Abstract
Transient receptor potential (TRP) ion channels are emerging as a new set of membrane proteins involved in a vast array of cellular processes and regulated by a large number of physical and chemical stimuli, which involves them with sensory cell physiology. The vanilloid TRP subfamily (TRPV) named after the vanilloid receptor 1 (TRPV1) consists of six members, and at least four of them (TRPV1-TRPV4) have been related to thermal sensation. One of the least characterized members of the TRP subfamily is TRPV2. Although initially characterized as a noxious heat sensor, TRPV2 now seems to have little to do with temperature sensing, but a much more complex physiological profile. Here we review the available information and research progress on the structure, physiology and pharmacology of TRPV2 in an attempt to shed some light on the physiological and pharmacological deorphanization of TRPV2.
Keywords: TRPV2, calcium signalling, somatosensation, neuroscience, immunology, cancer
Introduction
The transient receptor potential (TRP) superfamily is a multifunctional set of membrane proteins that function as ion channels and extend communication lines between the cell and its environment, what may be called sensory physiology or somatosensation. TRP channels in cellular membranes are modulated by a vast array of physical or chemical stimuli, including radiation (in form of temperature, infrared radiation, or light), pressure (osmotic or mechanical), and natural compounds. Excellent reviews about TRP channels are available [1–7], thus an overview of the TRP superfamily is beyond the scope of the present review. The subject of discussion here is Transient Receptor Potential Vanilloid 2 (TRPV2) channel, one of the most mysterious and intriguing members of this superfamily, with a paucity of data concerning the endogenous function of the channel. The intention of this review is to shed some light on the controversies concerning TRPV2 by integrating the latest reports on TRPV2 with historical data on this ion channel towards understanding the TRPV molecular mechanism from a more structural perspective.
The TRP channel superfamily
TRP channels were originally identified within the rhabdomeres of the photoreceptor in Drosophila [8] and the first member was cloned in the late 80s [9]. TRP channels constitute an extensive ion channel superfamily represented across the phylogenetic tree from yeast to human. It is indeed the second largest family of voltage-gated-like ion channels after the potassium channel family [10]. Many TRP channels are polymodal signal integrators that trigger cellular signalling pathways via non-selective cation flux (e.g. Ca2+, Mg2+, and Na+). Different TRP channels show different permeability and selectivity to cations, both monovalent and divalent, whereas most trivalent cations act as channel blockers. The specifics of cation permeability are physiologically important, since cations play roles in cell function regulation, such as fertilization, muscle contraction, or exocytosis.
As a relevant example of TRP channel function in mammals, several TRP channels located in the dorsal root ganglion (DRG) nociceptive sensory neurons respond to nociceptive stimuli to cause pain. From a pharmaceutical and biomedical point of view, there are vast and diverse implications including some still to be determined. For instance, TRPs are lead targets in the treatment of acute and chronic pain [11,12]. Nevertheless the role of TRP channels is not limited to the pain field. TRP channels have been implicated in several physiological and pathological processes such as cancer, genetic disorders, and other rare diseases, and not only restricted to somatosensation.
A set of common sequence features among TRPs translates to shared structural features. Mainly, these channels share a membrane topology of six transmembrane segments (S1 to S6), with a pore-forming loop between S5 and S6. Similarly to the tridimensional structure of potassium channels, TRPs are arranged in the membrane as tetramers as the basic functional unit. The S1–S4 segments and the N- and C-terminal cytoplasmic domains are modulating domains important to the gating of the channel, with the S5–S6 segments defining the pore and selectivity filter (Fig. 1A).
Fig. 1. The TRP channels molecular mechanism and classification.
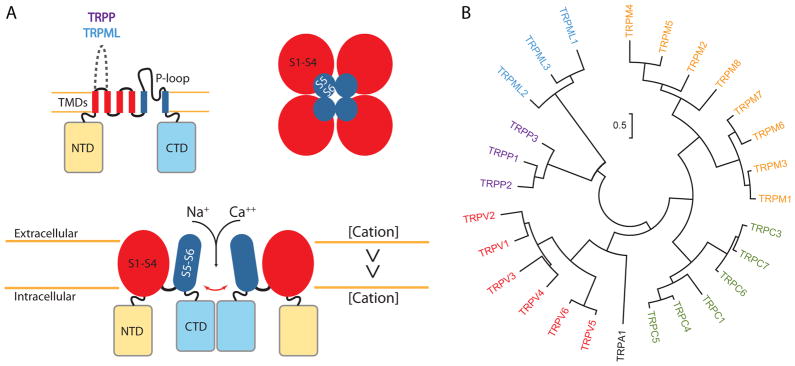
A. The six transmembrane segment topology of the monomer and tetrameric functional unit of TRP channels. The cytoplasmic N- and C-terminal domains, and the transmembrane domain constituting the pore are indicated. The long loop present between S1 and S2 in TRPP and TRPML is indicated by a dashed line. The transmembrane domain comprises segments 1 to 4 (red), distal to the pore, and the segments 5 and 6 (blue) proximal to the pore of the channel. B. Human classification of the TRP family representative of the mammal TRP subfamilies classification. Notice that in humans, TRPC2 is a non-coding pseudogene and it is not represented.
TRPs are not classified by their functional role, but based on amino acid sequence identity and similarity. The mammalian TRP channel superfamily is classified into six subfamilies with significant sequence similarity within the transmembrane domains, but very low similarity in their N- and C-terminal cytoplasmic regions (Fig. 1B). The six subfamilies are named based on founding members: TRPA (ANKTM1); TRPC (canonical); TRPM (melastatin); TRPML (mucolipin); TRPP (polycystin); and TRPV (vanilloid). A seventh subfamily, TRPN (NompC), is present in invertebrates and some vertebrates, although it is absent in mammals. Here we focus on the TRPV subfamily and how TRPV2 distinguishes itself from its closest TRPV homologs.
TRPV2 within the TRPV subfamily
The first identified TRP channel of the TRPV family was OSM-9 (osmotic avoidance abnormal family member 9) from Caenorhabditis elegans [13]. OSM-9 is required for olfaction, mechanosensation and olfactory adaptation. OCR-1 to OCR-4 (osm-9/capsaicin receptor related) are the other TRPV channels in C. elegans, for a total of five [14]. In Drosophila melanogaster, the TRPV members are Nanchung and Inactive, which are involved in sensory perception [15]. The TRPV mammal subfamily is divided in two groups based on sequence homology, TRPV1-4 and TRPV5-6 (Fig. 1B). The group formed by TRPV5 and TRPV6 share high sequence identity (~75%), but low identity with the TRPV1-4 group (~20%) [6,16]. TRPV5 and TRPV6 are calcium-selective channels important for general Ca2+ homeostasis and heterotetramers between them have been described [17]. All four channels within the TRPV1-4 group show temperature-invoked currents when expressed in heterologous cell systems, ranging from activation at ~25°C for TRPV4 to ~52°C for TRPV2. However, a physiological role in temperature sensing has not been demonstrated for TRPV2, and the thermal responses of the TRPV2 knockout mice have been shown to be similar to the wild type mice [18]. In contrast, TRPV1 has been convincingly shown to play an important role in thermosensation using knockout mice [19]. The original characterization of the TRPV3 knockout mice [20] and TRPV4 knockout mice [21] showed some impairment in thermosensation. However, more recent work showed that any thermosensory defects in the TRPV3 and TRPV4 knockout mice are minor and strain-dependent [22]. Therefore, a physiological thermosensory role is the exception (TRPV1) rather than the rule, for TRPV channels.
As detailed below, there is increasing support for a role of TRPV2 in various osmo- or mechanosensory mechanisms. The mechanosensory function of select TRPV channel family members seems to have been maintained through evolution. For instance, the Caenorhabditis elegans TRPV channels OSM-9 and OCR-2 are essential for osmo- and mechanosensation [23]. Similarly, Nanchung and Inactive, the TRPV-related proteins in Drosophila melanogaster, located in the mechanosensitive Johnston’s organ in the fly antennae, are responsible for the sense of hearing [15,24].
Two labs nearly simultaneously cloned TRPV2 orthologs. Human and rat TRPV2 were cloned based on their homology to TRPV1, and accordingly named vanilloid-receptor-like protein 1 (VRL-1) [25]. Mouse TRPV2 was cloned and named growth-factor regulated channel (GRC) based on the fact that it induces calcium currents upon stimulation of cells by insulin-growth-factor-1 (IGF-1) [26]. Of note, TRPV2 has also infrequently been referred to Osm-9-like TRP channel 2 (OTRPC2) [27]. TRPV2 mediates cationic currents, with higher divalent permeability (P) (Ca2+ > Mg2+ > Na+ ~Cs+ ~K+; PCa2+/PNa+=2.94; PMg2+/PNa+=2.40) [25]. The sequence identity among the best characterized orthologs (human, rat and mouse) ranges from 75 to 90% (Fig. 2). TRPV2 shares 50% sequence identity with TRPV1, the best characterized member of this subfamily. TRPV1 is a polymodal channel activated by anandamide, capsaicin, resiniferatoxin, heat, low pH, and eicosanoids [28]. TRPV3 is a thermosensor for non-noxious heat and responds to natural compounds found in plants such as oregano, camphor and thyme [29]. TRPV4 is a constitutively active Ca2+-permeable modulated by non-noxious heat [21], and also by pressure and hypotonicity [30,31]. TRPV4 has also be associated to hearing-loss [32].
