Abstract
This mini-review provides an overview of MRI applications to study rodent, cat, non-human primate and human retinas. These techniques include T1- and T2-weighted anatomical, diffusion, blood flow, blood volume, blood-oxygenation level dependent (BOLD), manganese-enhanced, physiological and functional MRI. Applications to study the retinas in diabetic retinopathy, glaucoma, and retinal degeneration are also reviewed. MRI offers some unique advantages compared to existing imaging techniques and has the potential to further our understanding of physiology and function in healthy and diseased retinas.
Keywords: BOLD, choroid, blood flow, blood volume, contrast-enhanced MRI, manganese-enhanced MRI, diabetic retinopathy, glaucoma, retinal degeneration, age-related macular degeneration, ocular imaging, optical imaging, eye motion, BOLD fMRI, visual stimulation
Retinal anatomy and physiology
Diabetic retinopathy, glaucoma, and age-related macular degeneration are the leading causes of blindness affecting the retina. The neural retina (~200µm thick) consists of 8 major layers: the ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, outer nuclear layer, inner segments, outer segments, and the retinal pigment epithelium (1).
The retina is nourished by two separate circulations (1, 2): the retinal and the choroidal vessels. The retinal vessels are localized in the ganglion cell layer with retinal capillaries projected down into the inner nuclear and inner plexiform layers. The choroidal vasculature is located outside the neural retina between the retinal pigment epithelium and the sclera. The avascular layer (~100µm) in between the retinal and choroid layers consists of the outer plexiform layer, outer nuclear retina, inner and outer photoreceptor segments. Retinal blood vessels have tight-gap junctions, constituting the blood-retinal barrier similar to the blood-brain barrier. By contrast, the choroidal vessels are porous, relying on the retinal pigment epithelium to act as a barrier. Basal retinal and choroidal blood flow, and their responses to stimuli differ substantially from each other (1). The arterio-venous oxygen saturation difference of the retinal circulation is similar to the brain (~50%) but that of the choroidal circulation is very small (3–5%). Moreover, the choroid is innervated by the autonomic system but the retina is not (2, 3).
Anatomical MRI
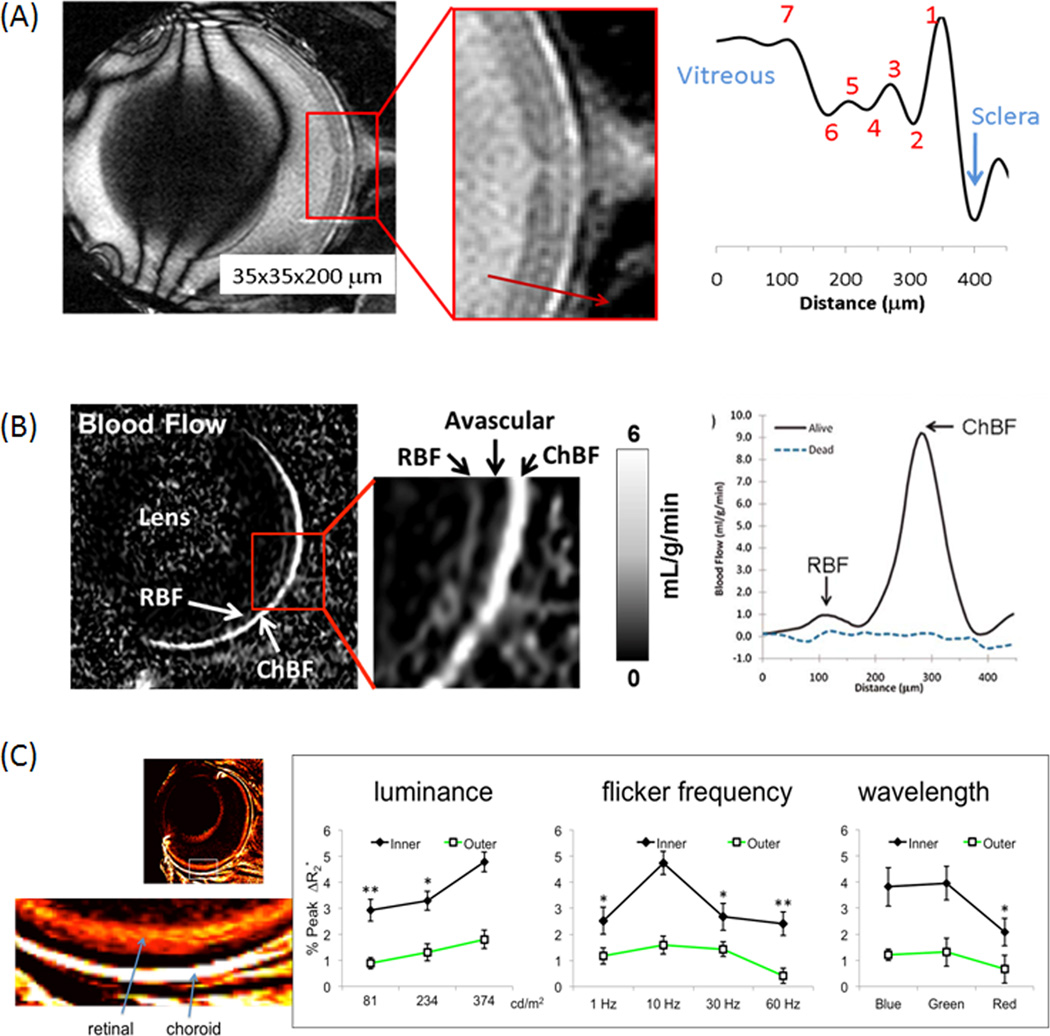
