Abstract
Induction of functional CTLs is one of the major goals for vaccine development and cancer therapy. Inflammatory cytokines are critical for memory CTL generation. Wnt signaling is important for CTL priming and memory formation, but its role in cytokine-driven memory CTL programming is unclear. We found that wnt signaling inhibited IL-12-driven CTL activation and memory programming. This impaired memory CTL programming was attributed to up-regulation of eomes and down-regulation of T-bet. Wnt signaling suppressed the mTOR pathway during CTL activation, which was different to its effects on other cell types. Interestingly, the impaired memory CTL programming by wnt was partially rescued by mTOR inhibitor rapamycin. In conclusion, we found that crosstalk between wnt and the IL-12 signaling inhibits T-bet and mTOR pathways and impairs memory programming which can be recovered in part by rapamycin. In addition, direct inhibition of wnt signaling during CTL activation does not affect CTL memory programming. Therefore, wnt signaling may serve as a new tool for CTL manipulation in autoimmune diseases and immune therapy for certain cancers.
Keywords: T cells, cytotoxicity, wnt, GSK3, cytokines, memory, mTOR, rapamycin
INTRODUCTION
Generation of functional memory is key for potential successful vaccination against many chronic virus infections such as HIV. Naïve CTLs recognize the antigen presented by MHC I and receive costimulation provided by antigen presentation cells such as DCs (Harty and Badovinac, 2008; Kaech et al., 2002; Mescher et al., 2006). Yet, a third signal – inflammatory cytokines, such as IL-12 and type I IFN – is required for full activation, development of effector functions and cytotoxicity, and establishment of memory for protection against the same or similar pathogens (Curtsinger et al., 2003b; Curtsinger et al., 1999; Curtsinger et al., 2005b; Kolumam et al., 2005; Mescher et al., 2006). It has been shown that short periods of IL-12 stimulation, together with antigen and costimulation, can program fully functional memory CTLs (Li et al., 2011; Rao et al., 2010; Xiao et al., 2009). More importantly, this IL-12-driven memory programming can be regulated by the inhibition of mTOR by rapamycin (Li et al., 2011; Rao et al., 2010), which is consistent with reports using pathogen infections (Araki et al., 2009; Pearce et al., 2009).
Wnt signaling is involved in embryonic development and homeostatic self-renewal of many tissues (Clevers, 2006) and is critical in T cell development (Yu et al., 2010). In the absence of wnt signaling, β-catenin in the canonical pathway is continually phosphorylated and degraded by Glycogen synthase kinase 3 (GSK-3) (Clevers, 2006). Activation of wnt signaling inhibits this process and intact β-catenin is released from the GSK3 complex without phosphorylation. β-catenin accumulates in the cytoplasm and translocates into the nucleus, forming a complex with two critical transcription factors, T-cell factor 1 (TCF1) and lymphoid enhancer-binding factor 1 (LEF1), which initiates the transcription of responsive genes (Clevers, 2006; Staal et al., 2008). Recently, wnt was elegantly shown to be important for CTL activation and memory development (Jeannet et al.; Willinger et al., 2006; Zhao et al.). Deficiency of TCF1 leads to impaired proliferation of CTLs and defects in the secondary memory CTL response (Jeannet et al., 2010a). Enforced expression of eomes partially rescues the memory lost (Zhou et al., 2010) and while enforced expression of TCF1 with β-catenin also improves memory formation and protection against pathogen challenge (Zhao et al., 2010). Recently, Gattinoni et al reported that wnt signaling activation can program self-renewing multipotent CD8 memory stem cells in vitro (Gattinoni et al., 2009a; Gattinoni et al., 2009b). However, expression of stable β-catenin impairs T cell activation (Driessens et al., 2011), suggesting GSK3 may work through other pathways in the regulation of memory CTLs (Driessens et al., 2010). Whether wnt signaling affects inflammatory cytokine-driven memory CTL programming is unknown.
In this report, we found that wnt signaling directly down-regulated CTL activation and subsequent memory programming. Despite up regulation of eomes and down regulation of T-bet, wnt signaling abolished IL-12-driven memory CTL programming. The resulting CTLs were unable to protect against pathogen challenge. The mTOR pathway was inhibited by wnt signaling, which is unlike the effects of wnt in other cell types (Inoki et al., 2006). However, rapamycin, an inhibitor of mTOR partially rescued impaired memory programming by wnt. Therefore, activation of wnt signaling may be a potential mechanism for immune evasion utilized by many cancers, which generate wnt ligands (Burgess et al., 2011; Chen et al., 2009; Najdi et al., 2011; Reya and Clevers, 2005; Song et al., 2011). In certain autoimmune diseases, activation of wnt signaling may prove useful for manipulating detrimental CTLs (Faustman and Davis, 2009; Friese and Fugger, 2009; Phillips et al., 2009; Skowera et al., 2008; Tsai et al., 2008; Tsai et al., 2010; Zaccone and Cooke, 2010).
MATERIALS AND METHODS
Mice, cell lines, and reagents
OT-I mice (a gift from Dr. Mescher, University of Minnesota) that have a transgenic TCR specific for H-2Kb and OVA257–264 (Hogquist et al., 1994) were crossed with Thy1-congenic B6.PL-Thy1a/Cy (Thy1.1) mice (Jackson ImmunoResearch Laboratories, Bar Harbor ME) and bred to homozygosity. The development of CD8 T cells in all strains appeared normal with respect to numbers, distribution and phenotype (data not shown). Mice were maintained under specific pathogen-free conditions at the University of Maryland, and these studies have been reviewed and approved by the Institutional Animal Care and Use Committee. C57BL/6 mice were purchased from the National Cancer Institute. All directly conjugated fluorescent antibodies were purchased from BD Biosciences, eBioscience or Biolegend. TWS119 and rapamycin were purchased from EMD (Gibbstown, NJ). The wnt signal inhibitor, ICG-100, was purchased from Selleckchem (Houston, TX).
Bacteria
Recombinant Listeria monocytogenes (a gift from Dr. Jameson, University of Minnesota) expressing full-length secreted ovalbumin (LM-OVA) was used for infection at 5 × 105 i.v. for re-challenge. The spleens from recipient mice were harvested 3 days after LM-OVA challenge. LM-OVA was cultured using TSB plates for the comparison of protection as in our previous reports (Li et al., 2011; Xiao et al., 2009).
Naive T cell purification
This was performed as previously reported (Li et al., 2011; Xiao et al., 2009). Briefly, inguinal, axillary, brachial, cervical, and mesenteric lymph nodes (LNs) were harvested from WT OT-I mice, pooled, and disrupted to obtain a single cell suspension. Cells were coated with FITC-labeled antibodies specific for CD4, B220, I-Ab, and CD44. Anti-FITC magnetic MicroBeads (Miltenyi Biotech) were then added and the suspension passed through separation columns attached to a MACS magnet. Cells that did not bind were collected, and were >95% CD8+ and <0.5% CD44hi. Purified naive OT-I cells were sorted to reach purity close to 100%.
Real-time RT-PCR
RNA was isolated (Qiagen RNeasy mini kit) and used to synthesize cDNA (Qiagen QuantiTech Reverse Transcription kit). Quantification was performed on a MyiQ™ Single-Color Real-Time PCR Detection System (Bio-Rad). Primers used were as follows: CD62L 5′ left primer, 5′-GCTGGAGTGACACCCTTTTC-3′; CD62L 3′ right primer, 5′ -GTTGGGCAAGTTAAGGAGCA-3′; GAPDH 5′ left primer, 5′ -TGTCTCCTGCGACTTCAACAGC-3′; GAPDH 3′ right primer, 5′ -TGTAGGCCATGAGGTCCACCAC-3′. Details of the real-time PCR conditions used are available upon request.
