Abstract
Cancer is a major risk factor for bone loss and fractures. This is due both to direct effects of cancer cells on the skeleton and to deleterious effects of cancer-specific therapies on bone cells. Marked improvements in survival for many cancers mean that strategies to limit bone loss and reduce fracture risk must be incorporated into the care plans for nearly all patients with cancer. The vast majority of effort thus far has focused on bone loss in patients with breast and prostate cancers, with comparatively few studies in other malignancies. Anti-resorptive therapies have proven nearly universally effective for limiting bone loss in cancer patients, although few studies have been powered sufficiently to include fractures as primary endpoints, and patients are frequently neither identified nor treated according to published guidelines. Non-pharmacologic approaches to limit falls, particularly in elderly patients, are also likely important adjunctive measures for most cancer patients.
Keywords: bone, cancer, osteoporosis, breast, aromatase inhibitor, prostate, myeloma, bisphosphonate, denosumab, exercise, vitamin D, calcium
Introduction
Nearly all cancers can have significant negative effects on the skeleton. Cancer is a major risk for both generalized and local bone loss, with bone loss as assessed by bone mineral density (BMD) testing substantially higher in cancer patients than in the general population, independent of cancer type.(1) Cancer-associated bone loss is the result of multiple, inter-related factors. These include both the direct effects of cancer cells, and the effects of therapies used in cancer treatment including chemotherapeutics, corticosteroids, aromatase inhibitors, and androgen deprivation therapy (Figure 1). Further, the skeleton is also the most common site of metastatic disease, as cancer cells growing within bone induce osteoblasts and osteoclasts to produce factors which stimulate further cancer growth.(2) Accordingly, the optimal management of skeletal health has become an increasingly important part of the care provided to cancer patients, as improved oncologic treatments have enhanced both patient survival and longevity.
Figure 1.
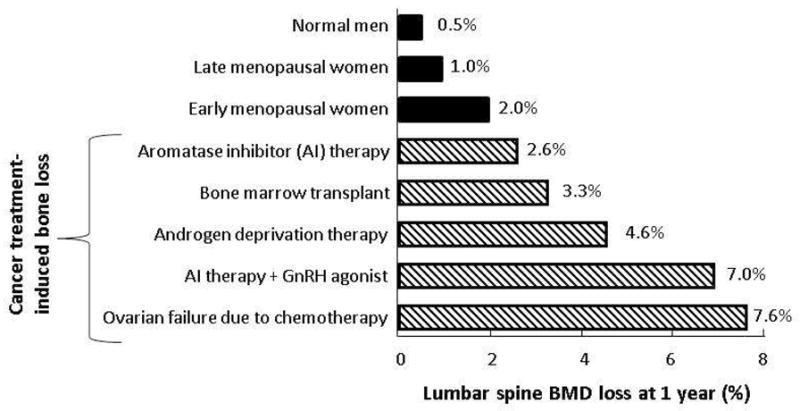
Comparison of annualized lumbar spine bone mineral density (BMD) losses associated with normal aging (top; solid bars) to BMD losses associated with different cancer therapies (bottom; hatched bars). AI indicates aromatase inhibitor; GnRH, gonadotropin-releasing hormone. Adapted from Postgraduate Institute for Medicine and Interlink Healthcare Communications, joint sponsors of the CME Lecture Series entitled Skeletal Complications Across the Cancer Continuum Slide/Lecture Kit. Released June 2005. Used with permission from Postgraduate Institute for Medicine.
Herein, recent work examining the relationship between cancer and bone loss, with an emphasis on breast and prostate cancers, will be reviewed.
Age-associated bone loss in men and women
Although cancer is not exclusively a disease of aging, cancer more commonly occurs in older individuals. Likewise, aging in both men and women is associated with increased rates of osteoporosis and fractures. Central to this bone loss is the decline in sex steroid (primarily estrogen and testosterone) levels that occurs in both sexes with aging.
In women, declining estrogen levels result from ovarian failure, with serum estradiol levels falling by 85–90% over the menopausal transition.(3) At present, there is little data to suggest a significant role for testosterone in the maintenance of postmenopausal skeletal integrity.
Men also undergo substantial changes in biologically available sex steroid levels, primarily due to the greater than two-fold age-related increase in sex hormone binding globulin levels over the male lifespan, making bioavailable estrogen and testosterone levels decline an average of 47% and 64%, respectively.(4) Although testosterone is the predominant sex steroid in men, evidence from cross-sectional and longitudinal studies has shown that male BMD is better correlated with circulating levels of bioavailable estradiol (made via aromatization of testosterone to estradiol) than testosterone.(5)
Thus in both men and women, decreases in sex steroid levels are central to the bone loss that occurs with normal aging (Figure 1). In patients with breast or prostate cancer, however, it is often precisely these sex steroid levels which are targeted for further reduction by hormonal therapies, an effect which can potentiate ongoing bone loss already occurring in aged patients.
