Abstract
Background
Estimated glomerular filtration rate (eGFR) and albuminuria are central for diagnosis, staging, and risk evaluation in chronic kidney disease (CKD). Universal thresholds regardless of age, sex, and race are recommended, but relatively little is known about how these demographic factors alter the relationship of eGFR and albuminuria to cardiovascular outcomes.
Study Design
Observational cohort study.
Setting & Participants
11,060 whites and blacks aged 52–75 years in the Atherosclerosis Risk in Communities (ARIC) Study with median follow-up of 11.2 years.
Predictors
eGFR by the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation (reference, 95 ml/min/1.73 m2) and urinary albumin-creatinine ratio (ACR) (reference, at 5 mg/g).
Outcomes
Cardiovascular events (coronary disease, stroke, and heart failure) and all-cause mortality.
Measurements
Adjusted HRs associated with eGFR and ACR in subgroups according to age, sex and race.
Results
Cardiovascular risk significantly increased at eGFR <70 ml/min/1.73 m2 in all subgroups according to age (< 65 vs. ≥65 years), sex, and race (P for interaction >0.2 for these subgroups; e.g., at eGFR 30 ml/min/1.73 m2, the adjusted HR was 2.19 [95% CI, 1.10–4.35] at age 52–64 years vs. 2.23 [95% CI, 1.33–3.72] at age 65–75 years). Results were similar for mortality. Log(ACR) was linearly associated with cardiovascular risk without threshold effects in all subgroups, with some quantitative interactions. HRs according to ACR tended to be lower in men vs. women (e.g., at ACR 40 mg/g, 1.18 [95% CI, 0.98–1.41] vs. 1.77 [95% CI, 1.45–2.15]) and in older vs. younger population (1.24 [95% CI, 1.04–1.49] vs. 1.73 [95% CI, 1.42–2.12]) (P for interaction <0.01 for sex and age). Less evident interactions were observed for mortality.
Limitations
Single measurement of eGFR with creatinine and ACR and relatively narrow age range.
Conclusions
The associations of eGFR and ACR with cardiovascular events were largely similar, with some quantitative interactions, among age, sex, and racial subgroups, generally supporting universal thresholds of GFR and ACR for CKD definition/staging.
INDEX WORDS: Chronic kidney disease, estimated glomerular filtration rate (eGFR), urinary albumin-creatinine ratio (ACR), cardiovascular disease, all-cause mortality
Chronic kidney disease (CKD) is a common condition that elevates the risk of adverse outcomes including cardiovascular disease.1 CKD is defined as either a decrease in kidney function (glomerular filtration rate [GFR] <60 ml/min/1.73 m2) or presence of kidney damage (usually represented by urinary albumin excretion ≥30 mg/day).2 Once CKD is defined, CKD stages are determined by the level of GFR and albuminuria.2 Although universal thresholds of these kidney measures are usually used for CKD definition and staging, some propose age-, sex-, and race-specific cut-points.3,4 However, little is actually known regarding whether these demographic characteristics modify the associations of GFR and albuminuria with clinical outcomes.
Regarding sex and race, most of previous studies have focused on sex or racial disparities in prognosis among the CKD population5–12 but not on potential effect modification of sex or race on the CKD-risk relationship.13 In terms of age, recent metaanalyses have shown that, although relative risk is slightly lower in older individuals than younger individuals, the pattern of relationship between kidney measures and cardiovascular mortality is largely consistent across age categories.1,14,15 Nevertheless, mortality can be affected by the healthcare system or different treatments across demographic subgroups, and thus it is preferable to investigate cardiovascular disease, including nonfatal events, to assess pathophysiological interaction. Also, to our knowledge, no studies have assessed potential effect modifications by age, sex, and race in the same study population.
Therefore, the objective of this study was to investigate whether the contribution of low eGFR and high albuminuria to increased risk of cardiovascular disease is consistent among subgroups by age, sex, and race in a community-based cohort, with implications for demographic-specific thresholds of GFR and albuminuria for defining and staging CKD. Since the new CKD guidelines recommend using both GFR and albuminuria categories for risk classification,2 we also evaluated the demographic interactions in the context of combined categories of GFR and albuminuria.
METHODS
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort study of 15,792 individuals between the ages of 45 and 64 years at baseline, recruited from four communities in the United States (Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland). The first examination was conducted during 1987–89, and then three follow-up examinations were conducted approximately every three years (visit 2, 1990–92; visit 3, 1993–95; and visit 4, 1996–98). Visit 4 was the only visit at which both serum creatinine and albuminuria were measured and was the baseline for the present study. Of 11,656 participants who attended visit 4, we excluded participants reporting race other than white or black (n = 30), missing values of either kidney measure (n = 215) or covariates (n = 351), leaving a final study population of 11,060 participants.
Exposures Measurement
GFR is estimated by the CKD Epidemiology Collaboration (CKD-EPI) equation using age, gender, race and serum creatinine.16,17 As recommended in clinical guidelines,2 albuminuria was assessed as urinary albumin-creatinine ratio (ACR) from spot urine sample. Urinary albumin was measured by a nephelometric method either on the Dade Behring BN 100 or the Beckman Nephelometer.
Covariates
The study participants provided comprehensive demographic, risk factor, and medical history information to a trained interviewer at each clinical examination. Blood pressure was taken twice with a random-zero sphygmomanometer by a certified technician and recorded as the average. Hypertension was defined as systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications. Height and weight were measured with the participants wearing a scrub suit and no shoes, and then body mass index (BMI) was calculated as weight (kg) divided by square of height (m). Smoking was categorized as current vs. former/never smoker. Total cholesterol was determined using enzymatic methods. Diabetes was defined as fasting blood glucose ≥126 mg/dl, non-fasting blood glucose ≥200 mg/dl, or a self-reported history of or treatment for diabetes. History of cardiovascular disease included history of coronary heart disease (CHD), stroke, and heart failure (HF). History of CHD and stroke at visit 4 was defined as self-reported history of CHD or stroke at visit 1 or any adjudicated events between visits 1 and 4. Prevalent HF was defined as self-reported treatment for HF or the Gothenburg stage 3, a status with dyspnea due to cardiac causes and under treatment with digitalis or loop diuretics,18,19 at visit 1 and hospitalization for HF between visits 1 and 4.
Outcomes Assessment
To maximize statistical power for assessing interactions, our primary outcome was composite cardiovascular disease including CHD, stroke and HF. We also analyzed specific cardiovascular disease separately. All-cause mortality was also investigated since cardiovascular disease is the leading cause of death in the United States.20 ARIC investigators conduct continuous, comprehensive surveillance for all cardiovascular disease-related hospitalizations and deaths in the four communities. All potential cardiovascular events are reviewed, and CHD and stroke are adjudicated by groups of experts.21–23 CHD events were defined as a definite or probable myocardial infarction, definite coronary death, or coronary revascularization procedure. Stroke was defined as sudden or rapid onset of neurologic symptoms lasting for 24h or leading to death in the absence of another cause22,23 and included definite or probable cases. HF was defined as hospitalization or death from HF with the International Classification of Diseases Code, Ninth Revision (ICD-9) 428 or Tenth Revision (ICD-10) I50 in any position on the hospital discharge list or the death certificate.24 These outcomes from visit 4 to December 31, 2008 were analyzed in the present study.