Ex vivo MRI can visualize most of the anatomical retinal layers (4) mentioned above. In live animals, protocols to achieve optimal animal preparation, anesthesia and post-processing motion corrections have been detailed (5, 6). In vivo anatomical MRI of the rodent retinas resolves multiple (3 to 5) anatomical layers based on T1 (7–10), T2 (7–10), diffusion (7,9,10), and balanced-steady-state free precession (bSSFP) (11) contrasts with an in-plane spatial resolution up to 20×20µm. A representative anatomical MRI with and without Gd-DTPA (gadolinium diethylenetriaminepentacetate) from a cat eye showed three layers on pre-contrast MRI. Post-contrast MRI and the difference image delineate the retinal and choroidal vascular layers, and the avascular in-between because the retinal vessels and the retinal pigment epithelium are impermeable to Gd-DTPA. The ciliary bodies are permeable to Gd-DTPA, and thus the anterior chamber is enhanced. With bSSFP acquisition, remarkable resolution and contrast among different retinal layers can be obtained without using an exogenous contrast agent (Figure 1A) (11).
Figure 1.
(A) In vivo bSSFP MRI of the mouse retina at 45×45×500µm. Contrasts among different retinal layers are observed without exogenous contrast agents. Adapted from (11). (B) Layer-specific blood-flow image and blood-flow profile from a live mouse under 1.1% isoflurane and a dead mouse in the same setting (same animal) at 42×42×400µm. Blood flow was acquired using continuous arterial spin labeling with a separate labeling coil at the neck position. chBF – choroid blood flow, rBF – retinal blood flow. Adapted from (14). (C) Layer-specific blood-volume (MION) weighted images and fMRI responses to graded luminance, flicker frequency and wavelength (color) at 60×60×1000µm nominal resolution (mean ± SEM, N=7). Adapted from (22).
T1, T2 and apparent diffusion coefficients of the neural retina are similar to those of the brain but those of the choroid are higher (7–10). Moreover, there are diffusion anisotropy differences among different layers of the retina (7,9,10). The thickness of the neural retina ranges from 200 to 300µm across different species and it has been reported in mouse (10,11), rat (8), cat (7), baboon (12), and human (13) by MRI. The thicknesses of the neural retinal and choroid are also dependent on location, with central retina being thicker than peripheral retina. The choroid layer thickness ranged from 90µm in rodents (8) to 600µm in humans (13) by MRI.
Blood-flow MRI
Arterial spin-labeling MRI can resolve two distinct blood-flow layers in the retina, separated by a region with little or no blood-flow signal (Figure 1B, 42×4242×42µm) (14–17). The “outer” layer corresponding to the choroid has very high blood flow, 7.7mL/g/min in rodents under isoflurane anesthesia (14–17), resulting in low oxygen extraction fraction and small arterio-venous oxygen saturation difference. The “inner” layer corresponding to the retinal vasculature has much lower blood flow, 1.3mL/g/min (14–16), similar to cerebral blood flow of 1mL/g/min (18). The avascular layer shows no blood-flow signal, albeit some partial volume effect.
