Abstract
Neuroimmune semaphorin 4D (Sema4D) was found to be expressed and function in the nervous and immune systems. In the immune system, Sema4D is constitutively expressed on T cells and regulates T cell priming. In addition, it displays a stimulatory function on macrophages, DC, NK cells, and neutrophils. As all these cells are deeply involved in asthma pathology, we hypothesized that Sema4D plays a critical non-redundant regulatory role in allergic airway response. To test our hypothesis, we exposed Sema4D−/− and WT mice to OVA injections and challenges in the well-defined mouse model of OVA-induced experimental asthma. We observed a significant decrease in eosinophilic airway infiltration in allergen-treated Sema4D−/− mice relative to WT mice. This reduced allergic inflammatory response was associated with decreased BAL IL-5, IL-13, TGFβ1, IL-6, and IL-17A levels. In addition, T cell proliferation in OVA323–339 - restimulated Sema4D−/− cell cultures was downregulated. We also found increased Treg numbers in spleens of Sema4D−/− mice. However, airway hyperreactivity (AHR) to methacholine challenges was not affected by Sema4D deficiency in either acute or chronic experimental disease setting. Surprisingly, lung DC number and activation were not affected by Sema4D deficiency. These data provide a new insight into Sema4D biology and define Sema4D as an important regulator of Th2-driven lung pathophysiology and as a potential target for a combinatory disease immunotherapy.
Introduction
Neuroimmune Sema4D (CD100), a class IV member of semaphorin family of glycoproteins, was found to be expressed and function in the nervous and immune systems (Bougeret et al. 1992; Puschel, Adams, Betz 1995; Furuyama et al. 1996). In the immune system Sema4D is constitutively expressed on T cells predominantly as a disulfide-linked homodimer (300 kDa) or a 150 kDa monomer (Bougeret et al. 1992; Kumanogoh et al. 2002; Kumanogoh, Kikutani 2003; Chabbert-de Ponnat et al. 2005; Suzuki, Kumanogoh, Kikutani 2008). However, low levels of Sema4D had been noticed also on macrophages, B cells, NK cells, and neutrophils (Chabbert-de Ponnat et al., 2005; Kumanogoh & Kikutani, 2003; Kumanogoh et al., 2002; Suzuki, Kumanogoh, & Kikutani, 2008; (Nkyimbeng-Takwi, Chapoval 2011) where it regulates cell migration and/or activation. In the inflammatory conditions Sema4D also exist in a soluble form after MT1-MMP-mediated cleavage (sCD100) which might have a distinct function from a membrane-bound molecule (Delaire et al. 2001; Elhabazi et al. 2001; Basile et al. 2007; Nkyimbeng-Takwi, Chapoval 2011). sCD100, for example, has been shown to downregulate immune cell migration and proinflammatory cytokine production acting through unknown receptor (Chabbert-de Ponnat et al. 2005). One more addition to the overall complex venue of Sema4D expression and function is its cell surface association with protein tyrosine phosphatase CD45 (Herold et al. 1996) which potentiates CD45-mediated signal in T-cell activation.
Sema4D utilizes two known receptors, the previously termed “non-lymphoid tissue receptor” Plexin B1 (epithelial and endothelial cells) (Tamagnone et al. 1999; Basile et al. 2004; Chabbert-de Ponnat et al. 2005; Fazzari et al. 2007) and “lymphoid tissue receptor” CD72 (APC) (Kumanogoh et al. 2000; Chabbert-de Ponnat et al. 2005). However, we and others have identified Plexin B1 expression on dendritic cells (lung and follicular) and activated T cells in the lymphoid tissue (Granziero et al. 2003; Smith et al. 2011). Moreover, we have shown that CD72 is also expressed on many lung resident cells (Smith et al. 2011). Thus, there is no clear restriction of two Sema4D receptors to immune or non-immune cells. To prove Plexin B1 functional significance for immune cell activation, a study by Granziero L and associates (Granziero et al. 2003) defined that Sema4D–Plexin B1 interaction prolongs B-cell life span and increases their proliferation activities. On the other hand, engagement of CD72, a constitutive negative regulator of APC activation, with Sema4D leads to B cell and DC activation through CD72 dephosphorylation followed by a dissociation of SHP-1 (Kumanogoh et al. 2000; Ishida et al. 2003; Kumanogoh, Kikutani 2003; Suzuki, Kumanogoh, Kikutani 2008). Thus, both these Sema4D receptors display a positive effect in immune cell function.
The Sema4D in vivo function has been examined using either blocking Ab administration or knock-out mouse response to different antigens. Sema4D−/− mice failed to develop the experimental autoimmune encephalomyelitis (EAE) (Okuno et al. 2010). Furthermore, blocking antibodies against Sema4D significantly inhibited neuroinflammation during EAE development. This effect of Sema4D was Plexin B1-dependent. Sema4D has been shown to play a pathogenic role in the experimental glomerulonephritis as Sema4D−/− mice were protected from histological and functional glomerular injury and reduced CD4+ cell activation (Li et al. 2006). The involvement of Sema4D in allergy and asthma is unknown.
We defined the role of Sema4D in allergen-induced experimental asthma by treating Sema4D−/− mice with allergen and assessing their lung inflammatory response. We have found that Sema4D−/− mice showed significantly lower airway inflammatory cell infiltration, including eosiniophilia, and local lung proinflammatory cytokine production such as IL-6 and TGFb as well as Th2 (IL-5, and IL-13) and Th17 cytokine (IL-17A). However, AHR to methacholine challenges in knock-out mice in either acute or chronic allergen exposure model was equal to that observed in WT allergen-treated control animals. The amplitude of a re-call spleen MNC proliferative response to immunodominant OVA peptide was also significantly affected by Sema4D deficiency suggesting the important role of this single member of semaphorin family proteins in Ag-dependent T cell activation. Moreover, the relative number of spleen Treg cells was increased in allergen-treated Sema4D−/− mice as compared to WT mice. However, lung DC were not affected by Sema4D deficiency. The additional studies are necessary to further delineate the multisided effects of Sema4D on lung allergic response as well as to define the receptor involved (or dominated) in the observed effects.
Materials and Methods
Mice
The Sema4D−/− mice were obtained from the laboratory of Hitoshi Kikutani (Osaka University) where they were generated as previously described (Shi et al. 2000). C57BL/6 mice (WT) were purchased from Taconic. Mice were bred and maintained under specific pathogen-free conditions within the animal facility at University of Maryland School of Medicine. All age- and sex-matched mice used in the experiments were between 8 and 12 weeks of age. All procedures on mice were performed according to the animal protocol approved by University of Maryland School of Medicine Animal Care and Use Committee.
