Abstract
BACKGROUND
Adult survivors of childhood cancer have an increased risk of cerebrovascular disease; little is known about early stroke risk in childhood cancer. Our objectives were to assess stroke prevalence in children with cancer; to establish cancer and stroke type; and to determine if modifiable risk factors for stroke were present.
METHODS
Children with stroke and cancer were compared to all children seen for cancer at a single institution from 2000–2009. An international classification of disease-9th version code search and search of existing pediatric oncology and stroke databases identified children <18 years with ischemic stroke, intracerebral hemorrhage and cerebral sinovenous thrombosis.
RESULTS
Of 1,411 children with cancer, 15 had stroke (1.1%, 95% CI: 0.6–1.7%). Strokes were 7 intracerebral hemorrhages, 5 ischemic strokes (1 of them followed by intracerebral hemorrhage), and 3 sinovenous thromboses. Stroke occurred at a median of 5 months post-cancer diagnosis. Ten children with strokes had hematologic malignancies and 5 had brain tumors. Thirteen patients died post-stroke, 8 via withdrawal of care. White blood cell count ≥48,000/mm3 was found in 4 children, all with intracerebral hemorrhage. Five of 7 children with intracerebral hemorrhage had platelets <50,000/mm3
CONCLUSIONS
Stroke has a prevalence of approximately 1% in children with cancer. Hemorrhagic stroke and ischemic stroke occur with approximately equal frequency; children with leukemia and brain tumors are at greatest risk.
Keywords: Pediatric Stroke, Pediatric Oncology, Leukemia, Brain tumors, Neurotoxicity of therapy
Introduction
Strokes result in increased morbidity and a high need for critical care services and are a known complication in patients with cancer1. Recent literature has demonstrated that adult survivors of childhood cancer have an increased risk of cerebrovascular disease2–7.
The Children's Oncology Group report on cerebrovascular disease in childhood cancer survivors showed that stroke risk is increased in survivors of pediatric central nervous system (CNS) tumors, Hodgkin lymphoma, and acute lymphoblastic leukemia (ALL) who received radiation to the brain and/or neck7. Specifically, the relative risk of stroke for leukemia survivors compared with sibling controls was 6.4 at a median of 9.8 years from cancer diagnosis; the relative risk of stroke for brain tumor survivors compared with siblings was 29 at a median of 13.9 years from cancer diagnosis3. Similarly, Campen et al. found that the incidence of neurovascular events in pediatric brain tumor survivors is 100-fold higher than in the general pediatric population and cranial irradiation is an important risk factor8. Haddy et al. found that among 5-year survivors of childhood cancer, the radiation dose to the brain during radiotherapy was significantly associated with long-term cerebrovascular mortality, namely children who received >50 Gray had a 17.8 fold higher hazard ratio of death from cerebrovascular disease at a median follow-up of 29 years5.
However, little is known about strokes within the first 5 years after diagnosis of childhood cancer. Packer et al. retrospectively analyzed 700 children newly diagnosed with systemic malignancy between 1979 and 1983 and found that 26 children had suffered cerebrovascular accidents (4%), but children with primary intracranial neoplasms were excluded9. Bowers et al. performed a 15 year retrospective review of 807 children with CNS tumors and found that 13 children (1.6%) suffered a non-perioperative stroke, defined as a new ischemic brain lesion10. In a 7-year retrospective analysis of 85 children with non-traumatic intracranial hemorrhage, Lo et al. found a higher than previously reported frequency of complex chronic illnesses, including 13 brain tumors (15%), as risk factors for pediatric intracranial hemorrhages11. Kyrnetskiy et al. identified 51 children with cancer and intracranial hemorrhages, which included subdural, epidural, subarachnoid and intracerebral (parenchymal or intraventricular) hemorrhages, in an 18-year retrospective review and found 30 children with brain tumors, 19 with leukemia and 2 with lymphoma12. There is literature focusing on stroke in children with selected neoplasms (either leukemia, specifically ALL, or brain tumors)13–15, or characterizing single stroke type (intracerebral hemorrhage, ischemic stroke or cerebral sinovenous thrombosis (CSVT) in children with cancer12, 16, 17.
