Summary
Background
Determining the optimal timing and progression of mobility exercise has the potential to affect functional recovery of critically ill adults. This study compared standard care with care delivered using a mobility protocol. We examined the effects of exercise on vital signs and inflammatory biomarkers and the effects of the nurse-initiated mobility protocol on outcomes.
Methods
Prospective, repeated measures study with a control (standard care) and intervention (protocol) period.
Results
75 heterogeneous subjects admitted to a Medical or Surgical intensive care unit (ICU) were enrolled. In <5% of exercise periods, there was a concerning alteration in respiratory rate or peripheral oxygen saturation; no other adverse events occurred. Findings suggested the use of a protocol with one 20 minute episode of exercise daily for 2 or more days reduced ICU length of stay. Duration of exercise was linked to increased IL-10, suggesting brief episodes of low intensity exercise positively altered inflammatory dysregulation in this sample.
Conclusion
A growing body of evidence demonstrates that early, progressive exercise has significant benefits to intubated adults. These results should encourage clinicians to add mobility protocols to the care of ICU adults and lead to future studies to determine optimal “dosing” of exercise in ICU patients.
Keywords: ICU, Exercise, Intubated, Mechanical ventilation, Inflammatory biomarkers
Introduction
There are multiple reports of safe implementation of rehabilitative activity among intubated and critically ill patients (Bailey et al., 2007; Burtin et al., 2009; Morris et al., 2008; Needham and Korupolu, 2010; Pohlman et al., 2010; Schweickert et al., 2009; Stiller et al., 2004; Thomsen et al., 2008; Zanni et al., 2009). However, guidelines are not established and establishing best practices for early, progressive mobility is still in progress. Determining the optimal timing and progression of mobility exercise has the potential to affect functional recovery of critically ill adults who experience prolonged mechanical ventilation (Herridge, 2009).
Both inflammation and immobility have been implicated in intensive care unit (ICU)-acquired weakness and subsequent prolonged functional impairment (Griffiths and Hall, 2010). There is limited information about the mechanisms through which progressive mobility exercises contribute to mitigation or prevention of ICU-acquired weakness (Griffiths and Hall, 2010). Circulating biomarkers related to both inflammation and ICU-acquired weakness include interleukin (IL)-6, a proinflammatory cytokine that influences muscle health, and IL-10, an anti-inflammatory cytokine that down-regulates the inflammatory cascade (Truong et al., 2009; Winkelman, 2007). Linking mechanisms of inflammation to a therapeutic “dose of mobility exercise” can support clinical decisions about starting and progressing mobility exercise.
The purpose of this study was to compare standard care versus an early mobility protocol in ICU adults. We examined the effects of exercise on vital signs and inflammatory biomarkers. We also quantified the effects of the nurse-initiated mobility exercise protocol according to outcomes. The four specific research questions were:
Is exercise associated with adverse changes in vital signs or unsafe events?
Is the mode of exercise or duration of exercise associated with change in IL-6 or IL-10?
Are there associations between change in IL-6 or IL-10 and patient outcomes?
Is there a difference in patient outcomes for the protocol of mobility therapy compared to standard care?
Methods
This was a prospective, repeated measures study with a control period (standard care), run-in period, and intervention period with protocol care (see Fig. 1). This study received approval from the hospital Institutional Review Board and all subjects or their surrogates provided informed consent. The study was registered at www.clinicaltrials.gov (NCT00787098).
Figure 1.
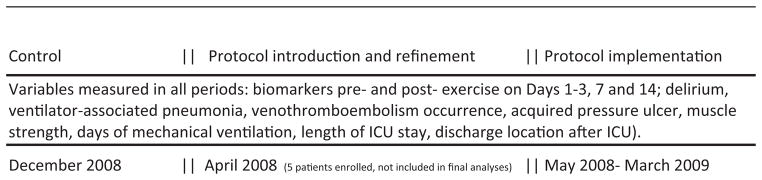
Experimental design and timeline. There were three study phases: (1) a control phase in which control subjects were enrolled; (2) a run-in phase to introduce the protocol to staff members and refine the protocol and (3) a protocol implementation period in which study participants were enrolled. Outcomes were measured during each phase, although the subjects enrolled during the run-in period were not included in analysis as the number of subjects is small and they received a combined control and protocol-directed delivery of exercise.
Setting and sample
We recruited patients from December 2007 through March 2009 from the medical and surgical ICUs at a large, urban, academic medical centre. Patients in both units are managed by intensivists, with surgical patients receiving co-management by surgeons. Subjects were assessed for enrolment if they experienced more than 48 hours of mechanical ventilation and were anticipated to continue receiving mechanical ventilation for the next 24 hours.
Subjects were excluded from the study if they had neurological, muscular or orthopaedic disorders that precluded the possibility of progressive mobility. Examples of exclusionary conditions included end-stage muscular dystrophy, myasthenia gravis, new quadriplegia, unexplained coma, increased intracranial pressure, unrepaired hip fracture and multiple lower extremity fractures. Patients with hospice care for high risk of death were also excluded, using criteria established by Norton et al. (2007): ICU admission following a hospital stay of >9 days in the past month; age >80 in the presence of two or more life-threatening illnesses (e.g., end-stage renal disease, severe heart failure); diagnosis of a metastatic malignancy; status post cardiac arrest with coma; and diagnosis of intracerebral haemorrhage requiring mechanical ventilation.
Patient and related measures
From the medical record, patient data were abstracted for age, gender, height, weight, admitting diagnosis, comorbid conditions (by number) and daily medications. Severity of illness was measured using the Acute Physiology and Chronic Health Evaluation (APACHE) 3 Score (Knaus et al., 1991), the Charlson Comorbidity Index (Charlson et al., 1987) from admission data and the ratio of partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) or P/F ratio on the day of exercise. The SpO2 was substituted for PaO2 when PaO2 was not available (Pnadharipande et al., 2009).
