Abstract
Background
Cystic fibrosis (CF) is a common genetic disease in Caucasians. Chronic pulmonary disease and progressive destruction of the pulmonary parenchyma are one of the major morbidities, but the relationship between clinical severity of CF and aortopulmonary collateral blood flow has not been assessed.
Objective
The purpose of this study is to measure changes in aortopulmonary collateral blood flow by phase-contrast magnetic resonance imaging (MRI) in children with CF across the spectrum of disease severity as measured by the forced expiratory volume in one second as percent predicted value (FEV1%).
Materials and methods
Sixteen patients with CF were prospectively evaluated. Eight were classified as having mild CF lung disease (FEV1 ≥80% predicted) and eight were classified as having moderate to severe CF lung disease (FEV1 <80% predicted). Seventeen age and gender matched non-CF subjects without cardiac or lung disease served as controls. Phase-contrast flow was measured at the ascending aorta, main pulmonary artery and both pulmonary arteries. Aortopulmonary collateral blood flow was calculated for each subject. The relationship between collateral flow and FEV1%P was modeled using nonparametric regression. Group differences were assessed by analysis of variance.
Results
Aortopulmonary collateral blood flow began to increase as FEV1%P in subjects with CF fell below 101.5% with significant further increase in the aortopulmonary collateral blood flow in the subjects with CF with moderate to severe lung disease compared to controls (0.89 vs. 0.20 L/min, (P<0.0001). Aortopulmonary collateral blood flow correlated negatively with FEV1%P (r, 0.70, P=0.0050) confirming its relationship to this established marker of disease severity. There was no statistically significant difference in results obtained from two independent observers.
Conclusion
These preliminary findings suggest that phase-contrast MRI can be performed reliably with consistent results and without interobserver variability. While the aortopulmonary collateral blood flow is within the normal range in subjects with mild CF disease, it begins to increase even when lung function is still in the normal range. A significant increase in the aortopulmonary collateral blood flow compared to controls is measured in patients with moderate to severe CF lung disease. The studies support the notion that aortopulmonary collateral blood flow may serve as a novel and sensitive biomarker of early pulmonary disease in cystic fibrosis.
Keywords: Phase-contrast MRI, Cystic fibrosis, Aortopulmonary ollateral blood flow, FEV1, Cardiac, Pulmonary hypertension, Bronchosystemic shunt, Children
Introduction
Cystic fibrosis (CF) is the most common inherited disease in Caucasians, occurring in one out of every 3,000 live births in the U.S.[1] CF is characterized by chronic pulmonary inflammation, bronchiectasis and progressive loss of gas exchange units, ultimately resulting in respiratory failure. Pulmonary vascular disease in CF typically presents with two distinct clinical pictures: pulmonary hypertension and hemoptysis [2]. Both are associated with a high risk of mortality but are the result of different pathological processes. Hemoptysis occurs due to vascular growth that results in markedly enlarged and tortuous bronchial/aortopulmonary collateral arteries, while pulmonary hypertension results from remodeling of pulmonary vessels and a reduction of the arterial tree. Therefore, CF pulmonary vascular disease is characterized by both vascular regression of the pulmonary vessels and concurrent systemic vascularization of the lungs by aortopulmonary collaterals.
The contribution of airway hypervascularity to airway disease is an area of research that remains under-investigated. In patients with asthma, the bronchial submucosa has an increased number of vessels, occupying a larger percentage of area than in normal subjects [3, 4], not only in the large, but also in the medium and small airways [5]. Biopsy specimens from subjects with mild asthma, as compared to healthy controls, have shown that remodeling of the airway wall involves significant changes of the bronchial vasculature including an increase in vessel numbers and in tissue vascularity [6] due to angiogenesis and, in the more acute setting, vasodilatation [7]. Furthermore, the increase in vascularity is directly proportional to asthma severity [8]. The causal relationship between airway blood flow and airway obstruction has yet to be confirmed, though in the context of asthma there is evidence that reduction of bronchial blood flow decreases antigen-induced airway obstruction [9]. Bronchial vascularity in CF, however, has not been investigated but is highly relevant due to the high morbidity and mortality associated with pulmonary vascular disease in this disorder. The primary objective of this study was to test the hypothesis that the flow through aortopulmonary collateral begins early in the course of CF lung disease and progresses with worsening lung function. Secondary objectives included determination of whether APCBF could be reliably measured with phase-contrast MRI, and to determine whether relationships were present between aortopulmonary collateral blood flow and lung function in CF.