Functional MRI (fMRI)
Layer-specific fMRI of hypercapnia (5%CO2), hyperoxia, carbogen (5%CO2+95%O2), hypoxia (10%O2) inhalation, and visual stimuli using BOLD (19,20), blood-volume (21,22), and/or bSSFP (11) contrasts have been reported. Blood-flow fMRI responses to pharmacological challenge have also been reported (23). Many of these studies show the retinal and choroidal vessels respond to various “stimuli” differently.
For example, in hyperoxia, BOLD increase was larger in the outer choroid layer (12±2%) than the inner retinal layer (7±2%, P<0.01) in rats (8). This is because retinal blood vessels are known to constrict during hyperoxia, which reduces blood flow and attenuates BOLD signal increase (24). In contrast, choroidal blood vessels constrict to a smaller extent during hyperoxia, leading to a larger BOLD increase in the choroidal layer. In hypercapnia, BOLD increase in the inner “layer” (10±2%, P<0.01) was substantially larger than the outer layer (1.6±1%) (8). This is likely because hypercapnia has smaller vasodilatory effect on choroidal vessels and/or the oxygen extraction in the choroid is small (24). These differential layer-specific BOLD responses are consistent with published literature using oxygen electrodes (24).
Mild hypoxia (10% O2) decreases BOLD signal but the magnitude changes are different with −12±2% in the inner (retinal) layer and −30±3% in the outer (choroid) layer (11).
Contrast-enhanced MRI
Anatomical MRI studies of the retina using contrast agents have been reported including those employing manganese-enhanced (4,25) and chromium-enhanced (26) MRI. The retinal and choroidal vascular layers and their respective blood barriers can be visualized by using Gd-DTPA (7,8) as described above.
Blood volume index can be measured by using the blood-pool contrast agent, monocrystalline iron oxide nanoparticles (MION). Blood-volume fMRI responses to hypercapnia and hyperoxia show differential responses between the retinal and choroidal layers (21). Blood-volume fMRI has been used to evaluate retinal and choroidal responses in rats at graded luminance, frequency, and wavelength of flickering light (22) (Figure 1C). In the retinal vasculature, increasing luminance increases fMRI signals, red light gives weaker response compared to blue and green, and 10Hz flicker gives maximal fMRI signals, as expected. The choroidal vascular response is weak and does not depend on flicker parameters, suggesting differential neurovascular coupling between the two vascular layers. Together, these findings offer a mean to probe the unique hemodynamic regulations in the eye.
Layer-specific fMRI of light and dark adaptation has been reported using manganese-enhanced (27,28) and diffusion (29) MRI contrasts. Vascular diameter and vessel intensity changes due to hyperoxia and carbogen inhalation can be detected on angiographic images without contrast agent (30).
Other contrasts
3D angiography (42×42×84µm) without and with contrast agent is capable of visualizing arterial and venous vessels in rats at 42×42×84µm (30), providing remarkable details of vessels in the eye and the choriocapillaris. Relative pO2 (ΔpO2) associated with carbogen inhalation in the vitreous next to the retina has been reported by T1-weighted MRI in which dissolved molecular oxygen acts as the endogenous contrast agent (31). This approach has also been used to map quantitative pO2 distribution in the human vitreous (32).
Retinal disease applications
MRI has been applied to study retinal diseases. Blood flow MRI of diabetic retinopathy (15), retinal degeneration (16,17) and glaucoma (33) in animal models show early blood-flow changes before retinal thickness changes by anatomical MRI. BOLD fMRI showed reduced responses to hyperoxia and hypercapnia in an animal model of retinal degeneration (8). These data show that these retinal diseases affect the two vasculatures differently at different stages. Manganese-enhanced MRI shows early abnormality in ion regulation in diabetic retinopathy (34), retinopathy of prematurity (35), glaucoma (36,37), and retinal degeneration (25,38,39). Permeability changes using contrast-enhanced MRI (40) and ΔpO2 MRI (41) have been reported in diabetic retinopathy in rats.