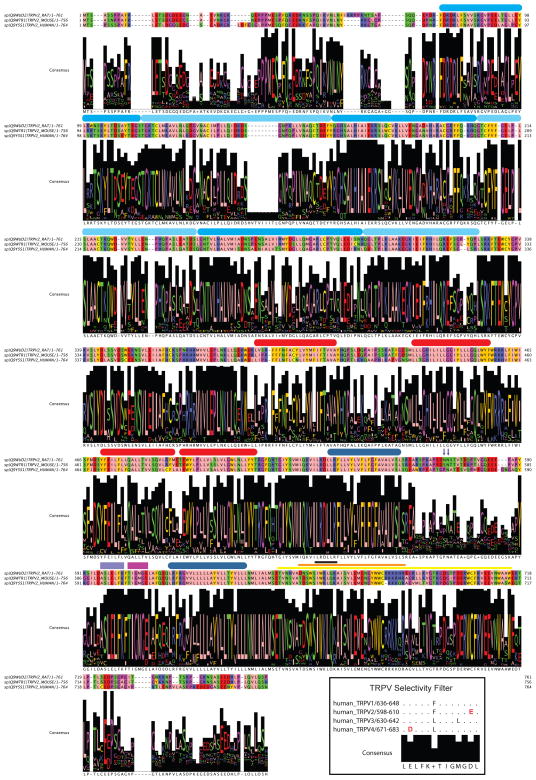
Fig. 2. TRPV2 sequence conservation and structural features.
Alignment of rat (rTRPV2), mouse (mTRPV2), and human (hTRPV2) orthologs. The most relevant sequence features are indicated using the following color code: ARD (light blue); S1–S4 (red); S5–S6 (dark blue); pore (violet); selectivity filter (magenta); PIP2 binding domain (yellow); Calmodulin binding domain (orange); TRP box (black). The N-glycosylation sites between S5 and S6 are indicated by black arrows. For details see the text. The alignment consensus indicates the conservation degree obtained by aligning the following UNIPROT codes: Q9WUD2, Q9WTR1, Q9Y5S, E2RLX8, F6WJB2, F7BDA4, F7A622, G1RXC4, G1SNM3, F1SDE4, G3T6Z2, G3GVL1, Q5EA32, G3WJU0, F6RMV4, F7A8J8, G1N455, F1NPJ9, F6UY90. The alignment has been built in MAFFT [136] and plotted using JalView [137] and the functional ZAPPO profile. Inset, the human TRPV1-4 pore region aligned highlighting the differences in respect to the pore and selectivity filter consensus sequence.
Heteromerization of TRPV1-4 channels has been documented, although mostly in heterologous expression systems. Heterotetramerization of TRPV2 with TRPV1 has been described in vitro in HEK cells [17,33,34]. TRPV1 and TRPV2 have also been shown to tightly colocalize in vivo in rat DRG [17,33,35]. However, physiological roles of any TRPV2 heterotetramerization have yet to be identified.
TRPV2 structure and evolution
TRPV2 presents a high conservation level of identity within mammalian orthologs (see Fig. 2 for human, rat and mouse sequence alignment). Specific TRPV2 orthologs are only found in tetrapod vertebrates, but not in fish, where there is a TRPV1/2 isoform. One evolutionary hypothesis is that a common ancestor between TRPV1 and TRPV2, referred to as TRPV1/2, duplicated generating two genes [36]. This gene duplication is ambiguous in time. One hypothesis is that it could have occurred in the common ancestor of between fishes and tetrapods, and then the copy for TRPV2 was lost in fishes. The second possibility argues for the gene duplication occurring after the divergence between tetrapods and vertebrates. Saito and Shingai argue that based on genomic organization of TRPVs the first possibility is more likely [36].
TRPV2 sequence features are best understood through comparisons with the other TRPV subfamily members. Excellent reviews on the structural organization of TRPV channels are available [7,37,38]. Briefly, TRPV2 consists of a large N-terminal cytoplasmic domain (~390 residues), followed by six transmembrane segments (~250 residues) containing a pore-forming loop, and a C-terminal cytoplasmic domain (~100 residues). Specific TRPV2 sequence features are summarized in Fig. 2.
The N-terminal cytoplasmic region of TRPV2 can be further subdivided into a distal N-terminal region, an ankyrin repeat domain, and a membrane-proximal linker region. The distal N-terminal cytoplasmic region comprises the first ~60–70 residues, which were not ordered and therefore not visible in the first crystal structure obtained for a human TRPV2 N-terminal region [39]. This distal N-terminal region is rather divergent in length, sequence and function, when comparing different TRPV channels. For instance, the TRPV4 N-terminus is longer, ~130 residues, and contains a proline-rich segment that interacts with the SH3 domain of PACSIN [40]. In rat TRPV2, deletion of up to 65 N-terminal residues did not significantly alter channel function as assessed in vitro, whereas deletion of 83 or more residues greatly reduced plasma membrane expression in HEK293 cells [41], supporting the assignment of a domain boundary around 60–70 residues from the N-terminus.
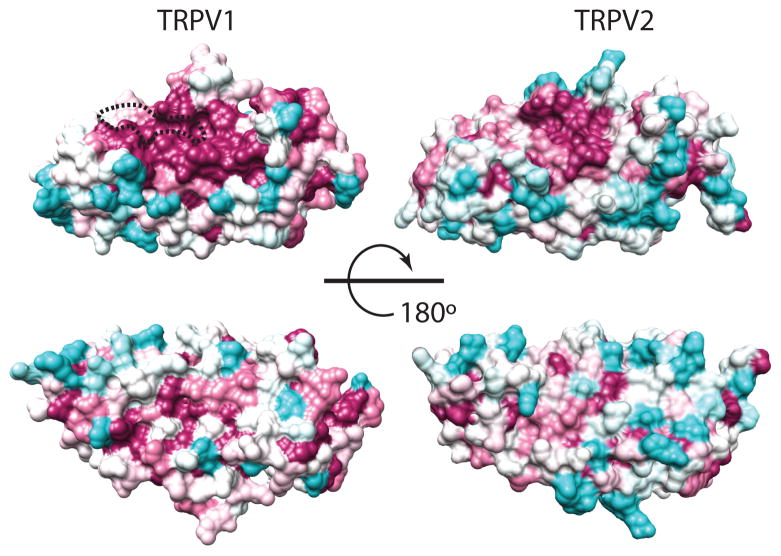
Residues ~70-320 of TRPV2 form the ankyrin repeat domain (ARD), which contains six ankyrin repeats. This soluble domain has yielded the only piece of atomistic tridimensional information currently available for TRPV2: the crystal structures of the human and rat TRPV2-ARD were published nearly simultaneously [39,42]. Ankyrin repeat domains are common protein-protein recognition domains, and although by no means unique to TRP channels, they are clearly important for the modulation of TRP channels from the TRPA, TRPC, TRPN and TRPV subfamilies [43]. Each TRPV2 ankyrin repeat (ANK1-6) is defined by two antiparallel helices (inner and outer) followed by a loop region linking to a β-hairpin structure (named Fingers 1–5). These motifs are arranged linearly, with repeats packing side-by-side and the β-hairpin structures projecting out ~90 degrees relative the helical axes. Each ankyrin repeat is typically rotated a few degrees counterclockwise relative to the previous repeat, leading to an overall helical arrangement of the repeats [44]. The concave surface thus generated is often used in protein-ligand interactions to modulate the function of ARD-containing proteins. This is an important observation when comparing the sequence conservation profiles the ARDs of TRPV1 and TRPV2 (Fig. 3). When the sequence conservation within a set of orthologous sequences is mapped onto the molecular surface of the ARD, TRPV2 shows a lower degree of conservation than TRPV1. The most conserved region on the TRPV2-ARD surface does map to the concave surface, partially overlapping with the larger conserved region on the TRPV1-ARD. As detailed below, the TRPV1-ARD binds to ATP, but the ATP-binding region, highly conserved in TRPV1, is not present in TRPV2 (Fig. 3). Overall, the different conserved surface profiles of TRPV1 and TRPV2 suggest that their ARDs bind to a different set of regulatory ligands.
Fig 3. The TRPV2-ARD concave surface has a small conserved region.
The sequence conservation of TRPV1 and TRPV2 orthologues is mapped onto the crystal structures of the TRPV1-ARD (left, [70]) and TRPV2-ARD (right, [42]), respectively, using ConSurf [138]. The degree of conservation follows a gradient from magenta, most conserved, to cyan, least conserved. The ATP binding region in TRPV1-ARD is indicated by a dashed outline.
Connecting the TRPV2-ARD to the first transmembrane segment (S1) is a highly conserved ~65-residue segment, which has been referred to as the membrane proximal domain. Functional analyses of modular chimeras between TRPV1 and TRPV2, and their functional analyses expressed in HEK cells, suggest that the membrane proximal domain is an important structural module for the temperature sensitivity of TRPV channels [45].
As mentioned above, the transmembrane domain (TMD; residues ~390 and ~640) of TRPV2 is predicted to contain six transmembrane segments (S1–S6) and a pore-forming loop between S5 and S6 (predictions by TMHMM [46] and ΔGpred [47]). Accordingly, we have experimentally refined the position of the TMD between residues for rat TRPV2 between residues 389 and 638 (unpublished results) as shown in Fig. 2. Heat, pH and voltage sensitivity and agonist binding sites for TRPV1 have been related to residues in the TMD (both at TM segments and loops) but none of the results obtained for TRPV1 could be translated for TRPV2 [48]. For example, residues in the S4–S5 linker region may be important for voltage sensitivity of TRPV1; however, no effect has been observed when mutating analogous residues in the TRPV2 S4–S5 linker [49].