Statistical Analysis
Our potential effect modifiers of interest were age, sex, and race (white vs. black). We dichotomized age at 65 years old, a threshold for older individuals applied in various clinical guidelines.25,26 Baseline characteristics were compared between subgroups determined by these potential effect modifiers. Continuous and categorical variables were compared between the subgroups using analysis of variance and chi-square tests, as appropriate. We defined follow-up time as the elapsed time to the first outcome during follow-up, loss to follow-up, or December 31, 2008 when administratively censored.
Cox proportional hazards models were used to quantify the association between eGFR and ACR with the outcomes in each subgroup according to age, sex, and race. Cox models were adjusted for age, sex, race, history of cardiovascular disease, smoking, systolic blood pressure, diabetes, total cholesterol, BMI, and eGFR or ACR, as appropriate. We first modeled eGFR and ACR as continuous variables with linear splines (knots at each 15 ml/min/1.73 m2 from 30 to 105 ml/min/1.73 m2 for eGFR and knots at 10, 30, and 300 mg/g for ACR).1,17 Based on previous literature,1,15 ACR was log-transformed. To evaluate interaction, models with and without product terms between kidney measures and dichotomized potential effect modifiers were tested. Using the models with the product terms, we computed the hazard ratios (HRs) for eGFR and ACR with the reference as eGFR 95 ml/min/1.73 m2 and ACR 5 mg/g within each subgroup. The eGFR reference value was selected at the lower range of optimal level of GFR (≥90 ml/min/1.73 m2)2 (but not at the knots for splines) due to higher mortality risk at high eGFR in previous studies.27 ACR 5 mg/g was arbitrarily chosen at the middle point of ACR category <10 mg/g, which includes approximately half of the healthy population.28 We evaluated the multiplicative interaction as the ratio of these HRs between subgroups at each point of eGFR (1 ml/min/1.73 m2 increment) and ACR (8% increment) (“point-wise interaction”). Overall interaction was tested based on a likelihood ratio test comparing the models with and without the product terms. To appreciate the main effect of age, gender, and race on cardiovascular and mortality risk, adjusted HRs in subgroups were also obtained using a single reference of eGFR and ACR in reference groups (young individuals, women, and whites). These adjusted HRs were used to present adjusted average incidence rates by combining with average incidence rates in the reference group at the range including reference point of eGFR (90–104 ml/min/1.73m2) and ACR (<10 mg/g). These adjusted average incidence rates allowed us to assess additive interaction in absolute scale.15 Standard errors for the comparison to the reference were calculated using the delta method and used for significance testing.29 We also tested multiplicative interaction based on clinical categories of the combination of eGFR (<30, 30–44, 45–59, 60–89, and ≥90 ml/min/1.73 m2) and ACR (<30, 30–299, and ≥300 mg/g).2 A p-value <0.05 (2-tailed) was considered statistically significant. Statistical analysis was performed using Stata 12 (StatCorp LP, College Station, TX).
RESULTS
Participant Characteristics
The mean age of our study population was 62.8 ± 5.7 (standard deviation) years. Of 11,060 participants, 6,178 (55.8%) were female and 2,428 (22.0%) were black (Table 1). As anticipated, older adults (65–75 years) and men were more likely to have comorbidities such as history of cardiovascular disease, hypertension, and diabetes as compared to their counterparts. In contrast, younger participants and women had higher BMI. Black participants were more likely to have hypertension, diabetes, and obesity compared with white participants. Lower eGFR was observed in older and white participants, while eGFR was comparable between men and women. Higher ACR was seen in older adults, women, and blacks. The higher prevalence of comorbidities in the older population, men, and blacks was reflected in a higher incidence of cardiovascular disease and mortality as compared to their counterparts (Table 2). One exception was higher incidence of CHD in whites as compared to blacks. As previously reported,30 the overall correlation between eGFR and log-ACR was weak (r=−0.13), and 538 (4.9%) and 721 (6.5%) of the participants met the definition of CKD by reduced GFR or high albuminuria alone, respectively (199 [1.8%] met both) (Table S1, available as online supplementary material).
Table 1.
Baseline Characteristics by age, sex, and race
| Overall | Age | Sex | Race | ||||
|---|---|---|---|---|---|---|---|
| 52–64 y | 65–75 y | Female | Male | White | Black | ||
| No. of participants | 11,060 | 6,674 | 4,386 | 6,178 | 4,882 | 8632 | 2428 |
| Age (y) | 62.8 ± 5.7 | 58.9 ± 2.6 | 68.8 ±3.2 | 62.5 ± 5.6 | 63.2 ± 5.7*** | 63.1 ± 5.6 | 61.8 ± 5.7*** |
| Female sex | 55.8% | 58.1% | 52.5%*** | 100% | 0% | 53.5% | 64.3%*** |
| Black race | 22.0% | 24.6% | 17.9%*** | 25.3% | 17.7%*** | 0% | 100% |
| History of CVD | 13.9% | 11.2% | 18.0%*** | 10.3% | 18.5%*** | 13.6% | 14.9% |
| Current smoker | 14.9% | 16.9% | 11.8%*** | 14.0% | 16.0%*** | 14.0% | 17.7%*** |
| Antihypertensive medication | 43.4% | 38.6% | 50.6%*** | 43.6% | 43.1% | 39.0% | 58.8%*** |
| Systolic BP (mmHg) | 127.6 ± 19.0 | 124.8 ± 18.1 | 131.8 ± 19.0*** | 128.1 ± 19.6 | 126.9 ± 18.2** | 125.8 ± 18.4 | 133.6 ± 19.9*** |
| Diastolic BP (mmHg) | 71.0 ± 10.3 | 72.2 ± 10.1 | 69.2 ± 10.4*** | 70.1 ± 10.3 | 72.2 ± 10.2*** | 69.7 ± 9.9 | 75.8 ± 10.5*** |
| Hypertension | 47.4% | 42.0% | 55.5%*** | 49.0% | 45.4%*** | 42.0% | 66.6%*** |
| Diabetes | 16.6% | 15.5% | 18.5%*** | 15.6% | 17.9%** | 13.9% | 26.4%*** |
| Total Cholesterol (mmol/L) | 5.2 ± 1.0 | 5.2 ± 1.0 | 5.2 ± 0.9 | 5.4 ± 1.0 | 5.0 ± 0.9*** | 5.2 ± 1.0 | 5.2 ± 1.0 |
| Body Mass Index (kg/m2) | 28.8 ± 5.6 | 29.1 ± 5.8 | 28.3 ± 5.4*** | 29.1 ± 6.3 | 28.4 ± 4.5*** | 28.3 ± 5.2 | 30.6 ± 6.4*** |
| eGFR (mL/min/1.73m2) | 84.1 ± 15.5 | 87.9 ± 14.8 | 78.3 ± 14.8*** | 84.5 ± 15.7 | 83.6 ± 15.2** | 82.6 ± 13.9 | 89.6 ± 19.5*** |
| ACR (mg/g) | 3.7 [1.8–7.8] | 3.4 [1.6–6.8] | 4.4* [2.0–10.0] | 4.3 [1.4–7.3] | 3.1 *[2.1–8.3] | 2.7 [0.8–8.9] | 4.0***[2.0–7.7] |
eGFR: estimated glomerular filtration rate; ACR: albumin-creatinine ratio. BP, blood pressure; CVD, cardiovascular disease;
Note: Values for categorical variables are given as percentages; values for continuous variables are given as mean ± standard deviation or median [interquartile range]. p-values were generated by ANOVA or Chi-square test, as appropriate.