Human applications
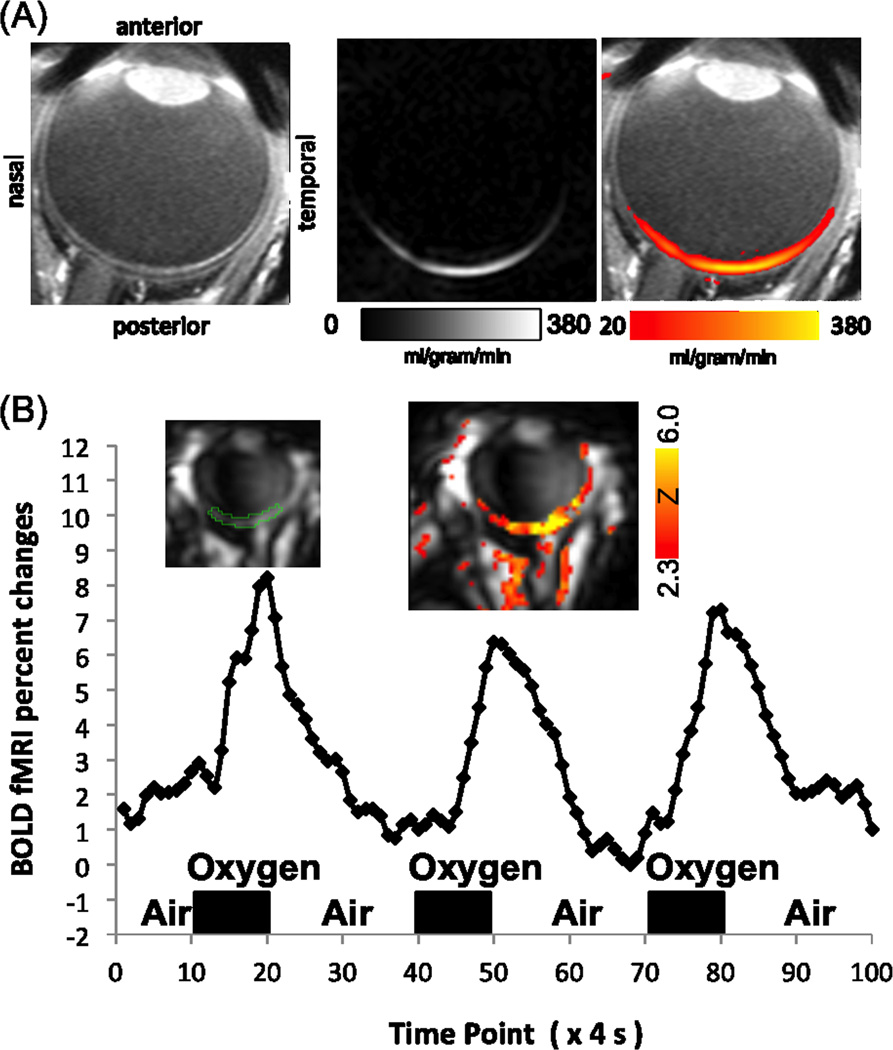
High-resolution anatomical, BOLD and blood-flow MRI on a human 3T clinical scanner is feasible on anesthetized baboon retinas where motion can be eliminated (12). The challenges in MRI application of the human retinas are eye motion and limited spatial resolution due to weaker magnetic field gradients. Eye fixation to a target with cued blinks every 4–8 s is comfortable and minimizes motion artifacts (13,42). A small eye radiofrequency coil, tailored pulse sequences and parameters can be used to improve signal-to-noise ratio (13). Anatomical (13,43), blood-flow (44,45) and BOLD (46) MRI applications to the human retina on 3T (Siemens and Phillips) clinical scanners are feasible with eye fixation and cued blink, albeit lacking layer resolution to date except for anatomical MRI. BOLD fMRI can detect hemodynamic changes in the retina due to hypercapnia and hyperoxia (46). Blood-flow fMRI can detect changes due to hypercapnia (44), hypercarbia (47) and isometric exercise (48). Figure 2A shows a representative basal blood-flow MRI, where blood flow is highest at the posterior pole of the retina (i.e., the macula) and a small indentation at the optic nerve head is apparent. Figure 2B shows the BOLD fMRI responses to hyperoxia of the human retina, delineating robust responses with large percent changes (due to inversion recovery suppression of the vitreous signal). In patients with retinitis pigmentosa, retina-choroidal blood flow is reduced and the extent of blood-flow reduction is correlated with electroretinography (49). Permeability changes using contrast-enhanced MRI (50) and ΔpO2 changes (42) have been reported in humans with diabetic retinopathy.
