Abstract
The protozoan parasite Leishmania experiences extreme environmental changes as it alternates between insect and mammalian hosts. In some species, differentiation of insect promastigotes into mammalian-infective amastigotes is induced by elevated temperature and low pH, conditions found within macrophage parasitophorous vacuoles (PVs). However, the signaling events controlling amastigote differentiation remain poorly understood. Recent studies revealed a novel role for iron uptake in orchestrating the differentiation of amastigotes, in a process that involves production of reactive oxygen species (ROS) and is independent from pH and temperature changes. ROS are generally thought to be deleterious for pathogens, but it is becoming increasingly apparent that they can also function as signaling molecules regulating Leishmania differentiation, in a process that is tightly controlled by iron availability.
Keywords: Leishmania, differentiation, iron, virulence, ROS, FeSOD
Signaling events drive differentiation of Leishmania virulent forms
The protozoan parasite Leishmania spp causes a broad spectrum of human diseases referred to as leishmaniasis. These intracellular parasites currently infect about 12 million people, and threaten more than 350 million globally. Depending on the Leishmania species, the disease pathology varies from self-healing cutaneous lesions to severe visceralizing disease. If left untreated, the visceral form of the disease has a high fatality rate, ranking next to malaria as the most deadly protozoan human disease [1].
Leishmania alternates between insect and vertebrate hosts, with inter-species transmission occurring through sand fly bites (Figure 1). During their life cycle the parasites encounter dramatic changes in environment, as they transition from the midgut and proboscis of Phlebotomus insects (sand flies) to phagolysosomal vacuoles in mammalian macrophages. Shifts in temperature, pH, O2/CO2, and availability of nutrients coincide with morphological and metabolic changes associated with virulence. However, how environmental conditions trigger Leishmania differentiation remains unknown.
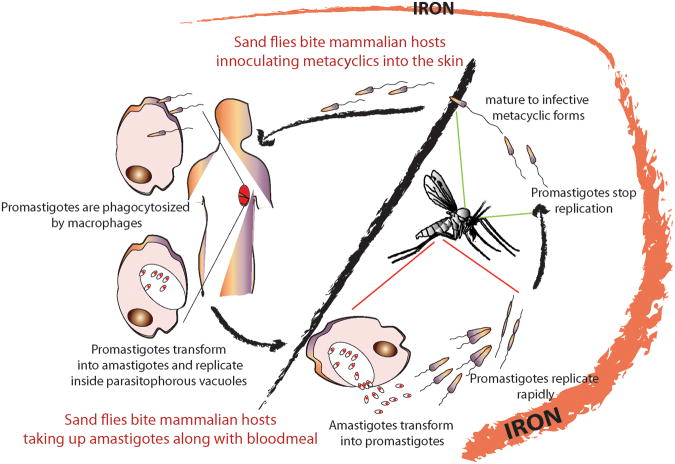
Figure 1. The availability of iron changes during the Leishmania life-cycle.
Iron is abundant in the sand fly midgut following blood meals, as result of the breakdown of hemoglobin. Amastigotes taken up in a blood meal transform into promastigotes within the digestive tract and multiply by binary fission. A few days after the initial feeding the parasites cease to replicate and transform into infective metacyclic promastigotes, during the ‘sugar-meal phase’, when iron availability becomes limited.. The transmissible metacyclic forms are regurgitated into the skin of a mammalian host by the flies, entering macrophages where they transform into amastigotes within lysosome-like parasitophorous vacuoles. The low availability of iron inside PVs in macrophages triggers upregulation of proteins involved in iron acquisition (see Figure 2).
A differentiation event of major clinical importance is the transformation of the flagellated metacyclic promastigotes, which are injected into the skin of mammalian hosts by sand flies, into rounded amastigotes, which replicate in macrophage phagolysosomes and can be generated axenically in some Leishmania species [2, 3, 4]. Transformation into amastigotes involves a shift to a higher energy production rate, thought to be required for driving proton pumps to counter the acidic environment of PVs [5]. Genome-wide changes in gene expression were observed between promastigotes and amastigotes of L. mexicana [6] and L. donovani [7], and proteomic studies revealed modulation of post-translational modifications [8, 9]. Understanding of the signaling pathways driving generation of Leishmania amastigotes has long been considered a ‘holy grail’ for the development of efficacious anti-leishmanial drug therapy. In this review we discuss recent advances in this area, including surprising new findings that point to iron acquisition as a process that drives differentiation of virulent amastigotes.
Metabolic and structural changes during amastigote differentiation are orchestrated at the post-transcriptional level
Leishmania amastigotes are highly adapted to function within the acidic environment of macrophage phagolysosomes, maintaining internal pH homeostasis and acquiring nutrients against a steep extracellular proton gradient. Residence inside host macrophages comes at a high cost, requiring significantly enhanced rates of ATP generation [5]. A major metabolic retooling appears to occur during amastigote differentiation, with the parasites replacing glucose by amino acids as the primary energy source and activating pathways such as amino acid catabolism, the tricarboxylic acid cycle, β-oxidation, the mitochondrial respiratory chain, and oxidative phosphorylation [10]. Protein synthesis is downregulated, a process proposed to be responsible for the slower growth rate of amastigotes [5].