Breast cancer
Despite the now widespread recognition that breast cancer therapies lead to bone loss and increased fracture risk, evidence indicates that a significant proportion of patients with breast cancer do not undergo routine BMD screening as recommended by the American Society of Clinical Oncology, likely contributing significantly to the skeletal morbidity(6) and leading to increased healthcare-associated costs in some women.(7)
Premenopausal women
On average, menopausal onset in women with breast cancer receiving chemotherapy occurs approximately a decade earlier than normal, (8) with advancing age (> 40 years) at the time of chemotherapy initiation the most significant factor associated with increased risk of ovarian failure (Figure 1). Due to both earlier breast cancer detection and continued improvements in chemotherapeutic options, however, many women diagnosed with breast cancer prior to menopausal onset have prolonged survivals. Accordingly, efforts to limit complications related to therapy, in particular the bone loss that occur secondary to the triumvirate of cytotoxic chemotherapy, corticosteroids used to limit chemotherapy-associated nausea, and hormonal therapies, is of paramount importance.
To address how intravenous bisphosphonate treatment affects bone loss in premenopausal women undergoing breast cancer therapy, Gnant and colleagues performed a sub-study of 404 women from a large randomized, phase III, four-arm trial in which subjects received the gonadotropin-releasing hormone agonist goserelin (which acts indirectly to suppress endogenous estrogen production) and either the estrogen receptor mixed agonist/antagonist tamoxifen or the aromatase inhibitor (AI) anastrozole, both with or without zoledronic acid infused every six months for three years.(9) BMD was then prospectively assessed by dual-energy x-ray absorptiometry (DXA). Relative to baseline, endocrine therapy alone resulted in a BMD declines (lumbar spine −11.3%; hip −7.3%) at 36 months. Further, in women not receiving zoledronic acid, average losses were greater in women treated with anastrozole compared to women receiving tamoxifen. In contrast, women treated with zoledronic acid in addition to endocrine therapy had stable BMD at 36 months (lumbar spine +0.4%; hip +0.8%) versus baseline. Similar results indicating a protective role for zoledronic acid on bone loss in premenopausal women receiving adjuvant chemotherapy for breast cancer have also been reported by others.(10)
In addition, it is useful to note that tamoxifen, which acts as an estrogen receptor antagonist in breast tissue and is frequently used in estrogen receptor positive breast cancer patients or alternatively as prophylaxis in women at high-risk for breast cancer, may lead to bone loss in premenopausal women who continue to menstruate after adjuvant chemotherapy.(11)
Postmenopausal women
Given that the majority of breast cancer is diagnosed in postmenopausal women, and that postmenopausal women are at increased risk for bone loss and fracture compared to premenopausal women, it is not surprising that the majority of work to date has focused on this group.
Whereas tamoxifen functions as an estrogen receptor antagonist prior to menopause and thereby increases bone loss, in postmenopausal women tamoxifen acts as a weak agonist at the estrogen receptor to reduce fracture risk.(12) When compared to AI therapies in particular, tamoxifen limits bone loss(13) and fracture risk(14), as does the closely related selective estrogen receptor antagonist arzoxifene.(15)
Due to their superior efficacy for reducing endogenous estrogen levels and their documented superior clinical efficacy, however, AI’s rather than tamoxifen are considered as first-line adjuvant therapy for most postmenopausal women with hormone-responsive tumors.(16) As would be expected by an intervention which further lowers estradiol levels in postmenopausal women, multiple studies have now clearly documented increased boss loss and fracture rates in breast cancer patients treated with AI therapy (Figure 1).(17, 18) As exemplified by the ATAC trial, which compared postmenopausal women treated with anastrozole, tamoxifen, either alone or in combination, five years of AI (anastrozole) treatment induced significant declines in BMD at both the lumbar spine (−6.1%) and total hip (−7.2%) versus tamoxifen treatment, which slightly increased BMD at the spine (+2.8%) and hip (+0.7%) at five years.(19) Notably, approximately similar rates of bone loss were also shown to occur in women treated with the AI exemestane for primary breast cancer prevention, even with concomitant supplementation with calcium and vitamin D.(20)
In light of the growing recognition of the rapid bone loss that occurs after AI initiation, a variety of pharmacologic agents have now been assessed in clinical trials for their abilities to halt such losses. Given their well-recognized role both in limiting both osteoporosis-associated bone loss and cancer-associated skeletal-related-events such as hypercalcemia and fractures,(21) bisphosphonates have also been studied extensively in postmenopausal breast cancer-associated bone loss.
