoxenus indagator (Diptera)* | larval feces* |
| O. bicornis | cocoon*, pollen provisions*, larval feces [23] | ||
| major | Europe | Anthophora pacifica | larval feces [34] |
| Apis mellifera | larvae [31], [33] | ||
| M. centuncularis | larval feces [7], leaf-lining of brood cell [7], cocoon [7] | ||
| M. inermis | cell lining [34] | ||
| O. bicornis * | pollen provisions* | ||
| proliperda | Denmark, N. America | M. centuncularis | larvae [9] |
| N. America | M. rotundata | larvae [35] | |
| O. bicornis * | larvae* | ||
| tenax | Denmark | M. rotundata | cocoon [24], larval feces [24], pollen provisions [24] |
| M. willughbiella | cocoon [24], larval feces [24], pollen provisions [24] |
newly reported in present study.
Acknowledgments
We thank Holger Philipsen and Louise Lee Munk Larsen for field assistance, the Land of Legends (Sagnlandet Lejre) for kindly allowing us to collect bees from their thatched roofs and Justin Wynns.
Funding Statement
This work was supported by the University of Copenhagen and the Danish National Research Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maasen A (1916) Über Bienenkrankheiten. Mitt k Biol Anst Land-Fortsw 16: 51–58. [Google Scholar]
- 2. Claussen P (1921) Entwichlungsgeschichtliche Untersuchungen über den Erreger der als Kalkbrut bezeichneten Krankheit der Bienen. Arb Biol Reichsanst f Land u Fortsw 10: 467–521. [Google Scholar]
- 3. Melville R, Dade HA (1944) Chalk brood attacking a wild bee. Nature 153: 112–112. [Google Scholar]
- 4. Anderson DL, Gibson NL (1998) New species and isolates of spore-cyst fungi (Plectomycetes: Ascosphaerales) from Australia. Aust Syst Bot 11: 53–722. [Google Scholar]
- 5. Bissett J (1988) Contribution toward a monograph of the genus Ascosphaera . Canadian Journal of Botany-Revue Canadienne De Botanique 66: 2541–2560. [Google Scholar]
- 6. Skou JP (1986) Notes on the habitats, morphology and taxonomy of spore cyst fungi. Apimondia 30: 260–264. [Google Scholar]
- 7. Holm SN, Skou JP (1972) Studies on trapping, nesting and rearing of some Megachile species (Hymenoptera, Megachilidae) and on their parasites in Denmark. Entomol Scand 3: 169–180. [Google Scholar]
- 8. Spiltoir CF, Olive LS (1955) A reclassification of the genus Pericystis-Betts. Mycologia 47: 238–244. [Google Scholar]
- 9. Skou JP (1972) Ascosphaerales. Friesia 10: 1–24. [Google Scholar]
- 10. Berbee ML, Taylor JW (1992) Two ascomycete classes based on fruiting body characters and ribosomal DNA sequence. Molecular Biology and Evolution 9: 278–284. [DOI] [PubMed] [Google Scholar]
- 11. Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, et al. (2006) Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98: 1053–1064. [DOI] [PubMed] [Google Scholar]
- 12. Spiltoir CF (1955) Life Cycle of Ascosphaera apis (Pericystis apis). American Journal of Botany 42: 501–508. [Google Scholar]
- 13. Wynns AA, Jensen AB, Eilenberg J, James R (2012) Ascosphaera subglobosa, a new spore cyst fungus from North America associated with the solitary bee Megachile rotundata . Mycologia 104: 108–114. [DOI] [PubMed] [Google Scholar]
- 14. Stephen WP, Vandenberg JD, Fichter BL (1981) Etiology and epizootiology of chalkbrood in the alfalfa leafcutting bee Megachile rotundata, with notes on Ascosphaera species. Oregon State University Agricultural Experimental Station Bulletin [Google Scholar]
- 15.James RR (2008) The problem of disease when domesticating bees. In: James RR, Pitts Singer TL, editors. Bee Pollination in Agricultural Ecosystems: Oxford University Press. pp. 124–141.
- 16. Aronstein KA, Murray KD, Saldivar E (2010) Transcriptional responses in honey bee larvae infected with chalkbrood fungus. BMC Genomics 11: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephen WP, Bohart GE, Torchio PF (1969) The Biology and External Morphology of Bees. CorvallisOregon: Oregon State University. 140 p.
- 18. Winfree R, Williams NM, Dushoff J, Kremen C (2007) Native bees provide insurance against ongoing honey bee losses. Ecology Letters 10: 1105–1113. [DOI] [PubMed] [Google Scholar]
- 19.Thiers B (2008 onwards) Index Herbariorum: A global directy of public herbaria and associated staff. continuously updated ed. New York Botanical Garden. [continuously updated]. http://sweetgum.nybg.org/ih/. Accessed 2013 Sep 3.