Our primary goals were 1) to assess the prevalence of stroke in children with all types of cancer 2) to determine which children with cancer were most likely to have early stroke and assess stroke type and 3) to determine whether stroke was simply a complication of aggressive cancer or if modifiable risk factors were present.
Study Design and Methods
We performed a retrospective review of children with intracerebral hemorrhage (ICH), ischemic stroke and CSVT who were also diagnosed with cancer and followed at a large pediatric tertiary care center. An arterial distribution of ischemic stroke was not required. Watershed and venous infarctions were included as was ischemic injury due to leukemia of the central nervous system. ICH was defined as parenchymal and/or intraventricular hemorrhage.
To identify children with stroke, an international classification of disease-9th version (ICD-9) code search of medical records was performed using codes for ICH, ischemic stroke, and CSVT. Upon reviewing the existing literature on accuracy and yield of ICD-9 codes for identifying children with stroke18, 19, as well as our institution's billing practices, we selected 14 ICD-9 codes. Included codes were: 325, 342, 430, 431, 432.9, 433, 434, 435, 436, 437, 438, 671.5, 747.81 and 767. Where no decimal places were included, our ICD-9 search included all variations so, 437 includes 437.XX. We also searched existing pediatric oncology and pediatric stroke databases at our center for children diagnosed with both stroke and cancer.
Children younger than 18 years of age seen between January 1, 2000 and December 31, 2009 were included. We excluded children with intra-tumoral hemorrhage, traumatic hemorrhage, subdural and epidural hemorrhage (typically classified as intracranial, not intracerebral hemorrhage), and bleeding related to surgical interventions. We abstracted information from the chart on patient demographics (age, sex, race/ethnicity), stroke type and potential etiologies, clinical features at presentation, time from cancer diagnosis to presentation, radiology reports, risk factors for stroke, such as abnormal platelet count, white blood cell count (WBC) and coagulation studies at the time of stroke, including disseminated intravascular coagulation (DIC), treatment (for cancer and stroke), including surgical treatment, length of follow-up and outcome.
Coagulopathy was defined as abnormal coagulation studies: prothrombin time (PT), international normalized ratio (INR) and activated partial thromboplastin time (aPTT). We defined coagulopathy using an INR >1.5, a PT >18sec, or an aPTT > 60sec, which is consistent with definitions used in previously published studies on acute traumatic coagulopathy, as there are no standard definitions in children with cancer20, 21. Since there is no gold standard for the diagnosis of DIC, we adapted the International Society of Thrombosis and Hemostasis DIC scoring system using a combination of prolonged PT or INR, hypofibrinogenemia (when an abnormal fibrinogen level was documented) and thrombocytopenia in the appropriate clinical setting22–24. Stroke was defined as documented clinical presentation consistent with stroke such as a focal neurological deficit of sudden onset and radiographic image(s), magnetic resonance imaging or computed tomography, showing cerebral parenchymal infarct(s) or ICH corresponding to the clinical manifestations25, 26. A pediatric neurologist with expertise in stroke reviewed the abstracted clinical information and neuroimaging to confirm strokes. A pediatric oncologist reviewed all abstracted clinical information to confirm cancer diagnoses. Children with more than one cancer type were classified according to the type of malignancy present at the time of stroke diagnosis. To define a denominator for period prevalence (how many children with cancer had stroke during the study time period)27, oncology billing records were searched for new pediatric patients < 18 years of age seen in our center for cancer care with at least 2 visits between January 1, 2000 and December 31, 2009. The time between first and last visit was calculated to define the duration of follow-up for each child.
All comparisons of proportions were analyzed using χ2 tests or Fisher exact tests when any value was less than five. Confidence intervals were calculated by exact methods. We conducted analyses using STATA 11.0 (College Station, TX) and considered a two-sided p-value of <0.05 to be significant for all analyses. This study was approved by the Institutional Review Board.