Comparative genome-wide analysis of the transcriptome and proteome of promastigotes and amastigotes have been critical in understanding the differentiation process. Studies of amastigotes derived from infected animals provided valuable information on cell structure and role in disease development [6, 11]. However, studying parasites isolated from an in vivo system poses two main disadvantages: (i) the limited amount of purified parasites that can be obtained and (ii) the difficulty in separating amastigotes from mammalian tissue contaminants. These problems were overcome by the use of axenically-differentiated amastigotes, which can be induced in several species of Leishmania by conditions that simulate the elevated temperature and low pH encountered by the parasites within macrophages [5, 12]. The use of axenic amastigotes also allowed precise monitoring of the progression of differentiation over time. Four major phases were identified in the promastigote-to-amastigote axenic transformation of L. donovani: (i) signal perception with no significant morphological change (0-5 h); (ii) cessation of movement and cell aggregation (5-10 h); (iii) loss of flagella and morphological transformation to rounded shape (10-24 h); and (iv) maturation into replicating amastigotes (24-120 h) [5]. During the initial 10 h extensive changes in RNA processing and turnover were observed, resulting in rapid downregulation of several gene products and upregulation of others [9, 10]. In addition to pH and temperature, factors reported to influence the differentiation of amastigotes include nutrient availability, CO2 levels, and hydrobiopterin concentration [13]. As discussed below, iron uptake was recently found to initiate differentiation of L. amazonensis amastigotes independently of the ‘classical’ pH and temperature cues [14].
Iron uptake regulates differentiation in Leishmania amazonensis
The Leishmania iron uptake machinery
Iron is a key trace element for virtually all organisms, functioning as an essential cofactor in many cellular processes. Due to its unique electrochemical properties, iron is an integral component of important physiological pathways such as oxygen delivery, acetyl CoA oxidation, ATP generation, nitrogen fixation, and photosynthesis, where iron is used as a sensor of the cellular redox status. However, iron is a double-edged sword. The soluble, free Fe+2 form can react with oxygen or nitrogen compounds generating highly toxic radicals: reactive oxygen species (ROS) and reactive nitrogen species (RNS) via the Fenton reaction. For this reason the cellular uptake, distribution, storage, and export of iron must be tightly regulated [15, 16].
Identification of the divalent cation transporter Nramp1 as a host susceptibility gene for infections with L. donovani and L. infantum [17] was among the first indications that Leishmania is critically dependent on iron availability for intracellular replication. Nramp1 translocates Fe2+ from late endocytic compartments into the cytosol [18], indicating that Leishmania amastigotes in these compartments have to compete with the host for the small amount of iron that enters by endocytosis. Leishmania can also influence the host cell's ability to internalize iron. Infection with L. donovani depletes the host cell labile iron pool and activates iron regulatory proteins, which in turn upregulate expression of transferrin receptors and stimulate iron uptake [19]. Preferential fusion of transferrin-containing endosomes with PVs was reported in L. amazonensis-infected macrophages, and proposed as a mechanism to ensure a constant supply of iron for replicating amastigotes [20]. Despite these strategies to increase their access to iron, the intracellular stages of Leishmania also must express specific surface transporters to acquire this essential element.
Leishmania parasites do not seem to express siderophore molecules capable of competing with host iron carrier proteins such as transferrin or lactoferrin or transferrin receptor-mediated uptake [21 22], leaving the question of how these parasites acquire iron unanswered until recently. We now know that Leishmania expresses two membrane proteins that play a central role in iron acquisition: (i) the ferric reductase LFR1, which reduces the insoluble Fe3+ to soluble Fe2+ [23] and (ii) the Fe2+ transporter LIT1, a member of the ZIP family of metal transporters (Figure 2) [24]. L. amazonensis null mutants in LFR1 and LIT1 are severely defective in intracellular replication as amastigotes, demonstrating the importance of this iron uptake machinery for survival in the iron-poor environment of macrophage phagolysosomes.
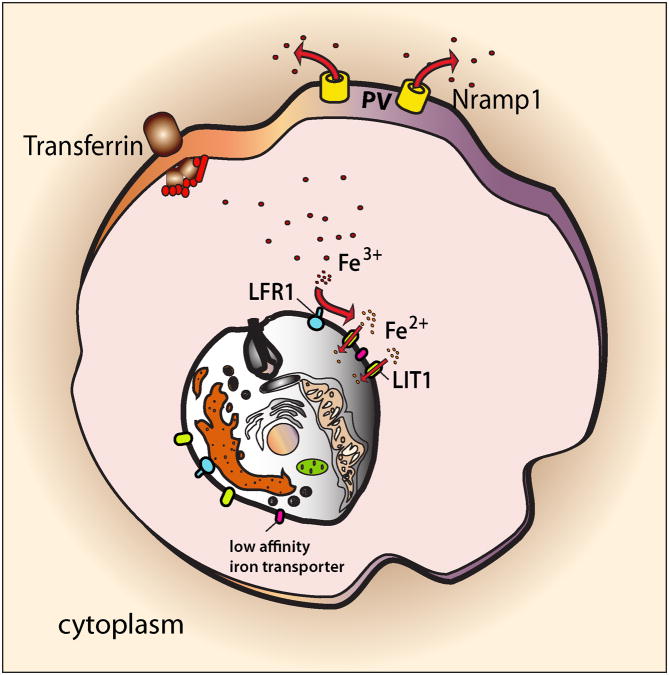
Figure 2. Leishmania must acquire iron from the host to survive inside PVs.
Within PVs of macrophages Leishmania upregulates an iron transport machinery that allows it to compete with the host iron transporter Nramp1 for iron. Intracellular amastigotes of Leishmania express on their plasma membrane the ferric iron reductase LFR1, which converts Fe3+ that enters the macrophage as a complex with transferrin into Fe2+, the soluble iron form. Fe2+ is then translocated across the membrane by LIT1, a ferrous iron transporter that is also upregulated in the iron-poor environment of the PV. When higher concentrations of iron are available inside the parasitophorous vacuole, low affinity iron transporters can compensate for the absence of LHR1 and LIT1, promoting parasite replication. Abbreviation: PV, parasitophorous vacuole.
