Abstract
The pelagic brown alga Sargassum forms an oasis of biodiversity and productivity in an otherwise featureless ocean surface. The vast pool of oil resulting from the Deepwater Horizon oil spill came into contact with a large portion of the Gulf of Mexico’s floating Sargassum mats. Aerial surveys performed during and after the oil spill show compelling evidence of loss and subsequent recovery of Sargassum. Expanding on the trends observed in the aerial surveys, we conducted a series of mesocosm experiments to test the effect of oil and dispersants on the vertical position and weight of the Sargassum complex (Sargassum natans and S. fluitans), as well as on the dissolved oxygen concentrations surrounding the algae. Dispersant and dispersed-oil had significant effects on the vertical position of both species of Sargassum over a period of 72 hours. Similarly, dissolved oxygen concentrations were lowest in dispersant and dispersed-oil treatments, respectively. Cumulatively, our findings suggest three pathways for oil-spill related injury: (1) Sargassum accumulated oil on the surface exposing animals to high concentrations of contaminants; (2) application of dispersant sank Sargassum, thus removing the habitat and potentially transporting oil and dispersant vertically; and (3) low oxygen surrounded the habitat potentially stressing animals that reside in the alga. These pathways represent direct, sublethal, and indirect effects of oil and dispersant release that minimize the ecosystem services provided by floating Sargassum – the latter two effects are rarely considered in assessing impacts of oil spills or response procedures.
Introduction
Given the vast literature demonstrating direct negative impacts of oil spills, understanding how response activities mitigate or exacerbate the impacts of an oil spill is of fundamental importance to understanding the ecological effects of the Deepwater Horizon oil spill (DWH) and most importantly, developing appropriate, multidimensional policy for future responses to oil spills. One of the most contentious response activities during the DWH was the use of dispersants [1]. Dispersants are typically used to reduce the impact of oil on shorelines, reduce impacts of oil on surface dwelling birds and mammals, and promote the biodegradation of oil in the water column [2]. In contrast to the deep-water injection of dispersant, the application of dispersants to surface oil was a traditional response, although the sheer magnitude of dispersant application (3.7 million L [3]) was likely never envisioned by ecosystem managers (officially termed an atypical response activity). While surface application appeared to have successfully dispersed a large fraction of the oil throughout the water column, it also transformed the traditional 2-D footprint of a surface oil spill into a dramatically more complex 3-D problem [1]. One key oceanic habitat affected by the DWH was floating Sargassum mats (Sargassum natans and S. fluitans). Given the extensive spatial and temporal scope of the DWH, the fate of Sargassum following the DWH in the Gulf of Mexico (GOM), which contains the second most productive Sargassum system in the world [4], [5], illustrates the profound ecological and socioeconomic tradeoffs that must be considered collectively and more fully when responding to oil spills.
Sargassum is a pelagic brown algae that represents an oasis of structure in the open ocean and supports a large and diverse assemblage of marine turtles [6], fish [7], [8], [9] and invertebrates [10]. In the northcentral GOM, Sargassum is composed almost exclusively of two species, Sargassum natans and S. fluitans [11]). Unlike other species of Sargassum, S. natans and S. fluitans are holopelagic, and are typically considered as a single complex (pelagic Sargassum, [12]). Pelagic Sargassum is a ubiquitous feature of the northcentral GOM where it occurs in three configurations depending on meteorological and in situ oceanic conditions: scattered clumps (during high winds), small and meso scale (1 m–10 km’s) convergence lines, and larger circular mats [13], [14]. Given its pelagic and ephemeral nature, systematic surveys of Sargassum abundance in the Gulf of Mexico are uncommon; thus we know of little historical abundance and distribution of this habitat.
The loss and degradation of another structurally complex brown algae Fucus in Prince William Sound, Alaska following the Exxon Valdez oil spill was responsible for a myriad of direct and indirect impacts on the food web, many of which persisted for years [15]. Fucus fulfills ecological functions similar to those of Sargassum; hence, we expect the consequences of any degradation of Sargassum to follow the responses documented for the loss of these brown algae. However, in contrast to Fucus, which supports a largely benthic and demersal community, Sargassum supports a pelagic ecosystem. Whereas Fucus represents one of a multitude of nearshore structurally complex habitats available for fish and invertebrates, Sargassum fulfills a unique position in the open ocean as the only naturally occurring biogenic habitat.
To quantify the potential impact of floating oil and dispersant application on pelagic Sargassum we first documented the distribution of Sargassum during and after the DWH in 2010–2012. Because of the clear overlap between Sargassum and oil as well as dispersant recorded in 2010, we then investigated the potential effects of Sargassum exposure to oil and dispersant via replicated mesocosm experiments.
