Abstract
Screening for critical congenital heart disease (CCHD) using pulse oximetry has been endorsed by the American Academy of Pediatrics and the American Heart Association. We sought to determine the incidence of undetected CCHD in Tennessee and the diagnostic gap of CCHD in Middle Tennessee prior to screening implementation. The Tennessee Initiative for Perinatal Quality Care (TIPQC) Undetected CCHD Registry is a quality improvement initiative established to identify neonates discharged from the nursery with undetected CCHD. The TIPQC database was queried and a simultaneous review of all neonates with CCHD in the Middle Tennessee region was performed to define the incidence and identify the pre-screen diagnostic gap of undetected CCHD at the time of hospital discharge. In 2011, of 79,462 live births in Tennessee, 12 newborns had undiagnosed CCHD (incidence 15 per 100,000; 95 % CI 9–26 per 100,000). Nine of 12 (75 %) had coarctation of the aorta (CoA). There were no deaths due to undiagnosed CCHD. In the Middle Tennessee region, 6 of 45 neonates with CCHD were missed, for a diagnostic gap of 13 % (95 % CI 6–26 %). Prior to implementation of CCHD screening using pulse oximetry, 12 Tennessee neonates with CCHD were missed by prenatal ultrasound and newborn examination. CoA was the most common lesion missed and is also the CCHD most likely to be missed despite addition of screening using pulse oximetry. Continued evaluation of the diagnostic gap with particular attention to missed diagnoses of CoA should accompany institution of CCHD screening programs.
Keywords: Coarctation of the aorta, Congenital heart disease, Newborn screening, Pulse oximetry
Introduction
Congenital heart disease (CHD) occurs in 7 to 9 of 1,000 live births in the United States [3, 19] One sixth to one fourth of these infants have critical congenital heart disease (CCHD), defined as severe and life-threatening disease requiring surgical or catheter intervention in the first month of life [25]. Congenital malformations are a leading cause of infant death in the United States, with CHD responsible for more infant deaths than any other congenital anomaly [26].
Due to advances in preoperative care and surgical technique, most congenital heart defects can be repaired or palliated, and mortality secondary to CHD has declined [2]. For infants with CCHD, intervention is commonly performed in the first few weeks of life to stabilize the circulation and to prevent end-organ damage. Delayed diagnosis places the infant at risk for hemodynamic compromise due to hypoxia, acidosis, and shock. Poor preoperative condition correlates with a poor operative outcome [4]. In addition, delayed diagnosis is associated with hypoperfusion, leading to end-organ damage including possible hypoxic/ischemic brain injury [13].
Prenatal diagnosis and postnatal clinical evaluation are imperfect in identifying all infants with CCHD, and a significant proportion of affected newborns remain undetected. This “diagnostic gap,” or the percentage of neonates with undetected CCHD at the time of hospital discharge, has been estimated at 25 % [21, 25].
Strategies for early diagnosis of CCHD have increasingly been evaluated because timely recognition of CCHD is known to improve outcome. Because subclinical hypoxemia is common in many forms of CHD, pulse oximetry has been proposed and studied for routine use in screening infants for CCHD [8, 11, 13, 18].
In 2011, the Department of Health and Human Services’ Secretary added CCHD screening to the Recommended Uniform Screening Panel. This recommendation has been endorsed by the American Academy of Pediatrics (AAP) and the American Heart Association (AHA) [14].
In 2012, the Tennessee General Assembly passed a bill directing the state’s Genetic Advisory Committee to develop a screening program for CCHD with the use of pulse oximetry [17]. Anticipating the implementation of routine newborn screening for CCHD, we sought to determine the prescreening incidence of undetected CCHD in the state of Tennessee and to determine the percentage of neonates falling into the diagnostic gap of CCHD in Middle Tennessee.
Materials and Methods
Statewide Data Collection
We performed a prospective statewide review of Tennessee neonates born between 1 January and 31 December 2011 who presented after nursery discharge with previously undiagnosed CCHD via a query of the Tennessee Initiative for Perinatal Quality Care (TIPQC) Undetected Critical Congenital Heart Disease Registry. This registry was facilitated by TIPQC, a statewide quality improvement collaborative established in 2008 and funded by the Tennessee Department of Health, to identify newborns discharged from the nursery with undiagnosed CCHD. To achieve statewide data collection, TIPQC promoted collaboration between pediatric cardiologists and neonatologists across the state [22].