Adoptive transfer and flow cytometric analysis
In vitro activated OT1 cells were adoptively transferred into normal C57BL/6NCr mice by i.v. (tail vein) injection at 106 cells/mouse and OT-I cells were identified as CD8+Thy1.1+ cells. Blood samples were drawn at indicated times, and the analysis of memory CTLs was based on the spleen and/or blood. Single cell suspensions were prepared, viable cell counts were performed (trypan blue) and the percent of OT-I cells in the sample was determined by flow cytometry. Background for determining OT-I cell numbers was determined by identical staining of cells from normal C57BL/6 mice (no adoptive transfer). Analysis was done using a FACSCalibur™ flow cytometer and CELLQuest™ software (BD Biosciences) to determine the percent and total OT-I cells in the samples. Flowjo software (Tree Star Inc.) was used for data analysis.
Intracellular cytokine staining after in vitro re-challenge
Spleen cells from adoptively transferred mice were incubated at 2 × 106 cells/ml in RP-10 with 0.2 µM OVA257–264 peptide and 1 µl Brefeldin A (Biolegend) for 3.5 hrs at 37°C. Cells were fixed in fixing buffer (Biolegend) for 15 min at 4°C, permeablized in Saponin-containing Perm/Wash buffer (Biolegend) for another 15 min at 4°C, and stained with PE-conjugated antibody to IFNγ for 30 min at 4°C. Cells were then washed once with Perm/Wash buffer, and once with PBS containing 2% FBS.
Intracellular staining for cell signaling molecules
Spleen cells from adoptively transferred mice were washed twice with cold PBS (4°C), and fixed with 2% paraformaldehyde for 20 min at 37°C. The cells were chilled on ice for 2 min, and washed twice with cold PBS. Permeablization was performed using 90% ice-cold methanol (stored at −20° C) on ice for 30 min. Permeablized cells were washed twice with cold PBS, and blocked for 10 min with 0.5% BSA-PBS at room temperature. Staining with primary and secondary antibodies was carried out for 30 min at 4°C. Cells were washed twice with 0.5% BSA-PBS after each staining.
In vitro stimulation of naïve OT-I T cells
Naïve OT-I.PL T cells were purified as described above and stimulated for a certain time in vitro in flat-bottom microtiter wells coated with antigen (DimerX H-2Kb:Ig fusion protein loaded with OVA257–264 peptide; BD Pharmingen) and recombinant B7-1/Fc chimeric protein (R&D Systems) as previously described (Li et al., 2011; Xiao et al., 2009). 3 × 105 cells in 1.5 ml Allos media were placed in each well and 2.5U/ml IL-2 was added to all wells (24-well plate). Where indicated, cultures were supplemented with 2U/ml of murine rIL-12 (R&D Systems) or universal type I IFN at 1000 U/ml (PBL Biomedical Laboratories). GSK3 inhibitor TWS119 and rapamycin stock (in DMSO) were diluted with corresponding culture medium as indicated. Cells that received IL-12 in vitro were termed 3 SI OT-I, and cells without IL-12 treatment were termed 2 SI OT-I. Transferred cells were identified by staining with anti-Thy 1.1 and anti-CD8 mAbs.
RESULTS
Wnt signaling inhibits CTL activation
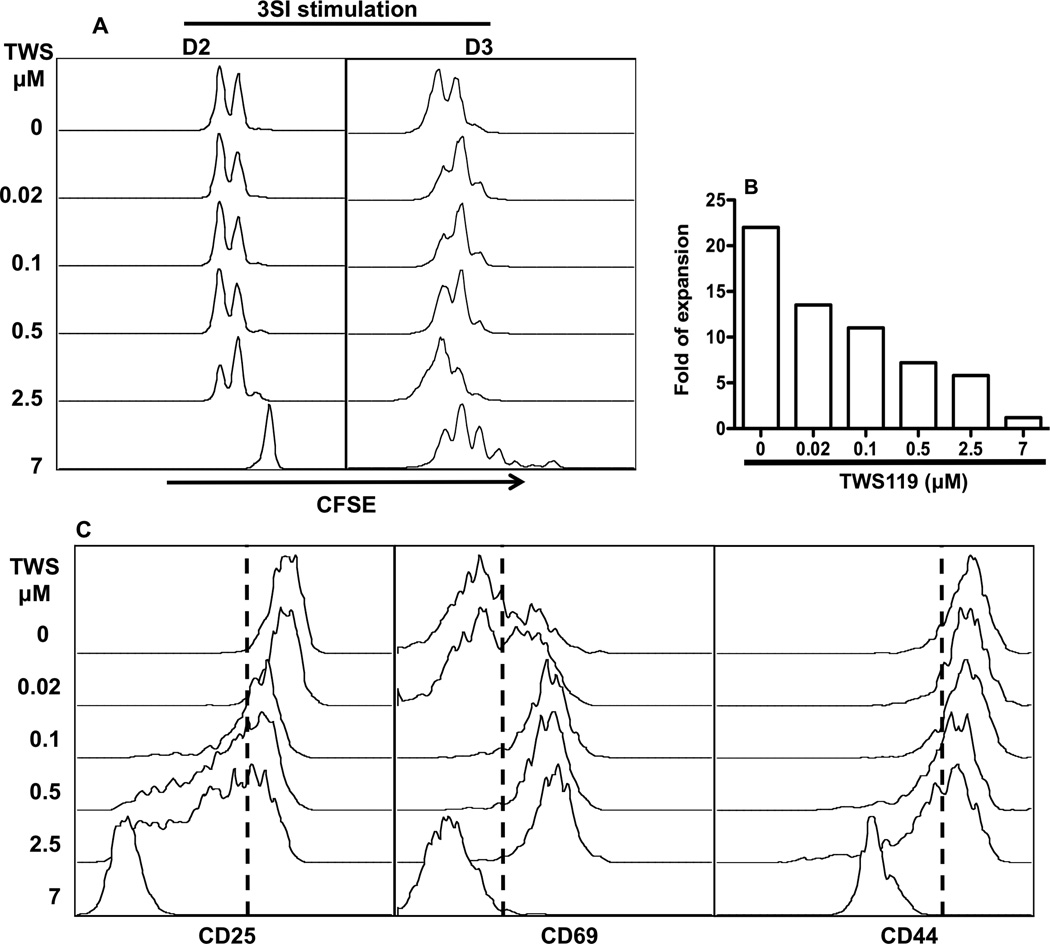
IL-12 is one of the third signal cytokines for CTL activation (Curtsinger et al., 2003b; Curtsinger and Mescher, 2010; Mescher et al., 2006; Xiao et al., 2009), and it drives efficient memory programming in vitro (Li et al., 2011; Rao et al., 2010). We sought to understand if this function of IL-12 in CTL activation could be affected by wnt signaling. Briefly, sorted naïve OT1 cells were stimulated with plate-bound antigen and B7 plus IL-12 (3 signal-3SI) for 3 days in the presence or absence of wnt signal (Xiao et al., 2009). Wnt signaling was activated by GSK3 inhibitor TWS119, which was added simultaneously at 5 different concentrations covering a 375-fold range from 0.02 to 7 µM (Gattinoni et al., 2009b). The division of CTLs was not inhibited by wnt signaling until 2.5 µM and complete inhibition was achieved at 7µM at day 2, although proliferation resumed at day 3 (Fig. 1A), which is in agreement with a recent report (Gattinoni et al., 2009b). However, the total expansion compared to the initial input was repressed in a dose-dependent way, and there was essentially no net increase at 7 µM of TWS119 after 3-day stimulation (Fig. 1B) even though CTLs continued to divide (Fig. 1A). These inhibitory effects of wnt signaling were further indicated by the down regulation of CD25 (IL-2 receptor α subunit) by TWS119 at high concentrations (2.5 and 7 µM) (Fig. 1C). CD69 expression was enhanced (Fig. 1C), which may suggest delayed activation by wnt signaling. CD44 was significantly down-regulated at 7 µM, consistent with the report by Gattinoni et al. (Gattinoni et al., 2009b). Thus, activation of wnt signaling inhibits IL-12 driven CTL activation through its effects on expansion and possible responsiveness to IL-2.