Results
Our initial ICD-9 code search produced 298 records. Upon chart review, 254 children had either a documented ischemic stroke or some type of intracranial hemorrhage (including subdural and epidural hemorrhages), whereas 44 children were incorrectly coded and had a different diagnosis. Among the 254 children, 189 had ICH (parenchymal or intraventricular hemorrhage), 38 had an ischemic stroke and 27 patients had CSVT. Thirty one of these 254 children also had cancer. The institutional pediatric stroke database and pediatric cancer database were searched as well and 3 additional children with cancer and stroke who met the study inclusion criteria were identified. We then reviewed the charts of the 34 pediatric patients diagnosed with both cancer and cerebral ischemia or ICH in detail.
Of the 34 children with cancer who had some form of cerebral ischemia or ICH, 19 were found to have intra-tumoral hemorrhage only. Intra-tumoral hemorrhage was typically found in children with a high-grade intracranial lesion. Excluding children with intratumoral hemorrhage, 15 children with cancer had stroke; stroke occurred at a median of 5 months (interquartile range (IQR) 0–19 months) after cancer diagnosis and the median length of follow up was 13 months (IQR 5–56 months).
Based on oncology billing records, there were 1,411 new pediatric patients seen and followed for cancer care at our institution between January 1, 2000 and December 31, 2009. Their median length of follow up was 47 months (IQR 14–76 months). Of these 1,411 children, 190 were diagnosed with ALL, 90 with acute myelogenous leukemia (AML), 122 with lymphoma and 389 with brain tumors. The remaining 620 children were diagnosed with other types of cancer. Therefore, 1.1% of children with a new diagnosis of cancer had stroke. Stroke prevalence in children with brain tumor was 1.3% (95% CI: 0.4–3%), in children with lymphoma was 1.6% (95% CI: 0.2–5.8%), and in children with leukemia was 2.9% (95% CI: 1.2–5.6%).
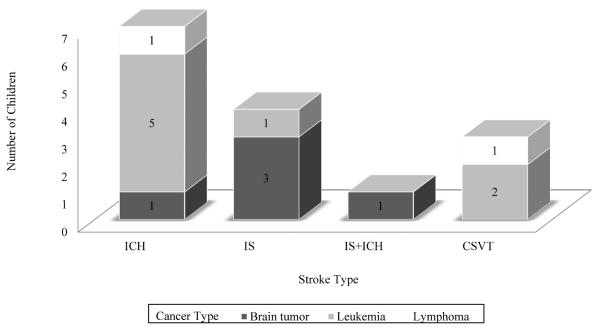
Seven children had ICH, 4 had ischemic stroke, 1 had ischemic stroke followed 5 months later by an ICH, and 3 children had CSVT. There were 10 strokes in children with hematologic malignancies and 5 in children with brain tumors.
Please see Table 1 for a description of patient characteristics (sex, age at time of stroke, time from cancer diagnosis to stroke, cancer types, stroke types, and outcomes). Table 2 provides details on all 15 children with stroke including risk factors for and presumed etiology. Figure 1 summarizes stroke types (ICH, ischemic stroke, or CVT) and type of cancer.
Table 1.
Characteristics of Children with Stroke (N=15)
| Characteristics | ICH (n=7) | Ischemic Stroke (n=4) | Ischemic Stroke and ICH (n=1) | CSVT (n=3) | Total Strokes (n=15) |
|---|---|---|---|---|---|
| Median (Interquartile Range) | |||||
| Age (years) | 12 (10–17) | 6 (5–6.5) | 13 | 9 (3–12) | 10 (6–13) |
| Cancer diagnosis to stroke (months) | 7 (0–19) | 21.5 (0–50.5) | 0 | 1 (0–10) | 5 (0–19) |
| Number (Percentage) | |||||
| Sex (males) | 6 (85.7%) | 1 (25%) | 0 | 2 (66.7%) | 9 (60%) |
| Brain tumors | 1 (14.3%) | 3 (75%) | 1 (100%) | 0 | 5 (33.3%) |
| Leukemia | 5 (71.4%) | 1 (25%) | 0 | 2 (66.7%) | 8 (53.3%) |
| Lymphoma | 1 (14.3%) | 0 | 0 | 1 (33.3%) | 2 (13.3%) |
| Stroke on presentation | 2 (28.6%) | 2 (50%) | 1 (100%) | 1 (33.3%) | 6 (40%) |
| Stroke recurrence | 0 | 1 (25 %) | 1 (100%) | 0 | 2 (13.3%) |
| Died | 7 (100%) | 3 (75%) | 1 (100%) | 2 (66.7%) | 13 (86.7%) |
| Support withdrawn | 4 (57.1%) | 2 (50%) | 0 | 2 (66.7%) | 8 (53.3%) |
Abbreviations:
ICH = intracerebral hemorrhage
CSVT = cerebral sinovenous thrombosis
Table 2.