Figure 2.
(A) Blood-flow MRI of a human retina acquired using pseudo-continuous arterial spin labeling at 0.5×0.8×8mm (N=1). Adapted from (44). (B) BOLD fMRI map and time course of the human retina associated with oxygen versus air inhalation at 1.6×2×4mm (N=1). Inversion pulses were used to suppress the otherwise strong vitreous signal. Adapted from (46).
Limitations and challenges
The major disadvantages of retinal MRI are its higher cost and lower spatiotemporal resolution compared to optical imaging techniques. The major advantages of MRI are its ability to image physiological and functional parameters and depth resolution. MRI is not competitive compared to optical coherence tomography (51) for measuring layer thickness because optical coherence tomography offers far better spatiotemporal resolution although MRI could provide some useful anatomical contrasts to study retinal diseases.
MRI offers some competitive advantages in measuring blood flow over existing retinal imaging techniques, such as laser speckle imaging (52,53), laser Doppler velocimetry and flowmetry (54), which generally provide a qualitative index of blood flow, limiting cross-subject comparison. Doppler optical coherence tomography has the potential to measure blood flow with substantial depth resolution but remains an active area of research (55). The use of optically based imaging techniques to probe functional responses of the retina is sparse (55). fMRI by comparison offers a unique means to probe “evoked” changes in the retina in a layer-specific manner, offering some competitive advantages over optical imaging techniques. As such, blood flow and functional MRI hold promise in providing competitive and valuable information compared to existing optically based imaging techniques.
The challenges and thus opportunities for further research in retinal MRI applications include the needs to improve: i) spatiaotemporal resolution, ii) routine 3D coverage, iii) novel contrasts to better visualize retinal layers, iv) eye fixation stability, v) further applications to preclinical and clinical retinal diseases, such as testing of therapeutic interventions, early detection and characterization of disease physiological processes. These advances can be facilitated by general improvement in MRI hardware including field gradient capability, preamplifiers, detectors, image acquisition speed (i.e., faster sequences and parallel imaging), and eye fixation stability.
MRI has the potential to be used in clinical settings although routine screening will likely be limited due to relatively high cost and low throughput at this time. It is reasonable to expect that MRI costs will decrease over time. That said, identifying patients at high risk of developing retinal diseases early and implementing of preventive measures should be cost effective considering the morbidity and economic burden of blindness from these diseases.
Conclusions
MRI can detect layer-specific anatomy, blood-flow, physiological and functional MRI, offering the potential to further advance our understanding of retinal physiology and function in health and diseased states. MRI has the potential to exert a sustained positive impact in retinal disease research by enabling objective early detection, longitudinal disease staging, and monitoring of interventions via detecting hemodynamic dysregulation in the retina with depth resolution. There remain many challenges before routine clinical retinal MRI applications become feasible.
Acknowledgement
This work was supported by the NIH/NEI EY014211, EY018855, and VA MERIT. I would like to thank all my current and former laboratory team members for their contributions to many of the works that made possible this review. I thank Dr. Bruce Berkowitz of Wayne State for helpful comments on the manuscript.