De-identified information collected by the TIPQC registry included the neonate’s diagnosis, age at diagnosis, presenting symptoms, type of nursery caring for the infant after birth (level 1 newborn nursery vs neonatal intensive care unit), and outcome. For the purpose of the registry, CCHD was defined as severe and life-threatening CHD requiring either surgical or catheter-based intervention in the first month of life. Reportable lesions included ductal-dependent lesions and lesions resulting in hypoxia. The specific lesions targeted are presented in Table 1. Acyanotic and non–ductal-dependent congenital heart defects requiring semi-elective surgical repair (tetralogy of Fallot without cyanosis, atrioventricular septal defect, atrial septal defect, ventricular septal defect, and patent ductus arteriosus) were excluded from the study.
Table 1.
Lesions targeted by the Tennessee Initiative for Perinatal Quality Care Undetected Critical Congenital Heart Disease Registry
| Ductal-Dependent Systemic Circulation |
| Critical aortic valve stenosis |
| Critical coarctation of the aorta |
| Interrupted aortic arch |
| Hypoplastic left heart syndrome |
| Ductal-Dependent Pulmonary Circulation |
| Critical pulmonary stenosis |
| Pulmonary atresia |
| Tetralogy of Fallot with cyanosis |
| Total anomalous pulmonary venous return |
| Transposition of the great arteries |
| Tricuspid atresia |
| Truncus arteriosus |
| Other complex congenital heart defects |
| Resulting in hypoxia with single-ventricle physiology |
Statewide death certificate data from 2011 also were reviewed by the state birth defects’ epidemiologist to ascertain whether any deaths in the first year of life were attributable to undetected CCHD. To determine the incidence, the numerator of infants with undetected CCHD was compared with a denominator of all live births statewide, including out-of-hospital births, provided by the Tennessee Department of Health [20].
Middle Tennessee Data Collection
A simultaneous prospective chart review of all Middle Tennessee neonates with CCHD also was undertaken. The purpose of the Middle Tennessee data collection was twofold. First, we sought to define the diagnostic gap of CCHD in Middle Tennessee. The “diagnostic gap,” as defined by Riede et al. [21], is the percentage of neonates with CCHD who are undiagnosed at the time of hospital discharge. Second, we re-examined the timing of CCHD diagnosis in Middle Tennessee to corroborate our previous research, which concluded that 49–66 % of infants with CCHD are prenatally diagnosed [9, 24].
The Middle Tennessee population was chosen because it comprises more than 2 million residents and encompasses one major metropolitan area, two pediatric cardiology groups, and one pediatric cardiothoracic surgery center, making case ascertainment feasible. Also, Middle Tennessee is composed of 40 counties, with both rural and urban medical centers. Middle Tennessee residency was determined by the mother’s home county.
The primary inclusion criterion was diagnosis of a cardiac defect requiring surgical or catheter-based intervention within the first month of life. Those infants with functionally univentricular hearts also were included regardless whether they required intervention within the first 30 days of life or not because they need frequent cardiology follow-up and close monitoring.
All Middle Tennessee infants with lesions mirroring those reportable to the statewide Undetected Critical Congenital Heart Disease Registry were tracked (Table 1). Review of cardiology admissions and consultations, neonatal intensive care unit admissions, pediatric intensive care unit admissions, and emergency department records was performed to identify cases with a diagnosis of CCHD. Only live births were included in the Middle Tennessee dataset.
The study was performed with approval of the Institutional Review Board. Study data were collected and managed using REDCap electronic data capture tools (Vanderbilt University, Nashville, TN) [7]. Descriptive statistics were used to evaluate registry data. The Statistical Package for Social Sciences, version 19 was used in data analysis (SPSS, Chicago, IL, USA), and Confidence Interval Analysis, version 2.2.0 was used to calculate confidence intervals (CIs).
Results
Statewide Incidence of Undetected CCHD
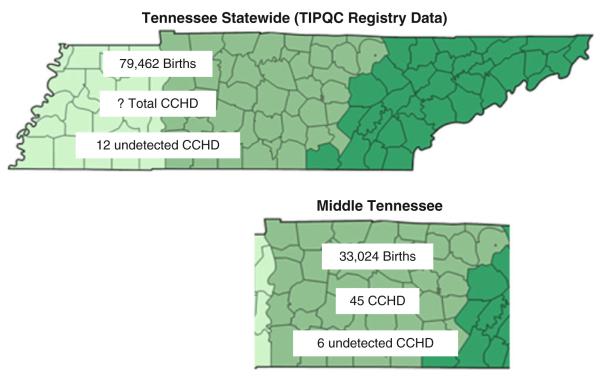
In 2011, before newborn screening for CCHD using pulse oximetry, 12 newborns (age, ≤30 days) were reported with undiagnosed CCHD out of 79,462 live births in the State of Tennessee, for an incidence of 15 per 100,000 (95 % CI, 9–26/100,000) (Fig. 1). No infant deaths attributable to undiagnosed CCHD were identified.