Anesthetic
Intraperitoneal injections of Pentobarbital in dose of 100 mg/kg or 200 mg/kg or Avertin in dose of 0.3 mg/kg or 2 mg/kg were used as previously described (Chapoval et al. 2011) to anesthetize or euthanize the mice, correspondingly. Cervical dislocation was performed on Avertineuthanized mice to ensure death.
Experimental model of acute asthma
Mice were treated with chicken OVA (Sigma) as described previously (Smith et al. 2011; Nkyimbeng-Takwi et al. 2012). Briefly, mice were injected i.p. with 100 µg OVA/2 mg Alum/200 µl on days 0 and 5. Control mice were injected with 200 µl of sterile endotoxin-free PBS/Alum. On days 12 and 14, mice received a 40 min aerosol challenge of either PBS or 1% (w/v) OVA (Smith et al. 2011; Nkyimbeng-Takwi et al. 2012). AHR in response to the methacholine challenges was measured 24 h after last nebulization. Mice were euthanized 24h thereafter for other analyses.
Experimental model of chronic asthma
Mice were treated with OVA as described above for an acute model of experimental asthma. After two OVA aerosol challenges on days 12 and 14 of the experimental protocol, mice received the additional challenges on days 21 and 28. AHR measurements were performed 24 h later.
Airway hyperreactivity measurements
AHR measurements to methacholine challenges were performed 24h after last Ag nebulization using non-invasive (BUXCO Electronics) technique as previously described (Hamelmann et al. 1997).
Histochemistry
Core Facility at the Center for Vascular and Inflammatory Diseases was used for histochemistry (H&E and PAS stains) of deparaffinized lung tissues (Chapoval et al. 2011).
Cellular composition and cytokine-chemokine content in bronchoalveolar lavages
Bronchoalveolar lavages (BAL) were performed 48h after last Ag nebulization, cells and BALF collected, cytospin prepared and cell counts performed as described earlier (Chapoval et al. 2011; Smith et al. 2011). BAL cytokine and chemokine levels were determined using Searchlight Proteome Array (Aushon), ELISA kits (R&D Systems), and CBA kits (BD Biosciences) (Chapoval et al. 2011; Smith et al. 2011; Nkyimbeng-Takwi et al. 2012). Array and CBA data were analyzed using the ArrayVision software and FlowJo softwares, correspondingly. Intact BAL fluids were used in the Proteome Array and ELISA. In the CBA assays, 2 times (2×) concentrated (10k Slide-A-Lyzer, Pierce) BAL fluids were used.
Proliferation assays
Cell proliferation was measured as described (Chapoval et al. 2011; Nkyimbeng-Takwi et al. 2012) using either [H]3 incorporation assay or Quick cell proliferation assay kit (ab65473, Abcam). Briefly, single cell suspensions were prepared from spleens of either PBS- or OVA-challenged mice on a day 5 post-challenge. Spleen MNC were plated to a density of 1 × 106 cells/200 µl in 96-well tissue culture plates (Cellstar, Greiner) and stimulated with either ConA (10µg/ml), LPS (100µg/ml), or OVA323–339 peptide (200 µg/ml) as previously described (Nkyimbeng-Takwi et al. 2012). After 72h of incubation, [H]3 thymidine (Perkinson-Maer) was added to the wells and plates were harvested on Packard Filtermate harvester (Packard Instruments) 24h later. In a colorimetric proliferation assay, 10 µl of tetrazolium salt WST-1 solution was added to each well followed by 4h of further incubation. The plates were read in the ELISA plate reader at 450 nm with a reference wavelength of 650nm.
Flow cytometry