References
- 1.Bill A. Circulation in the eye. In: Renkin EM, Michel CC, editors. Handbook of physiology Part 2 in Microcirculation. Bethesda, MD: American Physiological Society; 1984. pp. 1001–1035. [Google Scholar]
- 2.Kiel JW. The Ocular Circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 3.Schmetterer L, Kiel J. Ocular Blood Flow. Springer; 2012. [Google Scholar]
- 4.De La Garza BH, Muir ER, Shih YY, Duong TQ. 3D magnetic resonance microscopy of the ex vivo retina. Magn Reson Med. 2012;67:1154–1158. doi: 10.1002/mrm.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duong TQ, Muir ER. Magnetic resonance imaging of the retina. Jpn J Ophthalmol. 2009;53:352–367. doi: 10.1007/s10384-009-0688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair G, Kim M, Nagaoka T, Olson DE, Thule PM, Pardue MT, Duong TQ. Effects of common anesthetics on eye movement and electroretinogram. Doc Ophthalmol. 2011;122:163–176. doi: 10.1007/s10633-011-9271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Q, Cheng H, Pardue MT, Chang TF, Nair G, Vo VT, Shonat RD, Duong TQ. Magnetic resonance imaging of tissue and vascular layers in the cat retina. J Magn Reson Imaging. 2006;23:465–472. doi: 10.1002/jmri.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng H, Nair G, Walker TA, Kim MK, Pardue MT, Thule PM, Olson DE, Duong TQ. Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci USA. 2006;103:17525–17530. doi: 10.1073/pnas.0605790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair G, Shen Q, Duong TQ. Relaxation Time Constants and Apparent Diffusion Coefficients of Rat Retina at 7 Tesla. International Journal of Imaging Systems and Technology. 2010;20:126–130. [Google Scholar]
- 10.Chen J, Wang Q, Zhang H, Yang X, Wang J, Berkowitz BA, Wickline SA, Song SK. In vivo quantification of T(1), T(2), and apparent diffusion coefficient in the mouse retina at 11.74T. Magn Reson Med. 2008;59:731–738. doi: 10.1002/mrm.21570. [DOI] [PubMed] [Google Scholar]
- 11.Muir ER, Duong TQ. Layer-Specific Functional and Anatomical MRI of the Retina with Passband Balanced SSFP. Magn reson Med. 2011;66:1416–1421. doi: 10.1002/mrm.22935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Wey HY, Nateras OS, Peng Q, De La Garza BH, Duong TQ. Anatomical, blood oxygenation level-dependent, and blood flow MRI of nonhuman primate (baboon) retina. Magn Reson Med. 2011;66:546–554. doi: 10.1002/mrm.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, San O, Peng Q, Kuranov R, Harrison JM, Milner TE, Duong TQ. Lamina-specific anatomical magnetic resonance imaging of the human retina. Invest Ophthalmol Vis Sci. 2011;52:7232–7237. doi: 10.1167/iovs.11-7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muir ER, Duong TQ. MRI of Retinal and Choroid Blood Flow with Laminar Resolution. NMR in Biomedicine. 2011;24:216–223. doi: 10.1002/nbm.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muir ER, Renteria RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012;53:6488–6494. doi: 10.1167/iovs.12-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, De La Garza B, Shih YY, Muir ER, Duong TQ. Layer-specific blood-flow MRI of retinitis pigmentosa in RCS rats. Exp Eye Res. 2012;101:90–96. doi: 10.1016/j.exer.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muir ER, De La Garza B, Duong TQ. Blood flow and anatomical MRI in a mouse model of retinitis pigmentosa. Magn Reson Med. 2013;69:221–228. doi: 10.1002/mrm.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duong TQ, Ngan S-C, Ugurbil K, Kim S-G. Functional Magnetic Resonance Imaging of the Retina. Invest Ophthalmol Vis Sci. 2002;43:1176–1181. [PMC free article] [PubMed] [Google Scholar]