Methods
Sargassum for mesocosm experiments was collected in the offshore waters of the Gulf of Mexico. No specific permissions were required for this activity. Fish and invertebrates residing within the Sargassum were removed and returned to the water. None of these fish or invertebrates were endangered or protected, and no animals were killed specifically for this study.
Aerial Surveys of Sargassum Distribution
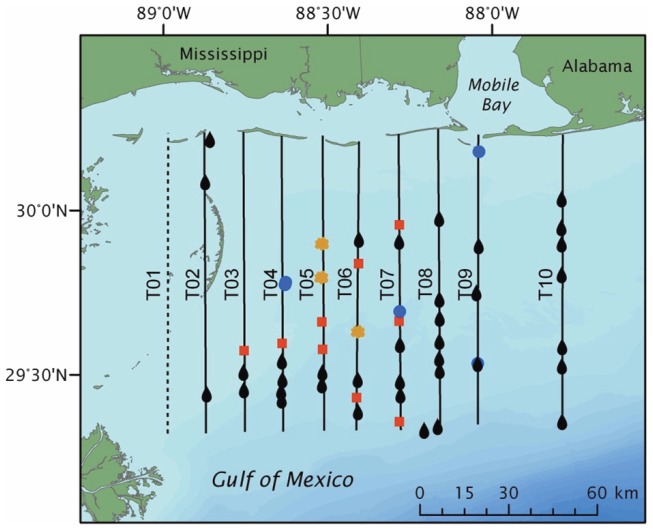
Sargassum can be patchily distributed in small clumps, or aggregated into large mats by Langmuir circulations or convergence along frontal zones. Strip-transect aerial surveys are commonly used to assess the abundance of surface-occurring marine fauna [16], [17]. We used a similar approach to locate Sargassum patches and convergence lines to estimate the quantity and distribution of Sargassum in relation to oil and dispersant application. Ten equally spaced transects were established within the area (the 9th transect in the eastern section was dropped during the project due to time constraints). Each transect started at the shoreline and extended perpendicularly to approximately 100 km offshore (Figure 1). Ten aerial surveys (5/28, 6/4, 6/9, 6/16, 6/23, 7/14, 7/21, 8/18, 9/8, 10/20) were conducted after the DWH in 2010. Follow up flights were also flown in 2011 (7 flights, 7/22, 7/26, 8/17, 9/9, 9/15, 9/21, 9/28) and 2012 (2 flights, 5/16, 7/5). A twin prop Cessna (or similar) aircraft was used for each flight and flown between 215–300 m elevation depending on ceiling restrictions imposed by the unified command and Federal Aviation Administration (FAA) during the DWH explosion. To ensure adequate detectability of Sargassum, all surveys were conducted on days where sea state was equal to or less than 4 on the Beaufort wind force scale. Two observers were seated in the rear of the aircraft and recorded the latitude and longitude (via GPS) of targets (Sargassum mats or convergence lines, whales, turtles, dolphins, fish schools, etc.) within 85° under the plane wing. The altitude for each aerial survey varied according to changing FAA restrictions; therefore, the detectable area ranged from 2.44–3.48 km, depending on the flight altitude.
Figure 1. Results of an aerial survey documenting the co-occurrence of oil and Sargassum, June 2010.
Results of one (6/16/2010) of 8 aerial surveys conducted during the summer of 2010 documenting oil presence (indicated by oil droplets), Sargassum (yellow floral symbols), dolphins (blue circles) and fish schools (red squares).
To examine temporal trends in abundance, observations from the aerial survey were used to create an index of Sargassum abundance. As is the case with many fishery-independent datasets, the aerial survey data had a high proportion of zero values (i.e. instances where no Sargassum was observed). As a result, we used a delta-lognormal approach [18], [19] that combines separate generalized linear models (GLM) of the probability of a non-zero observation (number of transects with positive sightings/total number of transects) and the observation rate along positive transects to construct a single standardized index of abundance, defined as:
| (1) |
where cy is the estimate of mean observations for positive observations for year y, and py is the estimate of mean probability of occurrence during year y. Data used to estimate abundance for positive catches (c) and probability of occurrence (p) were assumed to have a lognormal distribution and a binomial distribution, respectively, and modeled using the following equations:
| (2) |
and
| (3) |
where c is a vector of the positive observation data, p is a vector of the presence/absence data, X is the design matrix for main effects, β is the parameter vector for main effects, and ε is a vector of independent normally distributed errors with expectation zero and variance σ2. Coefficients of variation for the index were estimated using a jackknife routine. Year and season were used as covariates to control for seasonal effects on observation rates. Parameterization of each model was accomplished using R version 2.15.