The type and duration of exercise was recorded daily. Vital signs were obtained immediately preceding and during exercise: highest and lowest heart rate, respiratory rate, systolic blood pressure and SpO2. Unsafe events were recorded at the time of occurrence and monitored for potential occurrence in the 15 minutes following exercise. Patients were asked to separately rate fatigue and pain after an exercise session using a scale of 0 (no fatigue or pain) to 10 (worst possible fatigue or pain). Muscle strength was measured manually at discharge from the ICU, using the Medical Research Council Scale (0–5, with 5 being maximal strength) on four muscle groups: shoulder adduction, elbow extension, hip flexion, knee extension with a maximum score of 40 combining right and left extremity values (Fan et al., 2010). At the time of discharge from the ICU, function was measured using the Katz Activities of Daily Living scale (Katz et al., 1970) with either patient or proxy interview. At ICU discharge, delirium was measured using the Confusion Assessment Method for ICU (CAM-ICU) (Ely et al., 2001). Diagnosis of ventilator associated pneumonia (VAP) was determined by research staff using established criteria (Johanson et al., 1972), including bronchial lavage results when documented (Mayhall, 2001). Occurrence of venous thromboembolism (VTE) was extracted from daily progress notes and reports of related diagnostic tests (e.g., duplex sonography of lower extremities, computerised tomography of the chest and ventilation-perfusion scan). Outcomes of duration of mechanical ventilation, length of ICU stay and discharge location (including mortality) were abstracted from records within 24 hours of ICU discharge. Field notes were used to record changes in practice such as new equipment use, changes in nutrition delivery or changes in sedation. Research assistants (RAs) were not blinded to participant assignment to control or protocol care.
Data collectors were trained in medical record data abstraction, use of data collection forms, and the protocol before the study started. Data collectors were evaluated and achieved an inter-rater reliability of more than 95% at baseline and every six months for chart abstraction. Fidelity of treatment was reviewed quarterly by the principal investigator with direct observation.
Procedure
There were three phases of the study. During the control phase, standard care was observed and recorded for 20 subjects. During the run-in phase, five new subjects were enrolled, the intervention was refined for feasibility within the specific environment, and RAs were trained in the refined protocol. During the intervention period, a consistent research protocol was implemented for 55 new subjects and outcomes were measured. Identification and recruitment of patients were the same during each period. On the first day of eligibility, the patient was evaluated for physiologic stability before beginning the consent process. Physiologic stability was defined using the following parameters: P/F ratio > 100, FiO2 < 60% and positive end-expiratory pressure (PEEP) < 10 cm H2O, heart rate 50–125, mean arterial pressure (MAP) 60–100 mm Hg (SpO2 > 88%, and no active upward titration of vasoactive (e.g. dopamine, dobutamine, neosynepherine, epinephrine) or sedative (e.g., midazolam, propofol)) intravenous drugs in the previous four hours. If a patient was unstable on the first eligible day of enrolment, we followed his/her status daily until stability was achieved. When instability persisted beyond 14 days, we did not pursue consent, reasoning that “early” progressive mobility was not feasible. Following consent, demographic and resting data were collected. Patients were monitored for a 30–60 minute period of rest, and then either monitored during a period of exercise planned by the bedside nurse (control) or engaged in 20 minutes exercise by a RA using a protocol developed by Peter Morris (personal communication January, 2007; subsequently published; see Morris et al., 2008). Participants were followed until discharge from the ICU. While resting data could be collected pre-or post-exercise, it would always occur after a period of observed rest. As cytokines and vital signs (VS) typically return to baseline well within the minimal observed time of 30 minutes at rest (Winkelman et al., 2007; Vollman, 2012), one would not expect differing results from resting values as long as the values reflect a period of no exercise or other inflammatory intervention.
In the standard care (control) phase, research assistants observed an exercise period initiated by the bedside nurse. If no exercise was planned, then data were collected around a period of turning or repositioning. During the period of standard care, a common approach to mobility exercise was not in place in either unit, nor did participants routinely receive exercise from a physical therapist. In the run-in phase of the study, RAs trained to implement the protocol in a standard manner. Data from the five participants enrolled during the run-in period were not included in analysis as these participants received neither standard nor protocolised care.
During the intervention period, 55 subjects received 20 minutes of exercise once daily for 2–7 days. Research assistants initiated all in-bed exercise and either assisted with or initiated out-of-bed exercise based on the protocol’s criteria (Morris et al., 2008): ability to follow three out of five directions, lift both arms off the bed and/or lift each leg off the bed.
Throughout all exercise sessions, vital signs were continuously monitored. Biomarkers were collected from serum samples for three consecutive days after enrolment and again at day 7 after enrolment. Serum was analysed in the Clinical Research Unit using a Meso Scale Discovery technology, with established reliability and validity, custom-designed to simultaneously test both interleukins with an antibody luminescence signal (Mesoscale, 2012). Controls were included with each plate confirming detection limits and all patient samples were run in duplicate.
Statistical analysis
Data related to the sample are presented with descriptive statistics. The first three research questions were tested using a mixed model, repeated-measures multivariate analysis of variance. Chi square tests were used to examine differences in categorical outcomes (e.g., presence/absence of delirium and ventilator-associated pneumonia). Continuous variables were examined with t-tests (i.e., continuous variables of duration of mechanical ventilation and length of stay in the ICU), and analysis of variance (ANOVA) was used to evaluate differences in muscle strength between groups (SAS Analytics Pro; SAS, 2012).
Findings
Sample
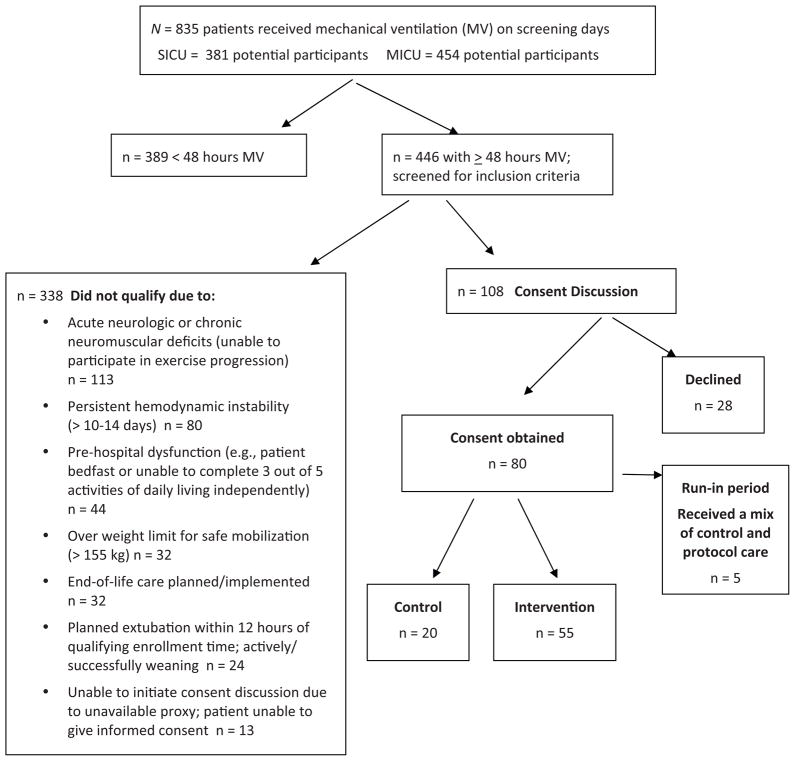
A total of 75 patients form the sample (Fig. 2) and their characteristics are summarised in Table 1. There were significantly more women and a significantly higher ICU admission acuity (APACHE 3 score) among patients enrolled during the intervention period. However, the P/F ratios for participants on enrolment day were similar in the control and intervention periods (225 versus 220, respectively). About 20% of enrollees in both control and intervention periods received continuous sedation during their participation in the study. Daily awakening/sedation holidays were not common practice during the data collection period (2009) although nonsedated participants did receive a spontaneous breathing trial daily per MICU and SICU practice. No changes in practice during the course of the study occurred in managing continuous sedation, VTE prophylaxis or weaning trials during the period of enrolment. However, about 30% of ICU beds were replaced during the study period. The new bed frames were closer to the floor, allowing greater ease in bringing patients to the standing position.