- 20.De La Garza BH, Muir ER, Li G, Shih YY, Duong TQ. Blood oxygenation level-dependent (BOLD) functional MRI of visual stimulation in the rat retina at 11.7 T. NMR Biomed. 2011;24:188–193. doi: 10.1002/nbm.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair G, Tanaka Y, Kim M, Olson DE, Thule PM, Pardue MT, Duong TQ. MRI reveals differential regulation of retinal and choroidal blood volumes in rat retina. Neuroimage. 2011;54:1063–1069. doi: 10.1016/j.neuroimage.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih YY, De la Garza BH, Muir ER, Rogers WE, Harrison JM, Kiel JW, Duong TQ. Lamina-specific functional MRI of retinal and choroidal responses to visual stimuli. Invest Ophthalmol Vis Sci. 2011;52:5303–5310. doi: 10.1167/iovs.10-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih YY, Li G, Muir ER, De La Garza BH, Kiel JW, Duong TQ. Pharmacological MRI of the choroid and retina: Blood flow and BOLD responses during nitroprusside infusion. Magn Reson Med. 2012;68:1273–1278. doi: 10.1002/mrm.24112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu D-Y, Cringle SJ, Su E-N, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmo Vis Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- 25.Nair G, Pardue MT, Kim M, Duong TQ. Manganese-enhanced MRI reveals multiple cellular and vascular layers in normal and degenerated retinas. J Magn Reson Imaging. 2011;34:1422–1429. doi: 10.1002/jmri.22719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan KC, Fan SJ, Zhou IY, Wu EX. In vivo chromium-enhanced MRI of the retina. Magn Reson Med. 2012;68:1202–1210. doi: 10.1002/mrm.24123. [DOI] [PubMed] [Google Scholar]
- 27.De La Garza BH, Li G, Shih YY, Duong TQ. Layer-specific manganese-enhanced MRI of the retina in light and dark adaptation. Invest Ophthalmol Vis Sci. 2012;53:4352–4358. doi: 10.1167/iovs.11-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkowitz BA, Roberts R, Goebel DJ, Luan H. Noninvasive and simultaneous imaging of layer-specific retinal functional adaptation by manganese-enhanced MRI. Invest Ophthalmol Vis Sci. 2006;47:2668–2674. doi: 10.1167/iovs.05-1588. [DOI] [PubMed] [Google Scholar]
- 29.Bissig D, Berkowitz BA. Light-dependent changes in outer retinal water diffusion in rats in vivo. Mol Vis. 2012;18:2561–2577. [PMC free article] [PubMed] [Google Scholar]
- 30.Shih YY, Muir ER, Li G, De La Garza BH, Duong TQ. High-Resolution 3D MR Microangiography of the Rat Ocular Circulation. Radiology. 2012;264:234–241. doi: 10.1148/radiol.12112033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkowitz BA, Kowluru RA, Frank RN, Kern TS, Hohman TC, Prakash M. Subnormal retinal oxygenation response precedes diabetic-like retinopathy. Invest Ophthalmol Vis Sci. 1999;40:2100–2105. [PubMed] [Google Scholar]
- 32.Muir ER, Zhang Y, San Emeterio Nateras S, Duong TQ. MRI of Oxygen Partial Pressure of the Human Vitreous Radiology. 2012 doi: 10.1148/radiol.12120777. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavery WJ, Muir ER, Kiel JW, Duong TQ. Magnetic resonance imaging indicates decreased choroidal and retinal blood flow in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2012;53:560–564. doi: 10.1167/iovs.11-8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkowitz BA, Roberts R, Stemmler A, Luan H, Gradianu M. Impaired apparent ion demand in experimental diabetic retinopathy: correction by lipoic Acid. Invest Ophthalmol Vis Sci. 2007;48:4753–4758. doi: 10.1167/iovs.07-0433. [DOI] [PubMed] [Google Scholar]
- 35.Berkowitz BA, Roberts R, Penn JS, Gradianu M. High-resolution manganese-enhanced MRI of experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2007;48:4733–4740. doi: 10.1167/iovs.06-1516. [DOI] [PubMed] [Google Scholar]
- 36.Calkins DJ, Horner PJ, Roberts R, Gradianu M, Berkowitz BA. Manganese-enhanced MRI of the DBA/2J mouse model of hereditary glaucoma. Invest Ophthalmol Vis Sci. 2008;49:5083–5088. doi: 10.1167/iovs.08-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan KC, Fu QL, Hui ES, So KF, Wu EX. Evaluation of the retina and optic nerve in a rat model of chronic glaucoma using in vivo manganese-enhanced magnetic resonance imaging. Neuroimage. 2008;40:1166–1174. doi: 10.1016/j.neuroimage.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Berkowitz BA, Gradianu M, Schafer S, Jin Y, Porchia A, Iezzi R, Roberts R. Ionic dysregulatory phenotyping of pathologic retinal thinning with manganese-enhanced MRI. Invest Ophthalmol Vis Sci. 2008;49:3178–3184. doi: 10.1167/iovs.08-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Song SK, Zhang H, Berkowitz BA, Chen S, Wickline SA, Chen J. Photoreceptor degeneration changes magnetic resonance imaging features in a mouse model of retinitis pigmentosa. Magn Reson Med. 2011;65:1793–1798. doi: 10.1002/mrm.22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berkowitz BA, Roberts R, Luan H, Peysakhov J, Mao X, Thomas KA. Dynamic contrast-enhanced MRI measurements of passive permeability through blood retinal barrier in diabetic rats. Invest Ophthalmol Vis Sci. 2004;45:2391–2398. doi: 10.1167/iovs.03-1381. [DOI] [PubMed] [Google Scholar]