Flow cytometry of lung and spleen single cell suspensions was performed as previously described (Chapoval et al. 2009; Nkyimbeng-Takwi et al. 2012) using the BD Biosciences Abs for the following cell markers: I-Ab (PE), CD4 (FITC), CD8 (PerCP), CD25 (PE), GR1 (FITC), CD11c (APC), B220 (FITC). Anti-mouse CD80 (PE-Cy5) and CD86 (PE-Cy5) Abs were obtained from Biolegend. Biotinylated armenian hamster IgG (eBioscience) followed by SA-PECy5 (BD Biosciences) staining and PE-Cy5 rat IgG2a (eBioscience) were used as isotype controls for corresponding costimulatory molecules. Intracellular staining for Foxp3 was done using anti-Foxp3 (APC) Ab or rat IgG2a (APC) isotype control Ab (both from eBioscience). Cells gated by forward- and side-scatter parameters were analyzed using either the CELLQuest or FlowJo software at the Flow Cytometry Facility, Center for Vascular and Inflammatory Diseases.
Statistics
Data were summarized as mean ± SEM. To calculate the significance levels between the experimental groups, Student’s t test (Microsoft Excel) and Mann-Whitney test (Prizm-4) were performed.
Results
Sema4D regulates allergic airway inflammation and antigen-specific IgE response
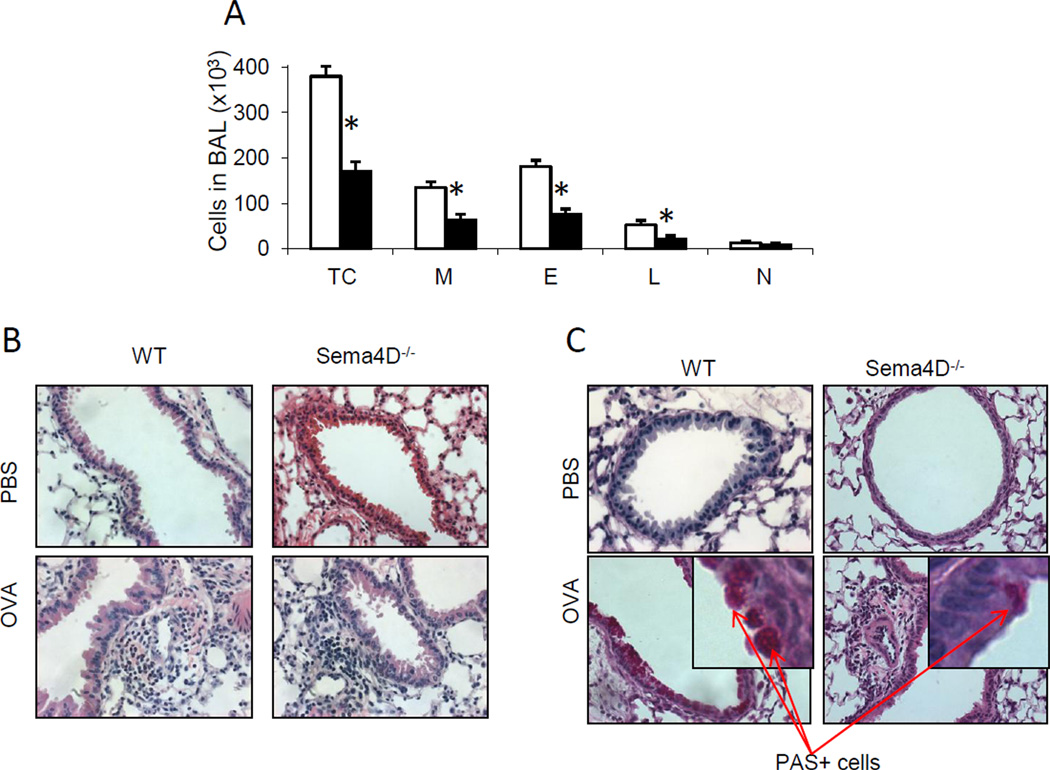
While the role of Sema4D in cancer (Basile et al. 2004; Basile, Afkhami, Gutkind 2005; Basile et al. 2007), EAE (Okuno et al. 2010), and EG (Li et al. 2006) was assessed previously, its regulation of an allergen-induced Th2 response has not been investigated. To define it, we subjected WT and Sema4D−/− mice to the allergen priming, boost and challenges as described in Material and Methods section. Control mice were treated with Alum/PBS and nebulized with PBS. A classical allergic airway response was observed in WT mice after OVA treatment (Fig. 1 A-B). This response consisted of prominent BAL and airway eosinophilic infiltration where more than 50% of BAL cells were eosinophils (Fig. 1 A). Eosinophils were also the predominant cells in the lung tissue multiple peribronchial and perivascular inflammatory infiltrates (Fig. 1 B). All these features of allergic response were significantly downregulated in Sema4D−/− mice (Fig. 1 A–B). Allergic inflammatory response was accompanied by mucus hypersecretion which was downregulated with Sema4D deficiency (19.2 – 20.7% vs 28 – 29.3% of PAS+ airways in WT lungs) (Fig. 1 C). In addition to that, Sema4D deficiency was associated with a significantly lower anti-OVA serum IgE concentration as compared to WT mice (819.4 ± 63.7 vs 994.1 ± 35.6 ng/ml, correspondingly, p<0.45, n=3/group).
Figure 1.
Sema4D deficiency regulates the severity of allergic airway response in mice. (A) WT and Sema4D−/− mice were immunized with OVA whereas control mice were challenge with PBS. (A) The average numbers (n=3–5) of BAL total cells (TC) macrophages (M), eosinophils (E), lymphocytes (L) and neutrophils (N) ± SEM in one of three representative experiments are shown. *, # p<0.05, differences in absolute numbers of TC, M, E, and L in BAL between OVAchallenged WT and Sema4D−/− mice. (B) Lung tissue sections were stained with H&E for inflammation and (C) Periodic acid stain (PAS) for mucus cell hyperplasia evaluation. Magnification used for pictures is 20×.
Sema4D regulates lung local Th2/Th17 cytokine response
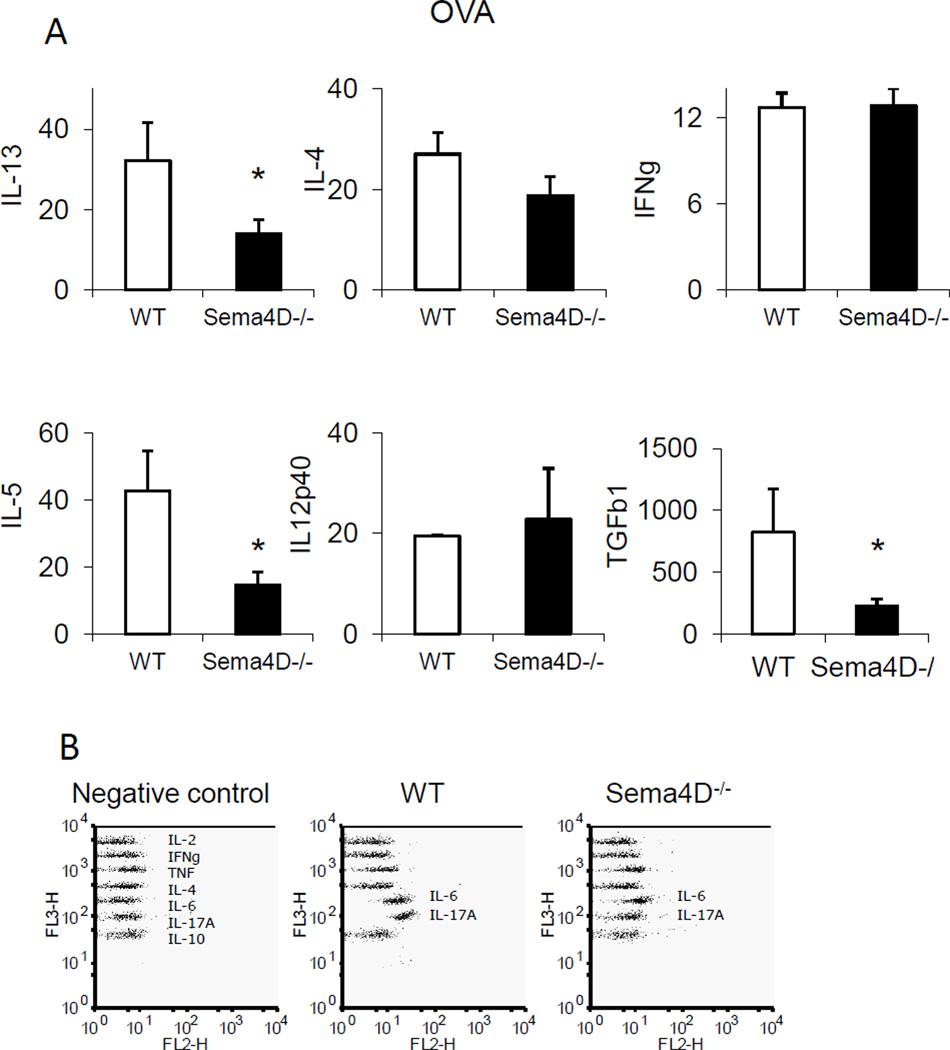
We evaluated lung cytokine dysregulation induced by allergen treatment and modulated by the Sema4D deficiency by comparing the levels of inflammatory and Th1/Th2/Th17 cytokines in the BAL of allergen-treated WT and Sema4D−/− mice. As shown in Fig, 2 A, we found a significant decrease in IL-5, IL-13 and TGFβ1 levels in Sema4D−/− mice when compared to WT mice. In addition, CBA data demonstrated downregulation of IL-6 and IL-17A levels with Sema4D deficiency (Fig. 2 B). Noteworthy, no substantial differences in the BAL contents of IFNγ and IL-12p40 were noted between WT and Sema4D−/− mice, whereas IL-4 levels were downregulated, although not significantly, with the absence of Sema4D. Of note, no significant differences in eotaxin, RANTES, MIP-1α, MIP-1β, JE, IL-10, and VEGF BALF levels were detected between WT and Sema4D−/− mice (data not shown).