Fig. 1.
Comparison of statewide data and the Middle Tennessee cohort. In 2011, the state of Tennessee had 79,462 live births, which included 12 neonates who had undetected critical congenital heart disease (CCHD) diagnosed within the first 30 days of life. The total number of infants with CCHD in the state is not known. In the Grand Division of Middle Tennessee, there were a total of 33,024 live births. Of these, 45 were diagnosed with CCHD. Six of the Middle Tennessee infants with CCHD were undetected by prenatal evaluation or neonatal physical examination
Of these 12 newborns, 9 (75 %) had coarctation of the aorta (CoA), 1 had hypoplastic left heart syndrome, 1 had total anomalous pulmonary venous return, and 1 had tetralogy of Fallot with severe pulmonic stenosis (Table 2). All 12 infants were cared for after birth in a level 1 newborn nursery. One of the neonates (case 9), had undergone an echocardiogram in the newborn nursery for murmur and was diagnosed with atrial septal defect and ventricular septal defect. This newborn was discharged home from the nursery but presented to the emergency department at 10 days of age in shock. Repeat echocardiogram confirmed CoA. All 12 neonates recovered well after surgical or catheter-based intervention and ultimately were discharged home.
Table 2.
Results of the 2011 TIPQC Undetected Critical Congenital Heart Disease Registry
| Case | Gender | Congenital heart disease | Age at diagnosis (days) |
Location of presentation | Symptoms at presentation |
|---|---|---|---|---|---|
| 1 | Male | Hypoplastic left heart syndrome | 3 | PCP’s office, referred for NICU admission |
Tachypnea, cyanosis, acidosis |
| 2a | Male | Total anomalous pulmonary venous return |
9 | PCP’s office, referred to emergency department |
Murmur, cyanosis |
| 3a | Female | Tetralogy of Fallot, severe pulmonary stenosis |
14 | PCP’s office, referred to cardiology clinic |
Murmur, cyanosis |
| 4a | Male | Coarctation of the aorta, bicuspid aortic valve |
7 | PCP’s office, referred for outpatient echo |
Murmur |
| 5 | Male | Coarctation of the aorta, bicuspid aortic valve |
7 | Emergency department | Poor feeding, respiratory distress |
| 6 | Male | Coarctation of the aorta, ventricular septal defect |
8 | PCP’s office, referred for outpatient echo |
Murmur |
| 7 | Male | Coarctation of the aorta | 9 | Emergency department | Shock |
| 8 | Female | Coarctation of the aorta, ventricular septal defect |
9 | Emergency department | Tachypnea |
| 9 | Male | Coarctation of the aorta, ventricular septal defect |
10 | Emergency department | Respiratory distress, acidosis |
| 10a | Male | Coarctation of the aorta, ventricular septal defect |
14 | Emergency department | Shock |
| 11a | Female | Coarctation of the aorta, bicuspid aortic valve |
23 | PCP’s office, referred to cardiology clinic |
Murmur, tachypnea |
| 12a | Male | Coarctation of the aorta, bicuspid aortic valve |
30 | Emergency department | Respiratory distress |
TIPQC Tennessee Initiative for Perinatal Quality Care, PCP primary care provider, NICU neonatal intensive care unit
Included in the Middle Tennessee cohort
An additional four infants were reported statewide who did not meet our strict inclusion criteria of requiring intervention within the first month of life. These infants presented at 33–63 days of age with potentially critical lesions, and all required early intervention. Of the four older infants who had undiagnosed CCHD, one had tetralogy of Fallot with severe pulmonary stenosis and presented with murmur and cyanosis. The remaining three infants had CoA and were hemodynamically stable at presentation.