p<0.05;
p<0.01;
p<0.001
Table 2.
Number of events and incidence rate by age, sex, and race
| Overall | Age | Sex | Race | ||||
|---|---|---|---|---|---|---|---|
| 52–64 y | 65–75 y | Female | Male | White | Black | ||
| No. of participants | 11060 | 6674 | 4386 | 6,178 | 4,882 | 8,632 | 2428 |
| Composite CVD | |||||||
| No. of events | 2607 | 1212 | 1395 | 1088 | 1519 | 1988 | 622 |
| IR (/1000 person-y) | 24.6 | 18.1 | 35.7*** | 17.4 | 35.0*** | 23.9 | 26.9** |
| CHD | |||||||
| No. of events | 1579 | 772 | 807 | 540 | 1039 | 1282 | 297 |
| IR (/1000 person-y) | 14.3 | 11.2 | 19.5*** | 8.3 | 22.9*** | 15.0 | 12.1** |
| Stroke | |||||||
| No. of events | 553 | 223 | 330 | 277 | 276 | 373 | 180 |
| IR (/1000 person-y) | 4.8 | 3.1 | 7.5*** | 4.2 | 5.6** | 4.1 | 7.2*** |
| Heart Failure | |||||||
| No. of events | 1284 | 508 | 776 | 607 | 677 | 931 | 353 |
| IR (/1000 person-y) | 11.3 | 7.2 | 18.3*** | 9.4 | 14.0*** | 10.5 | 14.4*** |
| All-cause mortality | |||||||
| No. of events | 1842 | 695 | 1147 | 774 | 1068 | 1373 | 469 |
| IR (/1000 person-y) | 15.7 | 9.6 | 25.6*** | 11.6 | 21.2*** | 15.0 | 18.3*** |
CVD: cardiovascular disease; CHD: coronary heart disease; IR, incidence rate
Note: p-values were generated by Chi-square test.
p<0.05
p<0.01
p<0.001
eGFR and Cardiovascular Risk in Age, Sex, and Race Subgroups
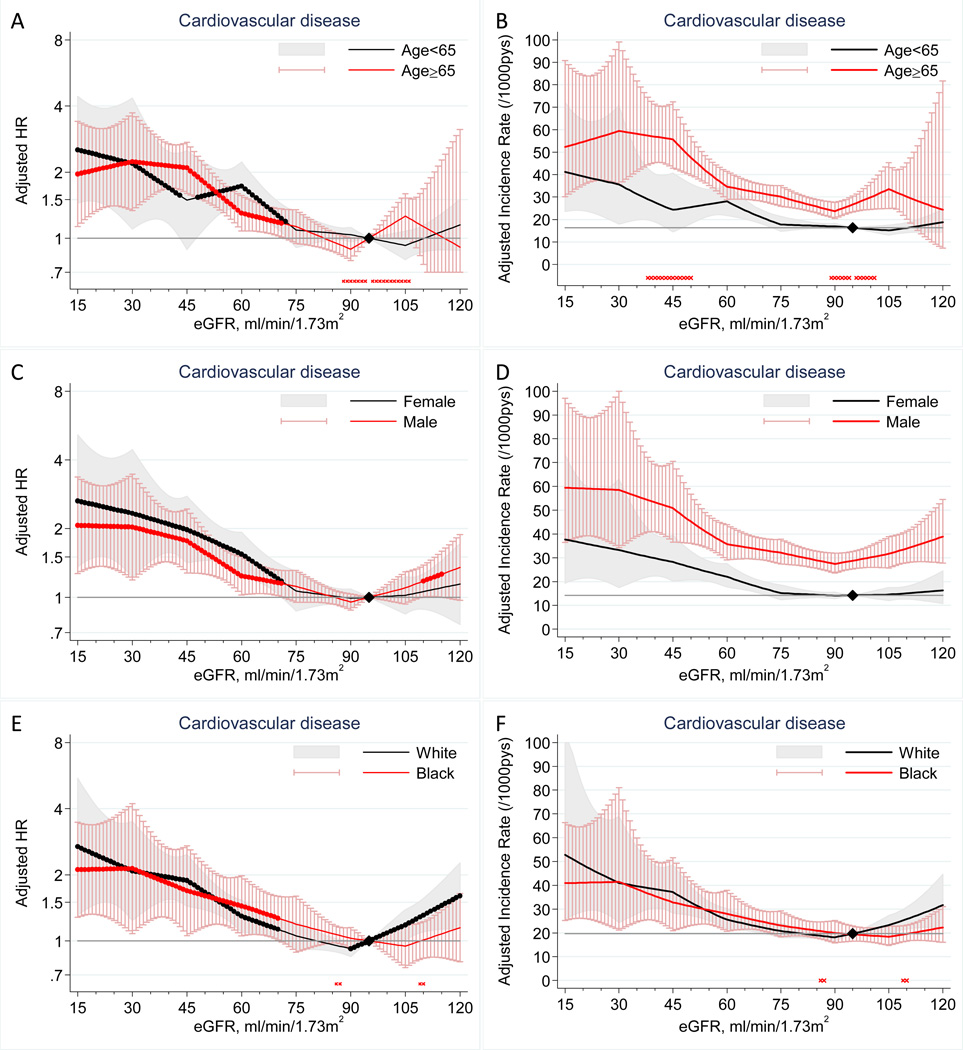
Lower eGFR was associated with increased risk of cardiovascular disease independently of potential confounders and ACR in all subgroups tested (Figure 1A–F). The lowest cardiovascular risk appeared to be in the range of eGFR 90–105 ml/min/1.73 m2 in all subgroups, with higher risk at eGFR above this range (most evident in whites). Cardiovascular risk according to low eGFR started to become significant at an almost identical level in all of the demographic subgroups (i.e., ~70 ml/min/1.73m2) (Figure 1A, C, E). The risk gradient for cardiovascular disease was similar between age (Figure 1A) and racial (Figure 1E) subgroups but was slightly shallower in men than in women (Figure 1C). However, we did not observe statistically significant point-wise or overall (P=0.7) interactions between sex and eGFR. Similar relative risk gradient was translated to significantly higher incidence rate at eGFR 38–50 ml/min/1.73m2 among older individuals compared to younger individuals (average cardiovascular incidence rate difference per 1,000 person-years at eGFR 45 versus 95 ml/min/1.73m2 was 29.0 vs. 8.0, respectively; p=0.03) given their higher risk at the reference eGFR range. We did not observe major additive interaction for sex and race (Figure 1D and F).