Significant similarities were identified between the Leishmania and plant iron acquisition machineries. The iron uptake strategy of Arabidopsis thaliana involves upregulation of AHA family H+-ATPases that promote acidification of the environment, thereby increasing the solubility of available iron [25]. This situation is comparable to the acidic environment where intracellular amastigotes of Leishmania replicate inside macrophages. In plants, Fe3+ is reduced to Fe2+ by a membrane-bound ferric-chelate reductase (AtFRO2 in Arabidopsis and PsFRO1 in peas) that shows significant homology to L. amazonensis LFR1. Reduction seems to be the rate-limiting step for iron uptake, as transgenic over-expression of ferric reductases in Arabidopsis, rice, tobacco, and soybean increases tolerance to low iron [25]. Similarly, over-expression of LFR1 rescues the intracellular replication of LIT1 null L. amazonensis [23]. In Arabidopsis Fe2+ is taken up by IRT1, a divalent cation transporter from the ZIP family that shares significant sequence identity with the L. amazonensis Fe2+ transporter LIT1 [15]. Arabidopsis IRT1 null mutants are severely chlorotic and seedling-lethal unless supplied with large amounts of exogenous iron, resembling the intracellular growth rescue of L. amazonensis LIT1 null mutants observed after loading macrophages with cationic ferritin [23].
Leishmania can also obtain iron from heme sources, including hemoglobin [26-28]. Recent studies showed that heme uptake in Leishmania is mediated by LHR1, a transmembrane transporter with limited similarity to C. elegans HRG-4 [28].
Iron uptake regulates amastigote differentiation and virulence
L. amazonensis promastigotes lacking the ferric iron reductase LFR1 are defective in generating infective metacyclic promastigotes and amastigotes [23]. This finding was the first indication that the iron acquisition pathway played an important role in signaling events regulating differentiation. Subsequent studies showed that specifically depleting the culture medium in iron (while maintaining all other divalent cations) induced strong upregulation in expression of the reductase LFR1 and the ferrous iron transporter LIT1. These experiments revealed that L. amazonensis responds to iron deficiency by rapidly upregulating the iron uptake machinery, which results in an increase in their iron content. Remarkably, the spike in iron uptake triggered by low iron was associated with growth arrest and a gradual change in morphology, with the generation of rounded forms expressing specific markers of the amastigote stage. Importantly, this transformation was also associated with expression of parasite genes regulating autophagy, a cell remodeling mechanism known to participate in promastigote-to-amastigote differentiation (Box 1) [29]. These were surprising findings, since all prior protocols for the generation of axenic amastigotes in L. mexicana/amazonensis involved a shift to acidic pH and 37°C, conditions mimicking the mammalian phagolysosome. Remarkably, the iron-regulated process of amastigote differentiation occurred at the neutral pH and low temperature (26°C) normally utilized to cultivate the insect promastigotes. Mouse infection experiments demonstrated that the amastigotes generated in these iron-deprived cultures were fully virulent, while parasites maintained in iron-containing medium as promastigotes were not, as expected, able to form cutaneous lesions [14].
Box 1.
Role of autophagy in Leishmania remodeling during differentiation
Autophagy serves a dual purpose: (i) a pathway for nutrient acquisition activated by cellular starvation, and (ii) a pathway for protein and organelle degradation during cellular differentiation [65]. The genesis of autophagosomes requires the activity of two protein conjugation systems, one involving the ubiquitin-like protein Atg8 that is proteolytically processed by the cysteine peptidase Atg4, and a second involving the ubiquitin-like protein Atg12, covalently attached to Atg5. In yeast and mammalian systems, formation of the Atg5-Atg12 complex is critical for generating the curved pre-autophagosomal membrane known as the phagophore. Conjugation of Atg5 and Atg12 is a multi-step process requiring ATP and interaction with several additional Atg proteins, which ultimately promote closure of the autophagosome and fusion with lysosomal compartments [66].
The autophagic machinery was shown to be involved in two Leishmania differentiation events: the transformation of procyclic promastigotes into metacyclic promastigotes within the sand fly, and the remodeling of metacyclic promastigotes into amastigotes within the macrophage [29]. Unlike yeast and humans that have only one Atg8 family, Leishmania has four families of Atg8-like genes. When over-expressed as GFP-fusions in L. major promastigotes, GFP-ATG8 and GFP-ATG8A showed a punctate pattern that was enhanced under nutrient starvation, consistent with a role of these isoforms in autophagosome formation. The function of Atg8 and Atg8A could not be investigated directly by creating null mutants because of multiple gene copies. However, mutants deficient in the Atg4.2 cysteine peptidase that activates Atg8 proteins show a pronounced virulence defect, and incomplete transformation into amastigotes [54]. Further highlighting the role of autophagy in Leishmania differentiation, a functional ATG5-ATG12 system was identified in L.major and Δatg5 null promastigotes were defective for differentiation and infectivity for macrophages and mice [67]. Furthermore, inhibition of the Leishmania lysosomal cysteine peptidases CPA and CPB also impaired autophagy and markedly reduced metacyclogenesis and differentiation into amastigotes [29].
There is evidence that ROS can activate autophagy in eukaryotic cells, which in turn protects cells by removing oxidized proteins [53]. In L. major, an increase in intracellular ROS coincided with the onset of autophagy during metacyclogenesis [54]. Δatg5 and Δatg4.2 null strains deficient in autophagy showed dysfunctional mitochondria and higher levels of ROS [54, 67], but the antioxidant N-acetyl cysteine reversed this phenotype restoring mitochondrial integrity and decreasing autophagosome formation. Thus, in Leishmania ROS also appear to regulate autophagy, a cell remodeling process that is essential for differentiation.