Figure 2.
Sema4D regulates cytokines. The levels of cytokines in BAL fluids of OVA-challenged WT (open bars) and Sema4D−/− (black bars) mice measured by either protein array (A) or CBA (B). Data are shown as mean ± SEM (n = 3–4 mice). *p<0.05, OVA-challenged WT versus Sema4D−/− mice. (F) For cytokine CBA CBA Th1/Th2/Th17 in lung lysates and 2× concentrated BALF data were acquired by BD FACS Calibur and shown are FlowJo generated dot plots for individual proteins for an individual mouse in one of 2 representative experiments (n=2/group).
Sema4D deficiency does not affect lung physiology
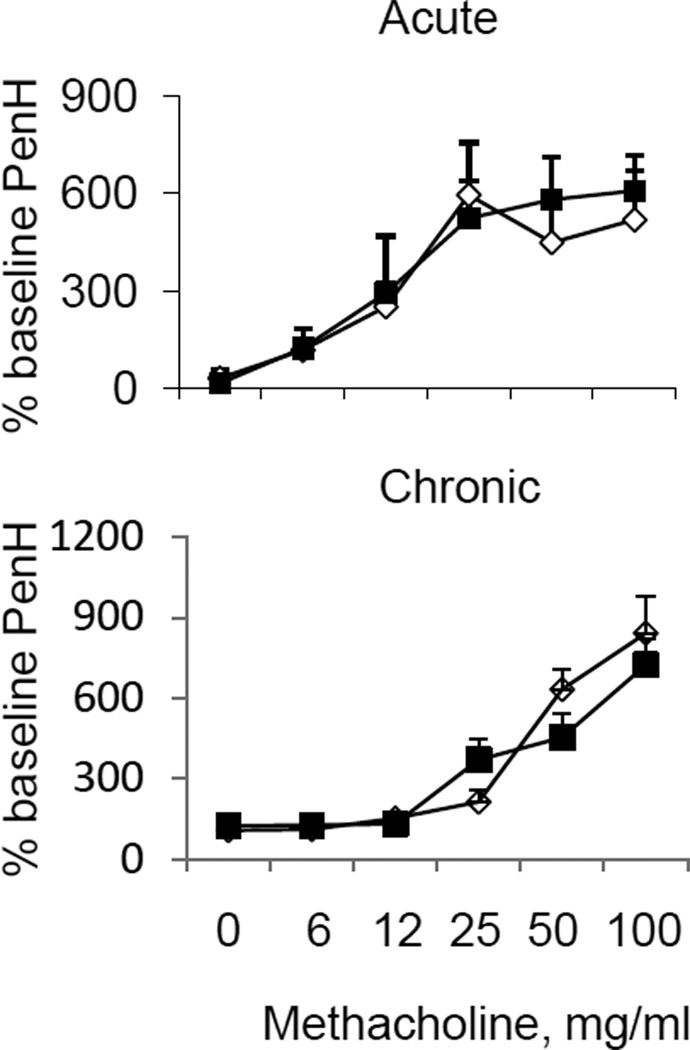
To assess the effect of Sema4D on the airway physiology, we performed the AHR measurements by a non-invasive technique (Fig. 3). We did not notice any differences in % baseline PenH, an index of airway obstruction, for five increasing doses of methacholine used (from 6 mg/ml to 100 mg/ml) between allergen-treated Sema4D−/− mice and WT control animals in either acute or chronic model of asthma. Based on these data, it was tempting to conclude that Sema4D has no effect in the airway physiology. However, additional studies using a more sensitive and reliable invasive Flexivent technique are necessary to make a final conclusion especially considering recent controversial reports about the results reproducibility with the use of a non-invasive Buxco technique for AHR measurements (Vanoirbeek et al. 2010; Berndt et al. 2011). The dissociation between AHR and inflammation in animal models of asthma due to different mechanisms of regulation of these different arms of allergic lung response had been noticed previously (Wilder et al. 1999; Makela et al. 2000; Hewitt et al. 2009; Short, Lipworth, Lipworth 2011).
Figure 3.
Sema4D has no effect on airway hyperreactivity in either acute or chronic model of experimental asthma. Airway reactivity is shown as the percent increase in PenH over a baseline (n = 3 – 4) for the corresponding doses of methacholine.
Sema4D is necessary for optimal inflammatory cell infiltration to the lung in allergic response
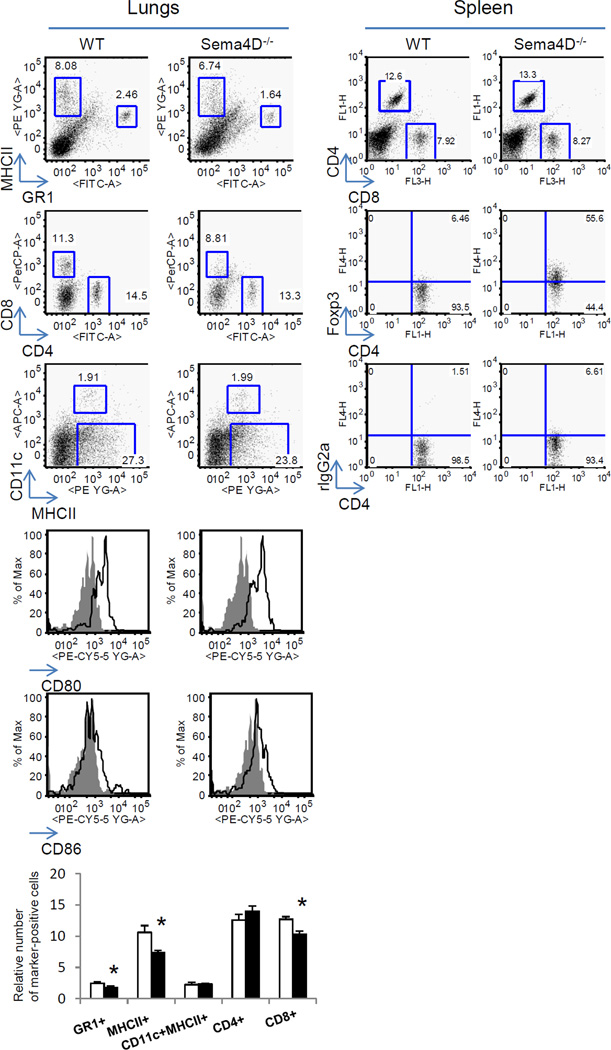
Lower relative numbers of MHCII+ (B cells) and GR1+ (eosinophils and neutrophils) cells were detected among lymphocyte-gated lung cells in Sema4D−/− mice as compared to WT mice (Fig. 4). Similarly, significantly lower relative numbers of CD8+ T cells were found in allergentreated mouse lungs with Sema4D deficiency whereas % of lung CD4+ T cells did not differ between WT and Sema4D−/− mice (dot plots shown were based on SSC-FSC gates of lymphocyte populations). However, neither relative numbers of lung myeloid DC (CD11c+/MHCII+/ autofluorescent macrophage negative cells) nor their activation stage as defined by costimulatory CD80 and CD86 molecule expression were affected. Considering an emerging critical role of Treg in the Th2 response suppression (Chapoval et al. 2010; Palomares et al. 2010), we analyzed the numbers of Treg in the spleens of WT and Sema4D−/− mice subjected to the allergen priming and challenges using corresponding Abs (Fig. 4). We observed a striking increase in Foxp3+CD4+ cells in Sema4D−/− mouse spleens as compared to WT spleens (55.6% compared to 6.46%, correspondingly). Similarly, we observed a substantial increase in the relative number of spleen Foxp3+CD25+ cells associated with Sema4D deficiency (data not shown). No significant differences were found in the relative numbers of both CD8+ and CD4+ T cells in the spleens of OVA-challenged Sema4D−/− mice as compared to WT mice (12.6 and 13.3%, correspondingly). This suggests that a defined regulatory role of Sema4D−/− in experimental allergic asthma is associated with decreases in lung inflammatory cell infiltration and increases in lymphoid tissue (and potentially in lung) Treg. This Sema4D effect could involve both cell activation and cell migration processes.