Summary of Cancer and Stroke Types (N=15)
| Cancer Diagnosis until Stroke (months) |
After Stroke until Death (days) |
Cancer Type | Outcome | WBC Count (103/mm3) |
Platelet Count (103/mm3) |
Abnormal Coagulation |
Cause of Stroke |
|---|---|---|---|---|---|---|---|
| Hemorrhagic Stroke | |||||||
| IP | 1 | Pre-T leukemia | Dead | 840 | 54 | Yes | Leukostasis, Thrombocytopenia, Coagulopathy/DIC |
| IP | 1 | AML | Dead | 52.5 | 28 | Yes | Hyperleukocytosis, Thrombocytopenia, Coagulopathy/DIC |
| 5 | 5 | Rhabdomyosar coma with secondary M5 AML | Dead | 10.2 | 39 | No | Thrombocytopenia |
| 7 | 1 | Acute T-cell lymphoblastic leukemia | Dead | 487 | 70 | Yes | Leukostasis, Coagulopathy/DIC |
| 18 | 470 | T-cell lymphoblastic lymphoma | Dead | 48 | 14 | N/A | Thrombocytopenia |
| 19 | 3 | Brainstem glioma | Dead | 4.4 | 30 | No | Thrombocytopenia |
| 58 | 1 | Pre-B ALL | Dead | 1.1 | 31 | Yes | Thrombocytopenia Coagulopathy/DIC |
| Hemorrhagic and Ischemic Stroke | |||||||
| IP | 325 | Anaplastic astrocytoma | Dead | 22 | 320 | No | Ischemic injury s/p mass effect; second stroke was idiopathic hemorrhage |
| Ischemic Stroke | |||||||
| IP | 34 | High grade thalamic glioma | Dead | 26 | 311 | Yes | Ischemic injury s/p tumor mass effect |
| IP | 409 | Grade 4 glioma, cerebellopontine angle | Dead | 17 | 237 | No | Ischemic injury s/p tumor mass effect |
| 58 | N/A | AML with CNS relapse | Alive | 40 | 369 | No | CNS relapse of leukemia presenting as diffuse ischemic stroke |
| 43 | 34 | Recurrent anaplastic astrocytoma with secondary AML | Dead | 4.5 | 8 | Yes | Ischemic stroke due to radiation induced vasculopathy |
| CSVT | |||||||
| 1 | N/A | Pre-B ALL | Alive | 1.2 | 90 | No | CSVT after L-asparaginase with ischemic venous infarction |
| IP | 256 | Burkitt lymphoma | Dead | 11.5 | 207 | No | CSVT due to tumoral infiltrative process |
| 10 | 181 | ALL | Dead | 5.9 | 167 | No | CSVT after L-asparaginase with ischemic venous infarction |
Abbreviations:
ALL = acute lymphoblastic leukemia
AML = acute myelogenous leukemia
CNS = central nervous system
CSVT = cerebral sinovenous thrombosis
DIC = disseminated intravascular coagulation
IP = initial presentation
N/A = not applicable
s/p = suspected
WBC = white blood cell
Figure 1.
Type of stroke and type of cancer for N=15 children with both diagnoses. ICH = intracerebral hemorrhage; IS = ischemic stroke; CSVT = cerebral sinovenous thrombosis.