Figure 1. Wnt signaling inhibits CTL activation.
Sorted OT1 cells were labeled with CFSE, then stimulated with 3 SI (antigen+B7 plus IL-12) in the presence of GSK3 inhibitor TWS119 at different concentrations. (A) Cell proliferation (CFSE dilution) was evaluated at day 2 and 3. (B) Fold expansion of CTLs 3 days after in vitro stimulation was calculated according to original input. Each sample included cells pooled from 5 to 30 individual wells of 24-well plates. (C) Expression of activation related molecules in CTLs cells at day 3 after stimulation. These are representatives of at least five independent experiments with similar results.
Wnt signaling suppresses CTL effector function
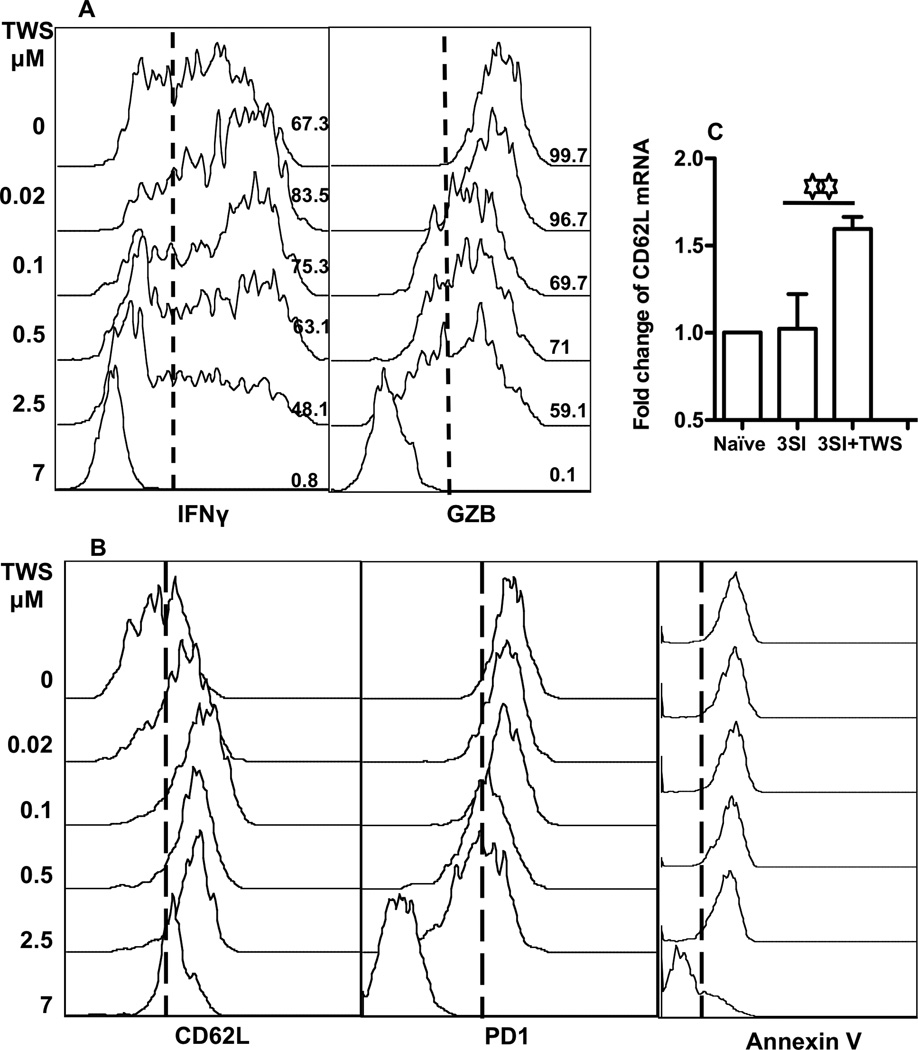
Next, we asked if wnt signaling influenced the effector function of CTLs. Sorted OT1 cells were stimulated for 3 days before analysis. Interestingly, at low concentrations of GSK3 inhibitor, TWS119, the production of IFNγ was slightly enhanced, while a further increase in TWS119 concentration reduced IFNγ production (Fig. 2A). This is consistent with a recent report on the negative effects of stabilized β-catenin on T cell activation (Driessens et al., 2011). Granzyme B (GZB), a molecule directly related to the killing function of CTLs (Curtsinger et al., 2003a; Curtsinger et al., 2005a; Curtsinger and Mescher, 2010), was not affected at low concentrations of TWS119, but was inhibited at high concentrations (Fig. 2A) and almost completely abolished at 7 µM. PD1, a receptor of the CD28 family, delivers inhibitory signals during CTL activation (Greenwald et al., 2005; Okazaki and Honjo, 2007; Riley, 2009; Sharpe et al., 2007). Exhausted CTLs express high amount of PD1, and blockage of PD1 can rescue CTL exhaustion during chronic LCMV infection (Barber et al., 2006; Blackburn et al., 2008). Despite the inhibitory effects of wnt signaling on CTL activation (Fig. 2A and B), the expression of PD1 decreased starting at 0.5 µM TWS119, and it was almost completely abolished at 7 µM (Fig. 2B). There was no shift in Annexin V staining induced by TWS119 except at 7µM. Annexin V binds to phosphatidylserine of apoptotic cells (Vermes et al., 1995). From this, it seems that PD1 and Annexin V binding are not related to inhibitory expansion by wnt signaling.
Figure 2. Wnt signaling suppresses CTL effector functions.
Sorted OT1 cells were stimulated with antigen+B7+IL-12 in the presence of TWS119 at different concentrations. Programmed CTLs were harvested at 72 hrs. (A) The expression of IFNγ and granzyme B (GZB). (B) The expression of CD62L, PD1 and Annexin V. (C) Transcriptional regulation of CD62L by TWS119. OT1 cells were stimulated for 3 days under 3 SI in the presence or absence of TWS119 at 2.5 µM, and then were harvested for real-time PCR examination. CD62L and housekeeping gene GAPDH were analyzed in triplicate in real-time RT-PCR assays. Relative mRNA amounts were normalized with respect to expression levels in Naïve OT1 control (fold change=1). The results are expressed as mean + SD of three independent experiments. Asterisks indicate statistical significance. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns: not significant. These will be followed in the rest of paper. These are representatives of three independent experiments with similar results.
CD62L is required for the migration of naïve CTLs to secondary lymphoid tissue for initial activation (Bevan, 2004; Harty and Badovinac, 2008; Jameson and Masopust, 2009). Wnt signaling up-regulated CD62L expression on the surface of CTLs (Fig. 2B), consistent with the report by Gattinoni et al. (Gattinoni et al., 2009b), but at the highest concentration (7µM) CD62L regulation was negatively affected (Fig. 2B). CD62L expression can be regulated by gene expression or shedding (Kusaba et al., 2005; Smalley and Ley, 2005; Wang et al., 2011; Wang et al., 2010). q-PCR was performed to confirm CD62L expression on the mRNA level. Wnt signaling increased transcription of CD62L by about 60% compared to the control (Fig. 2C). This suggests that wnt signaling controls CD62L surface expression at the transcription level. However, we could not exclude the possibllity that a reduction in CD62L shedding may have contributed to the observed increase in CD62L expression.