Figure 4.
Sema4D regulates inflammatory cell accumulation in the lung tissue and Treg cell in spleen. WT and Sema4D−/− mice were immunized with OVA as described in Materials and Methods section. Isolated lungs were subjected to enzymatic digestion to obtain single-cell suspensions. The cells were then stained with corresponding Abs to detect immune and inflammatory cells. Spleens were mechanically disrupted and processed to single cell suspensions involving a RBC lysis step. The spleen MNC were stained for T cells and Treg using corresponding Abs defined in Materials and Methods section. For detection of Foxp3+CD4+ cells in spleens, CD4+ cells were gated on SSC-FITC+ dot plots and then CD4+ cells were re-gated on Foxp3. The quadrants were drawn based on isotype control rat IgG2a staining results. *p<0.05, OVA-challenged WT vs Sema4D−/− mice.
Sema4D deficiency affects T cell activation
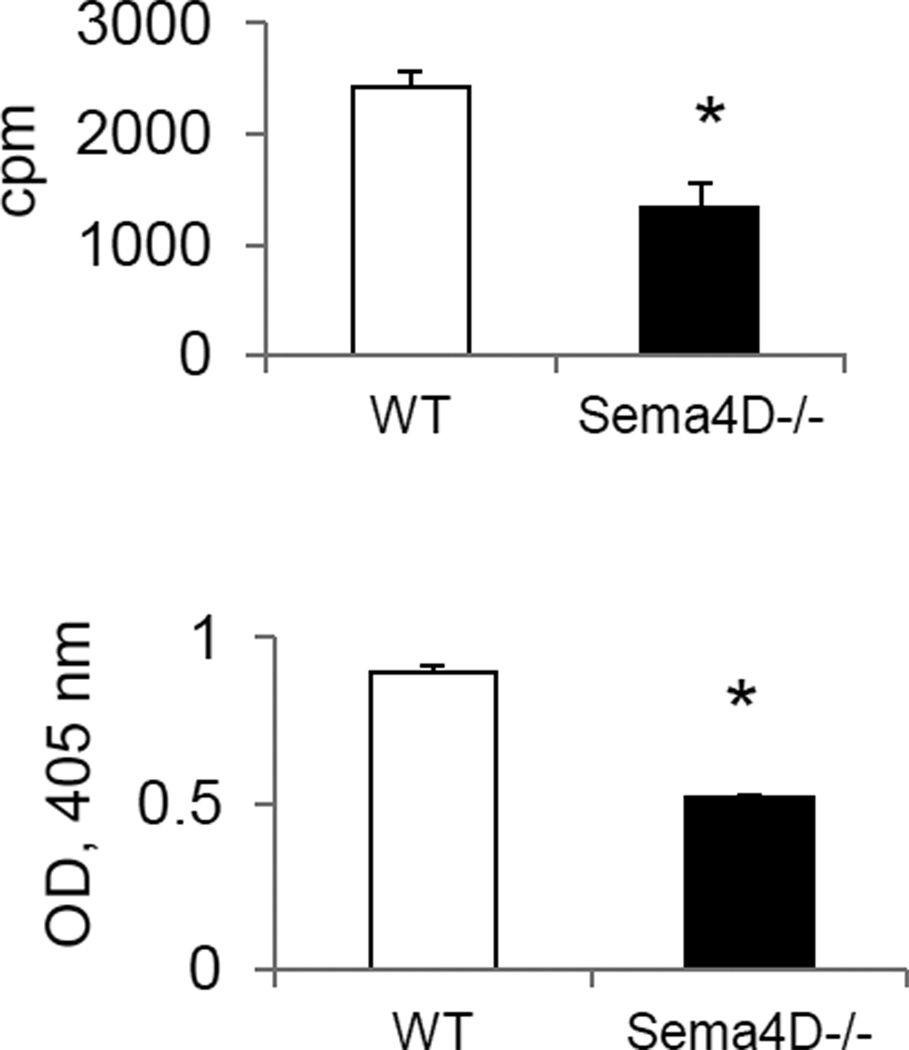
As Sema4D expression on T cells is reported to be a critical costimulator for Ag-specific T cell activation (Elhabazi et al. 2001), we analyzed the in vitro spleen MNC response to OVA323–339 restimulation (Fig. 5). Employing two distinct methods for T cell proliferation measurement, we have found that proliferative responses to OVA323–339 peptide were downregulated with Sema4D deficiency (Fig. 5). Therefore, Sema4D plays a critical costimulatory role in antigen-dependent T cell activation. Considering noted CD72 expression on a subset of peripheral T cells, this stimulation can be either direct (through Sema4D-CD72 autocrine mechanism) or indirect (through DC-dependent mechanism). The involvement of Plexin B1 in this process needs to be evaluated further.
Figure 5.
Sema4D deficiency affect the in vitro recall T cell response to OVA323–339 peptide challenge. Spleen MNC were obtained from allergen-treated WT (open bars) and Sema4D−/− (black bars) mice as described in the Materials and Methods. Cells were stimulated with 200 µg/ml of OVA323–339 peptide for 72h. Proliferation was measured in triplicate wells. [H]3 was added to the cultures for the last 24h before cell harvest. Data are presented as mean cpm ± SEM of [H]3 incorporation in 72h cultures. Means and SEM are shown for the measured O.D. of cells incubated with WST-1 solution for the colorimetric proliferation visualization. *p<0.05, WT vs Sema4D−/− mice.