Mesoscom Experiments
To complement our aerial survey data, mesocosm experiments were conducted to explore oil spill related effects on Sargassum. Sargassum was collected in September 2013 at three locations south of Dauphin Island. Location 1 was 29° 27.095′ N, 87° 46.518′ W, location 2 was 29° 48.442′ N, 87° 56.302′ W, and location 3 was 29° 43.246′ N, 88° 00.528′ W. At these locations, Sargassum was removed from the water using large dip nets, shook to remove fish and invertebrates, and placed in large coolers. Epiphytic algae were not removed from the Sargassum prior to the start of experiments.
We conducted two sets of experiments to determine the effects of oil and dispersant on floating Sargassum. For the first experiment, treatments were conducted and run under static conditions. The second set of experiments were run in an identical manner with the exception that air was bubbled slowly into the bottom of the tank to prevent low oxygen conditions from forming. Both sets of experiments were designed to test the hypothesis that exposure to moderate levels of oil or dispersant caused Sargassum to sink from its surficial habitat. In both experiments, four treatments were tested: 100 ml of LA sweet crude oil, 5 ml of Corexit 9500 A oil dispersant, dispersed-oil (dispersant+oil, 5 and 100 ml respectively), and a control (seawater). We chose 100 ml of crude oil after conducting preliminary trials where oil was released in varying concentrations on the surface. Based on visual examination, 100 ml of oil created distinct oil pockets that covered approximately 10% of the surface area – a realistic although conservative amount based on the authors’ on-water experience during the spill. MC 252 sweet crude oil was provided by BP Exploration and Production under agreement COC 20110512-HP1-053. Corexit 9500 A dispersant was supplied by Nalco. At the beginning of the trials, treatments (oil, dispersant, dispersed-oil and control) were measured with graduated cylinders and added to circular mesocosms (0.6 m diameter × 0.8 m depth) that contained 220 L of seawater (20–24 psu and 27–28°C) and were lined with Teflon bags (Figure 2). Four pre-weighed Sargassum colonies (2 S. natans and 2 S. fluitans) were then placed into the mesocosms. Both species of Sargassum co-occur in mats in the GOM and North Atlantic and we tracked the response of each species separately in case species-specific differences occurred. Four mesocosms, one for each of the four treatments, were placed in much larger circular seawater tanks (5000 L, 2 m tall filled to a level of 0.7 m) to maintain water temperature (+/−1°C). All treatments were replicated three times by placing 220 L circular mesocosms in three separate seawater tanks.
Figure 2. Evolution of experimental treatments.

Example of mesocosms (A) prior to addition of oil, (B) following addition of 100 ml of Louisiana sweet crude oil, (C) five minutes after application of Corexit 9500 A dispersant, and (D) 12 hours after dispersant application.
Vertical position of Sargassum, weight of Sargassum and dissolved oxygen concentrations were measured during both static and aerated experiments. During the experiments vertical position relative to the water surface of each of four colonies (2 S. natans and 2 S. fluitans) was measured every 24 hours for 72 hours. We chose 72 hours because our field observations during the oil spill suggested that this was a reasonable duration for oil and Sargassum to remain in close contact in surface waters. The four colonies used in each mesocosm (2 S. fluitans and 2 S. natans) were weighed and individually photographed at the beginning and end of each trial. All colonies had a small piece of fluorescent flagging tape (for ID) attached to the base of the colony. Colonies were photographed with their ID code showing. Prior to weighing, each colony was spun in a common household salad spinner for ten revolutions to remove excess water. Upon retrieval, colonies were spun again (in washed and de-oiled spinners) and weighed on a digital scale (+/−0.01 g). All material was wrapped in aluminum foil and frozen. Sargassum was weighed before and after the trials were completed and all trials were run for 72 hours. We monitored surface and bottom dissolved oxygen concentrations throughout both static and aerated experiments. Dissolved oxygen was monitored with a handheld Yellow Springs Instrument (YSI model #2030) dissolved oxygen meter.
A series of Analysis of Variance (ANOVAs) were used to test the response of Sargassum vertical position and weight change as well as dissolved oxygen concentrations in our experiments. We tested the hypothesis that the percent of colonies at the surface varied among treatments. Specifically, we used a repeated measures ANOVA with time (0, 24, 48 and 72 hour intervals) as a repeated measure and treatment (oil, dispersant, dispersed-oil and control) as an independent variable. The dependent variable in our analyses was the percent of Sargassum colonies in surface water. Finally, to examine the patterns in dissolved oxygen concentration, we performed a repeated measures ANOVA testing the effect of time (repeated measures 0, 24, 48, and 72 hours) and treatment (oil, dispersant, dispersed-oil, and control). Separate ANOVAs were performed for the static and aerated experiments. For all ANOVAs, no transformations of data were necessary to meet the requirements of homogeneity of variance (Cochran’s C test) or normality (K-S test).