Figure 1.
Adjusted hazard ratios (left panels) and adjusted incidence rates (right panels) and 95% CIs (shaded areas or whisker plots) of cardiovascular disease according to eGFR with 95 ml/min/1.73 m2 as a reference (diamond) in each subgroup (left panels) and in the reference subgroup (right panels) of age (A–B), sex (C–D), and race (E–F). Dots on each plotted line represent statistical significance (P<0.05). Stars placed just above the y axis represent significant point-wise interactions (P<0.05) between two subgroups in multiplicative (left panels) and additive (right panels) scale (absence of stars indicates no significant point-wise interaction). Adjustments were for age, sex, race, smoking, history of cardiovascular disease, systolic blood pressure, diabetes, total cholesterol oncentration, bo index, and log-ACR.
Similar findings were observed when specific cardiovascular disease was analyzed separately, with somewhat steeper risk gradient for HF than CHD and stroke (Figure S1 and S2). We also observed largely similar findings for all-cause mortality (Figure S3A–F). In contrast to similar cardiovascular risk between whites and blacks, blacks tended to have higher mortality risk compared with whites (Figure S3F). Nevertheless, eGFR-mortality association did not significantly differ between these two races for both relative and absolute risk (Figure S3E–F).
ACR and Cardiovascular Risk in Age, Sex, and Race Subgroups
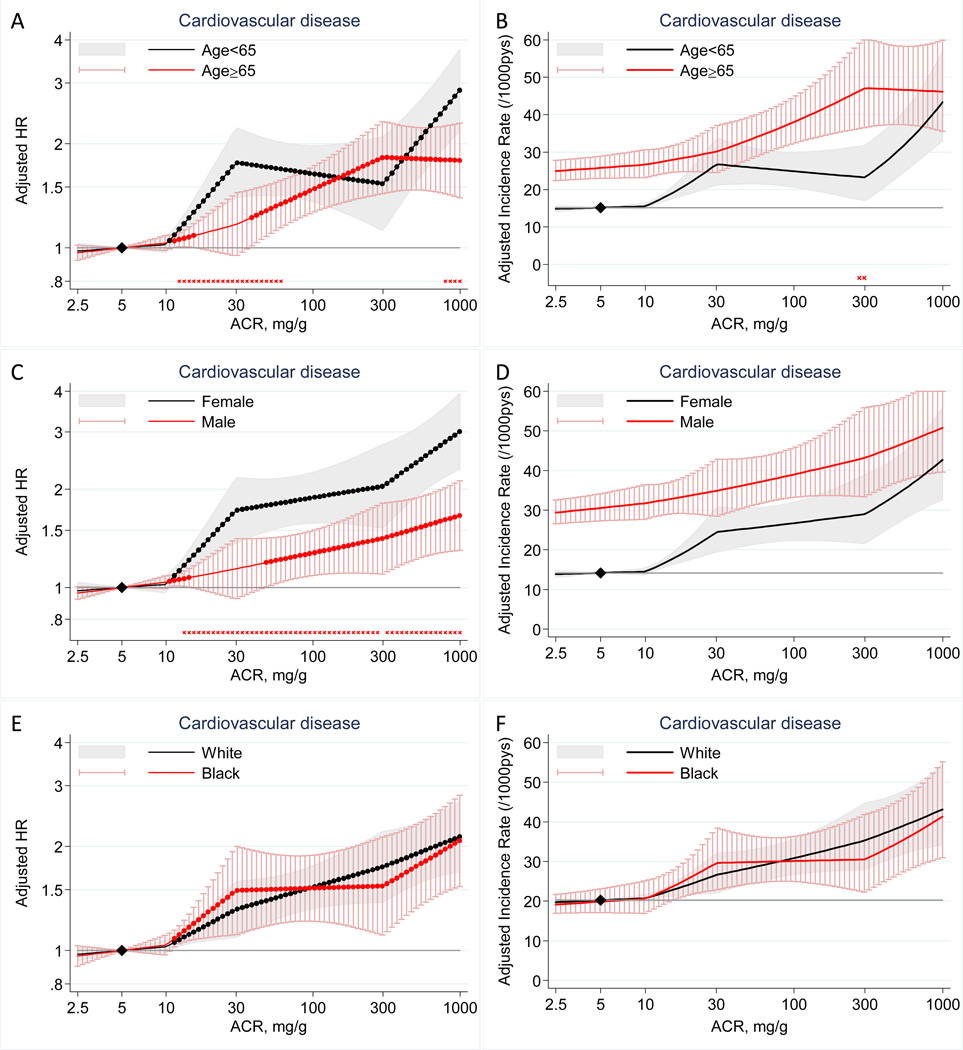
There was largely an independent linear relationship between log(ACR) and cardiovascular risk in each subgroup (Figure 2A–F). We observed some point-wise multiplicative interactions with age for cardiovascular disease (lower HR according to high ACR in older than in younger individuals) in some ACR ranges (12–60 mg/g and 794–1000 mg/g) (Figure 2A), leading to significant overall interaction (P=0.002). HR of cardiovascular disease according to high ACR was significantly lower in men than in women at a broad range of ACR ≥13 mg/g (overall P for interaction <0.001) (Figure 2C) The significantly lower relative risk according to high ACR in older individuals and men were canceled out in absolute scale by their higher baseline risk compared to their counter parts (Figure 2B, D).The cardiovascular risk according to high ACR was similar between whites and blacks in both multiplicative and additive scale (Figure 2E–F).
Figure 2.
Adjusted hazard ratios (left panels) and adjusted incidence rates (right panels) and 95% CIs (shaded areas or whisker plots) of cardiovascular disease according to ACR with 5 mg/g as a reference (diamond) in each subgroup (left panels) and in the reference subgroup (right panels) of age (A–B), sex (C–D), and race (E–F). Dots on each plotted line represent statistical significance (P<0.05). Stars placed just above the y axis represent significant point-wise interactions (P<0.05) between two subgroups in multiplicative (left panels) and additive (right panels) scale (absence of stars indicates no significant point-wise interaction). Adjustments were for age, sex, race, smoking, history of cardiovascular disease, systolic blood pressure, diabetes, total cholesterol concentration, body mass index, and eGFR splines.