Materials and methods
Subjects
Institutional review board approval was obtained prior to the initiation of the study and informed consent was obtained for all study subjects. The prospective study population consisted of 16 patients with CF. Inclusion criteria included a CF diagnosis based on sweat Cl− > 60 mmol/L or two CF-associated mutations in CF transmembrane conductance regulator with at least one organ system manifestation of CF, age > 6 years, FEV1 > 40% predicted absence of symptoms of acute pulmonary exacerbation, no antibiotic treatment in the 2 weeks that preceded the testing and normal glomerular filtration rate (GFR) measured by cystatin C. Each patient was categorized by lung disease severity based on his/her best FEV1% predicted in the 6 months prior to enrollment. Each patient was studied at her/his pulmonary function baseline, defined as an FEV1% predicted not more than 10% below his/her best value with the 6 months prior to enrollment. Each subject had a pulmonary function test performed within one month of the MRI. Patients with CF were equally divided into 2 groups; those with FEV1 ≥ 80% predicted and those with FEV1 < 80% predicted based on accepted standards defining mild and moderate CF.
Control patients were retrospectively selected from an unidentified general clinical cardiac MRI database. All patients who had undergone outpatient cardiac MRI with flow measurements in the ascending aorta, main pulmonary artery (MPA), right pulmonary artery (RPA) and left pulmonary artery (LPA) were selected; patients with congenital heart disease, abnormal cardiac echocardiogram, history of pulmonary disease, evidence of vascular shunting or poor imaging (artifacts, incorrect selection of velocity or patient motion) were then excluded, leaving 17 patients for comparison. The indication for imaging in the 17 control subjects were cardiomyopathy (6), aortic abnormality (3) and Turner syndrome (8). Turner syndrome can have partial anomalous pulmonary venous return, which was carefully excluded during MRI, but was also the reason thorough flow mapping was available in this group of patients. Additionally, the patients with cardiomyopathy all had normal echocardiograms.
Design and procedures
The design was a case-control study of patients with CF and historical controls. All MRI studies were performed without sedation on a 1.5-T imaging system (Signa Excite; GE Healthcare, Milwaukee, WI) with an 8-channel receive-only phased-array cardiac coil (USA Instruments, Aurora, OH). Free-breathing ECG-gated (retrospective) flow sensitive through plane phase-contrast gradient-echo sequences were obtained in the ascending aorta, MPA, and RPA and LPA (Fig. 1). Typical scan parameters for the phase-contrast images were TR/TE: 9.8/4.2, FOV: 36 cm, slice thickness: 5mm, matrix 256 × 128, NEX: 3, flip angle: 20°, views per segment: 2, receiver bandwidth 177.6 Hz/pixel, baseline resolution: 256, phase resolution: 128, and temporal resolution: 25 ms/frame. The phase-contrast sequence was performed through a plane perpendicular to each vessel (velocity encoding: 150 cm/sec). The 2D flow mapping requires approximately 10 min of imaging time.
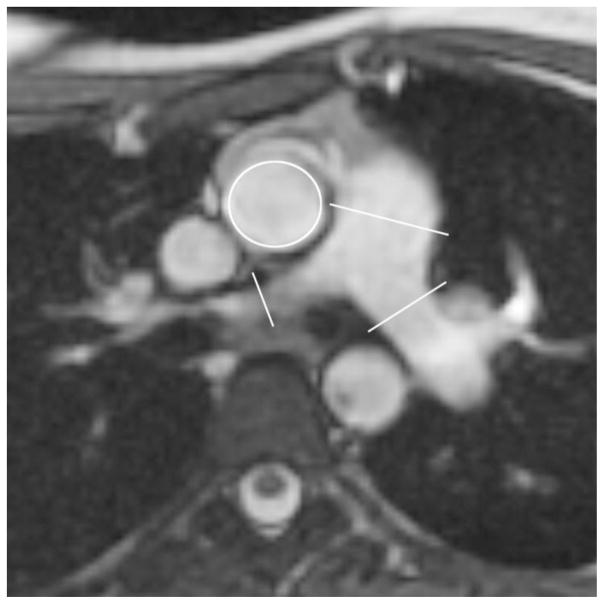
Fig. 1.