In a recent randomized, double-blinded placebo controlled study of the oral bisphosphonate clodronate in 3323 women with stage I-III breast cancer followed for a median of 91 months, subgroup analysis showed that clodronate treatment was associated with significantly higher spine (+1.9%) and hip (+1.3%) BMD compared to placebo,(22) although no difference in overall survival was found.(23) Recent studies have also examined the effectiveness of the oral bisphosphonate risedronate in limiting postmenopausal AI-induced bone loss, and concluded that once-weekly risedronate leads to maintenance of skeletal structural integrity(24) and increases in lumbar spine and hip BMD compared to placebo, an effect most pronounced in women with lower T-scores at baseline.(25, 26)
Relative to studies with oral bisphosphonates, comparatively more recent work has examined the role of intravenous zoledronic acid for the prevention of bone loss in postmenopausal women treated with AI’s. Although zoledronic acid was not shown to reduce the incidence of disease recurrence or death compared to placebo in a phase 3 study of women with early stage breast cancer,(27) provision of zoledronic acid every six months from time of AI therapy initiation lead to a significant increase (+4.3%) in lumbar spine BMD at 60 months compared to a significant decrease (−5.4%) in women in whom zoledronic acid was delayed (initiation only for on-study fragility fracture or pre-specified BMD decline).(28) These findings showing a substantial benefit for upfront versus delayed zoledronic acid therapy in limiting BMD losses in postmenopausal breast cancer patients have been confirmed by others.(29, 30) Given this confluence of data, recent guidelines are supportive of the early use of bisphosphonates for the management of AI-associated bone loss in postmenopausal women, with consideration for switching from oral to intravenous preparations if bone loss is noted by BMD assessment or if there are concerns regarding compliance.(31)
Lastly, it is useful to note that efforts have been made to evaluate whether denosumab, a fully-humanized monoclonal antibody directed against receptor activator of nuclear factor kappa-b ligand (RANKL) which acts by binding RANKL to prevent its association with the RANKL receptor (RANK) on osteoclasts, is active in limiting AI-associated bone loss in postmenopausal women. In a double-blind randomized controlled trial of postmenopausal women receiving AI therapy, denosumab increased BMD at both the hip and spine compared to placebo.(32) Notably, denosumab has also been shown to delay time to the composite endpoint of first skeletal-related-event in breast cancer patients.(33, 34), while a study designed to test whether denosumab compared to placebo will reduce the rate of first clinical fracture in women with breast cancer receiving AI therapy is currently underway (clinicaltrials.gov identifier: NCT00556374).
Non-pharmacologic approaches to limit bone loss in breast cancer
Although the majority of studies have focused on pharmacologic approaches to limit bone loss in women with breast cancer, a variety of physical fitness programs with skeletal endpoints have also been tested.(35) In a small study of breast cancer survivors, a moderate form of weight-bearing exercise (Tai Chi) performed three times/week for one hour increased bone formation and reduced bone resorption as assessed by biochemical markers of bone turnover.(36) In partial contrast, however, a twenty-four month randomized trial of weight training in 223 postmenopausal breast cancer survivors failed to show additional BMD or bone turnover marker benefit beyond that provided by treatment with risedronate, calcium, and vitamin D,(37) although a separate study of one year duration which combined impact with resistance training demonstrated BMD stabilization and improvements in biochemical markers of bone turnover when compared to control subjects enrolled in a low-intensity stretching program.(38) Whether exercise training programs are of greater benefit to premenopausal as compared to postmenopausal breast cancer survivors, however, has been recently suggested.(39) Thus although weight-bearing exercise is likely an important addition to efforts aimed at limiting the bone loss that occurs with breast cancer treatment, current evidence supports such activity in addition to, rather than in place of, concurrent pharmacologic approaches.