An interesting feature in the TRPV2 TMD is the presence of an N-glycosylation consensus motif (NXT/S) in the S5–S6 loop (Fig. 2). While the first asparagine is only found in rat and hamster TRPV2, the second one is conserved in nearly all TRPV2 orthologs. This glycosylation site is analogous to the N604 glycosylation site for TRPV1 [50]. An interesting study showed that in rat F11 DRG cells, heterologously expressed rat and mouse TRPV2 are mostly expressed in the plasma membrane in a glycosylated form, whereas the endogenous TRPV2 isoform (rat) remains in the cytoplasm in a non-glycosylated form [51]. These results suggest that glycosylation plays a role in TRPV2 trafficking towards the plasma membrane, as has been shown for other TRPs [52,53]. Recent reports indicate that the glycosylation of TRPV2 may play an anchoring role under the modulation of the Klotho factor ([54], further discussed below).
The TRPV2 pore-forming domain and selectivity filter, located in the S5–S6 region, is highly conserved (Fig. 2). Although the selectivity filter does not show a clear consensus sequence across the TRP channel superfamily [55,56], the TRPV1-4 group shows high overall conservation (Fig. 2 inset). TRPV2 does differ in divalent/monovalent cation permeability from the rest of the non-selective TRPV subfamily members (TRPV1, TRPV3 and TRPV4), with the lowest PCa2+/PNa+ at 2.94 (TRPV1 ~10, TRPV3 ~10, TRPV4 ~6) [3]. This difference in selectivity could be due to the conserved aspartate-to-glutamate substitution in TRPV2 (highlighted in Fig. 2 inset). TRPV4 shows a similar aspartate-to-glutamate substitution at a different location in the selectivity filter, which may similarly account for somewhat lower PCa2+/PNa+~6 selectivity compared to PCa2+/PNa+~10 for TRPV1 and TRPV3. Mutagenesis studies in the pore-forming region have indicated that mouse TRPV2 heterologously expressed in HEK cells may be constitutively active, inducing high cytotoxicity, since a change of charge mutant (E594K) reduced cytotoxicity [57].
The C-terminal cytoplasmic domain spanning residues 639 to 761 in rat TRPV2 contains relevant features for the assembly of the channel, such as oligomerization (TRP domain), phosphatidylinositol 4–5-bisphosphate (PIP2) binding and calmodulin-binding domains [58–60]. Although the sequence determinants driving TRPV2 oligomerization have not been proven directly, studies on TRPV4 [61] and TRPV1 [60] provide strong arguments for the role of a C-terminal coil-coiled domain in the homotetramerization of the channels. The predicted coil-coiled domain in TRPV1 overlaps with the TRP domain, just after the S6 segment. The TRP domain consists of ~20 amino acids, and for TRPV1 it has been shown that it participates in channel oligomerization and gating transduction [60,62,63].
TRPV2 regulatory interactions
Several regulatory interactions between TRPV2 cytoplasmic domains and various signalling molecules have been investigated. A two-hybrid screening using the first ~390 residues of human TRPV2, corresponding to the whole N-terminal cytoplasmic region, identified an interaction of TRPV2 with recombinase gene activator (RGA) [64] in the RBL2H3 mast cell line. Although it was later demonstrated that a physiological complex containing both TRPV2 and RGA is crucial for the cAMP-driven trafficking of TRPV2 to the plasma membrane, it remains unclear as to whether RGA and TRPV2 interact directly [65].
The TRPV2 N-terminal region has also been implicated in another protein-protein interaction. Mouse TRPV2 residues 1-167 were used as a bait to identify the human GSRP-56 (Golgi-localized spectrin-repeat containing protein 56), which is an alternative splicing isoform of Syne-1 (synaptic nuclear envelope protein-1) [66]. A physiological role for this interaction has yet to be elucidated.
The N-terminal ARD does play important roles in the regulation of the TRPV channels. For instance, mutations to its concave surface affect the activity of TRPV1, TRPV3 and TRPV4 [67–70]. In the case of TRPV1, the channel activity can be modulated by ATP or calmodulin, and these two ligands were found to bind competitively to the TRPV1-ARD concave surface [70]. This finding was extended to TRPV3 and TRPV4, although the details of the modulatory effects differ [68]. Intriguingly, TRPV2 is the only member of the TRPV1-4 group that shows no binding of ATP or calmodulin to its ARD, and is insensitive to changes in intracellular ATP concentration [67–70].
The TRPV2 Ca2+-dependent desensitization is dependent on PIP2 depletion from the membrane [58]. The C-terminal TRP domain contains the TRP box (I695WKLQR701 in rat TRPV1, I659WKLQK664 in rat TRPV2), which has been implicated in PIP2 and calmodulin binding. Alanine mutagenesis of TRPV1 residues I696, W697 and R701 (K in TRPV2) severely affected channel gating, raising the free energy of channel activation [62]. Mercado et al. defined a PIP2 binding domain in TRPV2 within the C-terminal proximal residues 647-715 (Fig. 2). It has been proposed that the phospholipase C hydrolysis of PIP2 is the main regulatory process for TRPV2 and other TRP channels [71]. Recently, David Julius and his team show compelling evidence that TRPV1 is intrinsically temperature sensitive by using an extremely elegant TRPV1 reconstitution model [72]. Besides being the first published report for TRPV1 activity in a biochemically defined reconstituted membrane system, they describe the inhibition of TRPV1 by C-terminal binding of phosphoinositides. A number of studies had previously provided evidence favoring a sensitizing role of phosphoinositides [58,71]. In contrast, the Julius lab had already previously described PIP2-mediated inhibition of TRPV1 in cells [73], and they now provide strong evidence of a similar inhibitory role in a reconstituted system. Julius and colleagues present a comprehensive and testable model of how physiology of phosphoinositide levels can regulate TRPV1 activity, which may be a conserved mechanism for TRPV2.
A C-terminal TRPV2 calmodulin-binding domain was initially predicted and later characterized in vitro by the Gordon Lab [58,74]. This calmodulin-binding domain was further characterized and restricted to the 654–683 region of human TRPV2, almost fully overlapping with the TRP domain [59]. Despite showing binding of calmodulin to a C-terminal fragment of TRPV2 (residues 684-753, Fig. 2) in a Ca2+-dependent manner, the Gordon lab could not show that calmodulin is a major player in TRPV2 desensitization [58].
A few additional predicted and/or characterized TRPV2-interacting proteins have been cataloged in the TRP channel interacting protein (TRIP) database [75,76], some of which are further detailed in later sections.
TRPV2 physiology and signalling pathways
TRPV2 is a homotetrameric N-glycosylated protein that translocates from intracellular membrane compartments to the plasma membrane after stimulation of the phosphatidylinositol 3-kinase (PI3K) and other kinase signalling pathways. TRPV2 proteins expressed heterologously in HEK293 cells show a species-dependent variability in thermal sensing and ligand activation (further described below in the Pharmacology section). From a physiological perspective, TRPV2 has been associated to several functions depending on the considered tissue. Although originally described as a noxious heat thermosensor, several reports point towards TRPV2 functioning instead as a mechanoreceptor and/or osmosensor. Strikingly, TRPV2 knockout mice display normal thermal and mechanical nociception responses [18]. Analysis of sensory ganglia development (DRG and TG) resulted in no significant differences when comparing wild type and knockout tissues. The analysis of acute nociception and hyperalgesia responses to thermal and mechanical stimuli in the knockout mice were normal. To discard compensation effects of other heat-gated channels on the TRPV2 knockout mice, the authors studied the TRPV1/TRPV2 double knockout mice, and the thermal phenotype was statistically indistinguishable from the single TRPV1 knockout. From a chemical ligand perspective, in contrast to most studies where TRPV2 was expressed heterologously, studies of TRPV2-null cultured neurons showed no differential calcium responses dependent of cannabinoid, probenecid or 2-APB when compared to wild type, although the wild type responses were highly inconsistent [18]. Therefore, the TRPV2 knockout mouse analyses indicate that TRPV2 is not sensitive to these compounds under physiological conditions.
The most distinctive features of the knockout mice are reduced perinatal viability, and embryo and adult body weight. Although the study of the knockout mice have thus far shed little light onto the physiological roles of TRPV2, studies derived from culturing knockout mice cells indicate that macrophage function is clearly affected [77], which may correlate with perinatal lethality relating TRPV2 to immune activity (discussed below and excellently reviewed recently in [78]). In summary, the physiological role of TRPV2 is probably one of the most unsettled and controversial among TRP channels. Below we discuss the available data on TRPV2 physiology and signalling pathways, but first, we introduce the pharmacological agents that have been shown to modulate TRPV2. The sparse pharmacology toolkit available to study TRPV2 is important context in evaluating our knowledge of TRPV2 physiology.
TRPV2 modulation and pharmacology
The pharmacology of TRP and TRPV channels has been extensively and excellently reviewed [2,28,79]. Several compounds have been shown to modulate TRPV2. However, few of them are specific, and most show species dependence in their effectiveness on TRPV2 (Table 1). The fact that TRPV2 was initially proposed as a noxious heat thermosensor involved in nociception may have misled the TRPV2 deorphanizing attempts to find endogenous and/or specific modulators. Other more physiological modulators will be described in subsequent sections. Here, we focus in the main chemical modulators for TRPV2 (listed in Table 1).
Table 1.