Results
Aerial Surveys of Sargassum Distribution
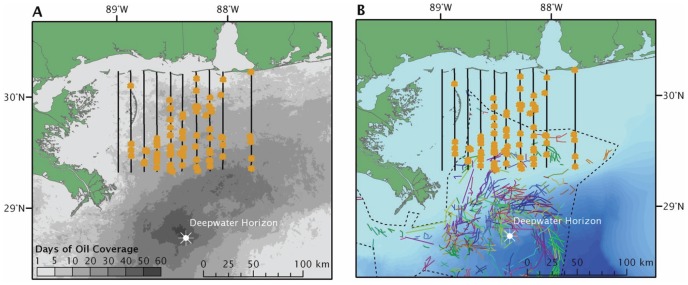
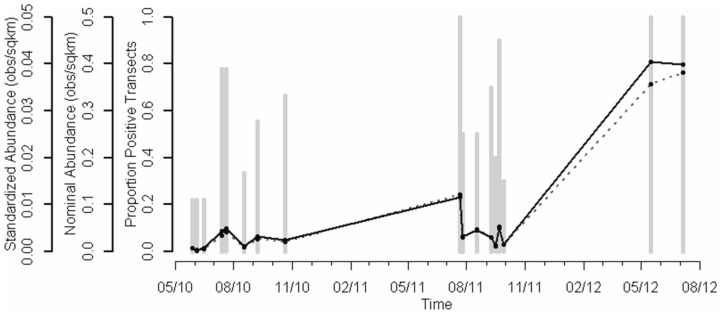
At the onset of the oil spill, we began flying aerial transects to quantify overlap between Sargassum and surface oil. Ten aerial surveys each covering ∼3100 km2 of ocean surface from the panhandle of FL to the Chandeleur Islands, LA were completed in 2010 and documented extensive co-occurrence of oil and Sargassum (Figure 3A). Our survey grid was also within a larger area that was actively sprayed with the dispersant Corexit 9500 A (Figure 3B); thus we documented that Sargassum was exposed to both oil and dispersants. Follow-up aerial surveys in 2011 and 2012 (n = 9) documented a four-fold increase in Sargassum abundance since the DWH (Figure 4).
Figure 3. Sargassum targets in relation to oil and dispersant.
Locations of Sargassum targets (yellow dots) identified along aerial flight transects (black lines) in 2010 relative to days of oil coverage (A) and application of dispersants (B). Transect 1, the dotted transect, was not flown in 2010 due to spill-related flight restrictions. In A, cumulative surface oiled was calculated through the analysis of NESDIS satellite imagery [*]. In B, the colored lines represent daily aerial spraying tracks and the dashed polygon is the full envelope of the effective area [2]. [*] Cumulative surface oiling of the Deepwater Horizon oil spill (2010) The Nature Conservancy, Coastal Resilience Gulf of Mexico Project (http://coastalresilience.org/gulfmex). Accessed November 7, 2012.
Figure 4. Index of relative Sargassum abundance.
Standardized (bold line) number of Sargassum targets based on a delta-log normal abundance index. Positive transect lines are shown in gray bars, and the nominal number of targets per square km are shown with a dashed line.
Experiment 1: Static Conditions
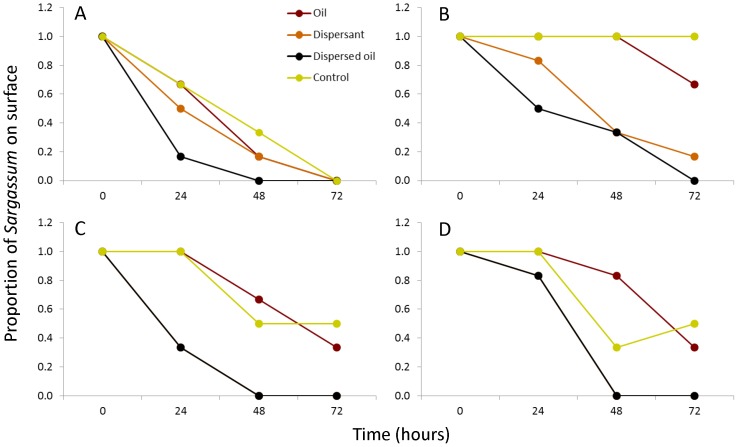
The proportion of Sargassum in surface water differed by oiling/dispersant treatments as well as over the course of the experiment as indicated by the significant treatment × time interaction in our ANOVA (Table 1, Figure 5A and B). This trend was most pronounced for S. natans, where all treatments started sinking after 24 hours. Sargassum in the control treatment sank slowest, followed by oil, dispersant, and dispersed-oil treatments (Figure 5A). An identical, yet less dramatic pattern was observed for S. fluitans, where all Sargassum in the control and oiled treatments remained at the surface through 48 hours (Figure 5B). Notably, for both S. natans and S. fluitans, no colonies in the dispersed-oil treatment were at the surface after 72 hours (Figure 5A and B).