Prostate cancer
Prostate cancer is the most common non-cutaneous cancer in men. As has been seen in women with breast cancer, earlier detection has increased survival length, such that prolonged periods of disease stability are increasingly commonplace. Again like breast cancer, prostate cancer disproportionately affects the elderly, the group most likely to already suffer from normal age-associated bone loss,(40) and like breast cancer, most prostate cancers are at least initially hormonally responsive. As such, androgen deprivation therapy (ADT) from either bilateral orchiectomy, or now more commonly gonadotropin-releasing hormone (GnRH) agonist-mediated therapy, is often used for the care of prostate cancer patients. As desired clinically, such treatments result in marked decreases in circulating total and free testosterone levels, with most ADT’s resulting in total testosterone values in the castrate range of < 20 ng/dL.(41) As a consequence of this severe suppression of testosterone levels, levels of bioavailable estradiol (normally made via aromatization of testosterone to estradiol) also plummet. It is particularly in subjects who undergo long-term androgen deprivation that fracture risk has been shown to be most markedly increased (Figure 1),(42) with intermittent ADT having an intermediate effect on bone loss.(43)
Despite these now well-recognized skeletal effects, however, the majority of prostate cancer patients remain inadequately treated,(44, 45) although the implementation of osteoporosis management programs to identify (by DXA screening) and treat men with newly diagnosed prostate cancer receiving ADT has recently been shown to profoundly reduce (by > 70%) fracture incidence in this population.(46)
As with breast cancer, bisphosphonates have been extensively studied for their effects on limiting ADT-associated bone loss in men with prostate cancer. Both oral and intravenous preparations have proven effective for this purpose. As example of oral bisphosphonate therapy, a recent double-blind, placebo-controlled, randomized study of 104 men with non-metastatic prostate cancer demonstrated that lumbar spine BMD decreased by 5.8% at 12 months and 13.6% at 24 months in ADT-treated men who received placebo, while remaining unchanged from baseline in risedronate-treated men.(47) Approximately similar results have also been seen with alendronate,(48, 49) as well as with the intravenous bisphosphonates pamidronate(50) and zoledronic acid.(51, 52) Consistent with these individual studies, a recent systematic review and meta-analysis of bisphosphonate therapy in men undergoing ADT for prostate cancer, which included 15 studies and 2634 subjects, concluded that bisphosphonate therapy significantly reduced both fracture risk [relative risk (RR) 0.80; P < 0.01] and osteoporosis (RR 0.39; P < 0.001), with zoledronic acid having the greatest effect among the bisphosphonates.(53)
More recently, a pivotal randomized, double-blind trial comparing denosumab (n=734) versus placebo (n=734) in men receiving ADT for prostate cancer was undertaken.(54) When subjects were provided with a subcutaneous 60 mg denosumab dose every six months, BMD assessed at 24 months increased significantly (difference from placebo: +6.7%, +4.8%, +3.9%, and +5.5%; all P values < 0.001) at the lumbar spine, total hip, and femoral neck, respectively, with significant differences between groups occurring as early as one month. Further, denosumab-treated patients had a significantly decreased risk for new vertebral fractures at 36 months (RR 0.38, P<0.01). Based on these findings, denosumab received approval from the United States Food and Drug Administration (FDA) for fracture risk reduction in men receiving ADT.
A subsequent study in which men with castration-resistant prostate cancer were treated with either denosumab (120 mg every four weeks; n=716) or placebo (n=716) showed that denosumab significantly increased metastasis-free survival and delayed time to first skeletal metastasis, although rates of osteonecrosis of the jaw (ONJ) were increased in subjects who received denosumab.(55) Finally, a trial which directly compared denosumab (n=950) to zoledronic acid (n=951) in men with castration-resistant prostate cancer and bone metastases demonstrated an increased time to first on-study skeletal-related event (20.7 versus 17.1 months; P<0.01 for superiority), although hypocalcemia was more frequent in the denosumab-treated subjects.(56)
Lastly, given the important role described above for estradiol in the maintenance of male skeletal health, rationale exists for the potential use of selective estrogen-receptor modulators (SERMs) to limit male bone loss. As recently described,(57) daily delivery of the oral SERM toremifene for up to 24 months decreased the risk for new vertebral compression fractures (by 50%; RR 0.50; P=0.05) versus placebo, while also increasing BMD at the lumbar spine, femoral neck, and total hip (all P<0.0001). Although venous thromboembolic events did occur more frequently in toremifene-treated subjects (2.6% versus 1.1%; P=0.26), this adverse effect was most common in men aged ≥ 80 years.(58) To date, however, no SERMS are approved for the treatment bone loss, regardless of etiology, in men.
Finally, it is also important to recognize that sarcopenia frequently occurs in parallel with bone loss in men receiving ADT,(59) a finding which might reasonably be expected to decrease stability and increase fall risk.(60) Accordingly, it seems prudent to encourage physical activity, and in particular weight bearing exercise, in men undergoing ADT, although only limited data exist to support such recommendations.(61) Ensuring that patients maintain adequate intakes of calcium and vitamin D also should be added to any comprehensive skeletal health program, although calcium and vitamin D by themselves are insufficient to limit ADT-associated bone loss.(62, 63)
Hematologic malignancies
Bone marrow transplantation
Bone loss and increased fracture risk are well-recognized consequences of solid organ transplantation, but are also common complications following bone marrow transplantation. In a recent study of 17 patients undergoing allogeneic stem cell transplantation for acute myeloid leukemia, prophylactic treatment with zoledronic acid both prior to transplant and six months post-transplant successfully prevented the early bone loss normally seen following transplant.(64)
Monoclonal gammopathies
Multiple myeloma is the second most common hematologic cancer, accounting for 10% of all hematologic malignancies.(65) In addition to developing focal osteolytic lesions characteristic of the disease, patients also suffer from generalized bone loss. Thus, nearly two-thirds have bone pain at presentation, and fracture rates are increased sixteen-fold relative to the general population in the year preceding diagnosis. (66) Further, even with disease remission, skeletal lesions rarely heal. Both pamidronate and zoledronic are FDA-approved for the treatment of myeloma bone disease, and have been shown on placebo-controlled trials to reduce hypercalcemia, bone pain, and fracture incidence.(67, 68)
Patients with monoclonal gammopathy of undetermined significance (MGUS), a common pre-malignant condition with an approximately 1% annual risk for progression to myeloma but in which patients do not have the characteristic osteolytic lesions of multiple myeloma, also have substantially increased fracture rates.(69) Interestingly, recent work suggests that subjects with MGUS have substantial alterations of both cortical and trabecular skeletal microarchitecture, as well as increases in circulating cytokines previously identified in patients with myeloma which are known to increase osteoclast activity and suppress osteoblast activity.(70) Treatment with both alendronate(71) and zoledronic acid(72) has been shown to increase BMD in patients with MGUS.