TRPV2 pharmacology
| Effect | Compound | Class | Concentration | System/Species | Reference |
|---|---|---|---|---|---|
| Agonist | 2-APB* | Diphenyl compound | 129 μM | HEK-293/rat | [82] |
| Agonist | diphenylboronic anhydride | Diphenyl compound | 100 μM | HEK-293/rat | [84] |
| Agonist | Cannabidiol* | Cannabinoid | 1.25 μM | HEK-293/rat | [81] |
| Agonist | Cannabigerol | Cannabinoid | 1.72 μM | HEK-293/rat | [81] |
| Agonist | Cannabinol | Cannabinoid | 39.9 μM | HEK-293/rat | [81] |
| Agonist | Cannabidivarin | Cannabinoid | 7.3 μM | HEK-293/rat | [81] |
| Agonist | Cannabigivarin | Cannabinoid | 1.41 μM | HEK-293/rat | [81] |
| Agonist | Δ9-tetrahydrocannabinol* | Cannabinoid | 0.65 μM | HEK-293/rat | [81] |
| Agonist | Tetrahydrocannabinol acid | Cannabinoid | 18.4 μM | HEK-293/rat | [81] |
| Agonist | Tetrahydrocannabivarin | Cannabinoid | 4.11 μM | HEK-293/rat | [81] |
| Agonist | Lysophosphatidyl choline | Lysophospholipid | 3.37 μM | HEK-293/rat | [81,132] |
| Agonist | Lysophosphatidyl inositol | Lysophospholipid | NA | HEK-293/rat | [132] |
| Agonist | Probenecid | Uricosuric compound | 31.9 μM | HEK-293/rat | [87] |
| Antagonist | Ruthenium Red | Inorganic dye | 0.6 μM | HEK-293/rat | [25] |
| Antagonist | SKF96365 | Alkylated imidazole | 100 μM | CHO-K1/mouse | [83] |
| Antagonist | Tranilast | Diphenyl compound | 10–100 μM | MCF-7/human | [89] |
Species dependence [41]
NA, not available
Cannabis sativa derivatives have proven the most potent TRPV2 activators thus far, with EC50 in the micromolar range, although they are not specific for TRPV2 [80] [81]. In a comparison of a panel of cannabinoids, TRPV2 is most strongly activated by (−)-trans-Δ9-tetrahydrocannabinol, cannabidiol, and Δ9-tetrahydrocannabivarin, whereas cannabinoic acids are least potent [81]. However, cannabinoids can also activate other TRP channels, for example cannabidiol is a potent activator of TRPV1 and TRPA1 [81]. Therefore, one has to be cautious in assigning the effect of these compounds to TRPV2 in a physiological context – in studies using live animal or native tissues, for example. Indeed, proof of direct interaction between TRPV2 and cannabidiol from biochemical and structural perspectives would promote better understanding on TRPV2’s activity.
2-aminoethoxydiphenyl borate (2-APB) was one of the first activators identified for TRPV2, with an EC50 of 129 μM in transfected HEK293 cells [82]. Later reports showed that human TRPV2 was insensitive to 2-APB, indicating species-dependent variability in 2-APB sensitivity [41,83]. Dyphenylboronic anhydride (DPBA) also activates mouse but not human TRPV2 [83,84]. After testing the activation by 2-APB and DPBA on chimeric TRPV2 constructs, Juvin et al. speculated that the 2-APB sensitivity region could be located in the cytoplasmic domains [83].
Blockers have been identified that can affect TRPV2 function, although most are promiscuous compounds that therefore have to be used with caution. The activation of mouse TRPV2 by 2-APB helped identify SKF96365 and amiloride as TRPV2 blockers [83]. Ruthenium red, trivalent cations and citral have also been identified as non-specific TRPV2 channel blockers [82,85,86].
From a specificity perspective, two compounds have shown promising results as more specific modulators of TRPV2: probenecid and tranilast, as activator and inhibitor, respectively. Probenecid (p-(di-n-propylsulfamyl)-benzoic acid), although not exclusive for TRPV2, showed high specificity for this TRP channel, compared to TRPV1, TRPV3, TRPV4, TRPA1 and TRPM8, with an EC50 = 32 μM in transiently transfected TRPV2-expressing HEK293 cells [87]. A recent report has shown the effect of probenecid on TRPV2 in vivo and ex vivo [88]. As an inhibitor, tranilast is an antiallergic drug described as an ion channel blocker by the Kojima lab [89]. Tranilast has been used recently in several reports as a TRPV2-specific antagonist [90–92], although it has not, to our knowledge, been fully validated as a direct TRPV2 blocker.
TRPV2 in the nervous system
During neural development, TRPV2 is expressed in developing mouse DRG and spinal motor neurons from embryonic day 10.5 and sustained until embryonic day 13.5[93]. This expression pattern and the localization of TRPV2 in developing growth cones pointed to the possible involvement of TRPV2 in axon outgrowth. Tominaga et al. indeed observed membrane stretch-induced TRPV2 activation and subsequent intracellular Ca2+ elevation exerted by axon outgrowth in developing neurons [93]. Of note, a role for TRPV2 in the developing nervous system could help explain the reduced perinatal viability observed in TRPV2 knockout mice.
There is also evidence for TRPV2 expression in the adult peripheral and central nervous systems. In DRG [25,94] and trigeminal ganglia (TG) [95], TRPV2 expression is concentrated in a population of medium- and large-diameter primary afferents that do not express TRPV1, primarily in myelinated A- and C-fiber sensory neurons [96–102]. Many of the neurons expressing TRPV2 are peptidergic neurons. For instance, in mice, TRPV2 co-localized with substance P [96]. Another study found that one third of TRPV2-expressing cultured rat DRG neurons were CGRP-positive, and activation of TRPV2 cannabinoid derivatives led to CGRP release [80]. In primary cultures of myenteric neurons, TRPV2-like currents were invoked by 2-APB, probenecid or lysophospholipids and stretch [92]. The same study also found that intestine relaxation is inhibited by nitric oxide synthase inhibitors and TRPV2 antagonists like tranilast. Furthermore, TRPV2 agonists inhibited muscle contraction, but not in the presence of a nitric oxide synthase inhibitor, indicating that TRPV2 is activated downstream nitric oxide. This last study therefore suggests that TRPV2 may be important in controlling the activity of sympathetic muscles like those of the intestinal track, and that the nitric oxide signalling pathway may be an important pathway that modulates TRPV2 function in vivo. One overarching conclusion, previously highlighted by the Basbaum and Julius groups, is that in contrast to TRPV1, the widespread distribution of TRPV2 in the spinal cord suggests that TRPV2 is a player in several physiological roles besides nociception [98].
A number of studies have also observed broad TRPV2 expression in important regions in rat, mouse and macaque brains [35,103–105]. A systematic approach analyzing the transcriptome of developing and mature mouse forebrain has identified TRPV2 as the highest expressing TRPV channel in the neurons of this brain region [106]. This is in contrast to TRPV1 for which a thorough study with GFP-reporter transgenic lines found that TRPV1 expression is essentially restricted to the hypothalamus within the mouse brain [107] Recently, a thorough characterization of the expression and distribution of TRPV2 in rat fore- and hindbrain has been published [108]. In this study, they observe TRPV2 expression in a number of brain structures relevant to osmoregulation and other autonomic functions. This detailed work provides important clues on TRPV2 function, suggesting, along with the previous studies, that TRPV2 is important in various osmosensory mechanisms, including osmotic balance, autonomous regulation, somatosensation, food and fluid intake, and cardiovascular functions [104,105,108].
TRPV2 in the endocrine system
TRPV2 has also been implicated in the endocrine system, more specifically in the pancreas and insulin secretion. In early studies, the Kojima lab cloned TRPV2 as a calcium-permeable channel that translocates from intracellular pools towards the plasma membrane in response to IGF-1 [26]. More recently [91], the same lab determined that TRPV2 is highly expressed in the β-type insulinoma cell line MIN-6, and in the core of mouse pancreatic islets, but not in α-cells. Using various inhibitors, knockouts and knockdowns, they determined that TRPV2 translocation is downstream of insulin signalling and upstream of insulin secretion and cell growth, suggesting that TRPV2 is part of an autocrine positive feedback loop for insulin secretion. Furthermore, glucose-induced insulin exocytosis is mediated by the TRPV2-dependent intracellular increase of Ca2+ concentration [90]. Of note, these studies have relied on the blocking effects of tranilast on TRPV2.
In β-cells, TRPV2 levels in the plasma membrane are also affected by Klotho, an antiaging protein, with TRPV2 downstream of Klotho and upstream of insulin secretion [54]. Interestingly, Klotho has a similar effect on the plasma membrane localization of TRPV5 in the kidney [109]. Klotho cleaves the terminal sialic acid in the glycan moiety of TRPV5, promoting the binding of Galectin-1 to the processed glycan, and thereby anchoring TRPV5 in the plasma membrane [110]. It is interesting to speculate as to whether TRPV2 translocation to the plasma membrane could be similarly mediated through modification of TRPV2 glycosylation (Fig. 2).
TRPV2 in immunity
TRPV2 is expressed in immune-related tissues such as the spleen [25], but additionally, TRPV2 transcripts have been detected in many resident macrophage populations, such as liver Kupffer cells, skin epidermal Langerhans cells and lung alveolar macrophages [77]. TRPV2 expression has also been identified in mast cells populations [65,111,112] and in lymphocytes [113].