Middle Tennessee Diagnostic Gap
In Middle Tennessee, CCHD was diagnosed for 45 neonates at the age of 30 days or younger from a total of 33,024 live births (incidence, 13.6 per 10,000 live births; 95 % CI, 10.1–18.2 per 10,000). Six of these neonates were discharged from the nursery with undiagnosed CCHD, for a diagnostic gap in Middle Tennessee of 13 % (95 % CI, 6–26 %). These neonates represented 6 of the 12 infants identified by the statewide TIPQC Undetected Critical Congenital Heart Disease Database (Table 2, denoted by a). Of the 45 Middle Tennessee neonates, 26 (58 %; 95 % CI, 43–71 %) were diagnosed prenatally, and 13 (29 %; 95 % CI, 18–43 %) were diagnosed in the newborn nursery or neonatal intensive care unit due to symptoms (Table 3).
Table 3.
2011 Middle Tennessee critical congenital heart disease: timing of diagnosis by lesion
| Lesion | Total | Prenatal diagnosis |
Postnatal clinical diagnosis |
Diagnosis after initial discharge |
Percentage with prenatal diagnosis |
|---|---|---|---|---|---|
| Coarctation of the aorta | 10 | 3 | 3 | 4 | 30 |
| Double-inlet left ventricle | 2 | 2 | 0 | 0 | 100 |
| Double-outlet right ventricle | 1 | 1 | 0 | 0 | 100 |
| Ebstein’s anomaly | 3 | 3 | 0 | 0 | 100 |
| Hypoplastic left heart syndromea | 10a | 7 | 3a | 0 | 70 |
| Interrupted aortic arch | 1 | 1 | 0 | 0 | 100 |
| Pulmonary atresia with intact ventricular septum | 1 | 1 | 0 | 0 | 100 |
| Single ventricle, NOS | 1 | 1 | 0 | 0 | 100 |
| Total anomalous pulmonary venous return | 3 | 0 | 2 | 1 | 0 |
| Tetralogy of Fallot with pulmonary atresia or critical pulmonary stenosis |
6 | 2 | 3 | 1 | 33 |
| Transposition of the great arteries | 2 | 0 | 2 | 0 | 0 |
| Tricuspid atresia | 4 | 4 | 0 | 0 | 100 |
| Truncus arteriosus | 1 | 1 | 0 | 0 | 100 |
| Total | 45 | 26 | 13 | 6 | 57 |
NOS not otherwise specified
Includes two patients with shone complex who underwent single-ventricle palliation
Discussion
This year-long, prospective statewide review of the TIPQC Undetected CCHD Registry documented an undetected CCHD incidence of 15 per 100,000 live births. Coarctation of the aorta was the most commonly missed diagnosis, accounting for 75 % of those with undetected CCHD.
This is the first study to define the incidence of undetected critical congenital heart defects prospectively in a large US population. Several recent retrospective studies of statewide hospital records and death registry data have variously reported the incidence of undetected or missed CCHDs as 4 to 9 per 100,000 live births [1, 12, 16]. Our undetected CCHD incidence of 15/100,000 is higher than in these retrospective reviews. This difference is likely related to differences in inclusion criteria (e.g., the age at death or hospital readmission and the specific lesions included) and in the mode of case identification (retrospective use of International Classification of Diseases [ICD] codes and/or death certificate data vs our prospective data collection). Consistent with our findings, critical left-sided obstructive lesions were the most common cause of hospital readmission or death related to undetected CCHD in the retrospective studies.
Delayed and missed diagnoses increase infant morbidity and mortality [4, 13]. As we have shown, undetected lesions comprise a significant proportion of all CCHD cases.
In 2009, the AHA and the AAP recommended that additional studies in large populations be conducted to determine whether screening for CCHD with the use of pulse oximetry should be implemented in the United States. Since the 2009 AHA and AAP statement, several large European multicenter prospective studies and a recent metaanalysis on the impact of screening for CCHD using pulse oximetry have been reported [5, 6, 21, 23]. These studies demonstrate a low false-positive rate (0.1–0.8 %), fair sensitivity (58–77 %), and high specificity (99 %), supporting the hypothesis that using pulse oximetry to screen for CCHD improves detection.
With mounting evidence confirming the potential benefits of CCHD screening via pulse oximetry, the Department of Health and Human Services’ Secretary followed the recommendation of the Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children and added CCHD to the Recommended Uniform Screening Panel. This recommendation has been endorsed by the AHA and the AAP [14].
The target population for CCHD screening is the subset of infants falling into the diagnostic gap (those with undetected CCHD at the time of hospital discharge). The goal of screening for CCHD is to reduce or eliminate this gap.