- 41.Luan H, Roberts R, Sniegowski M, Goebel DJ, Berkowitz BA. Retinal thickness and subnormal retinal oxygenation response in experimental diabetic retinopathy. Invest Ophthalmo Vis Sci. 2006;47:320–328. doi: 10.1167/iovs.05-0272. [DOI] [PubMed] [Google Scholar]
- 42.Berkowitz BA, McDonald C, Ito Y, Tofts PS, Latif Z, Gross J. Measuring the human retinal oxygenation response to a hyperoxic challenge using MRI: eliminating blinking artifacts and demonstrating proof of concept. Magn Reson Med. 2001;46:412–416. doi: 10.1002/mrm.1206. [DOI] [PubMed] [Google Scholar]
- 43.Richdale K, Wassenaar P, Teal Bluestein K, Abduljalil A, Christoforidis JA, Lanz T, Knopp MV, Schmalbrock P. 7 Tesla MR imaging of the human eye in vivo. J Magn Reson Imaging. 2009;30:924–932. doi: 10.1002/jmri.21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng Q, Zhang Y, Nateras OS, van Osch MJ, Duong TQ. MRI of blood flow of the human retina. Magn Reson Med. 2011;65:1768–1775. doi: 10.1002/mrm.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maleki N, Dai W, Alsop DC. Blood flow quantification of the human retina with MRI. NMR Biomed. 2011;24:104–111. doi: 10.1002/nbm.1564. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Peng Q, Kiel JW, Rosende CA, Duong TQ. Magnetic resonance imaging of vascular oxygenation changes during hyperoxia and carbogen challenges in the human retina. Invest Ophthalmol Vis Sci. 2011;52:286–291. doi: 10.1167/iovs.10-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maleki N, Alsop DC, Dai W, Hudson C, Han JS, Fisher J, Mikulis D. The effect of hypercarbia and hyperoxia on the total blood flow to the retina as assessed by magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2011;52:6867–6874. doi: 10.1167/iovs.10-6762. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, San Emeterio Nateras O, Peng Q, Rosende CA, Duong TQ. Blood flow MRI of the human retina/choroid during rest and isometric exercise. Invest Ophthalmol Vis Sci. 2012;53:4299–4305. doi: 10.1167/iovs.11-9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Harrison JM, San Emeterio Nateras S, Chalfin S, Duong TQ. Decreased Retinal/Choroidal Blood Flow in Retinitis Pigmentosa by MRI. Doc Ophthalmol. 2012 doi: 10.1007/s10633-013-9374-1. in press PMID:2340831.10.1007/s10633-013-9374-1 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trick GL, Liggett J, Levy J, Adamsons I, Edwards P, Desai U, Tofts PS, Berkowitz BA. Dynamic contrast enhanced MRI in patients with diabetic macular edema: initial results. Exp Eye Res. 2005;81:97–102. doi: 10.1016/j.exer.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Fujimoto JG, Brezinski ME, Tearney GJ, Boppart SA, Bouma B, Hee MR, Southern JF, Swanson EA. Optical biopsy and imaging using optical coherence tomography. Nat Med. 1995;1:970–972. doi: 10.1038/nm0995-970. [DOI] [PubMed] [Google Scholar]
- 52.Cheng H, Duong TQ. Simplified laser-speckle-imaging analysis method and its application to retinal blood flow imaging. Opt Lett. 2007;32:2188–2190. doi: 10.1364/ol.32.002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng H, Yan Y, Duong TQ. Temporal Statistical Analysis of Laser Speckle Image and its Application to Retinal Blood-Flow Imaging. Optics Express. 2008;16:10214–10219. doi: 10.1364/oe.16.010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riva CE, Logean E, Falsini B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Progress Retinal Eye Res. 2005;24:183–215. doi: 10.1016/j.preteyeres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, Fujimoto JG. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]