There are also several lines of evidence suggesting that TRPV2 function is important in various immune cell types. Studies from the Turner Lab [65,112] show that physical stimuli leading to mast-cell degranulation involve TRPV2 activation and calcium entry. They demonstrated a direct interaction of TRPV2 with an A kinase adapter protein (AKAP)-like protein, ACBD3 (Acyl CoA binding domain protein) and that TRPV2 can be phosphorylated by protein kinase A in vitro, suggesting that TRPV2 activation in mast cells could be mediated by a protein kinase A signalling pathway. Zhang et al. [111] show TRPV2-mediated mast-cell degranulation in a human cell line (HMC-1) in response to several stimuli, such as temperature (>50°C), red light, and mechanical stress. Of note, this TRPV2-mediated thermal response in a human cell line contradicts previous observations that human TRPV2 does not respond to noxious heat temperatures [41].
A study of TRPV2-deficient macrophages led to the conclusions that TRPV2 is involved in phagocytosis, and that TRPV2 recruitment towards the phagosome is driven by PI3K signalling, Src kinases, akt kinase and PKC, but not by PLC nor the Syk kinases [77]. A previous study had similarly shown the translocation of TRPV2 from the ER to the plasma membrane in macrophages after activation of the PI3K signalling pathway activated by the fMetLeu chemotactic peptide [114], similarly to the effect of the neuropeptide head activator in neuronal and neuroendocrine cell cultures [115]. Further studies on TRPV2 in macrophages showed that TRPV2 translocated to the podosome [116], where the plasma membrane calcium concentration seems to play a crucial role on the podosome assembly, an indispensable cytoskeleton tool for migration of cells such as macrophages, neutrophils, endothelial cells and malignant tumor cells [117].
As most of TRPV2’s physiology, the role in immunity is not exempt of controversy. Comparing wild-type and TRPV2-deficient macrophages led to the conclusion that lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF) release in macrophages is not mediated by TRPV2 [77]. However, Yamashiro et al. [118] showed evidence that TRPV2 was involved in TNF and interleukin-6 production induced by LPS in the RAW macrophage cell line. The authors argue that the differences between the Link et al. [77] and Yamashiro et al. [118] studies may derive from experimental conditions.
TRPV2 in circulatory organs and muscle function
TRPV2 is expressed in smooth muscle and endothelial cells of arteries in both rabbit [119] and human [120], and in smooth muscle cells of veins in rats [121], suggesting a role of TRPV2 in circulatory organs. Muraki et al. [105] found that ruthenium red and antisense oligos against TRPV2 decreased both the nonselective cation channel current and Ca2+ influx induced by hypotonic swelling of aortic myocytes. In contrast, inhibitors of L-type voltage-dependent calcium channels or treatment with caffeine had no effect, indicating that TRPV2 is activated by hypotonic induced cell swelling in arteries.
Studies in striated muscle are also providing new insights into the pathophysiological role of TRPV2. The Shigekawa Lab identified TRPV2 as the predominant TRP channel in heart [122]. Comparing the skeletal to cardiac muscle expression of TRPV2, it has been shown that the expression in cardiac muscle is 10-fold higher [122]. In skeletal muscle, the expression of TRPV2 in normal tissue is mostly located in the cell interior, and it is translocated towards the sarcolemma by addition of IGF-1. In dystrophic striated muscle, TRPV2 increases its expression level in the sarcolemma. This deregulated overexpression of TRPV2 in the sarcolemma may account for abnormal or leaky Ca2+ influx in dystrophic phenotypes [123]. Duchenne’s muscular dystrophy’s hallmark is the lack of expression of dystrophin disrupting the link between the extracellular matrix and the cytoskeleton, which is mediated by the interaction between dystrophin and dystro- and sarcoglycans (forming the dystrophin-glycoprotein complex, DGC) [124]. This DGC disruption affects stretch-modulated channels such as TRPV2. This hypothesis has been studied in eccentric contractions (lengthening) in dystrophic muscle where the endogenous expression of TRPV2 has been reduced by the use of dominant-negative TRPV2 transgenic mice [122,123,125].
TRPV2 in cancer
Overexpression of TRPV2 at the mRNA and protein levels has been observed in several cancer types and cell lines [126]. Nevertheless the role, if any, of TRPV2 in cancer remains poorly understood. With regards to liver cancers, TRPV2 shows high expression levels in human hepatocarcinoma cells (HepG2) [127]. Another study found high TRPV2 levels in cirrhotic liver and well-differentiated hepatic tumors, as compared to poorly differentiated tumors, suggesting a potential role for TRPV2 as prognostic marker [128].
TRPV2 has also been linked to bladder cancers. TRPV2 mRNA and protein levels were detected in both normal tissue and urothelial carcinoma patients and cell lines [129]. Intriguingly, mRNA was also detected for a short splice variant (s-TRPV2) in which removal of exons 10–11 would produce a protein without residues 529-663 – corresponding to the S5-S6 segments – although the presence of the corresponding protein was not confirmed. In non-tumor patients, mRNAs for both the full-length and short splice variants were found, but the s-TRPV2 variant was less abundant and the full-length mRNA more abundant in late-stage tumors [129]. A second study similarly observed higher TRPV2 mRNA levels in a poorly differentiated bladder cancer cell line compared to a well-differentiated line [130]. Furthermore, the carcinoma cells that expressed high levels of TRPV2 underwent apoptosis when exposed to the TRPV2 agonist cannabidiol, arguing for TRPV2 as a potential therapeutic target for the treatment of bladder cancer.
In prostate cancer, TRPV2 expression levels are higher in metastatic cancers compared to solid tumors, defining TRPV2 as a potential marker for advanced prostate cancer [131]. Lysophospholipids mediated the translocation of TRPV2 to the plasma membrane, stimulating prostate cancer cell migration, but not cell growth [132]. This suggests that TRPV2 plays a role in cancer cell migration that is analogous to its role in macrophages.
The Santoni group has investigated the expression of TRPV2 in gliomas. In primary glioma cells, mRNA TRPV2 expression is reduced compared to benign astrocyte control tissues, with a progressive reduction in high-grade gliomas [133]. Furthermore, TRPV2 silencing favors cell proliferation and survival of the U87MG cell line [133], whereas overexpressing TRPV2 in the MCZ glioma cell line resulted in reduced tumor diameter and glioma viability [134]. Altogether, the results suggest that TRPV2 functions as a negative regulator of glioma cell survival and proliferation. A very recent study similarly points to TRPV2 as an important therapeutic target for the treatment of glioblastoma, showing that cannabidiol enhances TRPV2 expression and activity, inducing apoptosis of human glioma cell lines [135]. The authors also suggest a role for TRPV2 in the uptake of chemotherapeutic agents, showing that a TRPV2 pore-less variant (deleting residues 572-609) reduces doxorubicin uptake in a dominant-negative manner [135]. Strikingly, a short TRPV2 splice variant (Δ551–663, corresponding to the pore region and the sixth transmembrane segment) has been identified in human leukemic cell lines [114], similar to the s-TRPV2 variant observed in bladder cells [129]. Nagasawa et al. speculate that short variants can act as dominant negative mutant, forming heteromers with full-length TRPV2 that do not traffic to the plasma membrane [114]. This hypothesis also fits the glioma results [135], as an explanation for chemotherapy resistance in tumor cells lines, and points to TRPV2 as a promising therapeutic target in several cancer types.
Conclusions and future perspectives
We have reviewed the current information about the sequence determinants and physiology of the TRPV2 ion channel. The TRPV2 tissue distribution is quite broad and the cellular signalling pathways that regulate its function seem to show some tissue-specific variations. In vivo, the knockout TRPV2 mouse model argues against the role of TRPV2 as a mechanosensor and thermosensor. The fact that TRPV1/TRPV2 double knockout mice shows a thermal phenotype that indistinguishable from the single TRPV1 knockout should discourage future classification of TRPV2 as a thermosensor, at least in mice. Most in vitro cellular studies have shown TRPV2 to be activated downstream of physical stimuli such as mechanical stretch, heat, osmotic swelling, and endogenous and exogenous chemical modulators such as hormones, growth factors, chemotactic peptides (fMetLeu, neuropeptide head activator, etc.), lysophospholipids and cannabinoids. Depending on the cell type, TRPV2 is regulated by general or specific signalling pathways involving phospholipase C, the PI3K pathway or other kinases. However, an important theme is emerging that TRPV2 is regulated through translocation and trafficking from internal cytosolic pools towards the plasma membrane, where the levels of PIP2 in the surroundings of TRPV2 seem to play a crucial role in TRPV2-mediated Ca2+ signalling. Concerning the translocation mechanism, heterologous expression studies of recombinant TRPV2 in HEK cells have shown that PI3K activation of TRPV2 does not drive TRPV2 trafficking towards the cell surface [57] in contrast to other studies [26,77,114,116,122]. The set of controversies related to TRPV2, such as thermosensation, translocation, LPS-dependence, etc. should call for extra attention on drawing definitive conclusions, since experimental conditions (e.g. expression systems, antibodies, detection tags/epitopes) seem to be a major source of conflicting observations for TRPV2 activation. Another major limitation in TRPV2 biochemistry and physiology studies is the scarcity of specific endogenous ligands and/or pharmacological drugs, making TRPV2 essentially an orphan TRP channel. The most reliable pharmacological modulators for TRPV2 are probenecid [87] and cannabidiol [135], as agonists, which are also known to activate/inhibit other TRP channels (e.g. TRPV1, TRPA1, TRPM8. [81,87]), which can lead to complications in interpreting experiments in physiological systems coexpressing several TRP channels. Regarding inhibition, tranilast has been identified, so far, as a TRPV2-specific antagonist [89].