Axial non-cardiac-gated FIESTA demonstrates the planes of phase-contrast MRI on the ascending aorta, MPA, RPA and LPA. Two orthogonal planes were used to plan each phase-contrast sequence to ensure measurements were perpendicular to the direction of flow
Flow quantification analysis of the phase-contrast flow sequence data was performed using QFLOW® version 5.1(Medis Medical Imaging Systems, Leiden, The Netherlands). Each vessel was contoured semiautomatically by two observers throughout the cardiac cycle, and then manually adjusted for phase variance and respiratory motion. The observers performed the contours independently and were blinded to the patient condition and clinical status. Blood flow in liters per minute was used to calculate the APCBF. Flow measurements in the ascending aorta were obtained above the coronary artery originations so 5 percent of the total measured ascending aorta flow was added to account for the coronary artery flow [10]. In a normal patient, the flow through the aorta and main pulmonary artery should be equivalent. When aortopulmonary collaterals develop, the flow from the aorta must increase to supply the blood flowing from the aorta to the pulmonary parenchyma. Therefore, the aortopulmonary blood flow is equal to the difference of aortic blood flow and pulmonary blood flow. The following equations were used to calculate APCBF: measured ascending aorta flow (L/min) × 1.05 – measured flow in the MPA, and measured ascending aorta flow (L/min) × 1.05 - measured flow (RPA +LPA). Both equations were used for each patient; the mean of the two values was treated as the observed APCBF. As an internal validation of flow measurements, the flow in the ascending aorta was compared to the sum of the flows in the descending aorta and the superior vena cava (SVC), which could easily be measured on the phase-contrast image of the ascending aorta (Fig. 2).
Fig. 2.
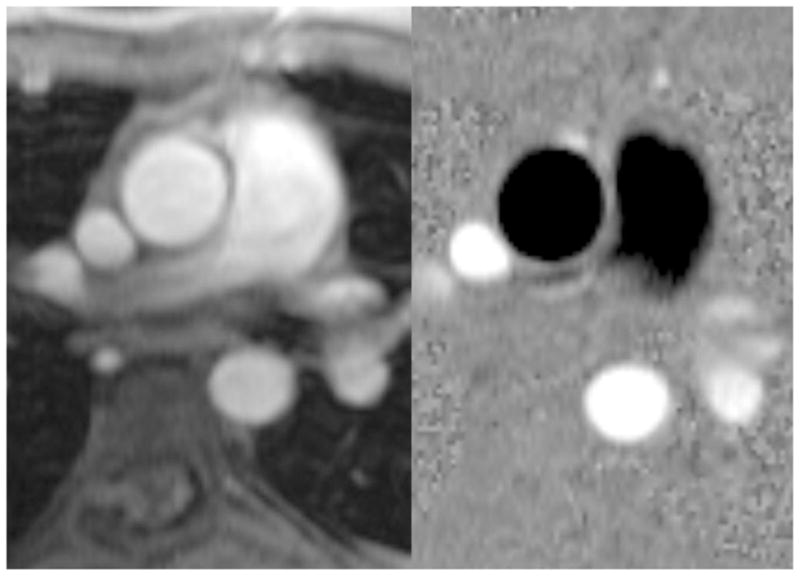
Phase-contrast anatomy (a) and flow (b) images in the axial plane perpendicular to the ascending aorta
Statistical analysis
Descriptive analysis was performed with calculation of means, standard deviations, medians for age, FEV1% predicted and APCBF. Proportion of males across groups was compared using Fisher exact test. Reliability of APCBF measurements between two observers was assessed using Bland-Altman analysis [11] and the intraclass correlation coefficient (ICC). The ICC was defined as the ratio of within-patient variance to the sum of within- and between-patient variances [12]. Internal validation of flow measurements was performed using bivariate analysis with Spearman correlations. We compared APCBF for each of the two groups of patients with CF (mild and moderate/severe) with the control group using analysis of variance (ANOVA). Pair-wise comparisons of least-squares means were made using t-tests. Exploratory analysis, including scatterplot smoothing, of the shape of the relationship between FEV1% predicted and APCBF indicated a curvilinear pattern. To account for the nonlinearity, FEV1% predicted was modeled as a function of APCBF using least-squares methods for regression splines [13].
Descriptive statistics, intraclass correlation and ANOVA models were implemented in SAS 9.2 (SAS Institute, Cary, NC). Scatterplot smoothing and least-squares fitting for regression splines were conducted in R 12.2.0 (The R Foundation for Statistical Computing, Vienna, Austria). Results are reported as mean ± standard error (SE) or 95% confidence interval (CI), unless stated otherwise. For this pilot study, p-values less than 0.05 were considered statistically significant; p-values between 0.05 and 0.10 were considered as demonstrating a trend toward statistical significance.