There were two children with recurrent stroke. One child had recurrent ischemic changes in a watershed pattern due to radiation-induced vasculopathy in a moyamoya pattern and presented with new onset refractory seizures. Recurrent stroke also occurred in one child who had an initial ischemic stroke related to tumor mass effect and then developed a new left thalamic hemorrhage five months later.
Of the 15 children with stroke and cancer, 13 children died. Of the children who died, 8 had care withdrawn for presumed futility related to both cancer and significant brain injury. The other 5 children died from complications due to progressive oncologic disease. Six of the 7 children with ICH died within 7 days of their stroke. The seventh child died 470 days later.
WBC count ≥48,000/mm3 was found in 4 children and 2 of these children also had low platelet counts <50,000/mm3. All of these children had ICH, not ischemic stroke. The median platelet count for all children with stroke was 70,000/mm3 (IQR 30–237/mm3). Five of 7 children with ICH had platelets <50,000/mm3, but this was not statistically significant. All seven children with ICH had platelet counts <100,000/mm3, compared to one child each with ischemic stroke and CSVT with low platelets (p=0.01).
Six out of fourteen children with documented INR and PTT at the time of their stroke had evidence of coagulopathy. Four of these children had clinical and laboratory findings consistent with DIC 22–24. One child's coagulation data could not be retrieved.
Potentially modifiable risk factors that might have prevented stroke if addressed by medical providers were not identified.
Discussion
In this single center study, we found that the prevalence of stroke in more than 1,400 children with new diagnoses of cancer over a 10-year period was 1.1% (95% CI: 0.6–1.7%). The prevalence of stroke in the 190 children treated for ALL during the same time period was 2.6%, the prevalence of stroke in the 90 children treated for AML was 3.3% and in the 122 children treated for various types of lymphoma was 1.6%. The stroke prevalence in the 389 children treated for brain tumors was 1.3%.
The low prevalence of stroke in our study confirms the findings in other published studies. Packer et al. evaluated 700 children with systemic malignancy over a 4 year period, excluding patients with primary intracranial neoplasms. He found that 26 children suffered cerebrovascular accidents (4%): 17 patients with lymphoreticular malignancy (4% ALL, 13% AML and 1% lymphoma) and 9 patients with solid tumors (6% neuroblastoma, 5% bone tumors 1% others) 9. DiMario et al. retrospectively reviewed 815 children with systemic cancer (excluding primary CNS tumors) over a 6-year period and identified 89 children with altered mental status (11%). Of those, 12 children had stroke (1.5%) and they were equally distributed amongst ALL, AML and lymphoma 28. Similarly low stroke prevalence was found in 4 other studies, all focused on patients diagnosed with ALL. Parasole et al. retrospectively assessed 27 neurologic events from 253 children enrolled in ALL treatment protocol during a 9-year period and found strokes in 5 children (1.97%). Three of the strokes were hemorrhagic and 2 were ischemic 14. Kuskonmaz et al. found that 20 of 203 ALL patients followed at a single institution developed neurologic complications; in total, 5 children had ischemic stroke or CSVT (2.46%) and two children had cerebral hemorrhage 13. Santoro et al. evaluated a group of 2,318 children with ALL and found the prevalence of ischemic stroke to be 0.47%, though in this study all children with ALL had cerebral ischemia due to CSVT 16. Umeda et al. evaluated a group of 541 children enrolled in ALL treatment protocol in Japan during a three year period and found that 15 patients developed 17 CNS complications; 3 of these were strokes (0.55%) 15. Whereas in these studies more strokes in children with ALL were ischemic, in our study 3 of the 5 strokes in children with ALL were ICH. A strength of our study is the fact that we did not limit our search to children with a particular type of malignancy. We collected data and calculated the prevalence of stroke in children with all types of cancer.