Unlike wild type promastigotes that respond to iron deprivation by increasing their iron content, slowing replication and differentiating into amastigotes, promastigotes lacking the iron transporter LIT1 maintain low intracellular iron and continue to grow exponentially, followed by a sudden catastrophic cell death. These findings indicated that the ability to acquire iron is necessary for signaling events that inhibit promastigote replication and trigger differentiation into virulent amastigotes [14]. These studies also suggested that entering a non-replicative stage is probably a necessary intermediate in the process of differentiation of promastigotes into amastigotes. Additional results revealed that the L. amazonensis iron uptake machinery is tightly regulated, consistent with the need to avoid toxicity from excessive intracellular iron accumulation: (i) expression of the LFR1 ferric reductase and the LIT1 ferrous iron transporter is very low in promastigotes and not required for their replication in iron-rich media; (ii) expression of LFR1 and LIT1 is upregulated in promastigotes exposed to low-iron conditions, causing an increase in intracellular iron concentration that slows growth and drives amastigote differentiation; and (iii) over-expression of the ferric reductase LFR1 is toxic for promastigotes [24, 23, 14].
Role of iron-dependent redox signaling in Leishmania differentiation
ROS as signaling molecules controlling the balance between proliferation and differentiation
ROS produced in vivo are largely viewed as deleterious for eukaryotic cells [30, 31]. However, it is becoming increasingly apparent that superoxide radicals (O2•−) and hydrogen peroxide (H2O2) also function as critical intermediates in cellular signaling pathways [32-35]. In particular, the intracellular balance between O2•− and H2O2 can influence cellular fate [36-38]. During normal cell growth, a tight balance between these two ROS species is maintained by the cells' antioxidant systems, and when these systems fail, a pro-oxidant environment is generated. Evidence from a variety of model systems underscores the relationship between a pro-oxidant state and cancer progression, and highlights a role for ROS in the control of cell proliferation and differentiation [39, 40, 41]. In Drosophila melanogaster, ROS prime hematopoietic progenitors for differentiation [34]. In Arabidopsis thaliana, superoxide (O2•−) and hydrogen peroxide (H2O2) exhibit distinct patterns of distribution in root tissues; proliferation of root cells requires O2•− accumulation, whereas cellular differentiation depends on elevated levels of H2O2 [42, 43]. Additional studies have confirmed a role for reduced superoxide dismutase (SOD) activity and increased O2•− in promoting cell proliferation [32], and of H2O2 in driving cellular differentiation [44]. Thus, it is becoming increasingly clear that the relative concentrations of O2•− and H2O2 can determine cell fate by altering the balance between cell proliferation and differentiation. As discussed below, recent findings indicate that ROS play an important role in the iron-regulated promastigote-to-amastigote differentiation of Leishmania.
Role of ROS in the regulation of Leishmania differentiation
Evidence has been accumulating that ROS generated during promastigote-to-amastigote differentiation is a key signaling event driving differentiation. Exposure to elevated temperature, a stimulus that induces promastigote-to-amastigote differentiation in L. infantum, triggers hyperpolarization of the parasite's mitochondrion, an increase in respiratory rate, and a ROS surge. Promastigotes in the stationary phase of growth in culture can tolerate this ROS surge and transform into amastigotes, whereas log-phase promastigotes accumulate higher levels of ROS and enter apoptosis, a process that can be reversed by over-expression of mitochondrial SOD [45].
The progression of promastigotes from logarithmic to stationary phase in culture is accompanied by an increase in resistance to oxidative stress [45, 46], increased virulence [47], and enhanced levels of SOD activity [48]. This was mostly interpreted as a protective measure to counter production of ROS by the host macrophage [49]. However, oxidative stress caused by heat shock or by treatment with H2O2 or the O2•−-generating drug menadione was found to increase the virulence of L. infantum (chagasi) for mice [47]. The importance of redox regulation in the differentiation of virulent forms was also suggested by studies of L. major mutants deficient in ascorbate-dependent peroxidase (APX) [50] or in components of the trypanothione reductase system [51, 52]. Interestingly, it is becoming increasingly clear that ROS plays an important role in the activation of autophagy [53], a process involved in Leishmania differentiation (Box 1). Consistent with these findings, recent work found a correlation between autophagy and ROS levels in L. mexicana [54], reinforcing the view that the regulation of oxidative signaling is critical for controlling life cycle transitions in these parasites.
Iron-dependent H2O2 generation drives amastigote differentiation in L. amazonensis
Direct evidence for ROS-mediated differentiation was recently obtained while exploring the responses of L. amazonensis to changes in iron availability [14]. The fact that SODs in trypanosomatid parasites exclusively contain iron as an essential co-factor (Box 2) suggested that this class of ROS-regulating enzymes might be particularly susceptible to changes in the intracellular iron content of L. amazonensis. Investigation of this issue revealed a sharp increase in SOD activity immediately preceding the promastigote-to-amastigote differentiation process that is triggered by iron removal from the culture medium. In agreement with an essential role for iron uptake in the activation of FeSOD capable of converting O2•− into H2O2, exposure of promastigotes to the O2•−-generating drug menadione initiated amastigote differentiation in wild type, but not in LIT1 null parasites. Importantly, bypassing the requirement for FeSOD by exposing the parasites to H2O2 alone was sufficient to initiate amastigote differentiation in both wild type and LIT1-deficient promastigotes [14], highlighting the key role played by this stable and diffusible ROS molecule.
Box 2.