Table 1. Test for between and within subject effects in repeated measures ANOVA with treatment (oil, dispersant, dispersed-oil and control) as fixed factors and time (0, 24, 48, and 72) as a repeated measures on the proportion of S. fluitans and S. natans colonies on the surface.
| Between subject effects | |||||
| Source | DF | SS | MS | F | Pr>F |
| Treatment | 3 | 2.714 | 0.905 | 13.838 | <0.001 |
| Error | 20 | 1.307 | 0.065 | ||
| Within subject effects | |||||
| Source | DF | SS | MS | F | Pr>F |
| Time | 3 | 8.807 | 2.936 | 92.654 | <0.001 |
| Treatment*Time | 9 | 1.229 | 0.137 | 4.311 | 0.001 |
| Error | 60 | 1.901 | 0.032 | ||
Figure 5. Percentage of surface Sargassum as a function of treatment.
Percent of Sargassum (S. natans and S. fluitans) colonies remaining on surface for four treatments: oil, dispersant, dispersed-oil and control under both static and aerated conditions. Panels are as follows: static, S. natans (A), static, S. fluitans (B), aerated, S. natans (C), aerated, S. fluitans (D). In panels C and D, the dispersant and dispersed-oil treatment data are identical.
Experiment 2: Aerated Conditions
Trends in the aerated experiments were similar to those observed during the static experiments. In the control treatment, 100% of colonies of both S. natans and S. fluitans were at the surface after 24 hours, after which time they began to sink. In the oil treatment, 67% of S. natans and 83% of S. fluitans were at the surface after 48 hours, and at 72 hours, only 33% of colonies of both species remained at the surface. For both Sargassum species in the dispersant and dispersed-oil treatments, no colonies were at the surface by 48 hours (Figure 5C and D).
Dissolved Oxygen
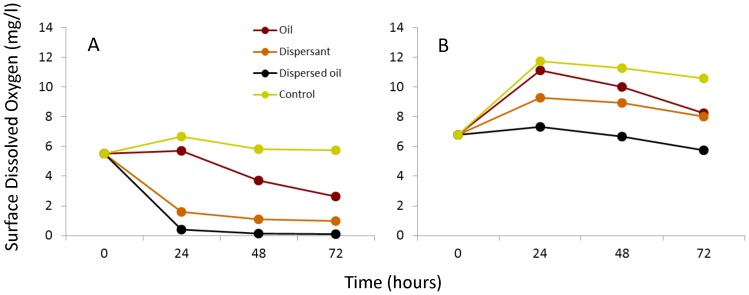
A significant interaction was detected in the repeated measures ANOVAs testing the effect of time (repeated measures 0, 24, 48, and 72 hours) and treatment (oil, dispersant, dispersed-oil, and control) under both static (Table 2) and aerated (Table 3) conditions. Oxygen concentrations were significantly different in each treatment for both static and aerated conditions (dispersed-oil<dispersant<oil<control) (Figure 6). Under static conditions hypoxic and anoxic conditions formed within 24 hours under dispersed-oil and dispersant only treatments (Figure 6A). During the aerated experiments, the same ordering of treatment effects was seen (dispersed-oil<dispersant<oil<control), but with higher overall values (all >6.0 mg/L, Figure 6B).
Table 2. Test for between and within subject effects in repeated measures ANOVA with treatment (oil, dispersant, dispersed-oil and control) as a fixed factor on surface dissolved oxygen concentrations in the first experiment (static conditions).
| Between subject effects | |||||
| Source | DF | SS | MS | F | Pr>F |
| Treatment | 3 | 143.292 | 47.292 | 100.409 | <0.001 |
| Error | 8 | 3.806 | 0.476 | ||
| Within subject effects | |||||
| Source | DF | SS | MS | F | Pr>F |
| Repetition | 3 | 128.138 | 42.713 | 378.399 | <0.001 |
| Treatment*Repetition | 9 | 56.688 | 6.299 | 55.801 | <0.001 |
| Error | 24 | 2.709 | 0.133 | ||
Table 3. Test for between and within subject effects in repeated measures ANOVA with treatment (oil, dispersant, dispersed-oil and control) as a fixed factor on surface dissolved oxygen concentrations in the second experiment (aerated conditions).