Results
Subjects
Table I summarizes the demographic factors for the study population. Sixteen subjects with CF were enrolled in the study. Half of the study population had FEV1 ≥ 80% predicted and the otherhalf had FEV1 < 80% predicted. APCBF measurements were obtained in all but one CF subject with FEV1 <80% predicted due to software failure during scanning. Another CF subject with FEV1 > 80% predicted had an outlying value of APCBF and was excluded from all subsequent analyses and the reason for exclusion is explained below.
Table 1.
Patient characteristics based on disease severity. Continuous data expressed as mean ± SD (n)
| Controls | Mild CF | Moderate/severe CF | |
|---|---|---|---|
| Male, % | 41.1% (7/17) | 85.7% (6/7) | 37.5% (3/8) |
| Age (years) | 18.6±6.9 (17) | 14.8±2.0 (7)^* | 18.0±0.8 (8) |
| FEV1 (% predicted) | --- | 103.6±10.4 (8)^^‡ | 56.6±11.6 (8) |
| Collateral Flow (L/min) | 0.20±0.13 (17) | 0.05±0.24 (7)^^‡ | 0.89±0.67 (7)^^† |
| Height (cm) | 143.1±21.4 (16) | 165.5±13.7 (7)^^* | 165.9±8.2 (8)^^† |
| Weight (kg) | 49.1±18.6 (16) | 57.7±11.6 (7) | 56.3±10.4 (8) |
| BMI | 23.1±4.8 (16) | 20.9±1.7 (7) | 20.4±2.6 (8) |
| BMI z-score | 0.65±0.90 (9) | 0.32±0.60 (7) | −0.57±1.07 (8) |
| BSA (m2) | 1.37±0.36 (16) | 1.63±0.23 (7)^* | 1.62±0.17 (8)^† |
| Heart rate (beats/min) | 84.1±17.8 (17) | 75.3±16.5 (6) | 90.6±15.1 (8) |
^0.05 ≤p <0.010 and ^^p < 0.05 for mild CF versus control (*), moderate/severe versus control (†), mild versus moderate/severe (‡)
In the group of patients with mild CF, FEV1 ranged from 90 to 116% predicted and in the group of patients with moderate/severe CF, FEV1 ranged from 40 to 77% predicted.
Inter-observer agreement
There was no statistically significant difference between the two independent observers in the measurement of APCBF. Interrater reliability was high (ICC, 0.85). Mean difference between raters was not significant based on Bland-Altman limits of agreement (difference, −0.15 L/min; 95%CI, −0.37 to 0.07; P=0.17).
Internal validation of flow measurements
Ascending aorta flow and SVC combined with descending aorta flow were significantly correlated (coefficient, 0.75, P=0.0018). Ascending aorta flow was also significantly correlated with cardiac output as measured by ventricular volume analysis (coefficient, 0.70, P=0.0034).
Agreement between equations to measure APCBF
There was no statistically significant difference in the APCBF values obtained by using the MPA flow measurement as opposed to using the sum of the LPA and RPA flow measurements. Agreement between flow measured in the MPA and (RPA+LPA) was also high (ICC, 0.92) and was within acceptable limits (difference, 0.03 L/min, −0.20 to 0.25; P=0.82).
Aortopulmonary collateral blood flow
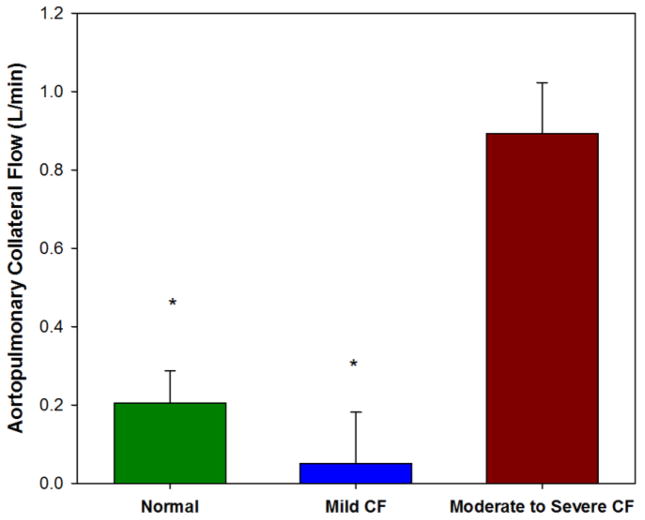
Subjects with moderate to severe disease had significantly higher APBCF compared to controls with least-square means difference of 0.69 ± 0.15, P=0.0001 and higher than subjects with mild disease with least-square means difference of 0.84±0.18, P<0.0001. These group differences remained statistically significant after adjusting for age, weight, height, BMI and BMI z-score. None of the covariates approached significance when included in the model (height: P=0.94, age: P=0.84, weight: P=0.99, BMI: P=0.75, BMI z-score: P=0.47). Subjects with mild disease had similar mean APCBF as controls with least-square means of 0.15 ± 0.0.15, P=0.33 (Fig. 3).