Other malignancies
Gastric cancer
Patients with gastric cancer who have undergone gastrectomy are at significantly increased risk for developing osteoporosis, likely due to a decrease in the gastric acidification necessary for optimal intestinal calcium absorption. In a small study of 47 post-gastrectomy patients, oral bisphosphonate treatment with alendronate or risedronate significantly reduced incident fractures and increased BMD at both the lumbar spine and femoral neck compared to no treatment (P<0.05 for all comparisons).(73)
Additional issues related to skeletal health in patients with cancer
In addition to pharmacologic interventions, conservative measures are extremely important for cancer patients to limit fracture risk. These include counseling about potentially high-risk activities such as falls and heavy lifting, the introduction of appropriately supervised physical therapy for muscle strengthening to limit fall risks, and ensuring adequate intake of calcium and vitamin D. Finally, it is important to recognize that cancer patients are often at increased fall risk due to concurrent use of analgesics and sedatives, and as such should be counseled accordingly.
Although beyond the scope of this review, it is now well-recognized that anti-resorptive therapies (both bisphosphonates and denosumab) are associated with increased risks both in the short- (such as hypocalcemia) and long- (such as ONJ and subtrochanteric femoral fractures) term. Such potential issues must be considered and discussed fully with patients considering initiating anti-resorptive therapy, particularly given that oncology patients generally receive more frequent anti-resorptive therapy dosing, leading to cumulative doses that are often at least a magnitude higher than those used for the routine treatment of osteoporosis.(21) Accordingly, the reader is referred to recent references more specific to such potential issues.(74–76)
Conclusion
Nearly all patients with cancer are at increased risk for bone loss and fractures due to a combination of factors including their underlying malignancy, an often advanced age, and therapeutic regimens which directly or indirectly affect bone cells. While therapeutic improvements for many cancers have substantially improved patient survival from diagnosis, skeletal health has often been a significantly affected, but too often neglected, bystander of this advancing care. Accordingly, optimal management of skeletal health has never been more important. In particular, all women and men initiating hormonal-based therapies (AI therapy in women and ADT in men) should be counseled on the risks for bone loss inherent with therapy. Comprehensive skeletal management plans which reflect these realities must become an essential component of our treatment for all patients with cancer.
Footnotes
Conflict of Interest
MT Drake declares no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Reuss-Borst M, Hartmann U, Scheede C, Weiss J. Prevalence of osteoporosis among cancer patients in Germany: prospective data from an oncological rehabilitation clinic. Osteoporos Int. 2012 Apr;23(4):1437–1444. doi: 10.1007/s00198-011-1724-9. [DOI] [PubMed] [Google Scholar]
- 2.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004 Apr 15;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 3.Khosla S, Atkinson EJ, Melton LJ, 3rd, Riggs BL. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study. J Clin Endocrinol Metab. 1997 May;82(5):1522–1527. doi: 10.1210/jcem.82.5.3946. [DOI] [PubMed] [Google Scholar]
- 4.Khosla S, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998 Jul;83(7):2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 5.Drake MT, Khosla S. Male osteoporosis. Endocrinol Metab Clin North Am. 2012 Sep;41(3):629–641. doi: 10.1016/j.ecl.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spangler L, Yu O, Loggers E, Boudreau DM. Bone mineral density screening among women with a history of breast cancer treated with aromatase inhibitors. J Womens Health (Larchmt) 2013 Feb;22(2):132–140. doi: 10.1089/jwh.2012.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K, Blinder VS, Elkin EB. Cost effectiveness of fracture prevention in postmenopausal women who receive aromatase inhibitors for early breast cancer. J Clin Oncol. 2012 May 1;30(13):1468–1475. doi: 10.1200/JCO.2011.38.7001. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso F, Loibl S, Pagani O, Graziottin A, Panizza P, Martincich L, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012 Dec;48(18):3355–3377. doi: 10.1016/j.ejca.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kassmann H, Piswanger-Solkner JC, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008 Sep;9(9):840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 10.Hershman DL, McMahon DJ, Crew KD, Shao T, Cremers S, Brafman L, et al. Prevention of bone loss by zoledronic acid in premenopausal women undergoing adjuvant chemotherapy persist up to one year following discontinuing treatment. J Clin Endocrinol Metab. 