Unfortunately, many strokes occurred at the time of presentation due to tumor mass effect, or as a complication of aggressive cancer in critically ill children. Most strokes occurred early in the course of cancer treatment with a median time to stroke presentation of 5 months after cancer diagnosis, though the range was broad. While none reached statistical significance, several known risk factors for stroke in patients with cancer were identified in our population: leukostasis leading to hyperviscous state, thrombocytopenia and DIC, radiation and chemotherapy (L-asparaginase is a known risk factor for CSVT) 1, 29–32.
Modifiable risk factors that if addressed by medical providers, might have prevented stroke were not clearly apparent. For example, of four children with hyperleukocytosis with high WBC ≥48,000/mm3, all had hemorrhagic not ischemic strokes. Two of these children also had low platelet counts <50,000/mm3. Additionally, all seven children with ICH had platelet counts <100,000/mm3 (p=0.01), and five of these seven children had platelets <50,000/mm3 (not statistically significant). While platelet count <50,000/mm3 is traditionally considered to be a risk factor for ICH, transfusion strategies vary widely among pediatric oncologists and many children with cancer are not transfused until their platelet count is less than 20,000/mm3 33, 34. These children likely had significant platelet dysfunction as well as thrombocytopenia.
Limitations of our work include the retrospective nature of our study and the use of ICD-9 code searches which may be inaccurate 18, 19. We feel that our case ascertainment was likely complete in this study because there were pre-existing prospective institutional databases for both pediatric stroke and pediatric oncology which confirmed our ICD-9 search results.
A small sample size due to the rarity of pediatric stroke in children with cancer is also a limitation. Our sample of children with cancer at a large tertiary medical center over a 10-year-period is of reasonable size for a single center study. Multicenter studies have been limited by patient-reported stroke 4 and by lack of imaging and medical record review 16. A particular strength of our study is that stroke was carefully defined as a focal neurological deficit of sudden onset with neuroimaging that showed a stroke in a location corresponding to the clinical manifestations.
Conclusions
Early stroke is a rare complication in children with cancer and has an overall prevalence of around 1%. Stroke affects children with leukemia (ALL and AML) and with brain tumors with approximately equal frequency. Hemorrhagic stroke and ischemic stroke each represent about 50% of strokes in children with cancer. We identified the risk factors known to be associated with stroke (hyperleukocytosis leading to hyperviscosity, thrombocytopenia, DIC, tumor mass effect, radiation, drugs); however we were unable to identify modifiable risk factors for stroke in this population.
Acknowledgments and Funding
We wish to thank Blair Anton for her help in retrieving bibliographical data, and Melissa Gindville for her assistance with formatting and editing the manuscript.
Lori Jordan is funded by NIH - K23-NS062110.
Footnotes
Conflict of Interest Statement The authors have no conflicts of interest to disclose.
References
- 1.Rogers LR. Cerebrovascular complications in patients with cancer. Semin Neurol. 2010;30(3):311–319. doi: 10.1055/s-0030-1255224. doi: 10.1055/s-0030-1255224. [DOI] [PubMed] [Google Scholar]
- 2.Bowers DC. Strokes among childhood brain tumor survivors. Cancer Treat Res. 2009;150:145–153. doi: 10.1007/b109924_10. doi: 10.1007/b109924_10. [DOI] [PubMed] [Google Scholar]