Figure 2.
Screened and enrolled participant flow diagram.
Table 1.
Patient characteristics.
| Demographics | Control, n = 20 | Intervention, n = 55 | All subjects, n = 75 |
|---|---|---|---|
| Age in years, mean (SD) | 66 (11.03) | 65 (13.27) | 666 (12.68) |
| Gender*: Male | 40% | 53% | 51% |
| Race | |||
| White | 60% | 58% | 60% |
| African American | 40% | 40% (2% Hispanic) | 39% |
| Body mass index; mean kg/m2 (SD) | 315 (7.99) | 31.0 (6.93) | 31 (7.21) |
| Admitting unit | |||
| MICU | 35% | 55% | 49% |
| SICU | 65% | 45% | 51% |
| Admitting diagnosis (category) | |||
| Respiratory failure/acute lung injury | 20% | 34% | 31% |
| Cardiovascular surgery | 40% | 11% | 21% |
| Cardiovascular medicine (endocarditis) | 0% | 5% | 2% |
| Gastrointestinal medicine or surgery (e.g., upper GI bleed, pancreatitis; perforated bowel repair, small bowel obstruction repair) | 25% | 22% | 21% |
| Cancer (i.e., thyroid, adrenal, breast and colon) | 5% | 13% | 12% |
| Sepsis | 5% | 9% | 3% |
| Other: acute renal failure and esophagotracheal fistula) | 5% | 2% | 3% |
| Number of comorbid conditions by mean number of categories (SD)/Charlson Score (SD) | 2 (1.46)/1.2 (.93) | 3 (1.67)/2.4 (1.99) | 3 (1.64)/2.1 (1.84) |
| APACHE* 3 score on admission, mean (SD) | 58 (18.49) | 75 (21.96) | 70 (22.31) |
| P/F ratio on admission, mean (SD) | 226 (81.9) | 222 (119.8) | 223 (110.5) |
| Receiving continuous sedation on enrolment | 15% | 9% | 11% |
Abbreviations: SD = standard deviation; P/F ratio = the partial pressure of arterial oxygen (PaO2)/the fraction of inspired oxygen (FiO2); APACHE 3 = acute physiology and chronic health evaluation, version 3; MICU = medical intensive care unit; SICU = surgical intensive care unit.
Significant differences between groups, p < .05.
Mobility exercises
For the 20 patients who received standard care, there were 46 potential episodes of once daily exercise over the three days subsequent to enrolment and 40 episodes were completed (i.e., 13% potential exercise sessions did not occur). In comparison, for the 55 patients who received the experimental intervention for three days after enrolment, there were 152 opportunities for daily exercise, and 143 of these exercises were completed (i.e., 6% potential exercise sessions did not occur).
There were contrasting reasons regarding why “no exercise” occurred on a given day for each of the two groups. For the patients receiving standard care, the most common reason for no exercise was “no activity planned” as reported by the bedside nurse. For patients who received the experimental intervention, the most common reason for not implementing daily exercise was “change in status or procedure” (e.g., newly unstable vital signs, new/upward titration of vasopressor, tracheostomy procedure; n = 5). A second reason was patient request/decline of intervention (n = 4).
Patients in the experimental group received their first exercise intervention on the day of enrolment. The most common reason for waiting for enrolment/exercise until day 6 was physiologic instability. In a few participants, the time needed to acquire informed consent (e.g., waiting until evening family visits or allowing family members time to think about the decision to enrol) necessitated starting the first exercise 12–24 hours after the patient met inclusion criteria. Patients in the control group received their first activity on average at day 9 after ICU admission; the most common reason voiced by staff nurses for starting activity was “the patient is ready now” citing vital stability over 24–48 hours as the trigger for readiness.
The most common mode of exercise in both groups was in-bed active and passive range of motion. Overall, 16/75 (21%) subjects experienced out-of-bed exercise within 3 days of enrolment, with a higher percentage of the experimental intervention group versus the control group experiencing chair-sitting or standing during the intervention period (25% or 14 enrollees in the intervention group versus 10% or two enrollees in the control group). Table 2 summarises activities during days 1–3 after enrolment. While patients were followed weekly after enrolment, the number of participants receiving the experimental intervention on days 4–7 dropped 80% due to discharge from ICU, limiting additional comparisons across those latter days.
Table 2.
Descriptive summary of mobility exercise.
| Duration in minutes | Control Mean 17 (range 5–25) |
Intervention Mean 20 (range 12–60) |
|---|---|---|
| Day of first exercise session after ICU admission | 9 | 6 |
| In-bed, number of exercise sessions (one/day/patient; not all patients were in ICU for 72 hours) | 37 | 123 |
| Episodes of passive ROM | 7 | 23 |
| Episodes of combined active/passive ROM or Active ROM | 30 | 100 |
| Out-of-bed, number of exercise sessions | 5 | 40 |
| Episodes of chair sitting | 4(2 patients) | 32(14 patients) |
| Episodes of standing or walking | 1(1 patient)a | 8(7 patients)a |
ROM = range of motion.
Patients who stood or walked all had a chair-sitting period.
Vital signs
The average differences between resting values and the highest values of heart rate (HR), respiratpry rate (RR) and sutolic blood pressure (SBP) and the lowest values of peripheral saturation (SpO2) during exercise are summarised in Table 3. Two patients were on low dose norepinephrine without titration during exercise in the intervention group and no clinically important changes in vital signs occurred despite vasopressor support.
Table 3.