Fig. 3.
Aortopulmonary collateral blood flow results in the control group and each of the two CF groups. The moderate to severe CF group had significantly greater aortopulmonary collateral flow than the control group and compared to the mild group (P<0.0001)
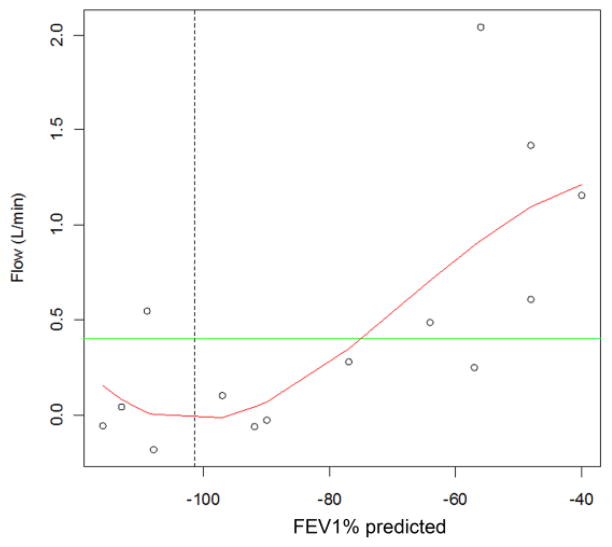
APCBF correlated negatively with FEV1% predicted (Spearman correlation: 0.70, P=0.0050). The observed increase in APCBF was not constant across lung function. The curvilinear fit based on regression splines was statistically significant for subjects with CF (R2=0.55, P= 0.0389) (Fig. 4). The regression line showed a point of inflection at FEV1 of 101.5% predicted where APCBF began to increase rapidly in a nearly linear fashion with worsening lung function (Fig. 4). A sensitivity analysis that included the aforementioned subject (a 13-year-old girl) with an outlying APCBF measurement showed similar results. The overall shape of the curve still showed an inflection point at the higher values of FEV1% predicted, but flow at this inflection point was higher. Aside from APCBF, statistical results that included data from this subject were similar to findings from the primary analyses. This child was discovered to have had hemoptysis severe enough to warrant a CT angiogram 11 months prior to this study. Review of the CT shows extensive right upper lobe disease with large aortopulmonary collaterals despite a FEV1% predicted of 95%. The discordant result of CT, which was not used as an inclusion/exclusion criterion, with FEV1% predicted leads us to believe this is a false-negative FEV1%; therefore, we excluded this patient.
Fig. 4.
Aortopulmonary collateral blood (APCBF) flow correlation to FEV1% predicted in CF patients. Curve represents 15 CF patients fit based on regression splines (adjusted R2 = 0.55, P=0.0389). Green line represents upper limits of normal collateral flow in the normal controls, vertical dashed line represents the inflection point at FEV1% predicted = 101.5%. There is a non-linear relationship of APCBF as the FEV1% predicted decreases
Discussion
In this study, we examined APCBF in a pilot cohort of patients with CF compared with non-CF historical controls. This included a new non-invasive MR-based approach to assess aortopulmonary collaterals. The results led to novel findings regarding the course of systemic vascularization of the lungs in patients with CF. The study identified significant differences in APCBF in patients with CF with less than normal function (FEV1 % predicted <80%) compared with non-CF controls, The data also suggests that the process leading to increased collateral blood flow begins very early in the course of the disease, before abnormalities in lung function were detected.