T-bet expression is repressed by wnt signaling
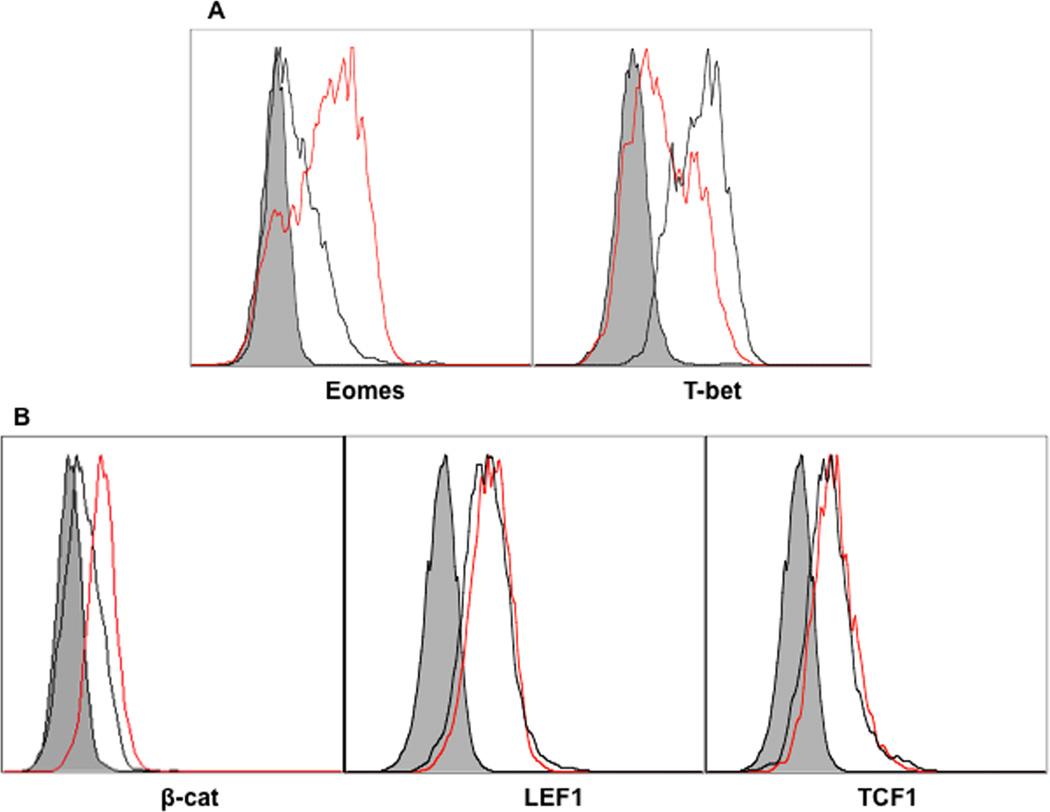
T-bet is a critical transcription factor responsible for the differentiation of Th1 and IFNγ production (Intlekofer et al., 2005; Szabo et al., 2000; Szabo et al., 2002). The balance of T-bet and eomes expression has implications for memory differentiation (Rao et al., 2010; Takemoto et al., 2006). To corroborate this in the presence of wnt signaling on CTLs, sorted OT1 cells were stimulated with 3SI in the presence or absence of TWS119 for 3 days. The expression of T-bet was inhibited by wnt signaling (Fig. 3A), which is consistent with the reduced production of IFNγ (Fig. 2A), but expression of eomes was enhanced (Fig. 3A). These results are consistent with the inverse regulation of T-bet and eomes by IL-12 (Rao et al., 2010; Takemoto et al., 2006). In the canonical wnt signaling pathway, phosphorylation of β-catenin and thus its eventual degradation, is induced by GSK3. The protein expression of β-catenin was only marginally increased by TWS119 (Fig. 3B), suggesting that GSK3 may work through other pathways to affect CTL activation (Driessens et al., 2010; Gattinoni et al., 2009b). Two transcription factors that bind to β-catenin to form a transcriptional complex, LEF-1 and TCF1, were also marginally up regulated by wnt activation (Fig. 3B). Therefore, wnt signal activation inhibits T-bet, but increases eomes expression during IL-12-driven CTL activation, possibly through pathways other than the canonical wnt pathway.
Figure 3. T-bet expression is repressed by wnt signaling.
Sorted OT1 cells were stimulated for 3 days with 3SI in the presence or absence of TWS119 at different concentrations. Red lines: samples treated with 3SI+TWS119. Black lines: 3SI only. Shaded histogram: isotype control.
CTL memory programming is abolished by wnt signaling
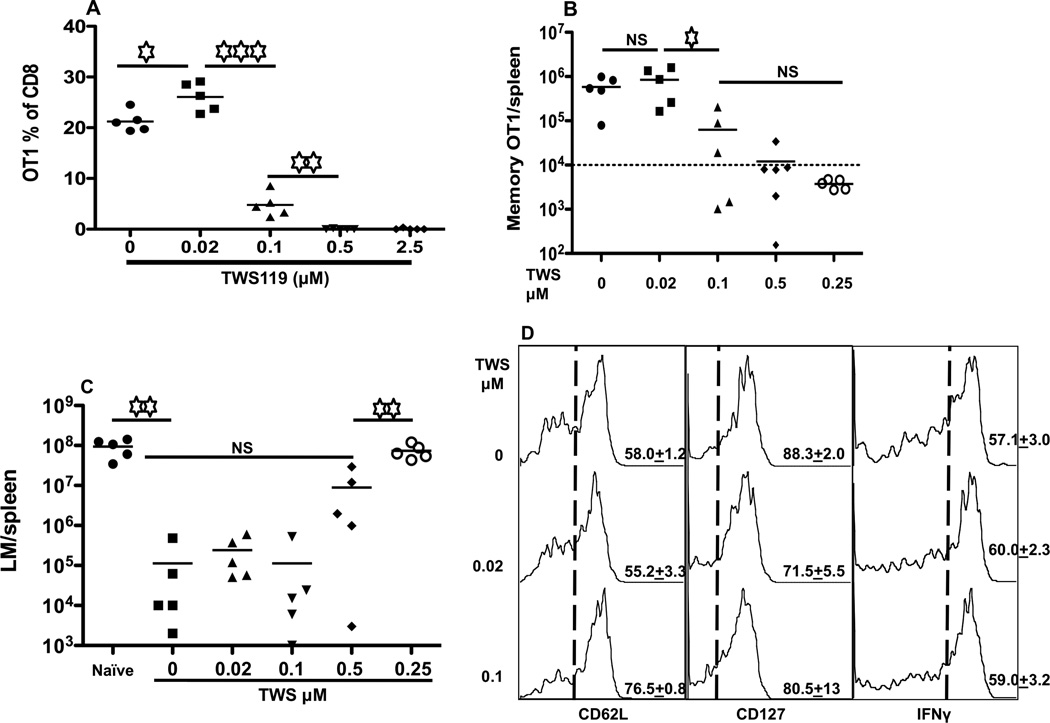
The inhibition of T-bet and enhanced expression of eomes would suggest that the memory programming of CTLs and CD62L expression should be increased by wnt signaling, similar to mTOR inhibition by rapamycin (Li et al., 2011; Rao et al., 2010; Takemoto et al., 2006). OT-1 cells were stimulated in the presence of IL-12 with or without GSK3 inhibitor TWS119, harvested after 3 days, and transferred into recipients. Surprisingly, 5 days after transfer, OT-1 cells in peripheral blood were reduced in a dose-dependent manner, dropping significantly at 0.1 µM TWS119 and undetectable at 0.5 µM TWS119 or higher (Fig. 4A). The same results occurred at day 40 after transfer. Memory OT-1 cells were undetectable in the spleens of transferred recipients at high concentration of TWS119 (Fig. 4B). The disappearance of memory CTLs at 2.5 µM TWS119 was similarly reflected in other tissues (data not shown). In addition, wnt signaling further reduced the barely detectable memory CTLs from 2SI to completely undetectable levels (Figure S1). To confirm the protection ability, recipients were challenged with LM-OVA (Li et al., 2011; Xiao et al., 2009). The extent of protection was associated with the number of memory CTLs in spleens—when memory CTLs were undetectable (2.5 µM), the protection was similar to the negative control (Fig. 4C). When CTLs were stimulated with TWS119 at 0.1 µM in vitro, the resultant memory CTLs after transfer had a typical central memory phenotype CD62Lhi/CD127hi/KLRG1lo (Fig. 4D and data not shown). The production of IFNγ in these memory CTLs was the same as the controls (Fig. 4D). In fact, CD62L expression on memory CTLs was significantly higher in TWS119 (0.1µM) group than the control (Fig. 4D). Therefore, wnt signaling quantitatively impairs memory programming. In addition, wnt signaling was also detrimental when another third signal cytokine type I IFN (Figure S2) was used instead of IL-12, suggesting that its negative effect on memory CTL programming may not be limited to IL-12.