Changes in vital signs during mobility exercise.
| Heart rate Mean change (SD) |
Respiratory rate Mean change (SD) |
Systolic blood pressure Mean change (SD) |
Peripheral oxygenation (SpO2) Mean change (SD) |
|
|---|---|---|---|---|
| Day 1 | ||||
| Control | 7(5) | 6(4) | 15(13) | 2(2) |
| Intervention | 6(5) | 5(4) | 13(13) | 2(2) |
| Total | 6(5) | 5(4) | 13(13) | 2(1) |
| Day 2 | ||||
| Control | 7(5) | 6(5) | 15(12) | 2(2) |
| Intervention | 6(5) | 5(4) | 14(13) | 2(2) |
| Total | 6(5) | 5(4) | 14(12) | 2(2) |
| Day 3 | ||||
| Control | 7(5) | 6(5) | 15(8) | 2(2) |
| Intervention | 6(4) | 5(4) | 14(13) | 2(3) |
| Total | 6(5) | 5(4) | 14(12) | 2(3) |
| Day 7 | ||||
| Control | 9(6) | 5(4) | 16(11) | 2(1) |
| Intervention | 7(6) | 5(4) | 16(20) | 2(1) |
| Total | 8(6) | 5(4) | 16(18) | 2(1) |
Abbreviations: SD = standard deviation.
No significant differences in resting values compared to intervention values, p > .10.
Adverse events
One adverse event occurred in the control group; specifically, the inadvertent removal of an arterial line. For the intervention group, when changes in vital signs occurred that caused concern (i.e., 20% change in VS), exercise was slowed or stopped. Concerning changes in vital signs occurred six times as an increased respiratory rate >35 breaths per minute and reduced SpO2 to 90% during exercise. These concerning changes all occurred while preparing the patient for a standing transfer to the chair for the first time; stopping the exercise resulted in a return to resting values within 3–5 minutes.
Post-exercise, there were no significant increases in pain or fatigue reported by the patients who were able to use a numeric scale to indicate these symptoms as shown in Table 4. There were no periods of hypotension or hypertension requiring cessation of exercise. There were no occurrences of new dysrhythmias concurrent with or immediately following exercise. There were no falls or near-falls during exercise at any time.
Table 4.
Changes in pain and fatigue; comparing pre-exercise and post-exercise self-reports in patients who were able to self-report using a scale of 0–10 (zero = none; 10 = worst/greatest possible).
| Day 1 | Day 2 | Day 3 | |
|---|---|---|---|
| n = 19 | N = 20 | N = 14 | |
| Pre-exercise fatigue | 5.8 | 4.91 | 5.6 |
| Post-exercise fatigue | 5.4 | 4.6 | 5.7 |
| Pre-exercise pain | 3.3 | 2.4 | 2.9 |
| Post exercise pain | 2.8 | 2.0 | 2.9 |
No significant differences between pre- and post-scores in either pain or fatigue on days 1–3 in those who were able to report.
Inflammatory biomarkers
Table 5 provides the values of IL-6 and IL-10 at rest and after exercise on days 1, 2, 3 and 7 after enrolment. The mean change was calculated for each patient, and then averaged. The mean change values (i.e. repeated values of exercise value minus resting values for days 1, 2, 3 and 7 after enrolment) were used in analyses for the research questions. Biomarkers had similar values during usual care compared to protocolised care (F = 1.98, p = .15). Each sample was run in duplicate to verify results; duplicates varied <.1 ng/mL.
Table 5.
Inflammatory biomarkers: interleukin (IL)-6 and IL-10.
| Cytokines (ng/mL) | Day 1a | Day 2a | Day 3a | Day 7a |
|---|---|---|---|---|
| Mean (SD) | n = 70 | n = 48 | n = 30 | n = 11 |
| IL-6 rest | 98 (135) | 70 (73) | 83 (110) | 107 139) |
| IL-6 after exercise | 100 (139) | 73 (74) | 83 (39) | 113 (140) |
| IL-10 rest | 31 (136) | 50 (261) | 74 (337) | 17 (19) |
| IL-10 after exercise | 33 (154) | 55 (304) | 75 (347) | 17 (19) |
Abbreviations: ng = nanogram; mL = millilitre; SD = standard deviation.
Day refers to day of enrolment: generally ICU day 3–16.
Outcomes
Outcomes are summarised in Table 6 for delirium, VAP, VTE, PU number of days of mechanical ventilation, length of ICU stay and discharge location after ICU. Measures of muscle strength and function are also summarised. Of the 75 enrolled subjects, 5 patients (7%) died while in the ICU; these participants were in the intervention group. All deaths occurred many hours (e.g., >14 hours) after exercise; review by the study monitoring committee members deemed deaths were not associated with the intervention.
Table 6.
Comparing outcomes between study periods.
| Control | Intervention | All subjects | Difference | |
|---|---|---|---|---|
| n = 20 | n = 55 | N = 75 | ||
| Muscle strength | 26/40 | 22.4/40 | 25.8/40 | F = .458 (p = .643) |
| n = 15 | n = 49 | n = 64 | ||
| Function (Katz total score) | 1.7 | 2.2/6 | 2.0/6 | χ2 = 1.146 (p = .327) |
| n = 17 | n = 44 | n = 61 | ||
| Delirium (number of occurrences) | 7 | 9 | 16 | χ2 = 1.299 (p = 0.52) |
| n = 14 | n = 48 | n = 63 | ||
| Ventilator associated pneumonia (number of occurrences) | 1 | 0 | 1 | χ2 = 2/259 (p = 0.133) |
| Venothromboembolism event (number of occurrences) | 2 | 11 | 13 | χ2 = 1.258 (p = 0.262) |
| Pressure ulcer (number of occurrences) | 4 | 5 | 9 | χ2 = 3.352 (p = 0.187) |
| Duration of mechanical ventilation in days | 12.4 (SD = 8.9) | 9.13 (SD = 6.6) | 10.5 (SD = 7.5) | t = 1.835 (p = .07) |
| Length of stay in the ICU in days | 19.6 (SD = 10.7) | 14.6 (SD = 8.7) | 16.1 (SD = 9.6) | t = 2.250 (p = .03) |
| Discharge location | χ2 = 7.155 (p = .07) | |||
| Mortality | 0 | 5 | 5 | |
| Acute care, rehabilitation | 12 | 41 | 54 | |
| Long-term care, skilled nursing facility | 8 | 7 | 15 | |
| Home | 0 | 2 | 2 |
Abbreviations: χ2 = Chi square; t = t-test; SD = standard deviation.
