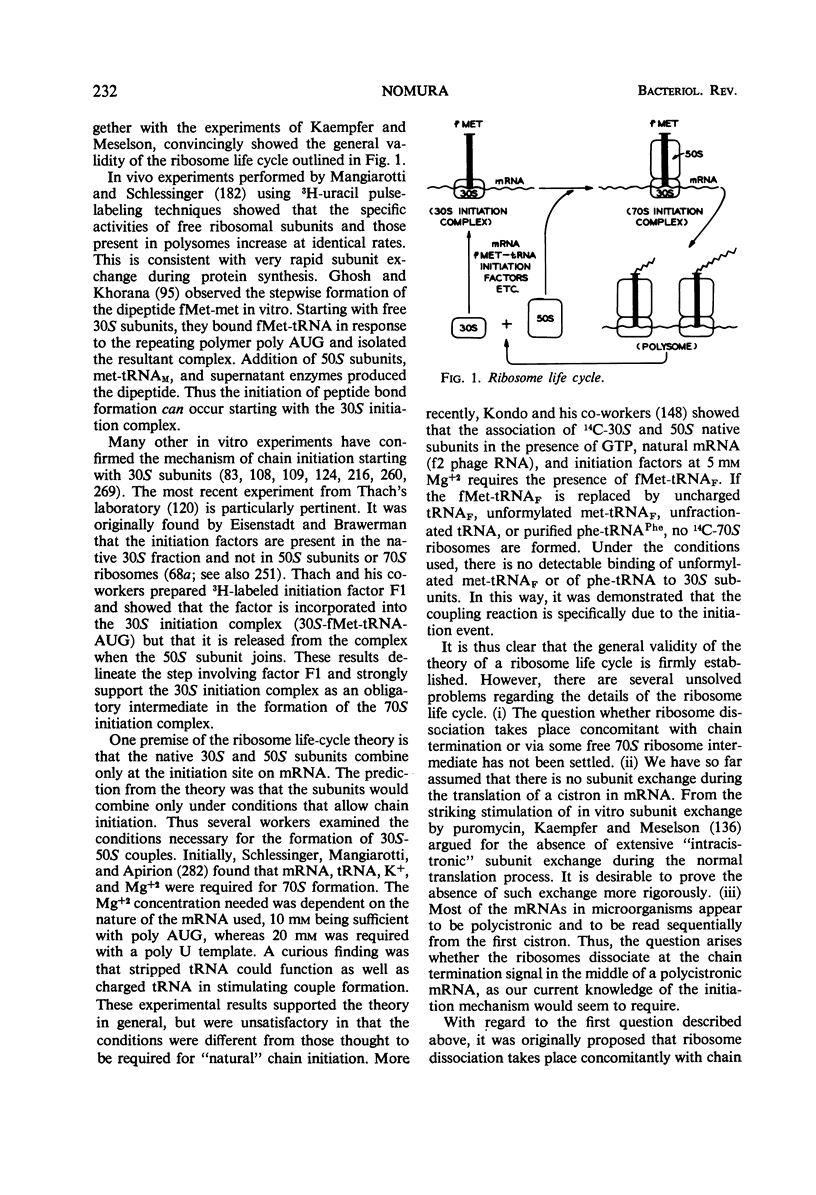
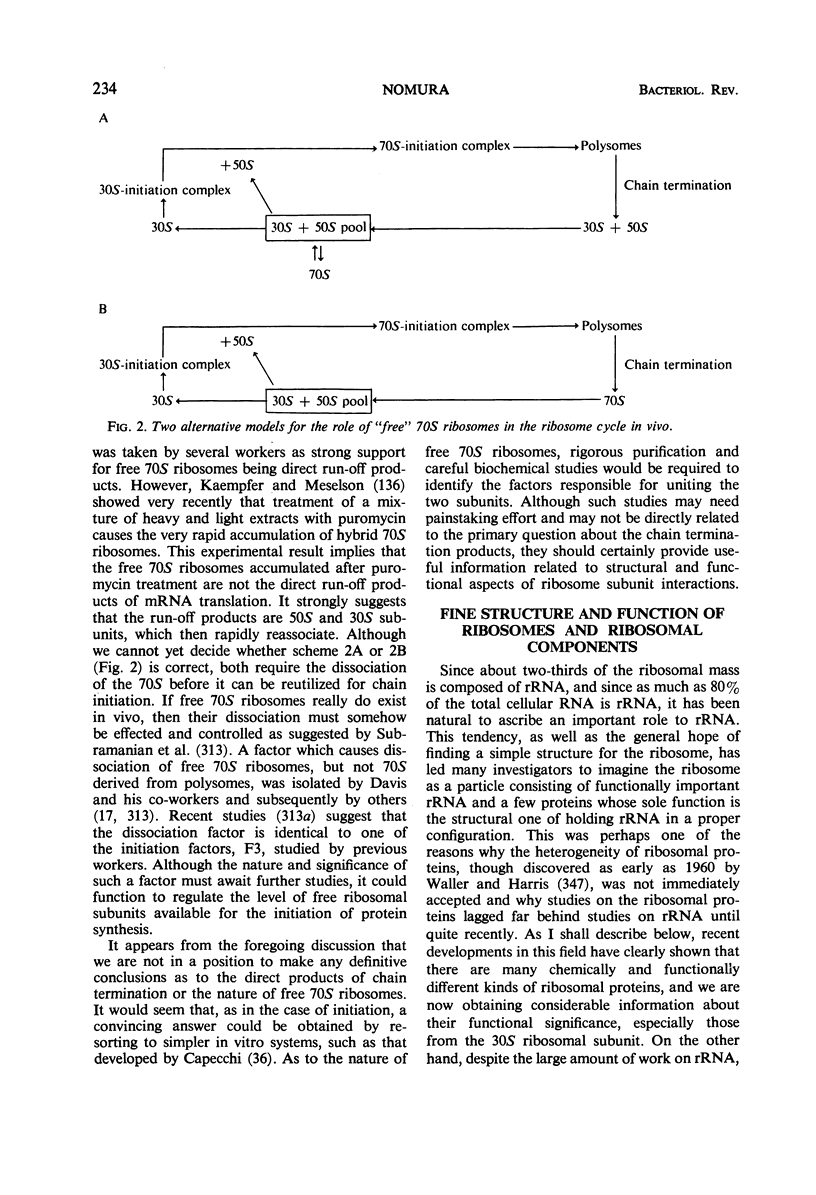