Figure 4. CTL memory programming is abolished by wnt signaling.
Sorted OT1 cells were stimulated for 3 days with 3SI in the presence or absence of TWS119 at different concentrations. Activated OT1 cells were then transferred into naïve B6 mice at 106/mouse. (A) Blood samples were drawn at day 5 after transfer. (B) Numbers of OT1 cells in spleen 40 days after transfer. The numbers on the bottoms of each panel (A and B) indicate the concentration of TWS119 during in vitro stimulation. (C) At 40 days after transfer, recipient mice were challenged with 5×105 CFU LM-OVA through i.v.. Bacterium counts were examined 3 days after LM-OVA challenge in spleen. (D) Comparison of the expression of CD62L, CD127 and IFNγ in memory OT1 cells from (B) at day 40 after transfer. Higher concentrations (0.5 and 2.5 µM) of TWS119 treatment had low frequency of events (data not shown). Dashed lines indicate the gating, and the number on each histogram indicates the percentage of positive cells. Experiments are representatives of at least three (A–C) or two (D) independent experiments with similar results. Each value in (D) represents the mean plus the SEM of 3–5 mice per group in D. Asterisks indicate statistical significance. *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS: not significant.
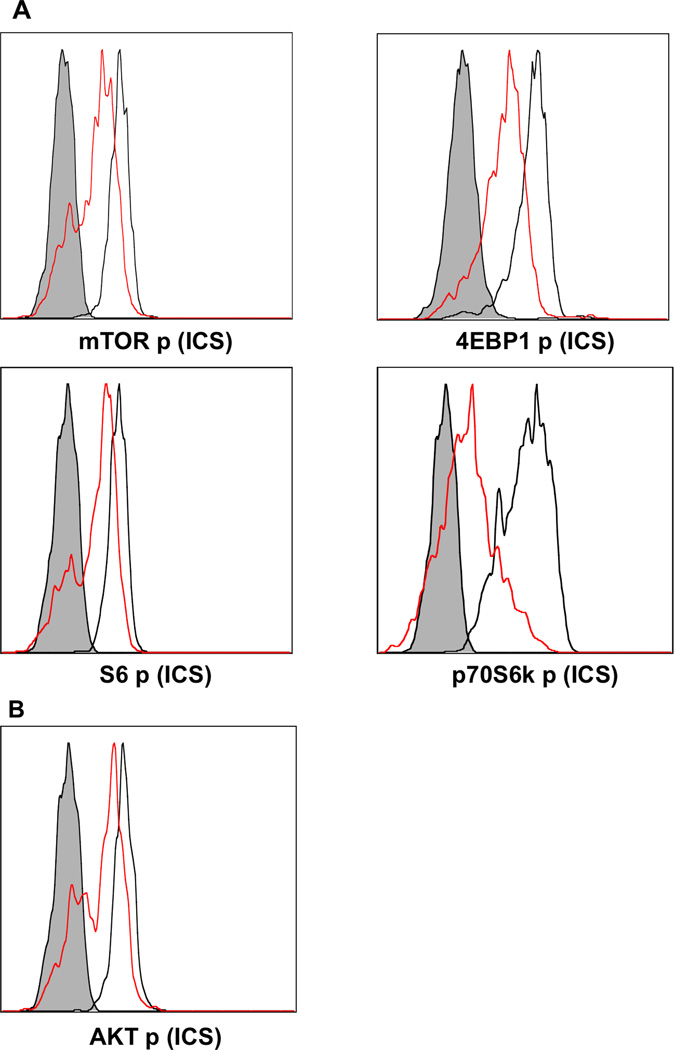
Wnt signaling inhibits mTOR pathway
Wnt signal activation has been shown to induce mTOR activation through GSK3 and TSC2 in embryonic fibroblasts, osteoblasts and bone marrow cells (Inoki et al., 2006). mTOR inhibition by rapamycin significantly enhances memory programming by IL-12 (Li et al., 2011; Rao et al., 2010). Thus, it would make sense that wnt signaling activated mTOR in CTLs, impairing memory CTL programming (Fig. 4). To investigate this possibility, sorted naïve OT-1 cells were stimulated with 3SI, in the presence or absence of 2.5 µM TWS119. In contrast to our expectation, mTOR activation was inhibited by wnt signaling after stimulation (Fig. 5), as the activation of the major downstream signaling molecules, factor 4E binding protein 1 (4EBP1) and p70 Ribosomal Protein S6 Kinase (p70S6K), was suppressed simultaneously (Fig. 5). Consistent with this, a downstream substrate of S6K, S6 ribosomal protein was repressed by wnt activation (Fig. 5). Although the inhibitory effects were similar to the effects of rapamycin (Li et al., 2011; Rao et al., 2010), wnt signaling resulted in the abolishment of memory programming (Fig. 4B – C). The opposite effects of rapamycin compared to wnt signaling may suggest that inhibition of mTOR is not necessary to enhance memory CTL programming, and rapamycin and wnt signaling may regulate CTL programming through different mechanisms, which are yet to be identified.
Figure 5. Wnt signaling inhibits mTOR pathway.
Sorted OT1 cells were stimulated for 2 days with 3SI in the presence or absence of TWS119 at different concentrations. Red lines: samples treated with 3SI+TWS119. Black lines: 3SI only. Shaded histogram: isotype control.