To deorphanize TRPV2 and to understand TRPV2’s molecular mechanisms, a combination of several approaches can be taken. First, integrating the current physiological knowledge of TRPV2 with structural biology approaches will open new perspectives on how this polymodal tetrameric channel is modulated. New structural knowledge on TRPV2 will also provide a more general knowledge on the TRPV subfamily, including its closest homolog TRPV1, and the TRP superfamily. For example, the structural characterization of the N-terminal ankyrin repeat domain has already yielded information about differential TRPV2 regulation compared to the rest of the TRPV subfamily. In line with the structural biology aspect, biochemical and/or structural evidences for TRPV2-modulators binding would be ideal for better understanding of TRPV2 molecular mechanism. Second, obtaining information on the TRPV2 interactome has already provided insightful information, such as protein-protein interactions implicated in regulation, translocation and trafficking. Further studies on the TRPV2 interactome may open new physiological perspectives on TRPV2’s elusive role(s). Finally, the development of methods to identify modulators of TRPV2 in a high-throughput screening fashion should open new therapeutic perspectives for a channel with a potential implication in several types of cancer, and other disorders such as muscular dystrophies, diabetes, and CNS disorders. Interestingly, several pharmaceutical companies have started to patent compounds and methods to screen properties of TRPV2 (reviewed in [28]). We can therefore anticipate some synergy in which pharmacological advances will enable experiments to deepen our understanding of TRPV2 physiology.
Acknowledgments
The authors want to acknowledge the funding from Spanish Government Young Researcher Grant (MICINN-SAF2010-21385 to A.P.-M.), a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme (PIOF-GA-2009-237120 to A.P.-M.), and the National Institutes of Health (R01GM081340 to R.G.).
Abbreviations
- TRPV2
transient receptor potential vanilloid 2
- TRP
transient receptor potential
- DRG
dorsal root ganglion
- TRPA
TRP subfamily ANKTM1
- TRPC
TRP subfamily canonical
- TRPM
TRP subfamily melastatin
- TRPML
TRP subfamily mucolipin
- TRPP
TRP subfamily polycystin
- TRPV
TRP subfamily vanilloid
- TRPN
subfamily NompC
- OSM-9
OSMotic avoidance abnormal family member 9
- OCR
osm-9/capsaicin receptor related
- IGF-1
insulin growth factor 1
- ARD
ankyrin repeat domain
- TMD
transmembrane domain
- PIP2
phosphatidylinositol 4-5-bisphosphate
- RGA
recombinase gene activator
- PI3K
phosphatidylinositol 3-kinase
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor
- TG
trigeminal ganglia
- CGRP
calcitonin gene-related peptide
References
- 1.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramsey IS, Delling M, Clapham DE. AN INTRODUCTION TO TRP CHANNELS. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu L-J, Sweet T-B, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 6.Montell C. The TRP superfamily of cation channels. Science’s STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 7.Latorre R, Zaelzer C, Brauchi S. Structure-functional intimacies of transient receptor potential channels. Quart Rev Biophys. 2009;42:201–246. doi: 10.1017/S0033583509990072. [DOI] [PubMed] [Google Scholar]
- 8.Minke B. Drosophila mutant with a transducer defect. Eur Biophys J. 1977;3:59–64. doi: 10.1007/BF00536455. [DOI] [PubMed] [Google Scholar]
- 9.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 10.Almén MS, Nordström KJV, Fredriksson R, Schiöth HB. Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 2009;7:50. doi: 10.1186/1741-7007-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Chung M-K, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Adv Exp Med Biol. 2011;704:615–636. doi: 10.1007/978-94-007-0265-3_33. [DOI] [PubMed] [Google Scholar]
- 13.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao R, Xu XS. Adv Exp Med Biol. 2010;704:323–339. doi: 10.1007/978-94-007-0265-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Chung YD, Park D-Y, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, Kernan MJ, Kim C. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 16.Clapham DE. Mammalian TRP Channels. Cell. 2007;129:220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 18.Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci. 2011;31:11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caterina MJ. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 20.Moqrich A. Impaired Thermosensation in Mice Lacking TRPV3, a Heat and Camphor Sensor in the Skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokolchik I, Tanabe T, Baldi PF, Sze JY. Polymodal sensory function of the Caenorhabditis elegans OCR-2 channel arises from distinct intrinsic determinants within the protein and is selectively conserved in mammalian TRPV proteins. J Neurosci. 2005;25:1015–1023. doi: 10.1523/JNEUROSCI.3107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang D-J, Kaang B-K, Cho H, Oh U, Hirsh J, Kernan MJ, Kim C. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–440. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 26.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 27.Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 28.Ferrer-Montiel A, Fernández-Carvajal A, Planells-Cases R, Fernández-Ballester G, González-Ros JM, Messeguer A, González-Muñiz R. Advances in modulating thermosensory TRP channels. Expert Opin Ther Pat. 2012;22:999–1017. doi: 10.1517/13543776.2012.711320. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 31.Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96–101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- 32.Tabuchi K, Suzuki M, Mizuno A, Hara A. Hearing impairment in TRPV4 knockout mice. Neurosci Lett. 2005;382:304–308. doi: 10.1016/j.neulet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 33.Rutter AR, Ma Q-P, Leveridge M, Bonnert TP. Heteromerization and colocalization of TrpV1 and TrpV2 in mammalian cell lines and rat dorsal root ganglia. Neuroreport. 2005;16:1735–1739. doi: 10.1097/01.wnr.0000185958.03841.0f. [DOI] [PubMed] [Google Scholar]
- 34.Cheng W, Yang F, Takanishi CL, Zheng J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J Gen Physiol. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci. 2005;22:825–834. doi: 10.1111/j.1460-9568.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 36.Saito S, Shingai R. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics. 2006;27:219–230. doi: 10.1152/physiolgenomics.00322.2005. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Yu Y, Yang J. Structural biology of TRP channels. Adv Exp Med Biol. 2011;704:1–23. doi: 10.1007/978-94-007-0265-3_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaudet R. TRP channels entering the structural era. J Physiol. 2008;586:3565–3575. doi: 10.1113/jphysiol.2008.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCleverty CJ, Koesema E, Patapoutian A, Lesley SA, Kreusch A. Crystal structure of the human TRPV2 channel ankyrin repeat domain. Protein Sci. 2006;15:2201–2206. doi: 10.1110/ps.062357206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuajungco MP, Grimm C, Oshima K, D’hoedt D, Nilius B, Mensenkamp AR, Bindels RJM, Plomann M, Heller S. PACSINs bind to the TRPV4 cation channel. PACSIN 3 modulates the subcellular localization of TRPV4. J Biol Chem. 2006;281:18753–18762. doi: 10.1074/jbc.M602452200. [DOI] [PubMed] [Google Scholar]
- 41.Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N. Activation properties of heterologously expressed mammalian TRPV2: evidence for species dependence. J Biol Chem. 2007;282:15894–15902. doi: 10.1074/jbc.M608287200. [DOI] [PubMed] [Google Scholar]
- 42.Jin X, Touhey J, Gaudet R. Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem. 2006;281:25006–25010. doi: 10.1074/jbc.C600153200. [DOI] [PubMed] [Google Scholar]
- 43.Gaudet R. A primer on ankyrin repeat function in TRP channels and beyond. Mol Biosyst. 2008;4:372–379. doi: 10.1039/b801481g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaely P, Tomchick DR, Machius M, Anderson RGW. Crystal structure of a 12 ANK repeat stack from human ankyrinR. EMBO J. 2002;21:6387–6396. doi: 10.1093/emboj/cdf651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao J, Liu B, Qin F. Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proc Natl Acad Sci. 2011;108:11109–11114. doi: 10.1073/pnas.1105196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krogh A, Larsson B, Heijne Von G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 47.Hessa T, Meindl-Beinker NM, Bernsel A, Kim H, Sato Y, Lerch-Bader M, Nilsson I, White SH, Heijne Von G. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature. 2007;450:1026–1030. doi: 10.1038/nature06387. [DOI] [PubMed] [Google Scholar]
- 48.Jordt S-E, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 49.Boukalova S, Marsakova L, Teisinger J, Vlachova V. Conserved residues within the putative S4–S5 region serve distinct functions among thermosensitive vanilloid transient receptor potential (TRPV) channels. J Biol Chem. 2010;285:41455–41462. doi: 10.1074/jbc.M110.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wirkner K, Hognestad H, Jahnel R, Hucho F, Illes P. Characterization of rat transient receptor potential vanilloid 1 receptors lacking the N-glycosylation site N604. Neuroreport. 2005;16:997–1001. doi: 10.1097/00001756-200506210-00023. [DOI] [PubMed] [Google Scholar]