Associations between vital signs and exercise
There were no significant differences in the changes for HR, RR, SBP or SpO2 between periods of rest or exercise (ANOVA, values not shown, p > .10). During exercise, there were no clinically important changes in vital signs, with the exception of 6 occurrences which concerned alterations in respiratory-related values that led to slowing/ceasing exercise (see adverse events, above). On average, HR increased 6–7 beats/minute, RR increased 5–6 breaths/minute, SBP increased 13–16 mm Hg and SpO2 typically decreased 2 (e.g., 96–94%).
Associations between biomarkers and exercise
There were no significant associations between patient characteristics (age, gender, race, body mass index [BMI], APACHE 3 score and number of comorbidities) and IL-6. There was a single, positive relationship between resting IL-10 and the patient characteristic of age (F = 2.17, p = .03). Changes in IL-6 and IL-10 were used to examine associations with mode (in-bed versus out-of-bed) and duration (time in minutes) of exercise. Using a mixed model of repeated measures of multivariate analysis of variance, there were no significant associations between change in IL-6 and either mode (p = .7) or duration of exercise (p = .9) after controlling for resting IL-6, age, group (control versus intervention), gender, race, BMI, APACHE 3 score and total number of comorbidities.
Using the same controlling factors for the IL-10 model, there was a statistically significant association between change in IL-10 and duration of exercise: the greater the duration of exercise, the lower the IL-10 in participants both during control and intervention periods (F = 7.03, p = .01). Mode of exercise was not associated with changes in IL-10 (p = .8).
Associations between biomarkers and outcomes
Using a third mixed model analysis, there was no association between the change in IL-6 and outcome measures of delirium, VAP, VTE, acquired PU, duration of mechanical ventilation and ICU length of stay (LOS) (p = .16 to .7) after controlling for resting IL-6, age, group, gender, race, BMI, APACHE 3 score and number of comorbidities (Table 7). Change in IL-6 was marginally associated with discharge location (F = 4.43, p = .07). Change in IL-6 was associated with self-reported fatigue (F = 6.78, p = .02) but not pain (F = 0.01, p = .9).
Table 7.
Results from examining associations between biomarkers, exercise and outcomes.
| Variables | F value | Degrees of freedom | p (significance) |
|---|---|---|---|
| IL-6 | |||
| Change in IL-6 and mode of exercise | .15 | 16 | .70 |
| Change in IL-6 and duration of exercise | 0 | 121 | .95 |
| Change in IL-6 and muscle strength | .03 | 31 | .87 |
| Change in IL-6 and function | .56 | 31 | .45 |
| Change in IL-6 and duration of mechanical ventilation | .78 | 65 | .47 |
| Change in IL-6 and ICU length of stay | 1.82 | 69 | .13 |
| Change in IL-6 and discharge location or mortality | 1.37 | 31 | .25 |
| IL-10 | |||
| Change in IL-10 and mode of exercise | .05 | 16 | .83 |
| Change in IL-10 and duration of exercise* | 7.03 | 121 | .01 |
| Change in IL-10 and muscle strength | .57 | 31 | .46 |
| Change in IL-10 and function | 1.27 | 31 | .27 |
| Change in IL-10 and duration of mechanical ventilation | .49 | 65 | .61 |
| Change in IL-10 and ICU length of stay | .59 | 69 | .67 |
| Change in IL-10 and discharge location or mortality | .08 | 31 | .79 |
Note: Too few instances of delirium, venousthromboembolism, ventilator-associated pneumonia and new pressure ulcer formation for analysis.
Abbreviations: IL = interleukin.
Significant interaction at p < .05
In separate mixed model analysis, the change in IL-10 was not associated with outcomes of delirium, VAP, VTE, acquired PU, duration of mechanical ventilation and ICU LOS (p = .16 to .8) after controlling for resting IL-10, age, group (control versus intervention), gender, race, body mass index, APACHE 3 score and number of comorbidities. There were no significant associations between fatigue or pain and resting IL-10 (p = 1).
Associations between control and intervention periods of exercise and outcomes
Outcome variables are summarised in Table 6. Significant differences occurred in the ICU length of stay. Participants enrolled in the intervention period experienced 5 fewer days of ICU hospitalisation despite higher acuity on admission to the ICU compared to the control group. The duration of mechanical ventilation was not different between groups (p = .07). Differences in discharge location did not demonstrate statistical significance (p = .07). There were few occurrences of delirium, VAP, VTE and pressure ulcers and no differences between patients enrolled during the control and intervention periods for these four outcomes (p > .13).
Discussion
This study extends the literature regarding early, progressive mobility for intubated patients in the ICU with three major findings. First, it confirms that exercise in relatively stable, intubated adults in the ICU is safe. Second, it illustrates that exercise does not appear to contribute to a pro-inflammatory milieu in serum. Instead, 20 minutes of low level exercise was associated with increase IL-10, an anti-inflammatory biomarker. Third, the use of a protocol promoted early and progressive exercise was associated with decreased length of stay.
In this study, once-daily exercise was not statistically associated with adverse changes in vital signs or unsafe events. These findings are consistent with research reports on similar populations (Bailey et al., 2007; Morris et al., 2008; Needham and Korupolu, 2010; Pohlman et al., 2010; Schweickert et al., 2009; Thomsen et al., 2008). As in our study, clinically important detrimental changes in respiratory status have been reported during <10% of exercise episodes (Stiller et al., 2004; Pohlman et al., 2010). No study has reported prolonged adverse sequelae from respiratory changes. A strategy to reduce respiratory-related derangements during exercise suggested by one research group is to increase FiO2 by .2 for intubated patients before initiating exercise (Bailey et al., 2007; Thomsen et al., 2008). While we did not increase FiO2 in our protocol, this seems a practical approach to use in patients with low respiratory reserve.
In the current study, participants demonstrated abnormally increased resting values for both pro- and anti-inflammatory biomarkers compared to values of healthy individuals. The high values and great variation appear congruent with inflammatory dysregulation common to ICU patients (Chien et al., 2006). In this study, the duration of exercise was associated with a statistically significant increase in the anti-inflammatory biomarker IL-10 and no increase in IL-6. It may be that exercise increased muscle-derived IL-6 not detected in serum samples, stimulating increased IL-10; IL-6 is associated with stimulation of IL-10 synthesis.
Rehabilitation in other ICU populations has been associated with improved patient outcomes of muscle strength and overall function (Burtin et al., 2009; Morris et al., 2011). It may be that exercise reduces the inflammatory milieu in ICU patients, mitigating muscle dysfunction. Further studies are needed to evaluate IL-6 and IL-10 effects on muscle strength and function during acute and chronic critical illness.