Inhibition of mTOR by rapamycin partially rescues impaired memory programming by wnt signaling
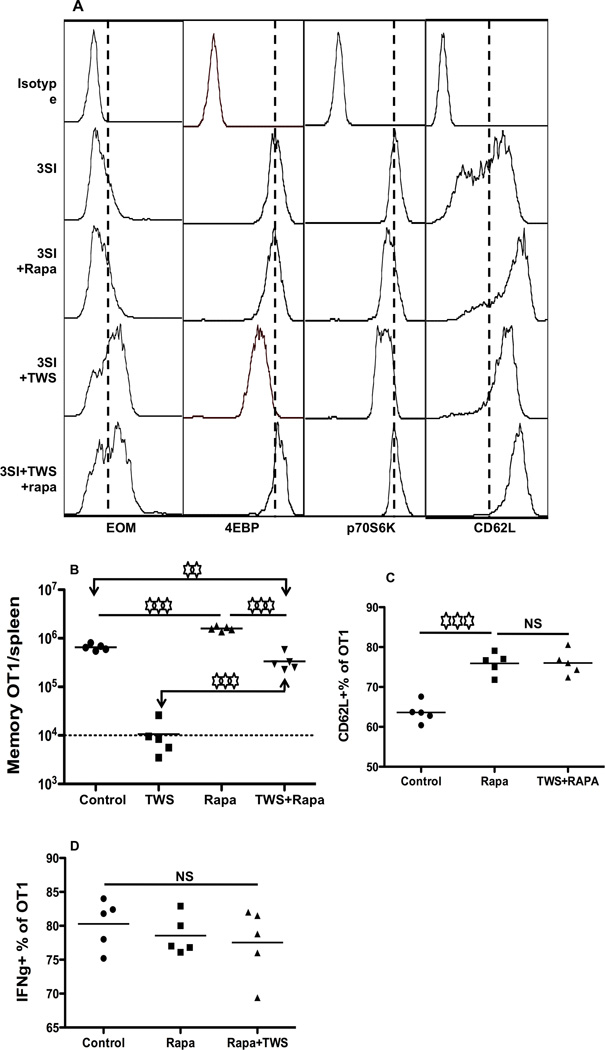
Since mTOR inhibition by rapamycin significantly enhances memory programming by IL-12 (Li et al., 2011; Rao et al., 2010), we hypothesized that the presence of rapamycin would rescue memory CTLs from the negative effects of wnt signaling. Sorted OT-1 cells were cultured with 3SI together with 2.5µM TWS119, in the presence or absence of rapamycin at 250 ηg/ml as we recently reported (Li et al., 2011). Three days after stimulation, OT-1 cells were harvested for analysis and transfer. As reported before, rapamycin slightly enhanced eomes expression (Rao et al., 2010), while the combination of rapamycin and wnt signaling did not change eomes expression compared to wnt signaling anlone (Fig. 6A). Surprisingly, although rapamycin and wnt signaling individually inhibited phosphorylation of mTOR downstream molecules, the application of both led to increased activation of 4EBP1 and p70S6k back to the level observed in the 3SI control (Fig. 6A). This suggests that the interactions between mTOR and wnt may be more complex than we thought. CD62L expression in CTLs treated by both rapamycin and TWS119 was similar to CTLs treated with rapamycin alone (Fig. 6A). All of these data indicate that the synergistic effects of wnt and rapamycin are different from the effects of each individually. In vitro activated OT1 cells were also transferred into naïve B6 mice for memory formation. 40 days after transfer, memory OT1 in spleens were examined. As a control, rapamycin enhanced memory programming by about 3-fold (Fig. 6B), consistent with our previous report (Li et al., 2011). Addition of TWS119 abolished memory programming (Fig. 6B), similar to Fig. 4B. Interestingly, rapamycin partially recovered the impaired memory programming caused by wnt signaling to half of the 3SI control and far less than the rapamycin plus 3SI (Fig. 6B). The partially rescued memory CTLs were of central memory phenotype CD62Lhi/CD127hi, with slightly higher expression of CD62L (Fig. 6C) but similar production of IFNγ compared to control and rapamycin alone (Fig. 6D). These results further support the notion that different molecular mechanisms are employed by rapamycin and wnt to inhibit the mTOR signaling pathway.
Figure 6. Inhibition of mTOR by rapamycin partially rescues suppression of memory programming by wnt signaling.
Sorted OT1 cells were stimulated with antigen+B7+IL-12 in the absence or presence of TWS119 at 2.5µM, or, rapamycin at 250ηg/ml, or both. (A) Comparison of the expression of EOMES, 4EBP, p70S6K and CD62L in OT1 cells 3 days after in vitro stimulation. (B) Programmed CTLs were harvested at 72 hrs, and were transferred into naïve B6 mice at 106/mouse through tail injection. Memory OT1 cells in spleen at day 40 after transfer. (C–D) Comparison of molecular expression in memory CTLs programmed by TWS119 (2.5µM) or rapamycin (250ηg/ml) or both. Controls were 3SI only. Results are representative of two independent experiments with similar results.
Inhibition of wnt signaling does not affect CTL memory programming
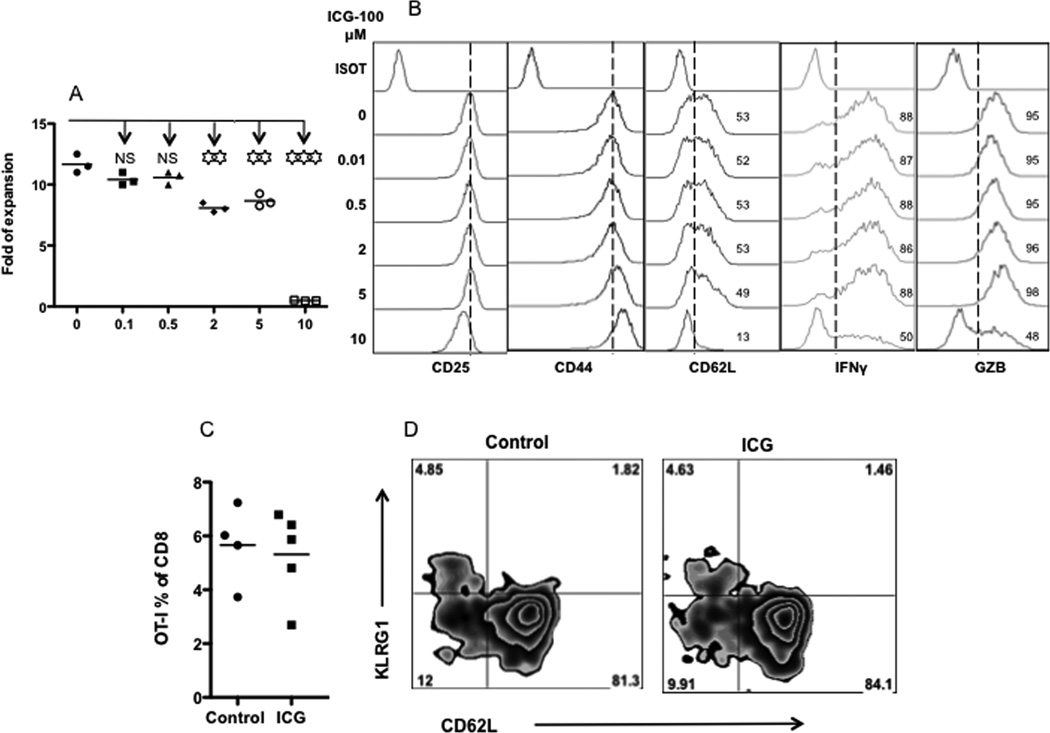
To further investigate the involvement of wnt signaling in CTL activation and memory programming, we used ICG-100, a small molecule that inhibits beta-catenin/T cell factor signaling by specifically binding to transcription factor CREB (Emami et al., 2004). ICG-100 was tested at a range of concentrations using the same system as in Figures 1 and 2. At 10 µM, ICG-100 became toxic, which was demonstrated by decreased viability (Fig. 7A) and down regulation of CD25, IFNγ and GZB. ICG-100 started to affect CTL expansion at 2 and 5 µM (Fig. 7A), but most surface markers and IFNγ and GZB production were not affected. In order to avoid potential toxicity, we chose 2 µM in wnt signal inhibition. OT-1 cells were stimulated with 3SI in the presence or absence of ICG-100 at 2 µM. Activated CTLs were harvested 3 days after stimulation, and transferred into recipient mice. There was no difference in the memory generation until 60 days after the transfer (Fig. 7C and data not shown). In addition, there was no significant difference in the expression of CD62L and KLRG1 (Fig. 7D). Therefore, wnt signal is not required during the CTL activation.
Figure 7. Inhibition of wnt signaling does not affect CTL memory programming.