Iron-dependent superoxide dismutases and Leishmania redox control
Iron plays a crucial role in regulating redox balance in all trypanosomatids, due to its essential role in the activity of superoxide dismutases (SODs). SODs are metalloenzymes that catalyze dismutation of superoxide (O2•−) to hydrogen peroxide (H2O2). Unlike mammals, which contain Cu/Zn and Mn-dependent SOD in the mitochondrial matrix and cytoplasm respectively, SODs in trypanosomatids are exclusively dependent on iron for activity [16]. For this reason, modulation in iron availability is expected to have a direct impact in cellular functions dependent on SOD and H2O2 generation, as recently demonstrated for the differentiation of Leishmania amazonensis amastigotes [14].
Of the three functionally characterized FeSODs in Leishmania, two isoforms have been localized in the glycosomes, compartments that house enzymes involved in the glycolytic pathway [16, 68]. The glycosomal FeSOD-B1 and FeSOD-B2 are developmentally regulated in Leishmania infantum (chagasi), with SODB1 transcript levels being more abundant in amastigotes and SODB2 in promastigotes. L. infantum (chagasi) SODB1 null mutant promastigotes were not viable, and single allele deletion strains showed reduced viability in macrophages or when exposed to the pro-oxidant paraquat [48]. Interestingly, in Trypanosoma brucei, which undergo pronounced glycosomal turnover during differentiation, both SODB1 and SODB2 were found to be essential for survival [16, 68].
The physiological role of Leishmania mitochondrial FeSOD A (LdFeSODA) has not yet been fully elucidated, but there is evidence that it can protect the parasites from oxidative stress and programmed cell death [69]. The FeSODA ortholog in T. brucei was found not to be essential for the bloodstream form of the parasites [16], but in T. cruzi upregulation of antioxidant enzymes including FeSODA observed in the infective metacyclic stage of were proposed to represent an important defense mechanism against the oxidative burst of host phagocytes, ROS production, and programmed cell death [70, 71].
A recent examination of changes in the proteome after oxidative stress provided independent evidence for ROS-induced differentiation in another Leishmania species, L. donovani. Promastigotes exposed to sub-lethal doses of menadione as a source of ROS and S-nitroso-N-acetylpenicillamine as a source of RNS showed upregulation of proteins involved in redox homeostasis including FeSODA and APX, and proteins of the amastin family that include amastigote-specific markers. Also consistent with the onset of amastigote differentiation, the observed changes in protein abundance were consistent with a shift from glucose to fatty acid oxidation as the main source of metabolic energy [55].
Collectively, these recent findings revealed an unexpected role for iron uptake and ROS generation in initiating autophagy and differentiation in Leishmania. Although the exact mechanism is still incompletely understood, a stress response triggered by low extracellular iron may set the process in motion by altering the parasite's mitochondrial redox balance. Iron deprivation may disrupt Fe-S clusters causing a leaky electron transport chain (ETC), generating O2•−. These highly reactive molecules may then further disrupt ETC Fe-S clusters and exacerbate O2•− production, unless the situation is remedied by SOD. Since all Leishmania SOD enzymes require iron as co-factor for activity, the ability to dismutate O2•− to H2O2 and restore the ETC depends critically on iron availability. Upregulation of the LIT1 iron transporter triggered by low extracellular iron could then provide a mechanism by which the parasites obtain the necessary iron for FeSOD assembly, fully consistent with the surge in activity of this enzyme that is observed following iron deprivation [14]. FeSOD-mediated dismutation of O2•− generates H2O2, which would correspond to the diffusible signaling molecule triggering differentiation (Figure 3).
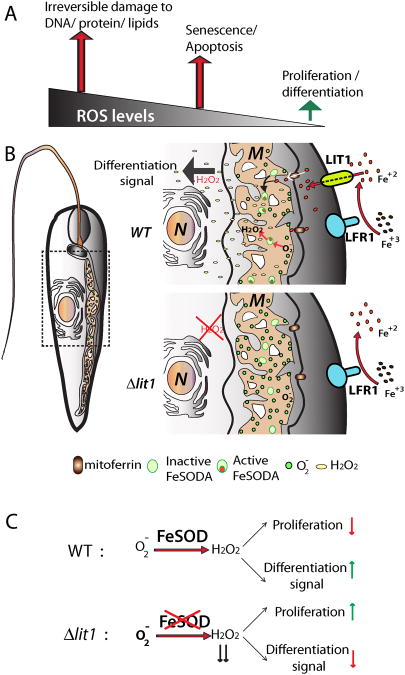
Figure 3. Model for the iron-regulated mechanism for ROS generation that triggers amastigote differentiation.
(A) Mitochondrial ROS levels determine cell fate. At low concentrations ROS molecules act as potent cell signals regulating the balance between proliferation and differentiation. Increasingly higher concentrations of ROS molecules trigger senescence and apoptotic death, and then irreversible damage to DNA, protein and lipid cellular components of the cell. (B) Low iron conditions cause an initial drop of intracellular iron, affecting Fe-S clusters and impairing the function of the mitochondrial electron transport chain, resulting in leaking of electrons that in the presence of molecular oxygen generates superoxide (O2•−). Iron transport is then activated by upregulation of the ferric reductase LFR1 and the ferrous iron transporter LIT1 on the parasite's plasma membrane. This results in an increase in intracellular iron concentration, activating FeSOD activity, that converts O2•− into H2O2. Being a highly diffusible molecule, H2O2 can oxidize cellular targets to initiate differentiation into amastigotes. In the absence of LIT1 mediated iron import, LIT1 null promastigotes are unable to activate FeSOD and H2O2. production, not generating a differentiation signal. M: Mitochondria; N: Nucleus. (C) FeSOD-mediated generation of H2O2 controls the balance between proliferation and differentiation.