Unlike previous reports in the literature, the current results showed that high levels of serum IL-6 were not associated with mortality (Dimopoulou et al., 2008; Jastrow et al., 2009; Lee et al., 2010). This finding is likely due to the few number of deaths in this study (i.e., <7%; insufficient power to detect differences). Low mortality is attributed to selection criteria of physiologic stability for enrolment. Alternatively, it may be that serum IL-6 is a less specific predictor than expression of IL-6 in activated white blood cells. The most likely explanation for greater mortality of participants enrolled during the intervention period is their greater illness severity (Table 2).
While neither inflammatory cascade molecule was associated with muscle strength at discharge from ICU, 21% of participants were unable to participate in manual muscle testing (see Table 6), due to cognitive impairment. The inability to measure muscle strength in a significant proportion of the sample limits the ability to draw conclusions from these results. Testing muscle strength is a challenge reported by other investigation ICU-acquired weakness (Griffiths and Hall, 2010). Further, pre-hospital muscle strength was unknown in this sample. Both inflammation and immobility have been identified as independent contributors to muscle weakness (Chambers et al., 2009; Truong et al., 2009). Pro-inflammatory biomarkers have been linked to both impaired muscle contractility and muscle atrophy (Brandt and Pedersen, 2010; Pedersen, 2011). Longitudinal methodology and multimodal measures of muscle strength may be able to better evaluate linkages between inflammatory biomarkers and muscle function.
In this study, the use of a protocol promoted early and progressive exercise compared to standard care. Improved progression to out-of-bed activity has been reported when a dedicated staff initiates exercise in intubated adults (Kasotakis et al., 2011; Morris and Herridge, 2007; Needham and Korupolu, 2010; Pohlman et al., 2010). The protocol used herein was clinically useful as evidenced by a number of results from the intervention group: reduced number of missed opportunities for exercise; increased duration of exercise; and more episodes of out-of-bed exercise. The first exercise session occurred on day 6 for the intervention group versus day 9 for the control group, despite greater admission acuity among intervention participants (see Table 2). Other reports of progressive mobility for intubated adults reported first out of bed activity on days 7–10 after ICU admission (Bailey et al., 2007; Pohlman et al., 2010; Thomsen et al., 2008).
Patients enrolled in the mobility protocol had significantly fewer ICU days. There were few complications typically associated with prolonged bed rest such as VTE or PU, but these findings may be attributed to VTE and pressure ulcer prevention protocols that were well-established in the setting.
Fewer participants exhibited delirium at discharge from the ICU than anticipated from recent reports (Vasilevskis et al., 2010a,b). There were no differences in the number of patients with delirium in each group. Very few participants in either group received continuous sedation at the time of enrolment in this study. While not all patients were able to be tested for delirium due to inability to assess (i.e., a Richmond Agitation Sedation Scale [RASS] score of −3 or −4), the finding of low incidence of delirium indirectly suggests that mobility exercises or the combination of exercise and limited sedation may contribute to reduced delirium. Alternatively, any exercise (standard or protocolised) provides an opportunity for patients to have verbal interaction with a staff member, and it may be that the psychosocial aspects of mobility (e.g., following directions or hearing conversational cues about sensation and movement) are beneficial to preserving cognitive function during prolonged critical illness and recovery.
Our inclusion criteria for physiological stability may be overly cautious. Based on one report, using a broader definition of physiological stability to implement the protocol within 48 hours of admission may result in further improvements in outcomes (Fan, 2010). Assessing patient readiness and response to mobility exercise is challenging. Progression of exercise requires that critically ill patients are alert and able to engage in activities (Kasotakis et al., 2011). Our protocol provided a focused exam to determine patient readiness and the mode of exercise (in-bed or out-of-bed); physiological parameters were monitored to maintain a relatively stable status. The protocol was consistently applied (fidelity maintained at >90%) and this indicates a potential clinical utility in that it was able to be used consistently with minimal training and no re-training required.
Providing mobility exercise is time-consuming and labour intensive (Winkelman et al., 2005). Turning and mobility are reported as one of the most commonly missed nursing interventions (Kalisch, 2006; Winkelman et al., 2005). Teamwork can reduce missed nursing care (Kalisch and Lee, 2010). Several mobility protocols recommend a 3–5 member team to implement exercise, including a registered nurse (RN), a respiratory therapist, a physical therapist, an occupational therapist and aide or nonprofessional assistant (Morris et al., 2008; Needham and Korupolu, 2010; Perme and Chandrashekar, 2009; Schweickert et al., 2009). In this current nurse-initiated protocol, all “teams” were ad hoc, with membership including the RN research assistant and available unit personnel, typically a RN and nursing assistant. It may be that the use of a RA (i.e., ICU RNs not employed in the setting) to initiate progressive mobility had unintended consequences, such as allowing patients or staff to decline participation. Several other studies report the value of a physical therapist to initiate and progress exercise. One setting uses a mobility team dedicated to assessing and implementing exercise. Nonetheless, in this protocol, both amount and duration of mobility exercise increased, despite limited resources.
Others have written about the influence of ICU culture on initiating and progressing exercise for intubated adults (Hopkins et al., 2007). The protocol was initiated by a RA, not a regular staff member of the ICUs. Use of a “clinical outsider” may not be an ideal strategy to facilitate early, progressive mobility (Hopkins and Spuhler, 2009; Perme and Chandrashekar, 2009; Vasilevskis et al., 2010a,b). Research staff anecdotally reported feeling welcomed in the ICUs. For example, ICU staff identified potential patients for enrolment and actively contributed to exercise regimens. While the impact of the initiating personnel is not clear regarding the influence on the success of mobility therapy, there is increasing evidence that the use of protocols for mobility (Hildreth et al., 2010; Kasotakis et al., 2011) improves quality indicators in a variety of institutions.
One serendipitous finding was the high level of acceptance by patients and their surrogates in this study. The consent rate was high (74%) and consistent through 14 months of data collection. Because this was a research project, patients could decline participation as well as individual sessions of exercise. While a few participants declined one exercise session, most patients were eager to engage in exercise at each opportunity. Some surrogates served as cheerleaders during a session and asked for instructions about exercise strategies to use after ICU discharge.
Limitations
This was a single site study. However, both surgical and medical patients were included and the heterogeneity of the sample is similar to generic ICU populations in the United States. While the numbers of participants in the control or experimental arms of the study varied (20 versus 55), the statistical methods used for analysis are robust to unequal group comparisons. There may have been ascertainment bias in identifying VTE as this complication may be undiagnosed but present in asymptomatic patients. At the time of the study, delirium was not assessed daily as part of ICU practice, so we are unable to comment on the progression or resolution of delirium as a result of daily exercise. This study provided an interrupted series of exercise (no exercise on Saturdays and Sundays) and we did not record additional exercise that may have occurred in addition to direct observations. The scope of the study did not include collection of data about readmission and post-ICU outcomes; inclusion of these variables in future work would add important information to the utility of early progressive mobility.