Sorted OT1 cells were stimulated for 3 days with 3SI in the presence or absence of ICG-100 at different concentrations (0.1–10 µM). (A) Fold expansion of CTLs 3 days after in vitro stimulation was calculated according to original input. (B) Expression of activation related molecules in CTLs cells at day 3 after stimulation. (C) Activated OT1 cells were then transferred into naïve B6 mice at 106/mouse 3 days after stimulation. OT1 cells in blood 60 days after transfer. (D) Representative plots of memory OT1 cells from (C) at day 60 after transfer. Asterisks indicate statistical significance. *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS: not significant.
DISCUSSION
Wnt signaling has been shown to be critical in the formation of memory CTLs (Jeannet et al., 2010a; Yu et al., 2010; Zhao et al., 2010; Zhou et al., 2010), which is also involved in the regulation of CD4 helper T cells, regulatory T cells and B cells (Ding et al., 2008; Reya et al., 2000; Yu et al., 2010; Zhao et al., 2010). We found that the activation of wnt signaling during CTL activation impaired effector function, and abolished memory CTL programming by IL-12. Although this is unexpected, it is not necessarily contradictory to previous publications (Gattinoni et al., 2009b; Jeannet et al., 2010b; Zhao et al., 2010; Zhou et al., 2010). When the wnt signal molecules are constitutively expressed, wnt signaling cannot be turned off (Driessens et al., 2011; Zhao et al., 2010). In our system, however, wnt signaling was activated for a short period of time. Our data did not disprove the function or requirement of wnt signal under physiological conditions. Instead, our results suggest that activation of wnt signaling during the window of CTL activation may not be beneficial for memory programming. Activation of CTLs only lasts for short period of time, and for most of the time CTLs are quiet. Since wnt signaling can be activated in quiet CTLs, it would be interesting to see if this could lead to a functional difference. Therefore, continuous wnt signaling may not be essential for memory CTL generation but may be required during certain phases of CTL development. Conditional knockouts could be used to elucidate the exact time window when wnt signaling is beneficial. Above all, caution must be taken when manipulating the wnt signal during vaccination.
Wnt signal activation has been associated with several kinds of cancer, such as colorectal cancers (Burgess et al., 2011; Chen et al., 2009; Huang et al., 2009; King et al., 2011; Najdi et al., 2011; Song et al., 2011). The observation is based on the accumulation of β-catenin in nuclei, which causes high activation of wnt signaling. Although a small number of these cancer cases are due to the mutation of β-catenin or APC loci (Najdi et al., 2011; Schmidt et al., 2009), many of them are attributed to the generation of wnt ligands and regulation of wnt receptors (King et al., 2011; Schmidt et al., 2009). These regulatory factors should be equally available for CD8 T cells, which are the critical controllers for malignant cells, and the secreted wnt ligands will activate wnt signaling in the tumor-specific CTLs. However, tumors induce immune suppressive environments which impair CTL activation. Thus, the presence of secreted wnt ligands could further inhibit tumor-specific CTLs, facilitating tumor progression. One strategy to control wnt activation would be inhibition of mTOR using rapamycin, which was shown partially reversed the negative effects of wnt signaling on memory CTL programming. Rapamycin is used in cancer therapy due to its ability to suppress tumor growth. More encouragingly, wnt signaling inhibitors have been extensively investigated in tumor therapy. Inhibiting both mTOR and wnt signaling in tumor patients may offer an effective strategy for tumor control targeting enhanced CTL activation.
Although wnt signaling inhibited mTOR and down-regulated 4EBP1 and p70S6K and S6 (Fig. 5), the addition of rapamycin enhanced the activation of 4EBP1 and p70S6k (Fig. 6A). These data suggest that wnt may suppress mTOR through pathways other than the canonical wnt pathway involving TSC2 and GSK3 (Inoki et al., 2006). Rapamycin partially reversed impaired memory CTL programming by wnt signaling, suggesting that rapamycin and wnt can affect memory programming through other pathways than mTOR. Akt is involved in wnt signaling by inhibiting GSK3 activity in different cell types (Anderson and Wong, 2010; Fukumoto et al., 2001; Lee et al., 2010; Naito et al., 2005). In this report, inhibition of GSK3 caused down regulation of Akt, suggesting that GSK3 may positively regulate Akt in CTLs. This seems to be a negative feedback loop, but more studies are needed.
The balance of T-bet and eomes expression is associated with memory differentiation (Intlekofer et al., 2005). T-bet and eomes are inversely regulated by IL-12 (Takemoto et al., 2006). Enhanced memory programming by inhibition of mTOR is associated with increased eomes expression, but decreased T-bet expression (Rao et al., 2010). Interestingly, enforced expression of eomes partially restored defective memory caused by TCF1 deficiency (Zhou et al., 2010), further confirming the critical role of eomes in memory CTL generation (D'Cruz et al., 2009; Intlekofer et al., 2005). Wnt activation during CTL activation did increase eomes expression (Fig. 3A), consistent with a recent report that wnt signal molecule TCF-1 binds to the eomes promoter region leads to its expression (Zhou et al., 2010). Wnt reduced T-bet expression (Fig. 3A), skewing the balance to eomes >T-bet (Rao et al., 2010). Although rapamycin also skewed the balance to eomes, it did not abolish memory programming by IL-12. Thus, the overall effect of rapamycin was the opposite of wnt activation which abolished the memory programming by IL-12 (Fig. 4B). There are two possibilities for this discrepancy. The ratio of eomes to T-bet may need to occur for longer than the 3-day window in our study or be present at a certain stage of activation and programming. Nevertheless, the balance of eomes to T-bet may not be an accurate indicator for memory differentiation (Rao et al., 2010; Takemoto et al., 2006). Lastly, the negative effects of wnt signaling on memory CTL programming have consequences for survival, as the activated CTLs disappeared shortly after transfer (day 5). This is similar to CTLs activated with only 2SI (Xiao et al., 2009). While the molecular mechanisms remain unclear, we found no obvious evidence of differential expression of PD1 and Annexin V binding (Fig. 2B). Annexin V binds to phosphatidylserine (PS) on the outside of the cell membrane. PS is unusually localized along the cytosolic side of plasma membrane through flippase-mediated inward-directed PS transportation (Devaux, 1992). Apoptotic cells lose their asymmetric PS distribution in the lipid bilayer, so PS can translocate to the extracellular side of plasma membrane, which can be detected by Annexin V binding (Zhou, 2007). However, the activation of human CTLs by antigens induces the exposure of PS on the cell surface by inhibiting the flippase-mediated transportation (Fischer et al., 2006). In addition, P53-induced Annexin V positive cells can be rescued (Geske et al., 2001) possibly through regulation of mRNA decay (Melanson et al., 2011). Although future research is warranted, we believe that the strong Annexin V staining for activated CTLs is not directly related to apoptosis. In summary, activation of wnt signaling inhibits CTL activation and memory CTL programming by IL-12, which could be one of the potential mechanisms for tumor evasion of CTL immunity. Targeting wnt signaling in cancer patients may be beneficial for the improvement of cancer-specific CTLs in addition to direct tumor control (Burgess et al., 2011; Chen et al., 2009; Song et al., 2011). Furthermore, activation of wnt signaling may be an alternative approach for manipulation of detrimental autoimmune CTLs such as in type I diabetes (Tsai et al., 2010; Wong et al., 2009; Zaccone and Cooke, 2010).
CONCLUSIONS
Wnt signaling impairs IL-12-driven CTL activation and memory programming, which leads to abolishment of memory CTLs. This may be related to down regulation of T-bet and mTOR pathway. This knowledge may have important implications for CTL manipulation in autoimmune diseases and immune therapy for certain cancers.
Highlights.
Wnt signaling inhibits CTL activation and memory programming.
Wnt signaling down regulates T-bet expression.