Concluding remarks and future directions
A stress-related response has long been assumed to initiate differentiation events in Leishmania, as the parasites transition from the insect to the mammalian environment. However, the underlying molecular mechanism triggering differentiation has remained very poorly understood. Recent work investigating iron uptake in Leishmania amazonensis led to the surprising discovery that changes in intracellular iron concentration activate a ROS-dependent signaling pathway that induces promastigotes to transform into infective amastigotes. This finding provided an unexpected connection between evidence for redox signaling in the development of infectivity, and the recent characterization of the Leishmania iron uptake machinery.
One aspect that still remains to be clarified is how Leishmania parasites sense low extracellular concentrations of iron, activating expression of genes required for iron uptake. Sensors that regulate expression of iron responsive genes have been extensively characterized in several systems [25, 56, 57, However, how this process is achieved in trypanosomatid protozoa is still unclear 58]. Given that mechanisms for transcript stabilization play a major role in regulating gene expression in Leishmania, the identification of iron-responsive cis-elements and trans-factors involved in this process should now be pursued to clarify this issue.
Another outstanding question is how Leishmania parasites store iron. Ferritin-like proteins that bind iron intracellularly and release it in a controlled fashion are found in bacteria, algae, higher plants, and animals, but are apparently absent in the Leishmania genome. Yeast cells also lack ferritin but contain frataxins, small mitochondrial proteins that bind and supply iron for assembly of Fe-S clusters and heme [59]. Frataxin orthologs are present in all trypanosomatids including Leishmania (LmjF.25.1050, LmxM.25.1050, LinJ.25.1090). In Trypanosoma brucei, RNAi-mediated knockdown of frataxin (Tb927.3.1000) rapidly decreased the activity of essential Fe-S dependent enzymes but did not cause iron accumulation in mitochondria, suggesting that this protein may not function in iron storage in trypanosomatids [60]. The role of frataxin in Leishmania still needs to be investigated, but the potential absence of a bona-fide iron sequestration mechanism in these parasites may have been overcome by the development of a tightly regulated iron transport machinery.
When appropriately controlled, ROS function as signaling molecules by modulating the activity of targets by oxidation. Thus, an important and still unanswered question is what are the targets of oxidation in the iron-dependent, H2O2-mediated promastigote-to-amastigote differentiation of Leishmania. Under tightly regulated concentrations, H2O2 can reversibly inhibit several enzymes, including cellular phosphatases that regulate major eukaryotic signaling cascades [61]. Analysis of the TriTryp database reveals the presence of more than 80 phosphatases in L. major. Kinetoplastids, when compared to humans, Saccharomyces cerevisiae, and plants, have a reduced number of tyrosine-specific phosphatases and an expanded number of serine/threonine phosphatases [62]. Several of these kinetoplastid phosphatases have novel domain architectures that suggest potentially novel functions. Despite their low number (two in T. brucei, two in Trypanosoma cruzi and three in L. major), phosphotyrosine protein phosphatases (PTPs) have been associated with infectivity in trypanosomatids. L. major PTP1 (LmPTP1), which has an ortholog in T. cruzi but not in T. brucei, is needed for virulence and intracellular survival of amastigotes [63]. A similar role for calcineurin, a Ca2+/calmodulin dependent serine/threonine-specific phosphatase was recently reported in L. major [64]. It remains to be determined if specific Leishmania phosphatases are targets for H2O2 oxidation under conditions that trigger amastigote differentiation. This possibility is reinforced by recent studies showing that a glycosomal tyrosine-phosphatase (TbPTP1) acts as a master-switch controlling differentiation in the African trypanosome T. brucei. TbPTP1 activity blocks the development of transmissible ‘stumpy’ bloodstream forms until the parasites enter the tsetse fly, where citrate and cis-aconitate signaling inactivates TbPTP1, triggering differentiation [62]. The recent developments in Leishmania linking iron uptake, FeSOD activity, H2O2 generation, and the potential oxidation of target enzymes provide an exciting new avenue of research that should rapidly expand our understanding of how environmental changes drive cellular remodeling in trypanosomatid parasites.
Highlights.
Iron uptake regulates differentiation of Leishmania promastigotes into virulent amastigote forms.
Iron uptake is essential for FeSOD activation and generation of H2O2.