Conclusion
The use of a mobility protocol promoted both earlier initiation and increased progression of exercise, avoiding clinician inertia and long periods of uninterrupted bed rest in intubated adults. Less than 5% of exercise periods were associated with a concerning increase in respiratory rate or peripheral oxygen desaturation. This report suggests a relatively limited intervention of one 20-minute episode of exercise daily for two or more days initiated by a nurse can demonstrate a significant reduction in ICU LOS. Duration of exercise was linked to an increase in IL-10, suggesting that brief episodes of low-intensity exercise positively altered inflammatory dysregulation in this sample. These results should encourage additional study of exercise therapies for the care of patients experiencing prolonged mechanical ventilation in the ICU.
Implications for clinical practice.
The use of a mobility protocol promoted both earlier initiation and increased progression of exercise, avoiding clinician inertia and long periods of uninterrupted bed rest in intubated adults.
Less than 5% of exercise periods were associated with a concerning increase in respiratory rate or peripheral oxygen desaturation further adding to the evidence that mobility in intubated and critically ill adults is safe.
This report suggests a relatively limited intervention of one 20-minute episode of exercise daily for two or more days initiated by a nurse can demonstrate a significant reduction in ICU length of stay.
A 20-minute daily period of exercise was linked to an increase in IL-10, an anti-inflammatory cytokine, suggesting that implementing a mobility protocol can improve inflammatory dysregulation in patients with prolonged critical illness.
Acknowledgments
This publication was made possible by the Case Western Reserve University/Dahms Clinical Resource Unit at University Hospitals, Case Medical Center M01 RR00080 and UL1 RR24989 from the National Center for Research Resources, a component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Dr. Daly was supported by the Department of Veterans Affairs, Office of Rehabilitation Research and Development, grant B5080S.
Hill-Rom provided salary support and funding for cytokine analyses; staff from Hill-Rom did not participate in the project design, implementation, data analysis or interpretation.
Staff in the Medical and Surgical ICUs at University Hospitals, Case Medical Center, including nurses, physical therapists and respiratory therapists, contributed to the implementation of the patient activities promoted in this study.
Emily Liou, PhD, served as the initial project manager, contributing to the design of the data collection forms and initial format of the database. We are grateful for her contributions and wish her well in her career in Taiwan.
Footnotes
Contributions
CW carried out the grant application, initiated study planning and participated in all aspects of study implementation, and drafted the manuscript. KJ participated in data management and analysis and helped to draft the manuscript. RH and JR participated in study design, coordination and oversight. JD participated in study design and study staff training and manuscript development. KP collected data, coordinated study implementation, assisted with data management and interpretation, and edited the draft and final manuscript. AL participated in study design and in interpreting the cytokine findings. All authors read, contributed to original and edited sections in the multiple manuscript revisions, and approved the final manuscript.
Conflict of interests
The authors state they have no competing interests related to this manuscript.
The study was funded by Hill-Rom; both CW and KP received salary support for the study. New Hill-Rom beds were purchased by ICUs during the study but neither CW nor KP were involved in the process of identifying or selecting ICU equipment, neither consulted for or received compensation from the hospital or Hill-Rom related to purchases. The study began prior to hospital equipment review and purchase.
Contributor Information
Chris Winkelman, Email: cxw26@case.edu, Chris.Winkelman@case.edu.
Kimberly D. Johnson, Email: Kimberly.D.Johnson@case.edu.
Rana Hejal, Email: Rana.Hejal@UHHosptials.org.
Nahida H. Gordon, Email: Nahida.Gordon@case.edu.
James Rowbottom, Email: James.Rowbottom1@UHHosptials.org.
Janis Daly, Email: Janis.Daly@case.edu.
Karen Peereboom, Email: Karen.Peereboom@case.edu.
Alan D. Levine, Email: Alan.Levine@case.edu.
References
- Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bexdihan L, et al. Early activity is feasible and safe in respiratory failure patients. Critical Care Medicine. 2007;35(1):139–45. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. Journal of Biomedicine and Biotechnology. 2010 doi: 10.1155/2010/520258. [Epub520258] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, et al. Early exercise in critically ill patients enhances short-term functional recovery. Critical Care Medicine. 2009;37(9):2499–505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- Chambers MA, Moylan JS, Reid MB. Physical inactivity and muscle weakness in the critically ill. Critical Care Medicine. 2009;37(10 Suppl):S337–46. doi: 10.1097/CCM.0b013e3181b6e974. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chien JY, Hseuh PR, Chen WC, Yu CJ, Yang PC. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11(6):715–22. doi: 10.1111/j.1440-1843.2006.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulou I, Orfanos S, Kotanidou A, Livaditi O, Giamarellos-Bourboulis E, Athanasiou C, et al. Plasma pro- and anti-inflammatory cytokine levels and outcome prediction in unselected critically ill patients. Cytokine. 2008;41(3):263–7. doi: 10.1016/j.cyto.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Ely EW, Inouye SK, Bernard GR, Francis J, May L, Truman B, et al. Derlirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Journal of the American Medical Association. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- Fan E. What is stopping us from early mobility in the intensive care unit? Critical Care Medicine. 2010;38(11):2254–5. doi: 10.1097/CCM.0b013e3181f8477d. [DOI] [PubMed] [Google Scholar]
- Fan E, Ciesla ND, Turong AD, Bhoopathi V, Zeger SL, Needham DM. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Medicine. 2010;36(6):1038–43. doi: 10.1007/s00134-010-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RD, Hall JB. Intensive care unit-acquired weakness. Critical Care Medicine. 2010;38(3):779–87. doi: 10.1097/CCM.0b013e3181cc4b53. [DOI] [PubMed] [Google Scholar]
- Herridge MS. Building consensus on ICU-acquired weakness. Intensive Care Medicine. 2009;35(1):1–3. doi: 10.1007/s00134-008-1305-3. [DOI] [PubMed] [Google Scholar]
- Hildreth AN, Enniss T, Martin RS, Miller PR, Mitten-Long D, Fasaway J, et al. Surgical intensive care unit mobility is increased after institution of a computerized mobility order set and intensive care unit mobility protocol: a prospective cohort analysis. American Surgeon. 2010;76(8):818–22. [PubMed] [Google Scholar]