Discussion
In this study we demonstrate that Sema4D acts as a critical positive regulator of the severity of inflammation observed in experimental allergic asthma. Its absence in vivo in Sema4D−/− mice led to a downregulation of many features of allergic response such as predominantly eosinophilic BAL and lung tissue infiltration, mucous cell hyperplasia, BAL Th2 cytokine (IL-5 and IL-13) levels. It affected T cell activation and function suggesting its positive costimulatory role. However, at least with the use of non-invasive technique for lung function measurements, we could not detect a significant Sema4D effect on AHR, suggesting a possible negligible role in lung physiology. Our studies show, for the first time, the functional effect of Sema4D in experimental allergic asthma. Considering a wide range of cells expressing this neuroimmune semaphorin and its receptors in the lung (Nkyimbeng-Takwi, Chapoval 2011; Smith et al. 2011) and immune system (Bougeret et al. 1992; Puschel, Adams, Betz 1995; Furuyama et al. 1996; Delaire et al. 2001; Kumanogoh et al. 2002; Kumanogoh, Kikutani 2003; Chabbert-de Ponnat et al. 2005; Basile et al. 2007; Suzuki, Kumanogoh, Kikutani 2008; Smith et al. 2011), there are several possible mechanisms of such Sema4D action in the lung tissue under allergen-induced inflammatory conditions.
First of all, as it has been shown previously in vitro and in vivo (Basile et al., 2007; Bougeret et al., 1992; Chabbert-de Ponnat et al., 2005; Delaire et al., 2001; Furuyama et al., 1996; Kumanogoh & Kikutani, 2003; Kumanogoh et al., 2002; Puschel et al., 1995; Suzuki et al., 2008), Sema4D is necessary for the optimal T cell activation. The human membrane-bound Sema4D provides a costimulatory signal to T cells through its engagement with membrane protein tyrosine phosphatase CD45 and intracellular serine kinase, whereas soluble Sema4D acts through CD72 and/or Plexin B1 to promote T cell priming (Elhabazi et al. 2001; Elhabazi et al. 2003). Previously published studies using in vitro cell cultures and Ab treatement (Herold et al. 1996) or employing Sema4D-deficient mice have shown a critical role of Sema4D in CD4+ T cell priming (Shi et al. 2000). In the latter study, the authors have shown that the in vitro proliferation of CD4+ T cells obtained from Sema4D−/− mice immunized with T cell-dependent antigen KLH in CFA was significantly lower to that measured in WT cells (Shi et al. 2000). Similarly, impaired generation of Ag-specific T cells and cytokine response (IL-4 and IFNγ) have been detected in the draining lymph nodes of either MOG peptide or OVA immunized Sema4D−/− mice as compare to WT control animals (Kumanogoh et al. 2002). Therefore, Sema4D plays an important role in antigen priming and sensitization phase of allergic disease. Here we show the effect of Sema4D deficiency in lung allergic disease outcome after airway allergen challenges.
It is well established that allergic asthma is a Th2-driven disease (Ingram, Kraft 2012) and activation of accessory cells, DC in particular, plays a critical role in its pathogenesis (Lambrecht, Hammad 2012). Sema4D has been shown to play an important costimulatory role in antigen-dependent T cell activation through activation of accessory cells (Kumanogoh et al. 2002; Granziero et al. 2003; Kumanogoh, Kikutani 2003; Li et al. 2006; Suzuki, Kumanogoh, Kikutani 2008). Its expression in the immune system was detected on T cells. Expression increases with T cell activation and enhances activation APC acting either through Plexin B1 or CD72 receptors (Granziero et al. 2003). APC, although at low levels, express Sema4D (Smith et al. 2011) suggesting the existence of a potential autocrine activation mechanism. Although CD72 is considered to be an inhibitory receptor, there is substantial evidence that CD100-CD72 interaction has a stimulatory effect (Kumanogoh et al. 2000; Ishida et al. 2003; Kumanogoh, Kikutani 2003). It has been suggested that in a microenvironment where two receptors co-exist, CD100 would preferentially bind the high affinity Plexin B1 receptor and this activation signal would dominate (Granziero et al. 2003). The importance of these two receptors in regulation of allergic response by Sema4D needs to be clearly defined. Nevertheless, the observed impaired T cell recall response to OVA-peptide restimulation (Fig. 5) suggests an important costimulatory role of Sema4D for regulation of Th2 response. However, we did not find any measurable effects of Sema4D on lung DC number or activation (Fig. 4), what suggests a potential autocrine T cell activation through, most probably, Sema4D-CD72 interaction and/or effect of Sema4D on lymphoid tissue DC. Indeed, we have noticed previously that lung DC express very low levels of Sema4D in a steady state condition (around 20%) and this level is significantly downregulated with OVA exposure (to 6%) what is associated with increased lung levels of soluble Sema4D (Smith et al. 2011). This contrasted with constitutively high levels of Sema4D on spleen DC further potentiated by allergen.
The role of Sema4D in vivo has been examined in EAE (Okuno et al. 2010) and immune glomerulonephritis (Li et al. 2006) models. Whereas WT mice developed a glomerulonephritis to horse afferitin injections, Sema4D−/− mice were protected from histological and functional kidney injury. This protection was associated with attenuated DC activation as measured by CD86 expression. Interestingly, transfer of activated Sema4D+ DO11.10 cells to Sema4D−/− mice resulted in a decreased T cell proliferation as compared to Sema4D+/+ mice. Therefore, Sema4D from other cellular sources besides T cells is also contributing to an optimal T cell activation. Indeed, we have demonstrated previously that Sema4D and especially CD72 were readily detected in the mouse lung tissue by IHC (Smith et al. 2011). Their expression was not restricted to immune cells only but was also found on different subsets of lung resident and inflammatory cells. Namely, besides T and B cells, Sema4D expression was found, although to a lower extend, on lung MHCII+ cells which, in addition to the lung professional APC, might include epithelial cells, fibroblasts, mast cells, macrophages, and, under inflammation, granulocytes. The detailed examination of lung cellular Sema4D and receptor distribution by multicolor flow cytometry is needed to clarify the picture of potential players in allergic response regulation. The in vitro corresponding cell cultures would provide the important data on the effect or the absence of such receptor-ligand interaction for different cells. We previously reported a complimentary cell distribution of these molecules (T cells, B cells, MHCII+ cells co-express a ligand and a receptor) what might be important for the corresponding cell autocrine regulation. Lung APC and alveolar type II-like cells were Sema4D- and CD72-positive. CD72 is also expressed on bronchial epithelial cells in large bronchi. Alveolar macrophages were also CD72-positive. Under OVA-induced inflammatory conditions, some additional cells such as subsets of lymphocytes, granulocytes, and inflammatory macrophages were also found to express CD72. All this shows a complex nature of allergic response regulation by a single semaphorin molecule.