When we looked at specific cardiovascular diseases (Figure S4 and S5), we observed increased risk of CHD according to high ACR in women but not in men (overall P for interaction=0.002) (Figure S4D). This interaction was significant at a broad range of high ACR in additive scale as well (Figure S5D). Additionally, the risk gradient was significantly shallower for HF in blacks compared to whites in both multiplicative (overall P for interaction=0.04) and additive scale (Figure S4I and S5I).
The risk gradient was very similar between age subgroups for all-cause mortality in relative scale (Figure S6A), translated to significant additive interaction at higher ACR ranges (average cardiovascular incidence rate difference per 1,000 person-years at ACR 100 versus 5 mg/g was 14.3 among older participants vs. 7.2 among younger participants p=0.02), given higher baseline risk among older individuals. The range of significant sex-ACR interaction was more restricted (12–65 mg/g) for all-cause mortality, and the overall multiplicative interaction did not reach significance (P=0.2) (Figure S6C). The mortality risk according to high ACR did not significantly differ between blacks and whites in both relative and absolute scale (Figure 6E–F).
Joint Categories of eGFR and ACR and Cardiovascular Risk in Age, Sex, and Race Subgroups
We subsequently investigated the joint effect of eGFR and ACR on cardiovascular disease in the demographic subgroups (Table 3). Cardiovascular risk largely increased multiplicatively along with lower eGFR and higher ACR in all subgroups. Indeed, the interactions between eGFR and ACR were not significant in all subgroups (P>0.05). Furthermore, there were no significant three-way interactions of eGFR, ACR, and the demographic variables (P>0.09). Of importance, GFR category 3a (45–59 ml/min/1.73 m2) without elevated albuminuria (ACR <30 mg/g) contributed to increased cardiovascular risk in each subgroup including older adults. Similar findings were observed for all-cause mortality (Table 4).
Table 3.
Hazard ratios of cardiovascular disease for categories of eGFR and ACR by subgroups according to age, sex, and race
| Age Subgroups | ||||||||
|---|---|---|---|---|---|---|---|---|
| eGFR category | Age < 65 y | Age ≥ 65 y | ||||||
| ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | |
| ≥90 | 1.00 (reference) | 1.49 (1.14–1.96) | 3.34 (2.20–5.08) | 1.00 (reference) | 1.00 (reference) | 1.45 (1.04–2.01) | 2.98 (1.65–5.36) | 1.00 (reference) |
| 60–89 | 1.17 (1.03–1.33) | 1.62 (1.19–2.21) | 4.60 (2.89–7.32) | 1.17 (1.04–1.31) | 1.09 (0.94–1.26) | 1.36 (1.06–1.74) | 1.56* (1.01–2.40) | 1.04 (0.91–1.19) |
| 45–59 | 1.43 (0.99–2.05) | 3.68 (2.16–6.27) | 3.80 (1.41–10.21) | 1.62 (1.21–2.17) | 1.57 (1.27–1.96)0 | 2.51 (1.82–3.45) | 2.82 (1.67–4.76) | 1.56 (1.29–1.87) |
| 30–44 | 0.78 (0.19–3.12) | 3.27 (1.54–6.92) | 4.39 (1.95–9.87) | 1.65 (0.98–2.77) | 2.04 (1.36–3.06) | 3.40 (1.89–6.09) | 7.00 (4.19–11.68) | 2.29 (1.71–3.08) |
| <30 | 4.04 (0.57–28.84) | -- | 5.77 (3.37–9.86) | 2.37 (1.38–4.07) | 1.96 (0.48–7.91) | 2.21 (0.70–6.93) | 4.12 (2.03–8.37) | 1.68 (0.95–2.97) |
| All | 1.00 (reference) | 1.50 (1.24–1.82) | 3.12 (2.37–4.09) | -- | 1.00 (reference) | 1.38 (1.18–1.62) | 2.00* (1.56–2.56) | -- |
| Sex Subgroups | ||||||||
| eGFR category | Female | Male | ||||||
| ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | |
| ≥90 | 1.00 (reference) | 1.64 (1.20–2.23) | 3.87 (2.45–6.13) | 1.00 (reference) | 1.00 (reference) | 1.28 (0.96–1.71) | 2.36 (1.42–3.92) | 1.00 (reference) |
| 60–89 | 1.08 (0.93–1.25) | 1.79 (1.35–2.38) | 2.92 (1.85–4.62) | 1.06 (0.92–1.21) | 1.05 (0.93–1.19) | 1.13* (0.88–1.46) | 1.64 (1.06–2.54) | 1.02 (0.91–1.15) |
| 45–59 | 1.48 (1.12–1.94) | 3.26 (2.17–4.89) | 3.97 (1.96–8.07) | 1.59 (1.27–2.00) | 1.39 (1.11–1.76) | 2.01 (1.41–2.88) | 2.28 (1.25–4.17) | 1.38 (1.13–1.68) |
| 30–44 | 1.68 (0.96–2.94) | 4.66 (2.54–8.54) | 8.94 (4.41–18.14) | 2.34 (1.63–3.38) | 1.83 (1.09–3.08) | 2.01 (1.00–4.07) | 4.66 (2.72–8.00) | 1.79 (1.27–2.53) |
| <30 | 1.67 (0.41–6.72) | 5.23 (0.73–37.29) | 5.38 (2.65–10.89) | 2.11 (1.14–3.89) | 4.68 (0.65–33.49) | 1.37 (0.34–5.52) | 4.41 (2.58–7.55) | 1.74 (1.05–2.89) |
| All | 1.00 (reference) | 1.77 (1.48–2.13) | 3.15 (2.41–4.12) | -- | 1.00 (reference) | 1.18* (1.00–1.39) | 1.92* (1.50–2.46) | -- |
| Race Subgroups | ||||||||
| eGFR category | White | Black | ||||||
| ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | |
| ≥90 | 1.00 (reference) | 1.46 (1.12–1.92) | 3.32 (2.06–5.35) | 1.00 (reference) | 1.00 (reference) | 1.43 (1.02–2.00) | 2.92 (1.79–4.77) | 1.00 (reference) |
| 60–89 | 1.01 (0.90–1.12) | 1.25 (0.99–1.57) | 1.72 (1.14–2.59) | 0.98 (0.88–1.08) | 1.26* (1.04–1.53) | 1.69 (1.20–2.38) | 3.01 (1.83–4.97) | 1.23* (1.04–1.47) |
| 45–59 | 1.39 (1.14–1.70) | 2.26 (1.64–3.12) | 3.06 (1.82–5.15) | 1.41 (1.19–1.68) | 1.51 (1.03–2.22) | 2.89 (1.78–4.71) | 2.12 (0.79–5.74) | 1.55 (1.15–2.10) |
| 30–44 | 1.52 (0.99–2.34) | 3.34 (1.83–6.09) | 6.41 (3.76–10.95) | 1.92 (1.42–2.60) | 3.22 (1.43–7.29) | 2.76 (1.36–5.62) | 4.98 (2.44–10.19) | 2.24 (1.43–3.49) |
| <30 | 1.99 (0.64–6.20) | 1.24 (0.17–8.87) | 5.76 (2.97–11.16) | 2.03 (1.16–3.56) | -- | 2.56 (0.63–10.39) | 4.50 (2.56–7.92) | 1.87 (1.09–3.22) |
| All | 1.00 (reference) | 1.39 (1.20–1.62) | 2.38 (1.89–3.01) | -- | 1.00 (reference) | 1.42 (1.15–1.76) | 2.33 (1.74–3.13) | -- |
Note: eGFRs are given in mL/min/1.73 m ; ACRs in mg/g. Associations given as hazard ratio (95% confidence interval).