- 51.Jahnel R, Bender O, Münter LM, Dreger M, Gillen C, Hucho F. Dual expression of mouse and rat VRL-1 in the dorsal root ganglion derived cell line F-11 and biochemical analysis of VRL-1 after heterologous expression. Eur J Biochem. 2003;270:4264–4271. doi: 10.1046/j.1432-1033.2003.03811.x. [DOI] [PubMed] [Google Scholar]
- 52.Morenilla-Palao C, Pertusa M, Meseguer V, Cabedo H, Viana F. Lipid raft segregation modulates TRPM8 channel activity. J Biol Chem. 2009;284:9215–9224. doi: 10.1074/jbc.M807228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erler I, Al-Ansary DMM, Wissenbach U, Wagner TFJ, Flockerzi V, Niemeyer BA. Trafficking and assembly of the cold-sensitive TRPM8 channel. J Biol Chem. 2006;281:38396–38404. doi: 10.1074/jbc.M607756200. [DOI] [PubMed] [Google Scholar]
- 54.Lin Y, Sun Z. Antiaging Gene Klotho Enhances Glucose-Induced Insulin Secretion by Up-Regulating Plasma Membrane Levels of TRPV2 in MIN6 -Cells. Endocrinology. 2012;153:3029–3039. doi: 10.1210/en.2012-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voets T, Nilius B. The pore of TRP channels: trivial or neglected? Cell Calcium. 2003;33:299–302. doi: 10.1016/s0143-4160(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 56.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 57.Penna A, Juvin V, Chemin J, Compan V, Monet M, Rassendren F-A. PI3-kinase promotes TRPV2 activity independently of channel translocation to the plasma membrane. Cell Calcium. 2006;39:495–507. doi: 10.1016/j.ceca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Mercado J, Gordon-Shaag A, Zagotta WN, Gordon SE. Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2010;30:13338–13347. doi: 10.1523/JNEUROSCI.2108-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holakovska B, Grycova L, Bily J, Teisinger J. Characterization of calmodulin binding domains in TRPV2 and TRPV5 C-tails. Amino Acids. 2011;40:741–748. doi: 10.1007/s00726-010-0712-2. [DOI] [PubMed] [Google Scholar]
- 60.García-Sanz N, Fernández-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sánchez E, Fernández-Ballester G, Ferrer-Montiel A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci. 2004;24:5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker D, Müller M, Leuner K, Jendrach M. The C-terminal domain of TRPV4 is essential for plasma membrane localization. Mol Membr Biol. 2008;25:139–151. doi: 10.1080/09687680701635237. [DOI] [PubMed] [Google Scholar]
- 62.Valente P, García-Sanz N, Gomis A, Fernández-Carvajal A, Fernández-Ballester G, Viana F, Belmonte C, Ferrer-Montiel A. Identification of molecular determinants of channel gating in the transient receptor potential box of vanilloid receptor I. FASEB J. 2008;22:3298–3309. doi: 10.1096/fj.08-107425. [DOI] [PubMed] [Google Scholar]
- 63.García-Sanz N, Valente P, Gomis A, Fernández-Carvajal A, Fernández-Ballester G, Viana F, Belmonte C, Ferrer-Montiel A. A role of the transient receptor potential domain of vanilloid receptor I in channel gating. J Neurosci. 2007;27:11641–11650. doi: 10.1523/JNEUROSCI.2457-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnhill JC, Stokes AJ, Koblan-Huberson M, Shimoda LMN, Muraguchi A, Adra CN, Turner H. RGA protein associates with a TRPV ion channel during biosynthesis and trafficking. J Cell Biochem. 2004;91:808–820. doi: 10.1002/jcb.10775. [DOI] [PubMed] [Google Scholar]
- 65.Stokes AJ, Wakano C, Del Carmen KA, Koblan-Huberson M, Turner H. Formation of a physiological complex between TRPV2 and RGA protein promotes cell surface expression of TRPV2. J Cell Biochem. 2005;94:669–683. doi: 10.1002/jcb.20331. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi Y, Katanosaka Y, Iwata Y, Matsuoka M, Shigekawa M, Wakabayashi S. Identification and characterization of GSRP-56, a novel Golgi-localized spectrin repeat-containing protein. Exp Cell Res. 2006;312:3152–3164. doi: 10.1016/j.yexcr.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 67.Phelps CB, Procko E, Lishko PV, Wang RR, Gaudet R. Insights into the roles of conserved and divergent residues in the ankyrin repeats of TRPV ion channels. Channels (Austin) 2007;1:148–151. doi: 10.4161/chan.4716. [DOI] [PubMed] [Google Scholar]
- 68.Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem. 2010;285:731–740. doi: 10.1074/jbc.M109.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myers BR, Bohlen CJ, Julius D. A yeast genetic screen reveals a critical role for the pore helix domain in TRP channel gating. Neuron. 2008;58:362–373. doi: 10.1016/j.neuron.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 71.Rohacs T, Thyagarajan B, Lukacs V. Phospholipase C mediated modulation of TRPV1 channels. Mol Neurobiol. 2008;37:153–163. doi: 10.1007/s12035-008-8027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 Channels Are Intrinsically Heat Sensitive and Negatively Regulated by Phosphoinositide Lipids. Neuron. 2013;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 74.Gordon-Shaag A, Zagotta WN, Gordon SE. Mechanism of Ca(2+)-dependent desensitization in TRP channels. Channels (Austin) 2008;2:125–129. doi: 10.4161/chan.2.2.6026. [DOI] [PubMed] [Google Scholar]
- 75.Shin Y-C, Shin S-Y, So I, Kwon D, Jeon J-H. TRIP Database: a manually curated database of protein-protein interactions for mammalian TRP channels. Nucleic Acids Res. 2011;39:D356–61. doi: 10.1093/nar/gkq814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shin Y-C, Shin S-Y, Chun JN, Cho HS, Lim JM, Kim H-G, So I, Kwon D, Jeon J-H. TRIP database 2.0: a manually curated information hub for accessing TRP channel interaction network. PLoS ONE. 2012;7:e47165. doi: 10.1371/journal.pone.0047165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santoni G, Farfariello V, Liberati S, Morelli MB, Nabissi M, Santoni M, Amantini C. The role of transient receptor potential vanilloid type-2 ion channels in innate and adaptive immune responses. Front Immunol. 2013;4:34. doi: 10.3389/fimmu.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharma. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- 80.Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharm. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu H-Z, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee L-Y, Wood JD, Zhu MX. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 83.Juvin V, Penna A, Chemin J, Lin YL, Rassendren FA. Pharmacological Characterization and Molecular Determinants of the Activation of Transient Receptor Potential V2 Channel Orthologs by 2-Aminoethoxydiphenyl Borate. Mol Pharmacol. 2007;72:1258–1268. doi: 10.1124/mol.107.037044. [DOI] [PubMed] [Google Scholar]
- 84.Chung MK, Güler AD, Caterina MJ. Biphasic currents evoked by chemical or thermal activation of the heat-gated ion channel, TRPV3. J Biol Chem. 2005;280:15928–15941. doi: 10.1074/jbc.M500596200. [DOI] [PubMed] [Google Scholar]
- 85.Leffler A, Linte RM, Nau C, Reeh P, Babes A. A high-threshold heat-activated channel in cultured rat dorsal root ganglion neurons resembles TRPV2 and is blocked by gadolinium. European Journal of Neuroscience. 2007;26:12–22. doi: 10.1111/j.1460-9568.2007.05643.x. [DOI] [PubMed] [Google Scholar]
- 86.Stotz SC, Vriens J, Martyn D, Clardy J, Clapham DE. Citral sensing by Transient [corrected] receptor potential channels in dorsal root ganglion neurons. PLoS ONE. 2008;3:e2082. doi: 10.1371/journal.pone.0002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bang S, Kim KY, Yoo S, Lee S-H, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007;425:120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 88.Robbins N, Koch SE, Tranter M, Rubinstein J. The History and Future of Probenecid. Cardiovasc Toxicol. 2011:1–9. doi: 10.1007/s12012-011-9145-8. [DOI] [PubMed] [Google Scholar]
- 89.Nie L, Oishi Y, Doi I, Shibata H, Kojima I. Inhibition of proliferation of MCF-7 breast cancer cells by a blocker of Ca(2+)-permeable channel. Cell calcium. 1997;22:75–82. doi: 10.1016/s0143-4160(97)90107-x. [DOI] [PubMed] [Google Scholar]
- 90.Aoyagi K, Ohara Imaizumi M, Nishiwaki C, Nakamichi Y, Nagamatsu S. Insulin/phosphoinositide 3-kinase pathway accelerates the glucose-induced first-phase insulin secretion through TrpV2 recruitment in pancreatic β-cells. Biochem J. 2010;432:375–386. doi: 10.1042/BJ20100864. [DOI] [PubMed] [Google Scholar]
- 91.Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes. 2009;58:174–184. doi: 10.2337/db08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mihara H, Boudaka A, Shibasaki K, Yamanaka A, Sugiyama T, Tominaga M. Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J Neurosci. 2010;30:16536–16544. doi: 10.1523/JNEUROSCI.4426-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M. TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J Neurosci. 2010;30:4601–4612. doi: 10.1523/JNEUROSCI.5830-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahluwalia J, Rang H, Nagy I. The putative role of vanilloid receptor-like protein-1 in mediating high threshold noxious heat-sensitivity in rat cultured primary sensory neurons. Eur J Neurosci. 2002;16:1483–1489. doi: 10.1046/j.1460-9568.2002.02231.x. [DOI] [PubMed] [Google Scholar]