- Hopkins RO, Spuhler J. Strategies for promoting early activity in critically ill mechanically ventilated patients. AACN Advanced Critical Care. 2009;20(3):277–89. doi: 10.1097/NCI.0b013e3181acaef0. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Spuhler VJ, Thomsen GE. Transforming ICU culture to facilitate early mobility. Critical Care Clinics. 2007;23(1):81–96. doi: 10.1016/j.ccc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jastrow KM, Gonzalez EA, McGuire MR, Sulibruk JW, Kozar RA, Iyengar S. Early cytokine production risk stratifies trauma patients for multiple organ failure. Journal of the American College of Surgeons. 2009;209(3):320–31. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Johanson WG, Pierce AK, Sanford JP, Thomas GD. Nosocomial respiratory infections with gram-negative bacilli. The significance of colonization of the respiratory tract. Annals of Internal Medicine. 1972;77:701–6. doi: 10.7326/0003-4819-77-5-701. [DOI] [PubMed] [Google Scholar]
- Kalisch BJ. Missed nursing care: a qualitative study. Journal of Nursing Care Quality. 2006;21(4):306–13. doi: 10.1097/00001786-200610000-00006. [DOI] [PubMed] [Google Scholar]
- Kalisch BJ, Lee KH. The impact of teamwork on missed nursing care. Nursing Outlook. 2010;58(5):233–41. doi: 10.1016/j.outlook.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Kasotakis G, Schmidt U, Perry D, Grosse-Sundrup M, Benjamin J, Ryan C, et al. The surgical intensive care unit optimal score predicts mortality and length of stay. Critical Care Medicine. 2011 doi: 10.1097/CCM.0b013e3182376e6d. [Epub ahead of print 2011/11/10] [DOI] [PubMed] [Google Scholar]
- Katz S, Down RD, Cash HR, Grotz RC. Progress in the development of the index of ADL. The Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- Knaus W, Wagner D, Draper E, Zimmerman J, Bergner M, Bastos P, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–36. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- Lee YL, Chen W, Chen CH, Lin YC, Liang SJ, Shih CM. Systemic and bronchoalveolar cytokines as predictors of in-hospital mortality in severe community-acquired pneumonia. Journal of Critical Care. 2010;25(1):176, e17–13. doi: 10.1016/j.jcrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Mayhall CG. Ventilator-associated pneumonia or not? Contemporary diagnosis. [accessed February 27, 2012];Emerging Infectious Diseases. 2001 7(2):200–4. doi: 10.3201/eid0702.010209. www.mesoscale.com. http://www.mesoscale.com/CatalogSystemWeb/WebRoot/products/plates.aspx; updated 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PE, Goad A, Thompson G, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility in the treatment of acute respiratory failure. Critical Care Medicine. 2008;36(8):2238–43. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- Morris PE, Griffin L, Berry M, Thompson C, Hite RD, Winkelman C, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. American Journal of Medical Science. 2011;342(5):373–7. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PE, Herridge MS. Early intensive care unit mobility: future directions. Critical Care Clinics. 2007;23(1):97–110. doi: 10.1016/j.ccc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Topics in Stroke Rehabilitation. 2010;17(4):271–81. doi: 10.1310/tsr1704-271. [DOI] [PubMed] [Google Scholar]
- Norton SA, Hogan LA, Holloway RG, Temkin-Greener H, Buckley MJ, Quill T. Proactive palliative care in the medical intensive care unit: effect on length of stay for selected high-risk patients. Critical Care Medicine. 2007;35(6):1530–5. doi: 10.1097/01.CCM.0000266533.06543.0C. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Muscles and their myokines. Journal of Exploratory Biology. 2011;214(Pt 2):337–46. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- Perme C, Chandrashekar R. Early mobility and walking program for patients in intensive care units: creating a standard of care. American Journal of Critical Care. 2009;18(3):212–21. doi: 10.4037/ajcc2009598. [DOI] [PubMed] [Google Scholar]
- Pnadharipande PP, Shintani AK, Hagerman HE, Jacques PJ, Rice TW, Sanders NW, et al. Derivation and validation of SpO2/FiO2 ratio to impute for PaO2/FiO2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Critical Care Medicine. 2009;37(4):1317–21. doi: 10.1097/CCM.0b013e31819cefa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlman MC, Schweickert WD, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. [accessed February 27, 2012];Critical Care Medicine. 2010 38(11):2089–94. doi: 10.1097/CCM.0b013e3181f270c3. www.sas.com. http://www.sas.com/technologies/analytics/statistics/; updated 2012. [DOI] [PubMed] [Google Scholar]
- Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Exbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller KA, Phillips AC, Lambert P. The safety of mobilisation and its effect on hemodynamic and respiratory status of intensive care patients. Physiotherapy in Theory and Practice. 2004;20(3):175–85. [Google Scholar]
- Thomsen GE, Snow GL, Rodriguez L, Hopkins RO. Patients with respiratory failure increase ambulation after transfer to an intensive care unit where early activity is a priority. Critical Care Medicine. 2008;36(4):1119–24. doi: 10.1097/CCM.0b013e318168f986. [DOI] [PubMed] [Google Scholar]
- Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit––from pathophysiology to clinical trials. Critical Care. 2009;13(4):216. doi: 10.1186/cc7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness––crossing the quality chasm. Chest. 2010a;138(5):1224–33. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevskis EE, Han JH, Shintani A, Girard TD, Ely WE. Delirium and mortality risk prediction: a story in evolution. Critical Care. 2010b;14(5):449. doi: 10.1186/cc9282. [author reply 449] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollman KM. Hemodynamic instability: is it really a barrier to turning critically ill patients? Critical Care Nurse. 2012;32(1):70–5. doi: 10.4037/ccn2012765. [DOI] [PubMed] [Google Scholar]
- Winkelman C. Inactivity and inflammation in the critically ill patient. Critical Care Clinics. 2007;23(1):21–34. doi: 10.1016/j.ccc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Winkelman C, Higgins PA, Chen YJ. Activity in the chronically critically ill. Dimensions in Critical Care Nursing. 2005;24(6):281–90. doi: 10.1097/00003465-200511000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman C, Higgins PA, Chen YJ, Levine AD. Cytokines in chronically critically ill patients after activity and rest. Biologic Research in Nursing. 2007;8(4):261–71. doi: 10.1177/1099800406298168. [DOI] [PubMed] [Google Scholar]
- Zanni JM, Korpolu R, Fan E, Pradham P, Janjua K, Palmer JB, et al. Rehabilitation therapy and outcomes in acute repiratory failure: An observational pilot. Journal of Critical Care. 2009;25(2):254–62. doi: 10.1016/j.jcrc.2009.10.010. [DOI] [PubMed] [Google Scholar]