- 3.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: A report from the childhood cancer survivor study. J Clin Oncol. 2006;24(33):5277–5282. doi: 10.1200/JCO.2006.07.2884. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 4.Bowers DC, McNeil DE, Liu Y, et al. Stroke as a late treatment effect of hodgkin's disease: A report from the childhood cancer survivor study. J Clin Oncol. 2005;23(27):6508–6515. doi: 10.1200/JCO.2005.15.107. doi: 10.1200/JCO.2005.15.107. [DOI] [PubMed] [Google Scholar]
- 5.Haddy N, Mousannif A, Tukenova M, et al. Relationship between the brain radiation dose for the treatment of childhood cancer and the risk of long-term cerebrovascular mortality. Brain. 2011;134(Pt 5):1362–1372. doi: 10.1093/brain/awr071. doi: 10.1093/brain/awr071. [DOI] [PubMed] [Google Scholar]
- 6.Jordan LC, Duffner PK. Early-onset stroke and cerebrovascular disease in adult survivors of childhood cancer. Neurology. 2009;73(22):1816–1817. doi: 10.1212/WNL.0b013e3181c33b10. doi: 10.1212/WNL.0b013e3181c33b10. [DOI] [PubMed] [Google Scholar]
- 7.Morris B, Partap S, Yeom K, Gibbs IC, Fisher PG, King AA. Cerebrovascular disease in childhood cancer survivors: A children's oncology group report. Neurology. 2009;73(22):1906–1913. doi: 10.1212/WNL.0b013e3181c17ea8. doi: 10.1212/WNL.0b013e3181c17ea8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campen CJ, Kranick SM, Kasner SE, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke. 2012;43(11):3035–3040. doi: 10.1161/STROKEAHA.112.661561. doi: 10.1161/STROKEAHA.112.661561; 10.1161/STROKEAHA.112.661561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packer RJ, Rorke LB, Lange BJ, Siegel KR, Evans AE. Cerebrovascular accidents in children with cancer. Pediatrics. 1985;76(2):194–201. [PubMed] [Google Scholar]
- 10.Bowers DC, Mulne AF, Reisch JS, et al. Nonperioperative strokes in children with central nervous system tumors. Cancer. 2002;94(4):1094–1101. [PubMed] [Google Scholar]
- 11.Lo WD, Lee J, Rusin J, Perkins E, Roach ES. Intracranial hemorrhage in children: An evolving spectrum. Arch Neurol. 2008;65(12):1629–1633. doi: 10.1001/archneurol.2008.502. doi: 10.1001/archneurol.2008.502;10.1001/archneurol.2008.502. [DOI] [PubMed] [Google Scholar]
- 12.Kyrnetskiy EE, Kun LE, Boop FA, Sanford RA, Khan RB. Types, causes, and outcome of intracranial hemorrhage in children with cancer. J Neurosurg. 2005;102(1 Suppl):31–35. doi: 10.3171/ped.2005.102.1.0031. doi: 10.3171/ped.2005.102.1.0031. [DOI] [PubMed] [Google Scholar]
- 13.Kuskonmaz B, Unal S, Gumruk F, Cetin M, Tuncer AM, Gurgey A. The neurologic complications in pediatric acute lymphoblastic leukemia patients excluding leukemic infiltration. Leuk Res. 2006;30(5):537–541. doi: 10.1016/j.leukres.2005.09.009. doi: 10.1016/j.leukres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Parasole R, Petruzziello F, Menna G, et al. Central nervous system complications during treatment of acute lymphoblastic leukemia in a single pediatric institution. Leuk Lymphoma. 2010;51(6):1063–1071. doi: 10.3109/10428191003754608. doi: 10.3109/10428191003754608. [DOI] [PubMed] [Google Scholar]
- 15.Umeda K, Yoshida M, Suzuki N, et al. Complications in the central nervous system during chemotherapy for childhood acute lymphoblastic leukemia: JACLS ALL-02 study. Rinsho Ketsueki. 2007;48(3):204–211. [PubMed] [Google Scholar]
- 16.Santoro N, Giordano P, Del Vecchio GC, et al. Ischemic stroke in children treated for acute lymphoblastic leukemia: A retrospective study. J Pediatr Hematol Oncol. 2005;27(3):153–157. doi: 10.1097/01.mph.0000157379.44167.b5. [DOI] [PubMed] [Google Scholar]
- 17.Reddingius RE, Patte C, Couanet D, Kalifa C, Lemerle J. Dural sinus thrombosis in children with cancer. Med Pediatr Oncol. 1997;29(4):296–302. doi: 10.1002/(sici)1096-911x(199710)29:4<296::aid-mpo11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Golomb MR, Garg BP, Saha C, Williams LS. Accuracy and yield of ICD-9 codes for identifying children with ischemic stroke. Neurology. 2006;67(11):2053–2055. doi: 10.1212/01.wnl.0000247281.98094.e2. doi: 10.1212/01.wnl.0000247281.98094.e2. [DOI] [PubMed] [Google Scholar]