The study uses a new non-invasive MR based approach to estimate aortopulmonary collaterals leading to novel findings about the course of systemic vascularization of the lungs in patients with CF. While differences in APCBF significantly exceed the normal range only in those patients with moderate to severe disease, the data suggest that the process of increasing collateral flow begins very early in the course of the disease when lung function is normal.
We demonstrated that measurements of APCBF obtained using phase-contrast MRI are reproducible, as shown by the consistency between two independent observers. However, there is no existing reference standard for the measurement of APCBF, but phase-contrast MRI may provide the least invasive and most reproducible technique. The primary advantage of CT over MRI to image pulmonary disease in CF is the higher resolution of lung anatomy that can be correlated to the patients’ clinical course. While MR anatomical imaging lacks the spatial resolution of CT, it can provide novel functional and physiological information (without radiation exposure) that may be essential in understanding the pathophysiology of chronic lung diseases. Specifically, MRI has the capacity to provide detailed knowledge about the hemodynamic changes of pulmonary and bronchial circulations [14], the two sources of blood supply to the lungs. In this report, we examined the role of phase-contrast MRI in measuring APCBF (bronchosystemic shunts) across the spectrum of severity of CF lung disease.
Our data revealed a significantly higher APCBF in patients with CF with a FEV1% predicted <80% compared to healthy controls. The magnitude of APCBF we observed in our study was smaller than that reported by Ley et al. [14] where the flow volumes reached up to 1.3 L/min. This difference may reflect the older average age of the study population in Ley’s study relative to our population (29 years vs. 16 years). By combining our data with that of Ley, we can assume that systemic vascularization of the lungs by aortopulmonary collaterals in CF is not only dependent on disease severity but also on age and body size. In subjects with FEV1% predicted >80%, APCBF was not significantly different from healthy controls, but began to accelerate at as lung function fell below 100%. Based on the data presented in this manuscript, this inflection point was around an FEV1%p of 101.5%p (Fig. 4). This observation is of major interest because it suggests that the processes that lead to increasing APCBF may begin well before significant pulmonary damage or systemic hypoxia can be detected. It is yet to be determined whether the early changes in APCBF are markers of disease progression in CF or actually contribute to progressive airway obstruction. In the context of lung transplantation, Sweet et al. [15] reported that the presence of aortopulmonary collateral vessels increased the risk of early mortality 20-fold post-lung transplantation. These prognostic APCBF data suggest that indeed collateral flow may serve as a disease marker and contribute to disease progression. The non-invasive estimation of aortopulmonary collaterals by MRI might prove to be a valuable parameter of disease progression before detectable decline in lung function. This may be particularly important in children (i.e.: in infants and children under age 6, where lung function measurements are impractical) since this technique can be applied to all ages. It also has the potential to serve as a future validated tool to investigate disease mechanisms.
There are multiple mechanisms of proliferation of aortopulmonary collateral vessels. Knowledge gained from animal models indicates that loss of pulmonary blood flow is associated with increased angiogenesis in the bronchial circulation [16–18]. Further, restoration of pulmonary blood flow leads to reversal of pathological angiogenesis and regression of the bronchial vessels [19–22]. In human diseases, such as pulmonary atresia or unilateral absence of the pulmonary artery, systemic-to-pulmonary collateral vessels are universal findings [23]. These observations lead us to the hypothesis that in CF, loss of pulmonary perfusion leads to the systemic vascularization of the lungs through the bronchial circulatory system. Inflammation, a central component of the CF lung pathology, is known to be an important mediator of vascular pathology. Several inflammatory mediators have the capacity to influence vascular growth. Among these, interleukin-8 is a pro-inflammatory cytokine that is present in high concentration in CF airway secretions and correlates with CF lung function. The angiogenic effect of interleukin-8 has been described in the seminal work described by Koch et al. [24]. It is also a well-described pro-angiogenic factor in other conditions, Interleukin-8 related angiogenesis has been described in human chronic lung diseases, such as in idiopathic pulmonary fibrosis [25]. Belperio et al. [26] extended this knowledge by not only demonstrating that interleukin-8 causes angiogenesis in bronchiolitis obliterans syndrome, but by also showing that angiogenesis predicted the future development of fibro-obliteration of the airways. Vascular endothelial growth factor (another factor that is upregulated in CF) also affects several functional properties of the endothelium such as nitric oxide and prostacyclin synthesis that can lead to pulmonary vasodilatation and angiogenesis [27–29]. These and additional signaling molecules in CF may contribute to the APCBF abnormalities observed, and studies of their angiogenic properties may provide insight to novel aspects of CF disease pathogenesis.