| Between subject effects | |||||
| Source | DF | SS | MS | F | Pr>F |
| Treatment | 3 | 77.741 | 25.914 | 15.168 | 0.001 |
| Error | 8 | 13.667 | 1.708 | ||
| Within subject effects | |||||
| Source | DF | SS | MS | F | Pr>F |
| Repetition | 3 | 67.295 | 22.432 | 61.035 | <0.001 |
| Treatment*Repetition | 9 | 27.779 | 3.087 | 8.398 | <0.001 |
| Error | 24 | 8.821 | 0.368 | ||
Figure 6. Surface water dissolved oxygen as a function of treatment.
Dissolved oxygen concentrations in surface water for four treatments: oil, dispersant, dispersed-oil and control under static (A) and aerated (B) conditions.
Discussion
Aerial surveys of Sargassum distribution documented exposure to oil and dispersants and subsequent increase of the alga in the years following the oil spill. The lack of pre-spill baseline data on Sargassum abundance renders it impossible to ascribe the higher abundance of Sargassum contacts in 2011 and 2012 to an oil spill affect; however, the pattern is suggestive of a potential response to the unique conditions in 2010. Our experiments demonstrated that oil and dispersant had significant effects on the vertical position of Sargassum and thus offer a potential explanation for the dramatic trend seen in our aerial survey data. Both experiments 1 (static) and 2 (aeration) demonstrated significant effects of dispersant on the vertical position of Sargassum. In both the dispersant only and dispersed-oil treatments, all Sargassum sank to the bottom within 24–48 hours. In contrast, Sargassum in the oil only and control treatments remained at the surface of the tank for the majority of the experiment. The effect was more pronounced for S. fluitans than S. natans, where the latter tended to sink towards the end of the experiments in the control and oil treatment. These findings support a body of literature documenting increased toxicity of chemically-dispersed compared to physically-dispersed oil (reviewed in [2]).In the GOM, S. natans and S. fluitans generally co-occur within neustonic mats and a mixed colony is likely buoyed by the broader leaf species S. fluitans. Thus, our experiments demonstrate two potential pathways for injury to Sargassum and its associated fauna. First, Sargassum mixing with oil and remaining on the surface exposes numerous invertebrates, fish [20], and sea turtles [6], which are attracted to the floating Sargassum, to elevated concentrations of oil. Additionally, contaminated Sargassum remaining on the ocean surface serves as a horizontal export mechanism, potentially extending the impact of the spill via the “Sargassum conveyor belt” from the central GOM to the North Atlantic Ocean [5]. Sargassum maintains it surface position via berry-like vesicles whose gas composition is maintained through diffusion with surrounding waters [21]. Once Sargassum sinks, its value as refuge for neustonic fauna disappears. Reemergence on the surface depends on the ability of Sargassum to “refill” its vesicles and the sinking depth [22]; however, most Sargassum that sinks will not return to the surface. In this second pathway, vertical sinking exposes mesopelagic and benthic fauna to oil and dispersant trapped in the Sargassum, and may represent a significant labile carbon source for microbial communities that can consume dispersed oil and dead plant material. The loss of surface refuge is further exacerbated when one considers the lost production by healthy Sargassum, which propagates via fragmentation and growth. Assuming a 1-year life span and growth rate of 0.04 d−1 [23], a km2 of lost Sargassum would translate to a total loss of 25 km2 of Sargassum per year. When one considers density estimates of sea turtles per km2 range from 0.9–6.9 post hatchlings and 4.8–7.2 juveniles [17], the potential injury from habitat loss could easily contribute to a year-class failure for some higher order consumers.
A third mechanism for injury is also evident in the dissolved oxygen dynamics under static conditions in our first experiment. The addition of dispersant to our mesocosms rapidly depleted oxygen with the greatest oxygen consumption observed in the dispersed-oil and dispersant only treatments, likely caused by increased microbial respiration [24]. Within 12 hours, hypoxic (dissolved oxygen <2 mg/L) conditions formed in the two treatments with dispersant and from 24–36 hours anoxia (0 mg/L) had developed in the dispersed-oil treatment. Dissolved oxygen conditions were higher in the oil only (>3 mg/L) and control (>5 mg/L) treatments (p<0.05 for post hoc contrast). A similar ranking of treatments was seen under aeration conditions (dispersed-oil<dispersant<oil<control) although aeration prevented the formation of hypoxic conditions. Negative effects (increased mortality, increased physiological stress, and decreased fitness) are well documented under episodic or chronic low oxygen conditions in marine waters [25], [26]. If winds are weak, as is the case when large Sargassum mats form [13], surface mixing would be reduced in the GOM and more conducive to the formation of water masses with low oxygen surrounding oil and dispersant mixtures. Surface water hypoxia/anoxia would be accentuated under oiled conditions because surface slicks form a physical barrier that resists wind stress leading to less mixing of the water column and retarding gas exchange from the ocean to atmosphere [27]. Under these conditions, the presence of low oxygen waters represents a major indirect effect that could lead to injury.