We also show here that Sema4D deficiency affects lung local Th2 cytokine production (Fig 2) which all have critical roles in inducing and maintaining allergic response at different levels (Barnes 2001; Chapoval et al. 2010; Chapoval et al. 2011; Ingram, Kraft 2012). Interestingly, lung IL-6 and IL-17A levels were also downregulated in OVA-treated Sema4D−/− mice as compared to WT counterparts. IL-6 is an important cytokine for Th2 regulation (La Flamme, MacDonald, Pearce 2000; Diehl, Rincon 2002; Dodge et al. 2003). It has been shown previously that IL-12-secreting DC together with external source of IFNγ from NK cells or memory T cells prime strong Th1 response, while IL-6-secreting DC together with an external source of IL-4 prime strong Th2 response (Dodge et al. 2003). Although we do not show here that DC were the primary source of lung IL-6, the lower level of this cytokine in Sema4D−/− allergic lungs suggest a potential mechanism of Sema4D regulation of allergic response by optimizing DC activation and IL-6 production. Another cytokine regulated by Sema4D is IL-17 (Fig. 2). IL-17 is important cytokine in more chronic asthmatic settings where it regulates AHR and neutrophilic inflammation (Nembrini, Marsland, Kopf 2009). But eosinophils were reported to be the active producers of this cytokine in human asthma (Molet et al. 2001). Based on all of the above, it is tempting to conclude that neuroimmune Sema4D regulates, either directly or indirectly, Th17 response to allergen in the lung and, what remains to be determined, systemically. Lung TGF-β1 content was also affected by Sema4D deficiency (Fig. 2). TGF-β1 has a downregulatory effect on Th2 responses by induction of Treg cells, directly suppressing Th2 cells and by mediating lung repair responses (Schmidt-Weber, Kunzmann, Blaser 2002), however, it also has a pathogenic role in asthmatic settings by mediating a tissue remodeling that aggravates the disease (Howell, McAnulty 2006). It is being produced by many cells in the lung and critically regulates their function. Moreover, several recent studies have shown that eosinophil-derived TGF-β1 production is one of the major mechanisms that drives the development of airway remodeling in allergic airways (Fattouh, Jordana 2008).
In conclusion, our previous observations (Nkyimbeng-Takwi, Chapoval 2011; Smith et al. 2011) and our new data reported here suggest that a regulatory effect of Sema4D in allergic asthma is a complex venue which can not be simplified by its role in DC – T cell interaction and involves other cells, lung resident cells and inflammatory cells, many of which express functional receptors for Sema4D (Kumanogoh et al. 2000; Ishida et al. 2003; Kumanogoh, Kikutani 2003; Nkyimbeng-Takwi, Chapoval 2011; Smith et al. 2011). Besides its role in immune cell activation and function (Kumanogoh, Kikutani 2003), Plexin B1 plays an important role in cell migration (Delaire et al. 2001; Basile, Afkhami, Gutkind 2005) and angiogenesis (Basile et al. 2004; Conrotto et al. 2005; Basile et al. 2007) which both are critical components of asthma pathogenesis (Lee et al. 2004; Velazquez, Teran 2011; Islam, Luster 2012; Meyer, Akdis 2012). Thus, despite the recent significant advances in the biology, distribution and function of Sema4D, the understanding of a complex venue of its regulation of different immunological diseases is still in the early stage in terms of understanding the exact role of each Sema4D-expressing cellular component in modulating allergic response.
Highlights.
We examined the changes in lung responses to allergen in Sema4D−/− and WT mice
Allergic lung response was downregulated in Sema4D−/− mice as compared to WT mice
Sema4D deficiency did not affect lung physiology measured by Buxco machine
We found a critical costimulatory role for Sema4D in T cell activation
Sema4D deficiency affects spleen Treg cells but not lung dendritic cells
Acknowledgements
We thank Dr. Hitoshi Kikutani (Osaka University) for providing us Sema4D−/− mice.
This work was supported by NIH grant R21AI076736 (to S.P.C.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25:6889–6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem. 2007;282:6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- Berndt A, Leme AS, Williams LK, et al. Comparison of unrestrained plethysmography and forced oscillation for identifying genetic variability of airway responsiveness in inbred mice. Physiol Genomics. 2011;43:1–11. doi: 10.1152/physiolgenomics.00108.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougeret C, Mansur IG, Dastot H, Schmid M, Mahouy G, Bensussan A, Boumsell L. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J Immunol. 1992;148:318–323. [PubMed] [Google Scholar]
- Chabbert-de Ponnat I, Marie-Cardine A, Pasterkamp RJ, Schiavon V, Tamagnone L, Thomasset N, Bensussan A, Boumsell L. Soluble CD100 functions on human monocytes and immature dendritic cells require plexin C1 and plexin B1, respectively. Int Immunol. 2005;17:439–447. doi: 10.1093/intimm/dxh224. [DOI] [PubMed] [Google Scholar]
- Chapoval S, Dasgupta P, Dorsey NJ, Keegan AD. Regulation of the T helper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J Leukoc Biol. 2010;87:1011–1018. doi: 10.1189/jlb.1209772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapoval SP, Dasgupta P, Smith EP, DeTolla LJ, Lipsky MM, Kelly-Welch AE, Keegan AD. STAT6 expression in multiple cell types mediates the cooperative development of allergic airway disease. J Immunol. 2011;186:2571–2583. doi: 10.4049/jimmunol.1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapoval SP, Lee CG, Tang C, Keegan AD, Cohn L, Bottomly K, Elias JA. Lung vascular endothelial growth factor expression induces local myeloid dendritic cell activation. Clin Immunol. 2009;132:371–384. doi: 10.1016/j.clim.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood. 2005;105:4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, Boumsell L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J Immunol. 2001;166:4348–4354. doi: 10.4049/jimmunol.166.7.4348. [DOI] [PubMed] [Google Scholar]
- Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- Dodge IL, Carr MW, Cernadas M, Brenner MB. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J Immunol. 2003;170:4457–4464. doi: 10.4049/jimmunol.170.9.4457. [DOI] [PubMed] [Google Scholar]
- Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol. 2001;166:4341–4347. doi: 10.4049/jimmunol.166.7.4341. [DOI] [PubMed] [Google Scholar]