Abbreviations: eGFR: estimated glomerular filtration rate; ACR: albumin-creatinine ratio.
significant interaction comparing HRs in the same category between subgroups based on coefficients for product terms of each potential effect modifier and categories of eGFR and ACR.
Bold indicates statistically significant as compared to reference category
-- indicates no event
Table 4.
Hazard ratios of all-cause mortality for categories of eGFR and ACR by subgroups according to age, sex, and race
| Age Subgroups | ||||||||
|---|---|---|---|---|---|---|---|---|
| eGFR category | Age < 65 y | Age ≥ 65 y | ||||||
| ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | |
| ≥90 | 1.00 (reference) | 1.75 (1.25, 2.45) | 3.00 (1.74, 5.17) | 1.00 (reference) | 1.00 (reference) | 1.32 (0.91, 1.91) | 3.34 (1.76, 6.35) | 1.00 (reference) |
| 60–89 | 1.03 (0.87, 1.23) | 2.15 (1.51, 3.06) | 3.95 (2.25, 6.94) | 1.06 (0.91, 1.24) | 1.04 (0.88, 1.22) | 1.41 (1.08, 1.86) | 2.70 (1.76, 4.13) | 1.04 (0.90, 1.21) |
| 45–59 | 1.91 (1.24, 2.95) | 3.33 (1.64, 6.73) | 3.08 (0.76, 12.42) | 1.84 (1.28, 2.65) | 1.45 (1.14, 1.84) | 2.80 (1.99, 3.94) | 2.11 (1.12, 4.01) | 1.47 (1.20, 1.80) |
| 30–44 | 2.71 (0.87, 8.47) | 6.49 (2.88, 14.63) | 8.07 (3.57, 18.23) | 3.28 (1.94, 5.56) | 1.68 (1.04, 2.73) | 8.25 (5.01, 13.59) | 5.16 (2.94, 9.06) | 2.42 (1.76, 3.32) |
| <30 | -- | -- | 6.23 (3.46, 11.20) | 2.35 (1.27, 4.33) | 8.34 (2.65, 26.23) | 7.10 (2.62, 19.29) | 6.86 (3.50, 13.46) | 3.60 (2.12, 6.13) |
| All | 1.00 (reference) | 1.93 (1.53, 2.43) | 2.85 (2.02, 4.00) | -- | 1.00 (reference) | 1.58 (1.33, 1.87) | 2.26 (1.72, 2.95) | -- |
| Sex Subgroups | ||||||||
| eGFR category | Female | Male | ||||||
| ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | |
| ≥90 | 1.00 (reference) | 1.38 (0.93, 2.04) | 2.41 (1.27, 4.57) | 1.00 (reference) | 1.00 (reference) | 1.51 (1.09, 2.09) | 3.48 (2.02, 6.01) | 1.00 (reference) |
| 60–89 | 0.97 (0.81, 1.15) | 1.56 (1.11, 2.20) | 3.01 (1.82, 4.97) | 1.00 (0.85, 1.17) | 0.92 (0.79, 1.07) | 1.39 (1.06, 1.82) | 2.40 (1.52, 3.80) | 0.91 (0.79, 1.05) |
| 45–59 | 1.42 (1.04, 1.94) | 3.16 (2.00, 4.97) | 1.23 (0.39, 3.87) | 1.45 (1.11, 1.90) | 1.22 (0.94, 1.59) | 2.01 (1.35, 3.01) | 2.64 (1.35, 5.15) | 1.21 (0.97, 1.51) |
| 30–44 | 1.91 (1.04, 3.51) | 8.34 (4.74, 14.67) | 6.12 (2.87, 13.07) | 2.85 (1.95, 4.18) | 1.51 (0.80, 2.85) | 5.01 (2.65, 9.45) | 4.52 (2.52, 8.10) | 1.95 (1.34, 2.84) |
| <30 | 5.92 (1.88, 18.59) | 8.05 (1.13, 57.57) | 6.16 (2.88, 13.17) | 3.22 (1.72, 6.04) | -- | 4.84 (1.54, 15.27) | 6.22 (3.61, 10.72) | 2.52 (1.50, 4.24) |
| All | 1.00 (reference) | 1.76 (1.43, 2.18) | 2.33 (1.69, 3.23) | -- | 1.00 (reference) | 1.58 (1.32, 1.89) | 2.46 (1.87, 3.24) | -- |
| Race Subgroups | ||||||||
| eGFR category | White | Black | ||||||
| ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | ACR < 30 | ACR = 30–299 | ACR ≥ 300 | Overall | |
| ≥90 | 1.00 (reference) | 1.52 (1.09, 2.11) | 3.09 (1.69, 5.68) | 1.00 (reference) | 1.00 (reference) | 1.39 (0.95, 2.04) | 2.81 (1.59, 4.99) | 1.00 (reference) |
| 60–89 | 0.93 (0.81, 1.07) | 1.48 (1.15, 1.90) | 2.31 (1.49, 3.57) | 0.93 (0.82, 1.06) | 0.96 (0.76, 1.20) | 1.37 (0.91, 2.06) | 3.39 (1.98, 5.79) | 0.98 (0.80, 1.20) |
| 45–59 | 1.27 (1.01, 1.61) | 2.06 (1.40, 3.01) | 2.28 (1.17, 4.45) | 1.24 (1.01, 1.52) | 1.40 (0.92, 2.12) | 3.35 (2.04, 5.48) | 1.58 (0.50, 4.98) | 1.50 (1.08, 2.07) |
| 30–44 | 1.34 (0.79, 2.25) | 7.76 (4.60, 13.08) | 4.46 (2.50, 7.96) | 2.01 (1.45, 2.79) | 4.85* (2.14, 11.03) | 4.97 (2.43, 10.17) | 6.53 (3.04, 14.01) | 3.30 (2.08, 5.23) |
| <30 | 4.15 (1.33, 12.96) | 6.15 (1.53, 24.83) | 10.48 (5.39, 20.40) | 4.18 (2.41, 7.27) | -- | 4.97 (1.22, 20.23) | 4.83 (2.67, 8.74) | 2.14 (1.20, 3.79) |
| All | 1.00 (reference) | 1.67 (1.41, 1.98) | 2.43 (1.86, 3.19) | -- | 1.00 (reference) | 1.60 (1.25, 2.03) | 2.36 (1.68, 3.32) | -- |
Note: eGFRs are given in mL/min/1.73 m2; ACRs in mg/g. Associations given as hazard ratio (95% confidence interval).