H2O2 promotes differentiation of avirulent promastigotes into virulent amastigotes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010:xii–xiii. 1–186. back cover. [PubMed] [Google Scholar]
- 2.Debrabant A, et al. Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int J Parasitol. 2004;34:205–217. doi: 10.1016/j.ijpara.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Gupta N, et al. In vitro cultivation and characterization of axenic amastigotes of Leishmania. Trends Parasitol. 2001;17:150–153. doi: 10.1016/s1471-4922(00)01811-0. [DOI] [PubMed] [Google Scholar]
- 4.Saar Y, et al. Characterization of developmentally-regulated activities in axenic amastigotes of Leishmania donovani. Mol Biochem Parasitol. 1998;95:9–20. doi: 10.1016/s0166-6851(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 5.Tsigankov P, et al. What has proteomics taught us about Leishmania development? Parasitology. 2012;139:1146–1157. doi: 10.1017/S0031182012000157. [DOI] [PubMed] [Google Scholar]
- 6.Holzer TR, et al. Expression profiling by whole-genome interspecies microarray hybridization reveals differential gene expression in procyclic promastigotes, lesion-derived amastigotes, and axenic amastigotes in Leishmania mexicana. Mol Biochem Parasitol. 2006;146:198–218. doi: 10.1016/j.molbiopara.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Saxena A, et al. Analysis of the Leishmania donovani transcriptome reveals an ordered progression of transient and permanent changes in gene expression during differentiation. Mol Biochem Parasitol. 2007;152:53–65. doi: 10.1016/j.molbiopara.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenzweig D, et al. Post-translational modification of cellular proteins during Leishmania donovani differentiation. Proteomics. 2008;8:1843–1850. doi: 10.1002/pmic.200701043. [DOI] [PubMed] [Google Scholar]
- 9.Lahav T, et al. Multiple levels of gene regulation mediate differentiation of the intracellular pathogen Leishmania. The FASEB Journal. 2011;25:515–525. doi: 10.1096/fj.10-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenzweig D, et al. Retooling Leishmania metabolism: from sand fly gut to human macrophage. FASEB J. 2008;22:590–602. doi: 10.1096/fj.07-9254com. [DOI] [PubMed] [Google Scholar]
- 11.Depledge DP, et al. Comparative expression profiling of Leishmania: modulation in gene expression between species and in different host genetic backgrounds. PLoS Negl Trop Dis. 2009;3:e476. doi: 10.1371/journal.pntd.0000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates PA, et al. Axenic cultivation and characterization of Leishmania mexicana amastigote-like forms. Parasitology. 1992;105(Pt 2):193–202. doi: 10.1017/s0031182000074102. [DOI] [PubMed] [Google Scholar]
- 13.Bates PA. Housekeeping by Leishmania. Trends Parasitol. 2006;22:447–448. doi: 10.1016/j.pt.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittra B, et al. Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J Exp Med. 2013;210:401–416. doi: 10.1084/jem.20121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh C, Andrews NW. Iron acquisition within host cells and the pathogenicity of Leishmania. Cellular microbiology. 2008;10:293–300. doi: 10.1111/j.1462-5822.2007.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor MC, Kelly JM. Iron metabolism in trypanosomatids, and its crucial role in infection. Parasitology. 2010;137:899–917. doi: 10.1017/S0031182009991880. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell JM, et al. SLC11A1 (formerly NRAMP1) and disease resistance. Cellular microbiology. 2001;3:773–784. doi: 10.1046/j.1462-5822.2001.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquis JF, Gros P. Intracellular Leishmania: your iron or mine? Trends Microbiol. 2007;15:93–95. doi: 10.1016/j.tim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Das NK, et al. Leishmania donovani depletes labile iron pool to exploit iron uptake capacity of macrophage for its intracellular growth. Cellular microbiology. 2009;11:83–94. doi: 10.1111/j.1462-5822.2008.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borges VM, et al. Subverted transferrin trafficking in Leishmania-infected macrophages. Parasitology research. 1998;84:811–822. doi: 10.1007/s004360050493. [DOI] [PubMed] [Google Scholar]
- 21.Wilson ME, et al. Acquisition of iron from transferrin and lactoferrin by the protozoan Leishmania chagasi. Infect Immun. 1994;62:3262–3269. doi: 10.1128/iai.62.8.3262-3269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson ME, Britigan BE. Iron acquisition by parasitic protozoa. Parasitol Today. 1998;14:348–353. doi: 10.1016/s0169-4758(98)01294-0. [DOI] [PubMed] [Google Scholar]
- 23.Flannery AR, et al. LFR1 Ferric Iron Reductase of Leishmania amazonensis Is Essential for the Generation of Infective Parasite Forms. J Biol Chem. 2011;286:23266–23279. doi: 10.1074/jbc.M111.229674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huynh C, et al. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J Exp Med. 2006;203:2363–2375. doi: 10.1084/jem.20060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hindt MN, Guerinot ML. Getting a sense for signals: regulation of the plant iron deficiency response. Biochim Biophys Acta. 2012;1823:1521–1530. doi: 10.1016/j.bbamcr.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos-Salinas J, et al. A new ATP-binding cassette protein is involved in intracellular haem trafficking in Leishmania. Mol Microbiol. 2011;79:1430–1444. doi: 10.1111/j.1365-2958.2010.07531.x. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho S, et al. Heme as a source of iron to Leishmania infantum amastigotes. Acta Trop. 2009;109:131–135. doi: 10.1016/j.actatropica.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Huynh C, et al. Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1. PLoS Pathog. 2012;8:e1002795. doi: 10.1371/journal.ppat.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams RA, et al. Cysteine peptidases CPA and CPB are vital for autophagy and differentiation in Leishmania mexicana. Mol Microbiol. 2006;61:655–674. doi: 10.1111/j.1365-2958.2006.05274.x. [DOI] [PubMed] [Google Scholar]
- 30.Balaban RS, et al. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buetler TM, et al. Role of superoxide as a signaling molecule. News Physiol Sci. 2004;19:120–123. doi: 10.1152/nips.01514.2003. [DOI] [PubMed] [Google Scholar]