There are several limitations present in this pilot study that should be noted. First, while mean ages for the CF and non-CF study groups are similar (adolescence), we did not age match with the historical control subjects. In addition, as the phase-contrast MRI data for the control group was retrospectively obtained from a clinical cardiac MRI database, these patients did not have lung function captured. Because none of the control patients had a documented history of pulmonary disease, it is reasonable to assume that as a group they had normal lung function. Second, measurement of pulmonary blood flow was not confirmed using an independent standard, but phase-contrast velocity encoded imaging is well accepted in cardiac imaging. We did perform an internal control measuring the flow in the descending thoracic aorta and the superior vena cava to correlate with the flow of the ascending aorta. Finally, we did not perform any baseline correction for phase offset errors, which have been identified as a possible source of error in the current literature [30, 31]. However, few cardiac imagers utilize phantom correction techniques in clinical practice. Despite these limitations, the results provide preliminary data describing changes in APCBF early in CF lung disease and support future prospective trials to validate these findings in CF and non-CF control populations. Based on these results, future examination of APCBF related to morphological pulmonary CF changes, including comparisons with MRI and concomitant CT, may be warranted. Larger studies with more patients, longer follow-up and baseline correction for phase offset errors or 3-D flow measurements simultaneously in all vessels could optimize technical performance.
Conclusion
Aortopulmonary collateral blood flow in CF is increased in CF patients with FEV1% predicted of <80%, and this increased flow begins as FEV1% falls below 100%. Phase-contrast MRI can be performed reliably with consistent results without interobserver variability. These data provide support for future studies to better define APCBF abnormalities in CF, both to evaluate its role in disease pathogenesis and as a potential biomarker of disease.
Abbreviations
- APCBF
aortopulmonary collateral blood flow
- ANOVA
analysis of variance
- CF
cystic fibrosis
- CI
95% confidence interval
- FEV1%
forced expiratory volume in one second (percent predicted)
- FEF25–75%
forced expiratory flow25–75% (percent predicted)
- FVC
forced vital capacity
- ICC
intraclass correlation coefficient
Footnotes
Conflicts of interest
None
Contributor Information
Robert Fleck, Email: Robert.Fleck@cchmc.org, Department of Pediatric Radiology, Cincinnati Children’s Hospital – MLC 5031, 3333 Burnett Avenue, Cincinnati, OH 45229, USA.
Gary McPhail, Department of Pulmonary Medicine, Cincinnati Children’s Hospital, Cincinnati OH.
Rhonda VanDyke, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital, Cincinnati, OH.
Joshua Knowlton, University of Missouri at Kansas City, School of Medicine, Children’s Mercy Hospital and Clinics, Kansas City, MO.
Rupa Radhakrishnan, Department of Radiology, University of Cincinnati College of Medicine, Cincinnati, OH.
John Clancy, Department of Pulmonary Medicine, Cincinnati Children’s Hospital, Cincinnati OH.
Raouf Amin, Department of Pulmonary Medicine, Cincinnati Children’s Hospital, Cincinnati OH.
References
- 1.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.Fraser KL, Tullis DE, Sasson Z, et al. Pulmonary hypertension and cardiac function in adult cystic fibrosis: role of hypoxemia. Chest. 1999;115:1321–1328. doi: 10.1378/chest.115.5.1321. [DOI] [PubMed] [Google Scholar]
- 3.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 4.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax. 2001;56:902–906. doi: 10.1136/thorax.56.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto M, Tanaka H, Abe S. Quantitative analysis of bronchial wall vascularity in the medium and small airways of patients with asthma and COPD. Chest. 2005;127:965–972. doi: 10.1378/chest.127.3.965. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 7.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 8.Vrugt B, Wilson S, Bron A, et al. Bronchial angiogenesis in severe glucocorticoid-dependent asthma. Eur Respir J. 2000;15:1014–1021. doi: 10.1034/j.1399-3003.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 9.Csete ME, Chediak AD, Abraham WM, et al. Airway blood flow modifies allergic airway smooth muscle contraction. Am Rev Respir Dis. 1991;144:59–63. doi: 10.1164/ajrccm/144.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Bogren HG, Klipstein RH, Firmin DN, et al. Quantitation of antegrade and retrograde blood flow in the human aorta by magnetic resonance velocity mapping. Am Heart J. 1989;117:1214–1222. doi: 10.1016/0002-8703(89)90399-2. [DOI] [PubMed] [Google Scholar]