Abbreviations eGFR: estimated glomerular filtration rate; ACR: albumin-creatinine ratio.
significant interaction comparing HRs in the same category between subgroups based on coefficients for product terms of each potential effect modifier and categories of eGFR and ACR.
Bold indicates statistically significant as compared to reference category
-- indicates no event
DISCUSSION
This study examines the interaction of three key demographic factors—age, sex, and race—with the entire range of eGFR and albuminuria on clinical risk in both relative and absolute scales. The relative risks of cardiovascular disease according to low eGFR were largely consistent in subgroups according to age (52–64 vs. 65–75 years), sex, and race (whites vs. blacks). Of note, the association of low eGFR with increased cardiovascular risk became significant at almost identical levels of eGFR (~70 ml/min/1.73 m2) in all six subgroups including older adults. Largely similar results were observed for high ACR, although there were some quantitative interactions of age and sex on cardiovascular risk. For specific cardiovascular diseases, we found a few significant interactions with ACR. Specifically, high ACR was significantly associated with CHD only in women but not in men and was more strongly associated with HF in whites as compared with blacks.
The main controversy of having uniform thresholds without accounting for demographic traits revolves around GFR.3 In this context, our findings that the contribution of reduced eGFR to cardiovascular risk is largely consistent in the demographic subgroups are clinically important. Among demographic characteristics, most people consider age as the most important potential effect modifier in CKD.31–33 Of note, GFR category 3a without elevated albuminuria, the category most controversial in CKD staging in the connection with aging,3 was significantly associated with increased cardiovascular risk in older (65–75 years) participants. Most previous studies reporting age-CKD interaction have used total mortality as the primary outcome,34–36 and, indeed, we observed lower risk in older compared with younger participants for all-cause mortality in relative, but not in absolute, scale. Similar relative risks for cardiovascular risk between young and old populations in our study are in line with a recent metaanalysis investigating cardiovascular mortality1,14,15 and were translated to higher risk difference in older individuals. These findings suggest that reduced GFR <60 ml/min/1.73 m2 even without kidney damage is not a benign state in older adults and needs strategies for risk reduction.
As compared to GFR, the interactions between demographic variables and albuminuria have been less intensively investigated.13,37,38 We observed significant overall age-ACR multiplicative interaction for cardiovascular disease but not for all-cause mortality. Significantly lower cardiovascular risk in older versus younger individuals was observed in a few segments of ACR (12–60 and 794–1000 mg/g). However, the interaction was quantitative, but not qualitative, and the largely linear relationship between log-ACR and risk was similar in both age groups. The lack of interaction between age and albuminuria on all-cause mortality in our study is in line with previous literature.1,15,35,36
We observed remarkable sex-albuminuria interaction on cardiovascular risk in relative scale. Specifically, high ACR contributed more to increased cardiovascular risk in women than in men (1.3- to 1.8-fold higher HRs in women at a given ACR above 30 mg/g). This sex interaction of albuminuria on cardiovascular outcomes was consistent with previous reports from US community-based cohorts.13,38 The reasons for the sex difference in albuminuria as a predictor of cardiovascular disease are unexplained. We found that this sex difference was derived from the interaction on CHD. Given that albuminuria is a marker of endothelial dysfunction and microvascular abnormalities,39 the sex-albuminuria interaction may reflect the fact that microvascular dysfunction is more involved in the pathophysiology of CHD in women than in men.40 Although some clinical guidelines4 —based on potential overestimation of albuminuria by ACR in women due to lower muscle mass and urinary creatinine excretion as compared to men41 —recommend a higher ACR threshold in women than in men, nevertheless, our findings along with those of others13,38 do not support this approach.
We observed race-albuminuria interaction on incident HF but not on composite cardiovascular disease. Overall, as previously reported,42 blacks were more predisposed to incident HF as compared with whites (Table 2) but the association with high albuminuria was weaker in both relative and absolute scales. The reasons for this racial difference in albuminuria-HF relationship are unclear. Given that there are sparse data about interactions between demographic traits and kidney measures on HF, further investigations and replication are warranted. Nevertheless, the interaction was quantitative and the continuous relationship between albuminuria and incident HF without thresholds remained similar in blacks and whites.
Age-, sex-, and race-specific thresholds for risk factors can induce complexity and confusion in clinical practice and epidemiological research. Although there were some statistical interactions between demographic variables and the two key kidney measures on cardiovascular and mortality risk in our study, overall, we did not observe clear evidence for the need of demographic-specific thresholds for both GFR and ACR in terms of their impact on prognosis. Quantitative interactions observed have implications for assessing individual risk but the overall pattern of association was more consistent than different across groups. Nevertheless, clinical cutoff points should be determined based on a wide range of considerations including prevalence of risk factors, incidence of outcomes of interest, and the cost-effectiveness of risk factor management.25,43,44 This is particularly the case for ACR with continuous risk gradient without threshold effects. Also, although it is beyond the scope of our study, future studies are needed to evaluate whether these kidney measures contribute to better cardiovascular prediction on top of conventional predictors as previous studies obtained conflicting results.45,46
There are several limitations in the present study. First, we used a single measurement of eGFR and ACR, leaving a possibility of misclassification. However, the ARIC Study collected and measured variables for these kidney measures based on standardized procedures. Second, we used the CKD-EPI creatinine equation for eGFR. Although this equation is more accurate and classifies risk better than the MDRD Study equation,16,17 further studies are needed for other GFR equations using other kidney filtration markers such as cystatin C. Third, our study population was aged between 52 and 75 years at baseline. Most women in our study were likely to be post-menopausal. Thus, our results may not be generalizable to individuals out of this age range (e.g., older populations [i.e., >75 years] or premenopausal women). Fourth, we did not have detailed information on medications during follow-up, and thus their impacts on our findings are unknown. Similarly, we did not have information on blood pressure over time. Fifth, as with any observational study, we cannot rule out the possibility of residual confounding despite rigorous adjustment for various cardiovascular risk factors at baseline. Finally, HF cases were identified based on ICD codes abstracted from hospital records and death certificates, which may underestimate HF incidence.24
In conclusion, the associations of eGFR and ACR with cardiovascular disease were generally consistent among subgroups according to age, sex, and race. Of note, relative risks of cardiovascular disease for low eGFR between young (<65 years old) and old (≥65 years old) populations largely were similar with significantly increased risk below eGFR <70ml/min/1.73 m2 after accounting for various cardiovascular risk factors and albuminuria. These findings generally support the use of universal thresholds of these kidney measures for CKD definition/staging and risk classification.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC Study for their important contributions.
Support: The ARIC Study is carried out as a collaborative study supported by
National Heart, Lung and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Supplementary Material
Table S1: Number of participants in each combined category of eGFR and ACR.