2010 Feb;95(2):559–566. doi: 10.1210/jc.2009-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. 2006 Feb 1;24(4):675–680. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005 Nov 16;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 13.Hadji P, Asmar L, van Nes JG, Menschik T, Hasenburg A, Kuck J, et al. The effect of exemestane and tamoxifen on bone health within the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial: a meta-analysis of the US, German, Netherlands, and Belgium sub-studies. J Cancer Res Clin Oncol. 2011 Jun;137(6):1015–1025. doi: 10.1007/s00432-010-0964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabaglio M, Sun Z, Price KN, Castiglione-Gertsch M, Hawle H, Thurlimann B, et al. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1–98 trial. Ann Oncol. 2009 Sep;20(9):1489–1498. doi: 10.1093/annonc/mdp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings SR, McClung M, Reginster JY, Cox D, Mitlak B, Stock J, et al. Arzoxifene for prevention of fractures and invasive breast cancer in postmenopausal women. J Bone Miner Res. 2011 Feb;26(2):397–404. doi: 10.1002/jbmr.191. [DOI] [PubMed] [Google Scholar]
- 16.Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol. 2013 Apr 10;31(11):1398–1404. doi: 10.1200/JCO.2012.44.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011 Sep 7;103(17):1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 18.Becker T, Lipscombe L, Narod S, Simmons C, Anderson GM, Rochon PA. Systematic review of bone health in older women treated with aromatase inhibitors for early-stage breast cancer. J Am Geriatr Soc. 2012 Sep;60(9):1761–1767. doi: 10.1111/j.1532-5415.2012.04107.x. [DOI] [PubMed] [Google Scholar]
- 19.Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008 Mar 1;26(7):1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 20.Cheung AM, Tile L, Cardew S, Pruthi S, Robbins J, Tomlinson G, et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol. 2012 Mar;13(3):275–284. doi: 10.1016/S1470-2045(11)70389-8. [DOI] [PubMed] [Google Scholar]
- 21.Drake MT, Cremers SC. Bisphosphonate therapeutics in bone disease: the hard and soft data on osteoclast inhibition. Mol Interv. 2010 Jun;10(3):141–152. doi: 10.1124/mi.10.3.5. [DOI] [PubMed] [Google Scholar]
- 22.McCloskey E, Paterson A, Kanis J, Tahtela R, Powles T. Effect of oral clodronate on bone mass, bone turnover and subsequent metastases in women with primary breast cancer. Eur J Cancer. 2010 Feb;46(3):558–565. doi: 10.1016/j.ejca.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Paterson AH, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012 Jul;13(7):734–742. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Londen GJ, Perera S, Vujevich KT, Sereika SM, Bhattacharya R, Greenspan SL. The effect of risedronate on hip structural geometry in chemotherapy-induced postmenopausal women with or without use of aromatase inhibitors: a 2-year trial. Bone. 2010 Mar;46(3):655–659. doi: 10.1016/j.bone.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010 Feb 20;28(6):967–975. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 26.Markopoulos C, Tzoracoleftherakis E, Polychronis A, Venizelos B, Dafni U, Xepapadakis G, et al. Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: results from the ARBI prospective clinical trial. Breast Cancer Res. 2010;12(2):R24. doi: 10.1186/bcr2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011 Oct 13;365(15):1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 28.Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013 Feb;24(2):398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 29.Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012 Mar 1;118(5):1192–1201. doi: 10.1002/cncr.26313. [DOI] [PubMed] [Google Scholar]
- 30.Llombart A, Frassoldati A, Paija O, Sleeboom HP, Jerusalem G, Mebis J, et al. Immediate Administration of Zoledronic Acid Reduces Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women With Early Breast Cancer: 12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer. 2012 Feb;12(1):40–48. doi: 10.1016/j.clbc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Hadji P, Aapro MS, Body JJ, Bundred NJ, Brufsky A, Coleman RE, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol. 2011 Dec;22(12):2546–2555. doi: 10.1093/annonc/mdr017. [DOI] [PubMed] [Google Scholar]
- 32.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Fan M, et al. Effect of denosumab on bone mineral density in women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: subgroup analyses of a phase 3 study. Breast Cancer Res Treat. 2009 Nov;118(1):81–87. doi: 10.1007/s10549-009-0352-y. [DOI] [PubMed] [Google Scholar]