Inconsistent definitions of CCHD make it inherently difficult to determine the success of screening with pulse oximetry and to compare the recent European studies with the Tennessee newborn population. In their scientific statement, the AHA and AAP used a broad definition of CCHD that included all lesions requiring surgical or catheter intervention within the first year of life [13]. Defining missed cases up to a year may include lesions that do not have potential for acute neonatal deterioration.
The seven AHA and AAP targets for CCHD screening using pulse oximetry (hypoplastic left heart syndrome, pulmonary atresia, tetralogy of Fallot, total anomalous pulmonary venous return, transposition of the great arteries, tricuspid atresia, and truncus arteriosus) do not precisely align with the lesions targeted in the large, multicenter European trials [10]. For example, de-Wahl Granelli et al. [5] targeted all ductal-dependent lesions, including aortic stenosis and CoA, but excluded tetralogy of Fallot. Using definitions very similar to those used in our study, the series by Ewer et al. [6] and a recent meta-analysis by Thangaratinam et al. [23] defined CCHD as lesions resulting in death or requiring surgery within the first 28 days of life.
In addition to the varied definitions of CCHD and the different lesions targeted by pulse oximetry screening, the diagnostic gap before pulse oximetry screening spans a large range, which is likely related to differences in prenatal diagnosis and neonatal care. In large studies of CCHD screening using pulse oximetry, the prenatal detection rate ranged from 3.3 to 60 %, and the diagnostic gap ranged from 16 to 26 % [5, 6, 15, 21].
In the setting of a low prenatal detection rate (3.3 %) due to the absence of routine fetal echocardiography, the diagnostic gap in the study by de-Wahl Granelli et al. [5] was 26 % in a region not screened with pulse oximetry versus 8 % in the region screened with pulse oximetry. In the study by Riede et al. [21] with a prenatal diagnosis rate of 60 %, the diagnostic gap of 20 % before pulse oximetry screening was reduced to 4.4 % with the use of pulse oximetry. The effectiveness of pulse oximetry in these studies has been primarily by detection of cyanotic lesions, including total anomalous pulmonary venous return and transposition of the great arteries, as well as lesions with ductal dependent pulmonary blood flow.
In contrast, the lesions with a false-negative screen were predominantly left-heart obstructive lesions. In the study by de-Wahl Granelli et al. [5] five infants with false-negative CCHD pulse oximetry screen results were discharged with undetected CCHD, three of whom had CoA. The remaining two infants had interrupted aortic arch. In the study by Riede et al. [21] three of four infants with false-negative screens had CoA. In the study by Ewer et al. [6], both infants with undetected CCHD had CoA (one with associated transposition of the great arteries and ventricular septal defect), and an additional three infants presented with CoA after the age of 28 days. Therefore, despite the institution of CCHD screening using pulse oximetry, up to 40 % of infants with CoA will remain undetected [6, 13, 9].
In Middle Tennessee, with a prenatal diagnosis rate of 58 %, the diagnostic gap of CCHD was 13 % (95 % CI, 6–26 %). Although this is less than the 16 to 26 % noted in large studies of CCHD screening using pulse oximetry, it is not significantly different and remains a clinically significant proportion of neonates with CCHD. Because our study confirms that CoA is the most common CCHD missed by prenatal ultrasound and neonatal physical examination, it is concerning that CoA also is the lesion most likely to be missed by pulse oximetry. Although CoA is not one of the seven primary targets of CCHD screening using pulse oximetry, it remains a critical defect that can be missed by prenatal and neonatal examination. This fact needs to be emphasized during the education of providers who participate in newborn CCHD screening programs.
Study Limitations
The primary limitation of our study in evaluating statewide data of undetected CCHD was voluntary registry reporting. Although we were in collaboration with pediatric cardiologists and neonatologists across the state, it is possible that a case was not reported. State death certificate data were queried to identify potential deaths secondary to undetected CCHD, but it is possible that a baby with CCHD could have died without a diagnosis at autopsy.
We were unable to collect data prospectively on all infants with CCHD in the state of Tennessee and thus were unable to obtain a diagnostic gap for the entire state. However, the vast majority of infants with CCHD born in the large Middle Tennessee region are referred to the region’s only pediatric cardiothoracic surgical center for care, and we used this data set to determine the missed percentage. It is possible that prenatal diagnosis could have resulted in referral to an out-of-state institution for birth and surgical palliation, which would have affected case ascertainment in the Middle Tennessee data set.