Our results illustrate the potential for direct, sublethal, and indirect effects as pathways for injury from oil spills and the application of dispersants with major implications for the management of ecosystem resources and services. Sargassum remained alive in our trials; therefore, traditional LC50 survival tests or EC10 assessments would have failed to detect a negative effect of oil and dispersant exposure. In fact, no significant change in weight was recorded during the experiment beyond a trend of heavier weight in the oil treatment, which is likely explained by fouling of plant material with oil. The sublethal effect of Sargassum’s loss of buoyancy and resulting sinking in the presence of dispersants means that Sargassum would have eventually died and immediately removed most of its ecological and socioeconomic benefits. The loss and degradation of structurally complex brown algae (Fucus) in Prince William Sound, AK following the Exxon Valdez oil spill resulted in ecosystem wide effects [15]. Interestingly, the loss of Fucus was also primarily a function of response activities (high pressure washing denuded nearshore rocky areas [28]).
As demand for fossil fuels remains high, so does the potential for oil spills. Response activities are a necessary component of mitigating the impacts of such spills. Although the intentions behind all response activities are good, the effects of these activities may further exacerbate injury. In the GOM, dispersants were applied to seven oil spills between 1990 and 1998; in each instance, their application was deemed successful to some extent [29]. In the case of the DWH, the efficacy of spraying vast quantities of Corexit 9500 A to disperse surface oil remains to be examined; however, this evaluation must include a careful consideration of the associated tradeoffs. For Sargassum in the GOM, contact with dispersant may result in loss of this unique habitat, while increasing exposure at depth to mesopelagic and benthic communities, illustrating the complex scenarios that must be considered. Additionally, Sargassum in the GOM and North Atlantic Ocean can account for as much as 60% of primary production in the oligotrophic expanses [30]. The loss of such high productivity in oligotrophic areas is a significant concern for the ecosystem. With more offshore drilling operations likely in regions that support Sargassum communities, understanding the ecological risks is a critical necessity. Full consideration of the tradeoffs associated with dispersants and oil spills are required for effective management and policy implementation. Here, we demonstrate novel and important pathways currently not considered during the DWH.
Acknowledgments
J. Jackson and L. Stone provided mesocosm support for the study.
Funding Statement
The study was funded by a NSF RAPID grant from the Division of Ocean Sciences (SPP and FJH), Gulf of Mexico Research Initiative Phase I (RHC) and III (SPP and FJH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Peterson CH, Anderson SS, Cherr GN, Ambrose RF, Anghera A, et al. (2012) A tale of two spills: novel science and policy implications of an emerging new oil spill paradigm. BioScience 62(5): 461–469. [Google Scholar]
- 2.Fingas M (2008) A review of literature related to oil spill dispersants 1997–2008. Prince William Sound Regional Citizens’ Advisory Council, Anchorage, Alaska.
- 3.Environmental Response Management Application (ERMA) website. After action report from the Deepwater Horizon MC 252 Aerial Dispersant Response. Available: http://gomex.erma.noaa.gov/layerfiles/18105/files/MASTER%20FINAL%20After%20Action%20Report%20Deepwater%20Horizon%20MC252%20Aerial%20Dispersant%20Response%20%202011-02-09a1.pdf. Accessed 2012 October 3.
- 4. Gower J, King S (2011) Distribution of floating Sargassum in the Gulf of Mexico and the Atlantic Ocean mapped using MERIS. Int J Remote Sens 32: 1917–1929. [Google Scholar]
- 5.Gower J, King S (2008) Satellite images show the movement of floating Sargassum in the Gulf of Mexico and Atlantic Ocean. Nature Proceedings hdl:10101/npre.2008.1894.1.
- 6. Witherington B, Hirama S, Hardy R (2012) Young sea turtles of the pelagic Sargassum-dominated drift community: habitat use, population density, and threats. Mar Ecol Prog Ser 463: 1–22. [Google Scholar]
- 7.Dooley J (1972) Fishes associated with the pelagic Sargassum complex, with a discussion of the Sargassum community. Contrib Mar Sci 1–31.