- 33.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 34.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinnula VL, Crapo JD. Superoxide dismutases in malignant cells and human tumors. Free Radic Biol Med. 2004;36:718–744. doi: 10.1016/j.freeradbiomed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Gius D, Spitz DR. Redox signaling in cancer biology. Antioxid Redox Signal. 2006;8:1249–1252. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- 38.Pervaiz S, Clement MV. Superoxide anion: oncogenic reactive oxygen species? Int J Biochem Cell Biol. 2007;39:1297–1304. doi: 10.1016/j.biocel.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 40.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 41.Theopold U. Developmental biology: A bad boy comes good. Nature. 2009;461:486–487. doi: 10.1038/461486a. [DOI] [PubMed] [Google Scholar]
- 42.Dunand C, et al. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol. 2007;174:332–341. doi: 10.1111/j.1469-8137.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsukagoshi H, et al. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Rigoulet M, et al. Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid Redox Signal. 2011;14:459–468. doi: 10.1089/ars.2010.3363. [DOI] [PubMed] [Google Scholar]
- 45.Alzate JF, et al. Mitochondrial superoxide mediates heat-induced apoptotic-like death in Leishmania infantum. Mol Biochem Parasitol. 2007;152:192–202. doi: 10.1016/j.molbiopara.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Miller MA, et al. Inducible resistance to oxidant stress in the protozoan Leishmania chagasi. J Biol Chem. 2000;275:33883–33889. doi: 10.1074/jbc.M003671200. [DOI] [PubMed] [Google Scholar]
- 47.Wilson ME, et al. Response of Leishmania chagasi promastigotes to oxidant stress. Infect Immun. 1994;62:5133–5141. doi: 10.1128/iai.62.11.5133-5141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plewes KA, et al. Iron superoxide dismutases targeted to the glycosomes of Leishmania chagasi are important for survival. Infect Immun. 2003;71:5910–5920. doi: 10.1128/IAI.71.10.5910-5920.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Assche T, et al. Leishmania-macrophage interactions: insights into the redox biology. Free Radic Biol Med. 2011;51:337–351. doi: 10.1016/j.freeradbiomed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Adak S, Pal S. Ascorbate Peroxidase Acts As a Novel Determiner of Redox Homeostasis in Leishmania. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4745. [DOI] [PubMed] [Google Scholar]
- 51.Iyer JP, et al. Crucial role of cytosolic tryparedoxin peroxidase in Leishmania donovani survival, drug response and virulence. Mol Microbiol. 2008;68:372–391. doi: 10.1111/j.1365-2958.2008.06154.x. [DOI] [PubMed] [Google Scholar]
- 52.Swenerton RK, et al. Leishmania subtilisin is a maturase for the trypanothione reductase system and contributes to disease pathology. J Biol Chem. 2010;285:31120–31129. doi: 10.1074/jbc.M110.114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Williams RA, et al. Distinct roles in autophagy and importance in infectivity of the two ATG4 cysteine peptidases of Leishmania major. J Biol Chem. 2013;288:3678–3690. doi: 10.1074/jbc.M112.415372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sardar AH, et al. Proteome changes associated with Leishmania donovani promastigote adaptation to oxidative and nitrosative stresses. J Proteomics. 2013;81:185–199. doi: 10.1016/j.jprot.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Fleischhacker AS, Kiley PJ. Iron-containing transcription factors and their roles as sensors. Curr Opin Chem Biol. 2011;15:335–341. doi: 10.1016/j.cbpa.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson CP, et al. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta. 2012;1823:1468–1483. doi: 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Weelden SW, et al. Procyclic Trypanosoma brucei do not use Krebs cycle activity for energy generation. J Biol Chem. 2003;278:12854–12863. doi: 10.1074/jbc.M213190200. [DOI] [PubMed] [Google Scholar]
- 59.Long S, et al. Ancestral roles of eukaryotic frataxin: mitochondrial frataxin function and heterologous expression of hydrogenosomal Trichomonas homologues in trypanosomes. Mol Microbiol. 2008;69:94–109. doi: 10.1111/j.1365-2958.2008.06260.x. [DOI] [PubMed] [Google Scholar]
- 60.Long S, et al. Mitochondrial localization of human frataxin is necessary but processing is not for rescuing frataxin deficiency in Trypanosoma brucei. Proc Natl Acad Sci U S A. 2008;105:13468–13473. doi: 10.1073/pnas.0806762105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groeger G, et al. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal. 2009;11:2655–2671. doi: 10.1089/ars.2009.2728. [DOI] [PubMed] [Google Scholar]
- 62.Szoor B, et al. A novel phosphatase cascade regulates differentiation in Trypanosoma brucei via a glycosomal signaling pathway. Genes Dev. 2010;24:1306–1316. doi: 10.1101/gad.570310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nascimento M, et al. Identification and characterization of a protein-tyrosine phosphatase in Leishmania: Involvement in virulence. J Biol Chem. 2006;281:36257–36268. doi: 10.1074/jbc.M606256200. [DOI] [PubMed] [Google Scholar]
- 64.Naderer T, et al. Calcineurin is required for Leishmania major stress response pathways and for virulence in the mammalian host. Mol Microbiol. 2011;80:471–480. doi: 10.1111/j.1365-2958.2011.07584.x. [DOI] [PubMed] [Google Scholar]
- 65.Tracy K, Baehrecke EH. The role of autophagy in Drosophila metamorphosis. Curr Top Dev Biol. 2013;103:101–125. doi: 10.1016/B978-0-12-385979-2.00004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klionsky D. An overview of autophagy: Morphology, mechanism and regulation. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams RA, et al. ATG5 is essential for ATG8-dependent autophagy and mitochondrial homeostasis in Leishmania major. PLoS Pathog. 2012;8:e1002695. doi: 10.1371/journal.ppat.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herman M, et al. Turnover of glycosomes during life-cycle differentiation of Trypanosoma brucei. Autophagy. 2008;4 doi: 10.4161/auto.5443. [DOI] [PubMed] [Google Scholar]
- 69.Getachew F, Gedamu L. Leishmania donovani mitochondrial iron superoxide dismutase A is released into the cytosol during miltefosine induced programmed cell death. Mol Biochem Parasitol. 2012;183:42–51. doi: 10.1016/j.molbiopara.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Atwood JA, 3rd, et al. The Trypanosoma cruzi proteome. Science. 2005;309:473–476. doi: 10.1126/science.1110289. [DOI] [PubMed] [Google Scholar]
- 71.Piacenza L, et al. Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem J. 2007;403:323–334. doi: 10.1042/BJ20061281. [DOI] [PMC free article] [PubMed] [Google Scholar]