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 12.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay JO. Functional data analysis. 2. Springer; New York: 2005. [Google Scholar]
- 14.Ley S, Puderbach M, Fink C, et al. Assessment of hemodynamic changes in the systemic and pulmonary arterial circulation in patients with cystic fibrosis using phase-contrast MRI. Eur Radiol. 2005;15:1575–1580. doi: 10.1007/s00330-005-2721-1. [DOI] [PubMed] [Google Scholar]
- 15.Sweet SC, Spray TL, Huddleston CB, et al. Pediatric lung transplantation at St. Louis Children’s Hospital, 1990–1995. Am J Respir Crit Care Med. 1997;155:1027–1035. doi: 10.1164/ajrccm.155.3.9116982. [DOI] [PubMed] [Google Scholar]
- 16.Remy J, Deschildre F, Artaud D, et al. Bronchial arteries in the pig before and after permanent pulmonary artery occlusion. Invest Radiol. 1997;32:218–224. doi: 10.1097/00004424-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 17.McColley SA, Stellmach V, Boas SR, et al. Serum vascular endothelial growth factor is elevated in cystic fibrosis and decreases with treatment of acute pulmonary exacerbation. Am J Respir Crit Care Med. 2000;161:1877–1880. doi: 10.1164/ajrccm.161.6.9905022. [DOI] [PubMed] [Google Scholar]
- 18.Charan NB, Carvalho P. Angiogenesis in bronchial circulatory system after unilateral pulmonary artery obstruction. J Appl Physiol. 1997;82:284–291. doi: 10.1152/jappl.1997.82.1.284. [DOI] [PubMed] [Google Scholar]
- 19.Watts KD, McColley SA. Elevated vascular endothelial growth factor is correlated with elevated erythropoietin in stable, young cystic fibrosis patients. Pediatr pulmonol. 2011;46:683–687. doi: 10.1002/ppul.21428. [DOI] [PubMed] [Google Scholar]
- 20.Verhaeghe C, Tabruyn SP, Oury C, et al. Intrinsic pro-angiogenic status of cystic fibrosis airway epithelial cells. Biochemical and biophysical research communications. Biochem Biophys Res Commun. 2007;356:745–749. doi: 10.1016/j.bbrc.2007.02.166. [DOI] [PubMed] [Google Scholar]
- 21.Fadel E, Mazmanian GM, Chapelier A, et al. Lung reperfusion injury after chronic or acute unilateral pulmonary artery occlusion. Am J Respir Crit Care Med. 1998;157:1294–1300. doi: 10.1164/ajrccm.157.4.9707063. [DOI] [PubMed] [Google Scholar]
- 22.Fadel E, Wijtenburg E, Michel R, et al. Regression of the systemic vasculature to the lung after removal of pulmonary artery obstruction. Am J Respir Crit Care Med. 2006;173:345–349. doi: 10.1164/rccm.200506-894OC. [DOI] [PubMed] [Google Scholar]
- 23.Norgaard MA, Alphonso N, Cochrane AD, et al. Major aorto-pulmonary collateral arteries of patients with pulmonary atresia and ventricular septal defect are dilated bronchial arteries. Eur J Cardiothorac Surg. 2006;29:653–658. doi: 10.1016/j.ejcts.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 24.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 25.Keane MP, Arenberg DA, Lynch JP, 3rd, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159:1437–1443. [PubMed] [Google Scholar]
- 26.Belperio JA, Keane MP, Burdick MD, et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115:1150–1162. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku DD, Zaleski JK, Liu S, et al. Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am J Physiol. 1993;265:H586–592. doi: 10.1152/ajpheart.1993.265.2.H586. [DOI] [PubMed] [Google Scholar]
- 28.He H, Venema VJ, Gu X, et al. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 29.Tsurumi Y, Murohara T, Krasinski K, et al. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- 30.Lew CD, Alley MT, Bammer R, et al. Peak velocity and flow quantification validation for sensitivity-encoded phase-contrast MR imaging. Acad Radiol. 2007;14:258–269. doi: 10.1016/j.acra.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chernobelsky A, Shubayev O, Comeau CR, et al. Baseline correction of phase contrast images improves quantification of blood flow in the great vessels. J Cardiovasc Magn Reson. 2007;9:681–685. doi: 10.1080/10976640601187588. [DOI] [PubMed] [Google Scholar]