Conclusion
The establishment of the TIPQC Undetected CCHD Registry allowed the data collection necessary to determine the incidence of undetected CCHD in Tennessee before implementation of statewide CCHD screening using pulse oximetry. In 2011, 12 neonates with CCHD were missed by prenatal ultrasound and newborn examination in Tennessee. Fortunately, there were no adverse outcomes prior to diagnosis. The most common diagnosis in these neonates was CoA, which is also the lesion most likely to be missed by CCHD screening using pulse oximetry. Thus, a crucial part of the institution of CCHD screening is educating providers that the lesions most likely to be missed by prenatal and neonatal evaluation may also be missed by pulse oximetry.
This study highlights a crucial component to implementation of any screening program, which is defining the baseline incidence of the targeted disease. As many states prepare to implement universal newborn screening for CCHD using pulse oximetry, comprehensive and prospective study to examine the pre- and postscreen incidence of undetected CCHD should be considered. Accurate incidence of disease detection before and after universal CCHD screening is necessary for evaluation of screening efficacy and determination of the risk–benefit ratio.
Acknowledgments
The first author’s fellowship training is supported by the T32HL 105334 Grant from the National Institutes of Health. The study was supported in part by REDCap database grant UL1 TR000445 from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health and Vanderbilt CTSA grant UL1 RR024975 from the National Center for Research Resources at the National Institutes of Health.
Abbreviations
- AAP
Academy of Pediatrics
- AHA
American Heart Association
- CHD
Congenital heart disease
- CCHD
Critical congenital heart disease
- CI
Confidence interval
- CoA
Coarctation of the aorta
- TIPQC
Tennessee Initiative for Perinatal Quality Care
Appendix.
The contributors to the TIPQC Undetected CCHD Registry are Drs Mark E. Anderson, Rajani Anand, H. Scott Baldwin, Jean Ballweg, William Devoe, Casilda Hermo, Jeffory Jennings, Michael Liske, Dennis Stokes, and Nisha Surenderanath. We thank Dr. Mouledoux’s mentor and manuscript editor, Dr. Stacy Killen; TIPQC Medical Director, Dr. Peter Grubb; TIPQC Project Director, Brenda Barker; and State of Tennessee Births Defects Epidemiologist, Dr. David Law.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
This research was conducted and manuscript compiled on behalf of the Tennessee Initiative for Perinatal Quality Care Undetected Critical Congenital Heart Disease Registry.
Registry contributors are listed in the Appendix.
Contributor Information
Jessica H. Mouledoux, Division of Pediatric Cardiology, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Vanderbilt Medical Center, Nashville, TN, USA
William F. Walsh, Division of Neonatology, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Vanderbilt Medical Center, Nashville, TN, USA
References
- 1.Aamir T, Kruse L, Ezeakudo O. Delayed diagnosis of critical congenital cardiovascular malformations (CCVM) and pulse oximetry screening of newborns. Acta Paediatr. 2007;96:1146–1149. doi: 10.1111/j.1651-2227.2007.00389.x. doi:10.1111/j.1651-2227.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 2.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–2381. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 3.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107:e32–e32. doi: 10.1542/peds.107.3.e32. doi:10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- 4.Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, Bull C. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart. 2006;92:1298–1302. doi: 10.1136/hrt.2005.078097. doi:10.1136/hrt.2005.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganas L, Eriksson M, Segerdahl N, Agren A, Ekman-Joelsson BM, Sunnegardh J, Verdicchio M. Impact of pulse oximetry screening on the detection of duct-dependent congenital heart disease: a Swedish prospective screening study in 39 821 newborns. BMJ. 2009;338:a3037–a3037. doi: 10.1136/bmj.a3037. doi:10.1136/bmj.a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewer AK, Middleton LJ, Furmston AT, Bhoyar A, Daniels JP, Thangaratinam S, Deeks JJ, Khan KS. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet. 2011;378:785–794. doi: 10.1016/S0140-6736(11)60753-8. doi:10.1016/S0140-6736(11)60753-8. [DOI] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. doi:10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoke TR, Donohue PK, Bawa PK, Mitchell RD, Pathak A, Rowe PC, Byrne BJ. Oxygen saturation as a screening test for critical congenital heart disease; a preliminary study. Pediatr Cardiol. 2002;23:403–409. doi: 10.1007/s00246-002-1482-8. [DOI] [PubMed] [Google Scholar]