- 19.Golomb MR, Garg BP, Williams LS. Accuracy of ICD-9 codes for identifying children with cerebral sinovenous thrombosis. J Child Neurol. 2007;22(1):45–48. doi: 10.1177/0883073807299959. [DOI] [PubMed] [Google Scholar]
- 20.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: Mechanism, identification and effect. Curr Opin Crit Care. 2007;13(6):680–685. doi: 10.1097/MCC.0b013e3282f1e78f. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 21.Mitra B, Cameron PA, Mori A, Fitzgerald M. Acute coagulopathy and early deaths post major trauma. Injury. 2012;43(1):22–25. doi: 10.1016/j.injury.2010.10.015. doi: 10.1016/j.injury.2010.10.015;10.1016/j.injury.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. british committee for standards in haematology. Br J Haematol. 2009;145(1):24–33. doi: 10.1111/j.1365-2141.2009.07600.x. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 23.Parker RI. Coagulopathies in the PICU: DIC and liver disease. Crit Care Clin. 2013;29(2):319–333. doi: 10.1016/j.ccc.2012.12.003. doi: 10.1016/j.ccc.2012.12.003; 10.1016/j.ccc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013 doi: 10.1111/jth.12155. doi: 10.1111/jth.12155;10.1111/jth.12155. [DOI] [PubMed] [Google Scholar]
- 25.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Recurrent hemorrhagic stroke in children: A population-based cohort study. Stroke. 2007;38(10):2658–2662. doi: 10.1161/STROKEAHA.107.481895. doi: 10.1161/STROKEAHA.107.481895. [DOI] [PubMed] [Google Scholar]
- 26.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: The importance of cerebrovascular imaging. Pediatrics. 2007;119(3):495–501. doi: 10.1542/peds.2006-2791. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 27.Gordis L. Epidemiology. 3rd illustrated ed. Elsevier Saunders; Philadelphia, PA: 2004. [Google Scholar]
- 28.DiMario FJ, Jr, Packer RJ. Acute mental status changes in children with systemic cancer. Pediatrics. 1990;85(3):353–360. [PubMed] [Google Scholar]
- 29.Reddy AT, Witek K. Neurologic complications of chemotherapy for children with cancer. Curr Neurol Neurosci Rep. 2003;3(2):137–142. doi: 10.1007/s11910-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 30.Elhasid R, Lanir N, Sharon R, et al. Prophylactic therapy with enoxaparin during L-asparaginase treatment in children with acute lymphoblastic leukemia. Blood Coagul Fibrinolysis. 2001;12(5):367–370. doi: 10.1097/00001721-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Kieslich M, Porto L, Lanfermann H, Jacobi G, Schwabe D, Bohles H. Cerebrovascular complications of L-asparaginase in the therapy of acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2003;25(6):484–487. doi: 10.1097/00043426-200306000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Kingma A, Tamminga RY, Kamps WA, Le Coultre R, Saan RJ. Cerebrovascular complications of L-asparaginase therapy in children with leukemia: Aphasia and other neuropsychological deficits. Pediatr Hematol Oncol. 1993;10(4):303–309. doi: 10.3109/08880019309029506. [DOI] [PubMed] [Google Scholar]
- 33.Wong EC, Perez-Albuerne E, Moscow JA, Luban NL. Transfusion management strategies: A survey of practicing pediatric hematology/oncology specialists. Pediatr Blood Cancer. 2005;44(2):119–127. doi: 10.1002/pbc.20159. doi: 10.1002/pbc.20159. [DOI] [PubMed] [Google Scholar]
- 34.Pisciotto PT, Benson K, Hume H, et al. Prophylactic versus therapeutic platelet transfusion practices in hematology and/or oncology patients. Transfusion. 1995;35(6):498–502. doi: 10.1046/j.1537-2995.1995.35695288769.x. [DOI] [PubMed] [Google Scholar]