- 20. James RR (2005) Temperature and chalkbrood development in the alfalfa leaf-cutting bee. Apidologie 36: 15–23. [Google Scholar]
- 21. Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- 22. Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- 23. Skou JP (1975) Two new species of Ascosphaera and notes on the conidial state of Bettsia alvei . Friesia 11: 62–74. [Google Scholar]
- 24. Skou JP, Holm SN (1989) Ascosphaera tenax species nova and a variant of Ascosphaera aggregata . Mycotaxon 35: 211–218. [Google Scholar]
- 25. Demendoza MH, Demendoza JH, Puerta F, Asensio E, Bustos M, et al. (1989) Ascosphaeriosis of the parasitic bee, Coelioxys rufocaudata, by Ascosphaera aggregata . Journal of Apicultural Research 28: 61–65. [Google Scholar]
- 26. Aronstein KA, Murray KD (2010) Chalkbrood disease in honey bees. J Invertebr Pathol 103 (Suppl 1) S20–29. [DOI] [PubMed] [Google Scholar]
- 27. Skou JP, Hackett K (1979) A new homothallic species of Ascosphaera . Friesia 11. [Google Scholar]
- 28. Vojvodic S, Jensen AB, James RR, Boomsma JJ, Eilenberg J (2011) Temperature dependent virulence of obligate and facultative fungal pathogens of honeybee brood. Veterinary Microbiology 149: 200–205. [DOI] [PubMed] [Google Scholar]
- 29.Westrich P (1989) Die Wildbienen Baden-Württembergs. Stuttgart: Ulmer Verlag.
- 30. Sedivy C, Muller A, Dorn S (2011) Closely related pollen generalist bees differ in their ability to develop on the same pollen diet: evidence for physiological adaptations to digest pollen. Functional Ecology 25: 718–725. [Google Scholar]
- 31. Prokschl H (1953) *Beitrage Zur Kenntnis Der Entwicklungsgeschichte Von Pericystis Apis Maassen. Archiv Fur Mikrobiologie 18: 198–209. [PubMed] [Google Scholar]
- 32. Maurizio A (1935) Beiträge zur Kenntnis der Pilzflora im Bienenstock. I. Die Pericystis-Infektion der Bienenlarven. Ber Schweiz Bot Ges 44: 676–677. [Google Scholar]
- 33. Hitchcock JD, Christensen M (1972) Occurrence of chalk brood (Ascosphaera apis) in honey bees in the United States. Mycologia 64: 1193–1198. [Google Scholar]
- 34. Baker GM, Torchio PF (1968) New records of Ascosphaera apis from North America. Mycologia 60: 189–190. [Google Scholar]
- 35. Youssef NN, Roush CF, Mcmanus WR (1984) In vivo development and pathogenicity of Ascosphaera proliperda (Ascosphaeraceae) to the alfalfa leafcutting bee, Megachile rotundata . Journal of Invertebrate Pathology 43: 11–20. [Google Scholar]
- 36. James RR, Skinner JS (2005) PCR diagnostic methods for Ascosphaera infections in bees. Journal of Invertebrate Pathology 90: 98–103. [DOI] [PubMed] [Google Scholar]
- 37. Evison SEF, Roberts KE, Laurenson L, Pietravalle S, Hui J, et al. (2012) Pervasiveness of parasites in Pollinators. Plos One 7: e30641 doi: 10.1371/journal.pone.0030641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goerzen DW, Dumouchel L, Bissett J (1992) Occurrence of chalkbrood caused by Ascosphaera aggregata Skou in a Native Leafcutting Bee, Megachile pugnata Say (Hymenoptera, Megachilidae), in Saskatchewan. Canadian Entomologist 124: 557–558. [Google Scholar]
- 39. Goerzen DW, Erlandson MA, Bissett J (1990) Occurrence of chalkbrood caused by Ascosphaera aggregata Skou in a Native Leafcutting Bee, Megachile relativa Cresson (Hymenoptera, Megachilidae), in Saskatchewan. Canadian Entomologist 122: 1269–1270. [Google Scholar]
- 40. Gilliam M, Lorenz BJ, Prest DB, Camazine S (1993) Ascosphaera apis from Apis cerana from South Korea. Journal of Invertebrate Pathology 61: 111–112. [Google Scholar]
- 41. Gilliam M, Lorenz BJ, Buchmann SL (1994) Ascosphaera apis, the chalkbrood pathogen of the honeybee, Apis mellifera, from larvae of a carpenter bee, Xylocopa californica arizonensis . Journal of Invertebrate Pathology 63: 307–309. [Google Scholar]
- 42.Wynns AA (2012) The bee specialist fungus family Ascosphaeraceae and its allies: systematics, ecology and co-evolution with solitary bees. PhD dissertation. University of Copenhagen. 90 p.