- 9.Israel SW, Roofe LR, Saville BR, Walsh WF. Improvement in antenatal diagnosis of critical congenital heart disease implications for postnatal care and screening. Fetal Diagn Ther. 2011;30:180–183. doi: 10.1159/000327148. doi:10.1159/000327148. [DOI] [PubMed] [Google Scholar]
- 10.Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow WR, Kelm K, Pearson GD, Glidewell J, Grosse SD, Howell RR. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128:e1259–e1267. doi: 10.1542/peds.2011-1317. doi:10.1542/peds.2011-1317. [DOI] [PubMed] [Google Scholar]
- 11.Koppel RI, Druschel CM, Carter T, Goldberg BE, Mehta PN, Talwar R, Bierman FZ. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics. 2003;111:451–455. doi: 10.1542/peds.111.3.451. [DOI] [PubMed] [Google Scholar]
- 12.Liske MR, Greeley CS, Law DJ, Reich JD, Morrow WR, Baldwin HS, Graham TP, Strauss AW, Kavanaugh-McHugh AL, Walsh WF. Report of the Tennessee Task Force on Screening Newborn Infants for Critical Congenital Heart Disease. Pediatrics. 2006;118:e1250–e1256. doi: 10.1542/peds.2005-3061. doi:10.1542/peds.2005-3061. [DOI] [PubMed] [Google Scholar]
- 13.Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, Gidding SS, Beekman RH, III, Grosse SD. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the AHA and AAP. Pediatrics. 2009;124:823–836. doi: 10.1542/peds.2009-1397. doi:10.1542/peds.2009-1397. [DOI] [PubMed] [Google Scholar]
- 14.Mahle WT, Martin GR, Beekman RH, III, Morrow WR. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129:190–192. doi: 10.1542/peds.2011-3211. doi:10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- 15.Meberg A, Andreassen A, Brunvand L, Markestad T, Moster D, Nietsch L, Silberg IE, Skalevik JE. Pulse oximetry screening as a complementary strategy to detect critical congenital heart defects. Acta Paediatr. 2009;98:682–686. doi: 10.1111/j.1651-2227.2008.01199.x. doi:10.1111/j.1651-2227.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 16.Ng B, Hokanson J. Missed congenital heart disease in neonates. Congenit Heart Dis. 2010;5:292–296. doi: 10.1111/j.1747-0803.2010.00418.x. doi:10.1111/j.1747-0803.2010.00418.x. [DOI] [PubMed] [Google Scholar]
- 17.Overbey D, Haynes R, translators. 107th General Assembly. Tennessee Code Annotated, Spring; Nashville, TN. Tennessee Office of Legal Services; 2012. HB 0373/SB 0065. [Google Scholar]
- 18.Reich JD, Connolly B, Bradley G, Littman S, Koeppel W, Lewycky P, Liske M. The reliability of a single pulse oximetry reading as a screening test for congenital heart disease in otherwise asymptomatic newborn infants. Pediatr Cardiol. 2008;29:885–889. doi: 10.1007/s00246-008-9214-3. doi:10.1007/s00246-008-9214-3. [DOI] [PubMed] [Google Scholar]
- 19.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153:807–813. doi: 10.1016/j.jpeds.2008.05.059. doi:10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Accessed 13 March 2013];Report of Tennessee Births 2011. http://health.state.tn.us/statistics/birth.htm.
- 21.Riede FT, Worner C, Dahnert I, Mockel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine: results from a prospective multicenter study. Eur J Pediatr. 2010;169:975–981. doi: 10.1007/s00431-010-1160-4. doi:10.1007/s00431-010-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed 6 Aug 2012];Tennessee Initiative for Perinatal Quality Care. http://tipqc.org/
- 23.Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379:2459–2464. doi: 10.1016/S0140-6736(12)60107-X. doi:10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]
- 24.Walsh W. Evaluation of pulse oximetry screening in Middle Tennessee: cases for consideration before universal screening. J Perinatol. 2011;31:125–129. doi: 10.1038/jp.2010.70. doi:10.1038/jp.2010.70. [DOI] [PubMed] [Google Scholar]
- 25.Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008;93:F33–F35. doi: 10.1136/adc.2007.119032. doi:10.1136/adc.2007.119032. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: final data for 2007. vol 58. US Department of Health and Human Services DoVS; 2010. 2010. [PubMed] [Google Scholar]