Impaired memory CTL programming by wnt can be partially rescued by mTOR inhibitor rapamycin.
ACKNOWLEDGEMENTS
We would like to thank Drs. MF Mescher and SC Jameson from the University of Minnesota for providing reagents. The authors have declared that no competing interests exist. This work was supported by National Institutes of Health Grants R21AI095715A (to X. Z.) and Startup from UMD (to X. Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson EC, Wong MH. Caught in the Akt: regulation of Wnt signaling in the intestine. Gastroenterology. 2010;139:718–722. doi: 10.1053/j.gastro.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess AW, Faux MC, Layton MJ, Ramsay RG. Wnt signaling and colon tumorigenesis--a view from the periphery. Exp Cell Res. 2011;317:2748–2758. doi: 10.1016/j.yexcr.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003a;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Lins DC, Johnson CM, Mescher MF. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J Immunol. 2005a;175:4392–4399. doi: 10.4049/jimmunol.175.7.4392. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003b;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005b;174:4465–4459. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- D’Cruz LM, Rubinstein MP, Goldrath AW. Surviving the crash: transitioning from effector to memory CD8+ T cell. Semin Immunol. 2009;21:92–98. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux PF. Protein involvement in transmembrane lipid asymmetry. Annual review of biophysics and biomolecular structure. 1992;21:417–439. doi: 10.1146/annurev.bb.21.060192.002221. [DOI] [PubMed] [Google Scholar]
- Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- Driessens G, Zheng Y, Gajewski TF. Beta-catenin does not regulate memory T cell phenotype. Nat Med. 2010;16:513–514. doi: 10.1038/nm0510-513. author reply 514-5. [DOI] [PubMed] [Google Scholar]
- Driessens G, Zheng Y, Locke F, Cannon JL, Gounari F, Gajewski TF. Beta-catenin inhibits T cell activation by selective interference with linker for activation of T cells-phospholipase C-gamma1 phosphorylation. J Immunol. 2011;186:784–790. doi: 10.4049/jimmunol.1001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman DL, Davis M. The primacy of CD8 T lymphocytes in type 1 diabetes and implications for therapies. J Mol Med. 2009;87:1173–1178. doi: 10.1007/s00109-009-0516-6. [DOI] [PubMed] [Google Scholar]
- Fischer K, Voelkl S, Berger J, Andreesen R, Pomorski T, Mackensen A. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood. 2006;108:4094–4101. doi: 10.1182/blood-2006-03-011742. [DOI] [PubMed] [Google Scholar]
- Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66:132–141. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci Transl Med. 2009a;1:11. doi: 10.1126/scitranslmed.3000302. ps12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009b;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geske FJ, Lieberman R, Strange R, Gerschenson LE. Early stages of p53-induced apoptosis are reversible. Cell death and differentiation. 2001;8:182–191. doi: 10.1038/sj.cdd.4400786. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Hogquist K, Jameson S, Heath W, Howard J, Bevan M, Carbone F. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010a;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010b doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- King TD, Zhang W, Suto MJ, Li Y. Frizzled7 as an emerging target for cancer therapy. Cell Signal. 2011 doi: 10.1016/j.cellsig.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba H, Ghosh P, Derin R, Buchholz M, Sasaki C, Madara K, Longo DL. Interleukin-12-induced interferon-gamma production by human peripheral blood T cells is regulated by mammalian target of rapamycin (mTOR) J Biol Chem. 2005;280:1037–1043. doi: 10.1074/jbc.M405204200. [DOI] [PubMed] [Google Scholar]
- Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM, Weber CR, Turner JR, He XC, Katzman RB, Li L, Barrett TA. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–881. 881, e1–e9. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Garcia K, Sun Z, Xiao Z. Temporal Regulation of Rapamycin on Memory CTL Programming by IL-12. PLoS ONE. 2011;6:e25177. doi: 10.1371/journal.pone.0025177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson BD, Bose R, Hamill JD, Marcellus KA, Pan EF, McKay BC. The role of mRNA decay in p53-induced gene expression. RNA (New York, N.Y.) 2011;17:2222–2234. doi: 10.1261/rna.030122.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H, Komuro I. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97:144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- Najdi R, Holcombe RF, Waterman ML. Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog. 2011;10:5. doi: 10.4103/1477-3163.78111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JM, Parish NM, Bland C, Sawyer Y, De La Pena H, Cooke A. Type 1 Diabetes Development Requires Both CD4+ and CD8+ T cells and Can Be Reversed by Non-Depleting Antibodies Targeting Both T Cell Populations. Rev Diabet Stud. 2009;6:97–103. doi: 10.1900/RDS.2009.6.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Reya T, O’Riordan M, Okamura R, Devaney E, Willert K, Nusse R, Grosschedl R. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity. 2000;13:15–24. doi: 10.1016/s1074-7613(00)00004-2. [DOI] [PubMed] [Google Scholar]
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Sievers E, Endo T, Lu D, Carson D, Schmidt-Wolf IG. Targeting Wnt pathway in lymphoma and myeloma cells. Br J Haematol. 2009;144:796–798. doi: 10.1111/j.1365-2141.2008.07503.x. [DOI] [PubMed] [Google Scholar]
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- Skowera A, Ellis RJ, Varela-Calvino R, Arif S, Huang GC, Van-Krinks C, Zaremba A, Rackham C, Allen JS, Tree TI, Zhao M, Dayan CM, Sewell AK, Unger WW, Drijfhout JW, Ossendorp F, Roep BO, Peakman M. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Christova T, Perusini S, Alizadeh S, Bao RY, Miller BW, Hurren R, Jitkova Y, Gronda M, Isaac M, Joseph B, Subramaniam R, Aman A, Chau A, Hogge DE, Weir SJ, Kasper J, Schimmer AD, Al-Awar R, Wrana JL, Attisano L. Wnt Inhibitor Screen Reveals Iron Dependence of beta-Catenin Signaling in Cancers. Cancer Res. 2011;71:7628–7639. doi: 10.1158/0008-5472.CAN-11-2745. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- Tsai S, Shameli A, Santamaria P. CD8+ T cells in type 1 diabetes. Adv Immunol. 2008;100:79–124. doi: 10.1016/S0065-2776(08)00804-3. [DOI] [PubMed] [Google Scholar]
- Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, Yang Y, Medarova Z, Moore A, Santamaria P. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Wang Y, Robertson JD, Walcheck B. Different Signaling Pathways Stimulate a Disintegrin and Metalloprotease-17 (ADAM17) in Neutrophils during Apoptosis and Activation. J Biol Chem. 2011;286:38980–38988. doi: 10.1074/jbc.M111.277087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang AC, Ni Z, Herrera A, Walcheck B. ADAM17 activity and other mechanisms of soluble L-selectin production during death receptor-induced leukocyte apoptosis. J Immunol. 2010;184:4447–4454. doi: 10.4049/jimmunol.0902925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176:1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- Wong FS, Siew LK, Scott G, Thomas IJ, Chapman S, Viret C, Wen L. Activation of insulin-reactive CD8 T-cells for development of autoimmune diabetes. Diabetes. 2009;58:1156–1164. doi: 10.2337/db08-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sharma A, Sen JM. TCF1 and beta-catenin regulate T cell development and function. Immunol Res. 2010;47:45–55. doi: 10.1007/s12026-009-8137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccone P, Cooke A. Harnessing CD8(+) regulatory T cells: therapy for type 1 diabetes? Immunity. 2010;32:504–506. doi: 10.1016/j.immuni.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue HH. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue HH. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. New phosphatidylserine receptors: clearance of apoptotic cells and more. Developmental cell. 2007;13:759–760. doi: 10.1016/j.devcel.2007.11.009. [DOI] [PubMed] [Google Scholar]