- Elhabazi A, Marie-Cardine A, Chabbert-de Ponnat I, Bensussan A, Boumsell L. Structure and function of the immune semaphorin CD100/SEMA4D. Crit Rev Immunol. 2003;23:65–81. doi: 10.1615/critrevimmunol.v23.i12.40. [DOI] [PubMed] [Google Scholar]
- Fattouh R, Jordana M. TGF-beta, eosinophils and IL-13 in allergic airway remodeling: a critical appraisal with therapeutic considerations. Inflamm Allergy Drug Targets. 2008;7:224–236. doi: 10.2174/187152808786848388. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Penachioni J, Gianola S, et al. Plexin-B1 plays a redundant role during mouse development and in tumour angiogenesis. BMC Dev Biol. 2007;7:55. doi: 10.1186/1471-213X-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Inagaki S, Kosugi A, Noda S, Saitoh S, Ogata M, Iwahashi Y, Miyazaki N, Hamaoka T, Tohyama M. Identification of a novel transmembrane semaphorin expressed on lymphocytes. J Biol Chem. 1996;271:33376–33381. doi: 10.1074/jbc.271.52.33376. [DOI] [PubMed] [Google Scholar]
- Granziero L, Circosta P, Scielzo C, Frisaldi E, Stella S, Geuna M, Giordano S, Ghia P, Caligaris-Cappio F. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood. 2003;101:1962–1969. doi: 10.1182/blood-2002-05-1339. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- Herold C, Elhabazi A, Bismuth G, Bensussan A, Boumsell L. CD100 is associated with CD45 at the surface of human T lymphocytes. Role in T cell homotypic adhesion. J Immunol. 1996;157:5262–5268. [PubMed] [Google Scholar]
- Hewitt M, Creel A, Estell K, Davis IC, Schwiebert LM. Acute exercise decreases airway inflammation, but not responsiveness, in an allergic asthma model. Am J Respir Cell Mol Biol. 2009;40:83–89. doi: 10.1165/rcmb.2008-0172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JE, McAnulty RJ. TGF-beta: its role in asthma and therapeutic potential. Curr Drug Targets. 2006;7:547–565. doi: 10.2174/138945006776818692. [DOI] [PubMed] [Google Scholar]
- Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012;130:829–842. doi: 10.1016/j.jaci.2012.06.034. quiz 843-824. [DOI] [PubMed] [Google Scholar]
- Ishida I, Kumanogoh A, Suzuki K, Akahani S, Noda K, Kikutani H. Involvement of CD100, a lymphocyte semaphorin, in the activation of the human immune system via CD72: implications for the regulation of immune and inflammatory responses. Int Immunol. 2003;15:1027–1034. doi: 10.1093/intimm/dxg098. [DOI] [PubMed] [Google Scholar]
- Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18:705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanogoh A, Kikutani H. Immune semaphorins: a new area of semaphorin research. J Cell Sci. 2003;116:3463–3470. doi: 10.1242/jcs.00674. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Suzuki K, Ch'ng E, et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J Immunol. 2002;169:1175–1181. doi: 10.4049/jimmunol.169.3.1175. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Watanabe C, Lee I, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- La Flamme AC, MacDonald AS, Pearce EJ. Role of IL-6 in directing the initial immune response to schistosome eggs. J Immunol. 2000;164:2419–2426. doi: 10.4049/jimmunol.164.5.2419. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- Lee CG, Link H, Baluk P, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, O'Sullivan KM, Jones LK, Semple T, Kumanogoh A, Kikutani H, Holdsworth SR, Kitching AR. CD100 enhances dendritic cell and CD4+ cell activation leading to pathogenetic humoral responses and immune complex glomerulonephritis. J Immunol. 2006;177:3406–3412. doi: 10.4049/jimmunol.177.5.3406. [DOI] [PubMed] [Google Scholar]
- Makela MJ, Kanehiro A, Borish L, Dakhama A, Loader J, Joetham A, Xing Z, Jordana M, Larsen GL, Gelfand EW. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci U S A. 2000;97:6007–6012. doi: 10.1073/pnas.100118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N, Akdis CA. Vascular Endothelial Growth Factor as a Key Inducer of Angiogenesis in the Asthmatic Airways. Curr Allergy Asthma Rep. 2012 doi: 10.1007/s11882-012-0317-9. [DOI] [PubMed] [Google Scholar]
- Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–994. doi: 10.1016/j.jaci.2009.03.033. quiz 995-986. [DOI] [PubMed] [Google Scholar]
- Nkyimbeng-Takwi E, Chapoval SP. Biology and function of neuroimmune semaphorins 4A and 4D. Immunol Res. 2011;50:10–21. doi: 10.1007/s12026-010-8201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkyimbeng-Takwi EH, Shanks K, Smith E, Iyer A, Lipsky MM, Detolla LJ, Kikutani H, Keegan AD, Chapoval SP. Neuroimmune semaphorin 4A downregulates the severity of allergic response. Mucosal Immunol. 2012;5:409–419. doi: 10.1038/mi.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Nakatsuji Y, Moriya M, et al. Roles of Sema4D-plexin-B1 interactions in the central nervous system for pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:1499–1506. doi: 10.4049/jimmunol.0903302. [DOI] [PubMed] [Google Scholar]
- Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol. 2010;40:1232–1240. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Adams RH, Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Schmidt-Weber, CB, Kunzmann S, Blaser K. TGF-beta-mediated control of allergen-specific T-cell responses. Curr Allergy Asthma Rep. 2002;2:259–262. doi: 10.1007/s11882-002-0028-8. [DOI] [PubMed] [Google Scholar]
- Shi W, Kumanogoh A, Watanabe C, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–642. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- Short PM, Lipworth SI, Lipworth BJ. Relationships between airway hyperresponsiveness, inflammation, and calibre in asthma. Lung. 2011;189:493–497. doi: 10.1007/s00408-011-9328-x. [DOI] [PubMed] [Google Scholar]
- Smith EP, Shanks K, Lipsky MM, DeTolla LJ, Keegan AD, Chapoval SP. Expression of neuroimmune semaphorins 4A and 4D and their receptors in the lung is enhanced by allergen and vascular endothelial growth factor. BMC Immunol. 2011;12:30. doi: 10.1186/1471-2172-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Vanoirbeek JA, Rinaldi M, De Vooght V, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2010;42:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- Velazquez JR, Teran LM. Chemokines and their receptors in the allergic airway inflammatory process. Clin Rev Allergy Immunol. 2011;41:76–88. doi: 10.1007/s12016-010-8202-6. [DOI] [PubMed] [Google Scholar]
- Wilder JA, Collie DD, Wilson BS, Bice DE, Lyons CR, Lipscomb MF. Dissociation of airway hyperresponsiveness from immunoglobulin E and airway eosinophilia in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1999;20:1326–1334. doi: 10.1165/ajrcmb.20.6.3561. [DOI] [PubMed] [Google Scholar]