- 33.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010 Dec 10;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 34.Martin M, Bell R, Bourgeois H, Brufsky A, Diel I, Eniu A, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012 Sep 1;18(17):4841–4849. doi: 10.1158/1078-0432.CCR-11-3310. [DOI] [PubMed] [Google Scholar]
- 35.Kilbreath S, Refshauge KM, Beith J, Ward L, Sawkins K, Paterson R, et al. Prevention of osteoporosis as a consequence of aromatase inhibitor therapy in postmenopausal women with early breast cancer: rationale and design of a randomized controlled trial. Contemp Clin Trials. 2011 Sep;32( 5):704–709. doi: 10.1016/j.cct.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Peppone LJ, Mustian KM, Janelsins MC, Palesh OG, Rosier RN, Piazza KM, et al. Effects of a structured weight-bearing exercise program on bone metabolism among breast cancer survivors: a feasibility trial. Clin Breast Cancer. 2010 Jun;10(3):224–229. doi: 10.3816/CBC.2010.n.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waltman NL, Twiss JJ, Ott CD, Gross GJ, Lindsey AM, Moore TE, et al. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: a 24-month randomized controlled trial. Osteoporos Int. 2010 Aug;21(8):1361–1369. doi: 10.1007/s00198-009-1083-y. [DOI] [PubMed] [Google Scholar]
- 38.Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat. 2011 Jun;127(2):447–456. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saarto T, Sievanen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. 2012 May;23(5):1601–1612. doi: 10.1007/s00198-011-1761-4. [DOI] [PubMed] [Google Scholar]
- 40.Brown JE, Sherriff JM, James ND. Osteoporosis in patients with prostate cancer on long-term androgen deprivation therapy: an increasing, but under-recognized problem. BJU Int. 2010 Apr;105(8):1042–1043. doi: 10.1111/j.1464-410X.2010.09251.x. [DOI] [PubMed] [Google Scholar]
- 41.Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007 Jan 1;13(1):241–245. doi: 10.1158/1078-0432.CCR-06-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005 Jan 13;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 43.Yu EY, Kuo KF, Gulati R, Chen S, Gambol TE, Hall SP, et al. Long-term dynamics of bone mineral density during intermittent androgen deprivation for men with nonmetastatic, hormone-sensitive prostate cancer. J Clin Oncol. 2012 May 20;30(15):1864–1870. doi: 10.1200/JCO.2011.38.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alibhai SM, Yun L, Cheung AM, Paszat L. Screening for osteoporosis in men receiving androgen deprivation therapy. Jama. 2012 Jan 18;307(3):255–256. doi: 10.1001/jama.2011.2022. [DOI] [PubMed] [Google Scholar]
- 45.Morgans AK, Smith MR, O’Malley AJ, Keating NL. Bone density testing among prostate cancer survivors treated with androgen-deprivation therapy. Cancer. 2013 Feb 15;119(4):863–870. doi: 10.1002/cncr.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhumkhawala AA, Gleason JM, Cheetham TC, Niu F, Loo RK, Dell RM, et al. Osteoporosis management program decreases incidence of hip fracture in patients with prostate cancer receiving androgen deprivation therapy. Urology. 2013 May;81(5):1010–1015. doi: 10.1016/j.urology.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 47.Choo R, Lukka H, Cheung P, Corbett T, Briones-Urbina R, Vieth R, et al. Randomized, double-blinded, placebo-controlled, trial of risedronate for the prevention of bone mineral density loss in nonmetastatic prostate cancer patients receiving radiation therapy plus androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2013 Apr 1;85(5):1239–1245. doi: 10.1016/j.ijrobp.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Greenspan SL, Nelson JB, Trump DL, Wagner JM, Miller ME, Perera S, et al. Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapy. J Clin Oncol. 2008 Sep 20;26(27):4426–4434. doi: 10.1200/JCO.2007.15.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klotz LH, McNeill IY, Kebabdjian M, Zhang L, Chin JL. A phase 3, double-blind, randomised, parallel-group, placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: the cancer and osteoporosis research with alendronate and leuprolide (CORAL) study. Eur Urol. 2013 May;63(5):927–935. doi: 10.1016/j.eururo.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001 Sep 27;345(13):948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 51.Bhoopalam N, Campbell SC, Moritz T, Broderick WR, Iyer P, Arcenas AG, et al. Intravenous zoledronic acid to prevent osteoporosis in a veteran population with multiple risk factors for bone loss on androgen deprivation therapy. J Urol. 2009 Nov;182(5):2257–2264. doi: 10.1016/j.juro.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 52.Campbell SC, Bhoopalam N, Moritz TE, Pandya M, Iyer P, Vanveldhuizen P, et al. The use of zoledronic acid in men receiving androgen deprivation therapy for prostate cancer with severe osteopenia or osteoporosis. Urology. 2010 May;75(5):1138–1143. doi: 10.1016/j.urology.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 53.Serpa Neto A, Tobias-Machado M, Esteves MA, Senra MD, Wroclawski ML, Fonseca FL, et al. Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2012 Mar;15(1):36–44. doi: 10.1038/pcan.2011.4. [DOI] [PubMed] [Google Scholar]