- 95.Ichikawa H, Sugimoto T. Vanilloid receptor 1-like receptor-immunoreactive primary sensory neurons in the rat trigeminal nervous system. Neuroscience. 2000;101:719–725. doi: 10.1016/s0306-4522(00)00427-9. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto Y, Taniguchi K. Immunolocalization of VR1 and VRL1 in rat larynx. Auton Neurosci. 2005;117:62–65. doi: 10.1016/j.autneu.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 97.Ma Q-P. Vanilloid receptor homologue, VRL1, is expressed by both A- and C-fiber sensory neurons. Neuroreport. 2001;12:3693. doi: 10.1097/00001756-200112040-00018. [DOI] [PubMed] [Google Scholar]
- 98.Lewinter RD, Skinner K, Julius D, Basbaum AI. Immunoreactive TRPV-2 (VRL-1), a capsaicin receptor homolog, in the spinal cord of the rat. J Comp Neurol. 2004;470:400–408. doi: 10.1002/cne.20024. [DOI] [PubMed] [Google Scholar]
- 99.Koike S, Uno T, Bamba H, Shibata T, Okano H, Hisa Y. Distribution of vanilloid receptors in the rat laryngeal innervation. Acta Otolaryngol. 2004;124:515–519. doi: 10.1080/00016480310000674. [DOI] [PubMed] [Google Scholar]
- 100.Hamamoto T, Takumida M, Hirakawa K, Takeno S, Tatsukawa T. Localization of transient receptor potential channel vanilloid subfamilies in the mouse larynx. Acta Otolaryngol. 2008;128:685–693. doi: 10.1080/00016480701669489. [DOI] [PubMed] [Google Scholar]
- 101.Hamamoto T, Takumida M, Hirakawa K, Tatsukawa T, Ishibashi T. Localization of transient receptor potential vanilloid (TRPV) in the human larynx. Acta Otolaryngol. 2009;129:560–568. doi: 10.1080/00016480802273108. [DOI] [PubMed] [Google Scholar]
- 102.Gibbs JL, Melnyk JL, Basbaum AI. Differential TRPV1 and TRPV2 channel expression in dental pulp. J Dent Res. 2011;90:765–770. doi: 10.1177/0022034511402206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wainwright A, Rutter AR, Seabrook GR, Reilly K, Oliver KR. Discrete expression of TRPV2 within the hypothalamo-neurohypophysial system: Implications for regulatory activity within the hypothalamic-pituitary-adrenal axis. J Comp Neurol. 2004;474:24–42. doi: 10.1002/cne.20100. [DOI] [PubMed] [Google Scholar]
- 104.Nedungadi TP, Carreño FR, Walch JD, Bathina CS, Cunningham JT. Region-specific changes in transient receptor potential vanilloid channel expression in the vasopressin magnocellular system in hepatic cirrhosis-induced hyponatraemia. J Neuroendocrinol. 2012;24:642–652. doi: 10.1111/j.1365-2826.2011.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 106.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nedungadi TP, Dutta M, Bathina CS, Caterina MJ, Cunningham JT. Expression and distribution of TRPV2 in rat brain. Exp Neurol. 2012;237:223–237. doi: 10.1016/j.expneurol.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 110.Cha S-K, Ortega B, Kurosu H, Rosenblatt KP, Kuro-O M, Huang C-L. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang D, Spielmann A, Wang L, Ding G, Huang F, Gu Q, Schwarz W. Mast-cell degranulation induced by physical stimuli involves the activation of transient-receptor-potential channel TRPV2. Physiol Res. 2012;61:113–124. doi: 10.33549/physiolres.932053. [DOI] [PubMed] [Google Scholar]
- 112.Stokes AJ, Shimoda LMN, Koblan-Huberson M, Adra CN, Turner H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J Exp Med. 2004;200:137–147. doi: 10.1084/jem.20032082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saunders CI, Kunde DA, Crawford A, Geraghty DP. Expression of transient receptor potential vanilloid 1 (TRPV1) and 2 (TRPV2) in human peripheral blood. Mol Immunol. 2007;44:1429–1435. doi: 10.1016/j.molimm.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 114.Nagasawa M, Nakagawa Y, Tanaka S, Kojima I. Chemotactic peptide fMetLeuPhe induces translocation of the TRPV2 channel in macrophages. J Cell Physiol. 2007;210:692–702. doi: 10.1002/jcp.20883. [DOI] [PubMed] [Google Scholar]
- 115.Boels K, Glassmeier G, Herrmann D, Riedel IB, Hampe W, Kojima I, Schwarz JR, Schaller HC. The neuropeptide head activator induces activation and translocation of the growth-factor-regulated Ca(2+)-permeable channel GRC. J Cell Sci. 2001;114:3599–3606. doi: 10.1242/jcs.114.20.3599. [DOI] [PubMed] [Google Scholar]
- 116.Nagasawa M, Kojima I. Translocation of calcium-permeable TRPV2 channel to the podosome: Its role in the regulation of podosome assembly. Cell Calcium. 2012;51:186–193. doi: 10.1016/j.ceca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 117.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 118.Yamashiro K, Sasano T, Tojo K, Namekata I, Kurokawa J, Sawada N, Suganami T, Kamei Y, Tanaka H, Tajima N, Utsunomiya K, Ogawa Y, Furukawa T. Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem Biophys Res Comm. 2010;398:284–289. doi: 10.1016/j.bbrc.2010.06.082. [DOI] [PubMed] [Google Scholar]
- 119.Park KS, Kim Y, Lee Y-H, Earm YE, Ho W-K. Mechanosensitive cation channels in arterial smooth muscle cells are activated by diacylglycerol and inhibited by phospholipase C inhibitor. Circ Res. 2003;93:557–564. doi: 10.1161/01.RES.0000093204.25499.83. [DOI] [PubMed] [Google Scholar]
- 120.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JXJ. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1233–45. doi: 10.1152/ajplung.00445.2002. [DOI] [PubMed] [Google Scholar]
- 121.Peng G, Lu W, Li X, Chen Y, Zhong N, Ran P, Wang J. Expression of store-operated Ca2+ entry and transient receptor potential canonical and vanilloid-related proteins in rat distal pulmonary venous smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2010;299:L621–L630. doi: 10.1152/ajplung.00176.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Gen. 2009;18:824–834. doi: 10.1093/hmg/ddn408. [DOI] [PubMed] [Google Scholar]
- 124.Gailly P. TRP channels in normal and dystrophic skeletal muscle. Curr Op Pharm. 2012;12:326–334. doi: 10.1016/j.coph.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 125.Zanou N, Iwata Y, Schakman O, Lebacq J, Wakabayashi S, Gailly P. Essential role of TRPV2 ion channel in the sensitivity of dystrophic muscle to eccentric contractions. FEBS Lett. 2009;583:3600–3604. doi: 10.1016/j.febslet.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 126.Lehen’kyi V, Prevarskaya N. TRPV2 (transient receptor potential cation channel, subfamily V, member 2) Atlas Genet Cytogenet Oncol Haematol. 2012;16:563–567. [Google Scholar]
- 127.Vriens J, Janssens A, Prenen J, Nilius B, Wondergem R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium. 2004;36:19–28. doi: 10.1016/j.ceca.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 128.Liu G, Xie C, Sun F, Xu X, Yang Y, Zhang T, Deng Y, Wang D, Huang Z, Yang L, Huang S, Wang Q, Liu G, Zhong D, Miao X. Clinical significance of transient receptor potential vanilloid 2 expression in human hepatocellular carcinoma. Cancer Genet Cytogenet. 2010;197:54–59. doi: 10.1016/j.cancergencyto.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 129.Caprodossi S, Lucciarini R, Amantini C, Nabissi M, Canesin G, Ballarini P, Di Spilimbergo A, Cardarelli MA, Servi L, Mammana G, Santoni G. Transient Receptor Potential Vanilloid Type 2 (TRPV2) Expression in Normal Urothelium and in Urothelial Carcinoma of Human Bladder: Correlation with the Pathologic Stage. Eur Urol. 2008;54:612–620. doi: 10.1016/j.eururo.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 130.Yamada T, Ueda T, Shibata Y, Ikegami Y, Saito M, Ishida Y, Ugawa S, Kohri K, Shimada S. TRPV2 activation induces apoptotic cell death in human T24 bladder cancer cells: a potential therapeutic target for bladder cancer. Urology. 2010;76:509.e1–7. doi: 10.1016/j.urology.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 131.Monet M, Lehen’kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, Gkika D, Pourtier A, Bidaux G, Slomianny C, Delcourt P, Rassendren F, Bergerat J-P, Ceraline J, Cabon F, Humez S, Prevarskaya N. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;70:1225–1235. doi: 10.1158/0008-5472.CAN-09-2205. [DOI] [PubMed] [Google Scholar]
- 132.Monet M, Gkika D, Lehen’kyi V, Pourtier A, Abeele FV, Bidaux G, Juvin V, Rassendren F, Humez S, Prevarsakaya N. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim Biophys Acta - Mol Cell Res. 2009;1793:528–539. doi: 10.1016/j.bbamcr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 133.Nabissi M, Morelli MB, Amantini C, Farfariello V, Ricci-Vitiani L, Caprodossi S, Arcella A, Santoni M, Giangaspero F, De Maria R, Santoni G. TRPV2 channel negatively controls glioma cell proliferation and resistance to Fas-induced apoptosis in ERK-dependent manner. Carcinogenesis. 2010;31:794–803. doi: 10.1093/carcin/bgq019. [DOI] [PubMed] [Google Scholar]
- 134.Morelli MB, Nabissi M, Amantini C, Farfariello V, Ricci-Vitiani L, di Martino S, Pallini R, Larocca LM, Caprodossi S, Santoni M, De Maria R, Santoni G. The transient receptor potential vanilloid-2 cation channel impairs glioblastoma stem-like cell proliferation and promotes differentiation. Int J Cancer. 2012;131:E1067–E1077. doi: 10.1002/ijc.27588. [DOI] [PubMed] [Google Scholar]
- 135.Nabissi M, Morelli MB, Santoni M, Santoni G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2013;34:48–57. doi: 10.1093/carcin/bgs328. [DOI] [PubMed] [Google Scholar]
- 136.Katoh K, Misawa K, Kuma KI, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38:W529–33. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]