- 8. Bortone S, Hastings P, Collard S (1977) The pelagic-Sargassum ichthyofauna of the eastern Gulf of Mexico. Northeast Gulf Sci 1(2): 60–67. [Google Scholar]
- 9. Wells RJD, Rooker J (2004) Spatial and temporal patterns of habitat use by fishes associated with Sargassum mats in the northwestern Gulf of Mexico. Bull Mar Sci 74(1): 81–99. [Google Scholar]
- 10.Coston-Clements L, Settle LR, Hoss DE, Cross FA (1991) Utilization of the Sargassum habitat by marine invertebrates – a review. NOAA Technical Memorandum NMFS-SEFSC-296, 32 p. [Google Scholar]
- 11. Parr AE (1939) Quantitative observations on the pelagic Sargassum vegetation of the western North Atlantic. Bull Bingham Oceanogr Coll 6: 1–94. [Google Scholar]
- 12.South Atlantic Fishery Management Council (2002) Fishery management plan for pelagic Sargassum habitat of the South Atlantic region. 1–153.
- 13. Faller AJ, Woodcock AH (1964) The spacing of windrows of Sargassum in the ocean. J Mar Res 22: 22–29. [Google Scholar]
- 14.Szekielda KH, Marorino GO (2010) High spatial resolution spectrometry of rafting macroalgae (Sargassum). J Appl Remote Sens 4(1) 043529-043529-13.
- 15. Peterson CH, Rice SD, Short JW, Esler D, Bodkin JL, et al. (2003) Long-term ecosystem response to the Exxon Valdez oil spill. Science 302(5653): 2082–2086. [DOI] [PubMed] [Google Scholar]
- 16. Craig BA, Reynolds JE (2004) Determination of manatee population trends along the Atlantic coast of Florida using a Bayesian approach with temperature-adjusted aerial survey data. Mar Mamm Sci 20: 386–400. [Google Scholar]
- 17. Houghton JDR, Doyle TK, Wilson MW, Davenport J, Hays GC (2006) Jellyfish aggregations and leatherback turtle foraging patterns in a temperate coastal environment. Ecology 87: 1967–1972. [DOI] [PubMed] [Google Scholar]
- 18. Lo NC, Jackson LD, Squire JL (1992) Indices of relative abundance from fish spotter data based on delta-lognormal models. Can J Fish Aquat Sci 49: 2515–2526. [Google Scholar]
- 19.Maunder MN, Punt AE (2004) Standardizing catch and effort data: a review of recent approaches. Fish Res 70(2–3) 141–159.
- 20. Rooker JR, Turner JP, Holt SA (2006) Trophic ecology of Sargassum-associated fishes in the Gulf of Mexico determined from stable isotope and fatty acid analysis. Mar Ecol Prog Ser 313: 249–259. [Google Scholar]
- 21. Hurka H (1971) Factors influencing the gas composition in the vesicles of Sargassum . Mar Biol 11: 82–89. [Google Scholar]
- 22. Johnson DL, Richardson PL (1977) On the wind-induced sinking of Sargassum . J Exp Mar Biol Ecol 28: 255–267. [Google Scholar]
- 23. LaPointe BE (1995) A comparison of nutrient-limited productivity in Sargassum natans from neritic vs. oceanic waters of the western North Atlantic Ocean. Limnol Oceanogr 40: 625–633. [Google Scholar]
- 24. Ortmann AC, Anders J, Shelton N, Gong L, Moss AG, et al. (2012) Dispersed oil disrupts microbial pathways in pelagic food webs. PLoS ONE 7(7): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321(5891): 926–929. [DOI] [PubMed] [Google Scholar]
- 26. Powers SP, Peterson CH, Christian RR, Sullivan E, Powers MJ, et al. (2005) Effects of eutrophication on bottom habitat and prey resources of demersal fishes. Mar Ecol Prog Ser 302: 233–243. [Google Scholar]
- 27. Abbriano RM, Carranza MM, Hogle SL, Levin RA, Netburn AN, et al. (2011) Deepwater Horizon oil spill: A review of the planktonic response. Oceanography 24(3): 294–301. [Google Scholar]
- 28. Driskell WB, Ruesink JL, Lees DC, Houghton JP, Lindstrom SC (2001) Long term signal of disturbance: Fucus gardneri after the Exxon Valdez oil spill. Ecol Appl 11: 815–827. [Google Scholar]
- 29. Henry C (2005) Review of Dispersant Use in US Gulf of Mexico waters since the Oil Pollution Act of 1990. Proceedings of the 2005 International Oil Spill Conference, American Petroleum Institute, Washington, D. C: 4. [Google Scholar]
- 30. Carpenter EJ, Cox JL (1974) Production of pelagic Sargassum and a blue-green epiphyte in the western Sargasso Sea. Limnol Oceanogr 19: 429–436. [Google Scholar]