- 54.Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009 Aug 20;361(8):745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012 Jan 7;379(9810):39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011 Mar 5;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith MR, Morton RA, Barnette KG, Sieber PR, Malkowicz SB, Rodriguez D, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2013 Jan;189(1 Suppl):S45–50. doi: 10.1016/j.juro.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Smith MR, Malkowicz SB, Brawer MK, Hancock ML, Morton RA, Steiner MS. Toremifene decreases vertebral fractures in men younger than 80 years receiving androgen deprivation therapy for prostate cancer. J Urol. 2011 Dec;186(6):2239–2244. doi: 10.1016/j.juro.2011.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TL, Ke C, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2012 Sep 10;30(26):3271–3276. doi: 10.1200/JCO.2011.38.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clegg A, Barber S, Young J, Forster A, Iliffe S. The Home-Based Older People’s Exercise (HOPE) trial: study protocol for a randomised controlled trial. Trials. 2011;12:143. doi: 10.1186/1745-6215-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Culos-Reed SN, Robinson JW, Lau H, Stephenson L, Keats M, Norris S, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Support Care Cancer. 2010 May;18(5):591–599. doi: 10.1007/s00520-009-0694-3. [DOI] [PubMed] [Google Scholar]
- 62.Datta M, Schwartz GG. Calcium and vitamin D supplementation during androgen deprivation therapy for prostate cancer: a critical review. Oncologist. 2012;17(9):1171–1179. doi: 10.1634/theoncologist.2012-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alibhai SM, Mohamedali HZ, Gulamhusein H, Panju AH, Breunis H, Timilshina N, et al. Changes in bone mineral density in men starting androgen deprivation therapy and the protective role of vitamin D. Osteoporos Int. 2013 Apr 6; doi: 10.1007/s00198-013-2343-4. [DOI] [PubMed] [Google Scholar]
- 64.Ganguly S, Divine CL, Aljitawi OS, Abhyankar S, McGuirk JP, Graves L. Prophylactic use of zoledronic acid to prevent early bone loss is safe and feasible in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation. Clin Transplant. 2012 May-Jun;26(3):447–453. doi: 10.1111/j.1399-0012.2011.01527.x. [DOI] [PubMed] [Google Scholar]
- 65.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008 Mar 15;111(6):2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melton LJ, 3rd, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2005 Mar;20(3):487–493. doi: 10.1359/JBMR.041131. [DOI] [PubMed] [Google Scholar]
- 67.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996 Feb 22;334(8):488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 68.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001 Sep-Oct;7(5):377–387. [PubMed] [Google Scholar]
- 69.Melton LJ, 3rd, Rajkumar SV, Khosla S, Achenbach SJ, Oberg AL, Kyle RA. Fracture risk in monoclonal gammopathy of undetermined significance. J Bone Miner Res. 2004 Jan;19(1):25–30. doi: 10.1359/JBMR.0301212. [DOI] [PubMed] [Google Scholar]
- 70.Ng AC, Khosla S, Charatcharoenwitthaya N, Kumar SK, Achenbach SJ, Holets MF, et al. Bone microstructural changes revealed by high-resolution peripheral quantitative computed tomography imaging and elevated DKK1 and MIP-1alpha levels in patients with MGUS. Blood. 2011 Dec 15;118(25):6529–6534. doi: 10.1182/blood-2011-04-351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pepe J, Petrucci MT, Mascia ML, Piemonte S, Fassino V, Romagnoli E, et al. The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int. 2008 Jun;82(6):418–426. doi: 10.1007/s00223-008-9145-2. [DOI] [PubMed] [Google Scholar]
- 72.Berenson JR, Yellin O, Boccia RV, Flam M, Wong SF, Batuman O, et al. Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res. 2008 Oct 1;14(19):6289–6295. doi: 10.1158/1078-0432.CCR-08-0666. [DOI] [PubMed] [Google Scholar]
- 73.Lim JS, Jin SH, Kim SB, Lee JI. Effect of bisphosphonates on bone mineral density and fracture prevention in gastric cancer patients after gastrectomy. J Clin Gastroenterol. 2012 Sep;46(8):669–674. doi: 10.1097/MCG.0b013e31824f1af4. [DOI] [PubMed] [Google Scholar]
- 74.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007 Oct;22(10):1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 75.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010 Nov;25(11):2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 76.Khosla S, Bilezikian JP, Dempster DW, Lewiecki EM, Miller PD, Neer RM, et al. Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metab. 2012 Jul;97(7):2272–2282. doi: 10.1210/jc.2012-1027. [DOI] [PubMed] [Google Scholar]