Figure S1: Adjusted HRs of CHD, stroke, and heart failure, by eGFR.
Figure S2: Adjusted incidence rates of CHD, stroke, and heart failure, by eGFR.
Figure S3: Adjusted HRs and adjusted incidence rates of all-cause mortality, by eGFR.
Figure S4: Adjusted HRs of CHD, stroke, and heart failure, by ACR.
Figure S5: Adjusted incidence rates of CHD, stroke, and heart failure, by ACR.
Figure S6: Adjusted HRs and adjusted incidence rates of all-cause mortality, by ACR.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
References
- 1.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;(Suppl. 3)(1):1–150. [Google Scholar]
- 3.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009 May;75(10):1009–1014. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 4.IDF Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. Brussels: International Diabetes Federation; 2012. [Google Scholar]
- 5.Barbour SJ, Er L, Djurdjev O, Karim M, Levin A. Differences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patients. Nephrol Dial Transplant. 2010 Nov 1;25(11):3663–3672. doi: 10.1093/ndt/gfq189. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003 Nov;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 7.Jafar TH, Schmid CH, Stark PC, et al. The rate of progression of renal disease may not be slower in women compared with men: a patient-level metaanalysis. Nephrol Dial Transplant. 2003 Oct;18(10):2047–2053. doi: 10.1093/ndt/gfg317. [DOI] [PubMed] [Google Scholar]
- 8.Jolly SE, Burrows NR, Chen SC, et al. Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP) Clin J Am Soc Nephrol. 2011 Aug;6(8):1858–1865. doi: 10.2215/CJN.00500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000 Feb;11(2):319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 10.Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006 Oct;17(10):2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
- 11.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI. Revisiting survival differences by race and ethnicity among hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol. 2006 Oct;17(10):2910–2918. doi: 10.1681/ASN.2005101078. [DOI] [PubMed] [Google Scholar]
- 12.de Zeeuw D, Ramjit D, Zhang Z, et al. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int. 2006 May;69(9):1675–1682. doi: 10.1038/sj.ki.5000326. [DOI] [PubMed] [Google Scholar]
- 13.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002 Mar;13(3):745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011 Jul;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 15.Hallan SI, Matsushita K, Sang Y, et al. Age and Association of Kidney Measures With Mortality and End-stage Renal Disease. JAMA. 2012 Oct 30;308(22):2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012 May 9;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for use: the Study of Men Born in 1913. Eur Heart J. 1987 Sep;8(9):1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelmsen L, Eriksson H, Svardsudd K, Caidahl K. Improving the detection and diagnosis of congestive heart failure. Eur Heart J. 1989 Aug;10(Suppl C):13–18. doi: 10.1093/eurheartj/10.suppl_c.13. [DOI] [PubMed] [Google Scholar]
- 20.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years experience. J Clin Epidemiol. 1996 Feb;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 22.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke. 2002 Nov;33(11):2718–2721. doi: 10.1161/01.str.0000035286.87503.31. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999 Apr;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita K, Blecker S, Pazin-Filho A, et al. The association of hemoglobin a1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes. 2010 Aug;59(8):2020–2026. doi: 10.2337/db10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–3421. [PubMed] [Google Scholar]
- 26.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011 May 17;57(20):2037–2114. doi: 10.1016/j.jacc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Tonelli M, Klarenbach SW, Lloyd AM, et al. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011 Dec;80(12):1306–1314. doi: 10.1038/ki.2011.280. [DOI] [PubMed] [Google Scholar]
- 28.National Kidney Foundation: KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 29.Xu J, Long JS. Confidence intervals for predicted outcomes in regression models for categorical outcomes. Stata Journal. 2005;5(4):537–559. [Google Scholar]
- 30.McCullough PA, Li S, Jurkovitz CT, et al. CKD and cardiovascular disease in screened high-risk volunteer and general populations: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kid Dis. 2008 Apr;51(4) Suppl 2:S38–S45. doi: 10.1053/j.ajkd.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Abdelhafiz AH, Brown SH, Bello A, El M. Chronic kidney disease in older people: physiology, pathology or both? Nephron Clin Pract. 2010;116(1):c19–c24. doi: 10.1159/000314545. [DOI] [PubMed] [Google Scholar]
- 32.McCullough PA, Li S, Jurkovitz CT, et al. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J. 2008 Aug;156(2):277–283. doi: 10.1016/j.ahj.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 2012 Mar 21;:1–8. doi: 10.1038/ki.2012.65. Advance Online Publication(21 March 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.OHare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007 Oct;18(10):2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 35.O'Hare AM, Bertenthal D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol. 2006;17(3):846–853. doi: 10.1681/ASN.2005090986. [DOI] [PubMed] [Google Scholar]
- 36.Raymond NT, Zehnder D, Smith SC, Stinson JA, Lehnert H, Higgins RM. Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol Dial Transplant. 2007 Nov;22(11):3214–3220. doi: 10.1093/ndt/gfm396. [DOI] [PubMed] [Google Scholar]
- 37.OHare AM, Hailpern SM, Pavkov ME, et al. Prognostic implications of the urinary albumin to creatinine ratio in veterans of different ages with diabetes. Arch Intern Med. 2010 Jun 14;170(11):930–936. doi: 10.1001/archinternmed.2010.129. [DOI] [PubMed] [Google Scholar]
- 38.Jassal SK, Langenberg C, von Muhlen D, Bergstrom J, Barrett-Connor E. Usefulness of microalbuminuria versus the metabolic syndrome as a predictor of cardiovascular disease in women and men>40 years of age (from the Rancho Bernardo Study) Am J Cardiol. 2008 May 1;101(9):1275–1280. doi: 10.1016/j.amjcard.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdelhafiz AH, Ahmed S, El Nahas M. Microalbuminuria: marker or maker of cardiovascular disease. Nephron Exp Nephrol. 2011;119(Suppl 1):e6–e10. doi: 10.1159/000328015. [DOI] [PubMed] [Google Scholar]
- 40.Arthur HM, Campbell P, Harvey PJ, et al. Women, cardiac syndrome X, and microvascular heart disease. Can J Cardiol. 2012 Mar-Apr;28(2) Suppl:S42–S49. doi: 10.1016/j.cjca.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983 Mar;37(3):478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 42.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. The New England journal of medicine. 2009 Mar 19;360(12):1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004 May 1;159(9):882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 44.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003 Nov;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 45.Clase CM, Gao P, Tobe SW, et al. Estimated glomerular filtration rate and albuminuria as predictors of outcomes in patients with high cardiovascular risk: a cohort study. Ann Intern Med. 2011 Mar 1;154(5):310–318. doi: 10.7326/0003-4819-154-5-201103010-00005. [DOI] [PubMed] [Google Scholar]
- 46.Nerpin E, Ingelsson E, Riserus U, et al. The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrology Dialysis Transplantation. 2011 Sep;26(9):2820–2827. doi: 10.1093/ndt/gfq848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.