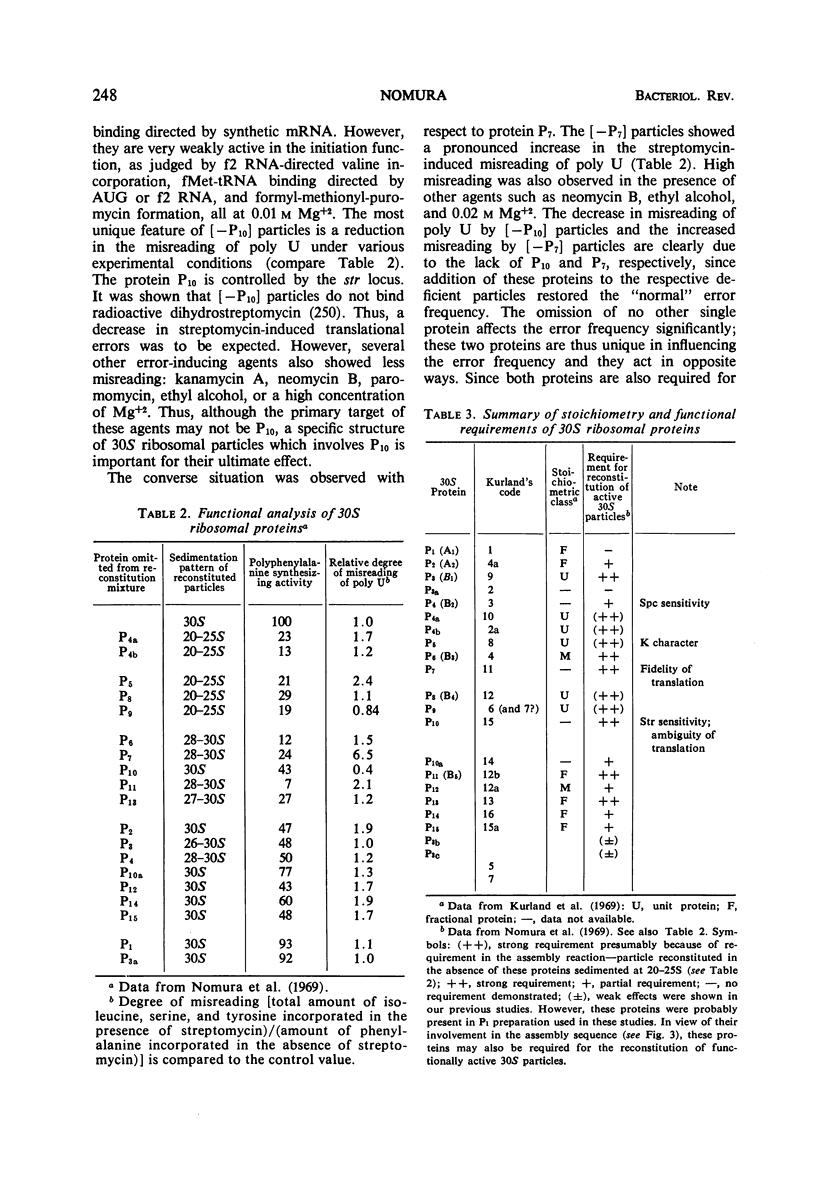
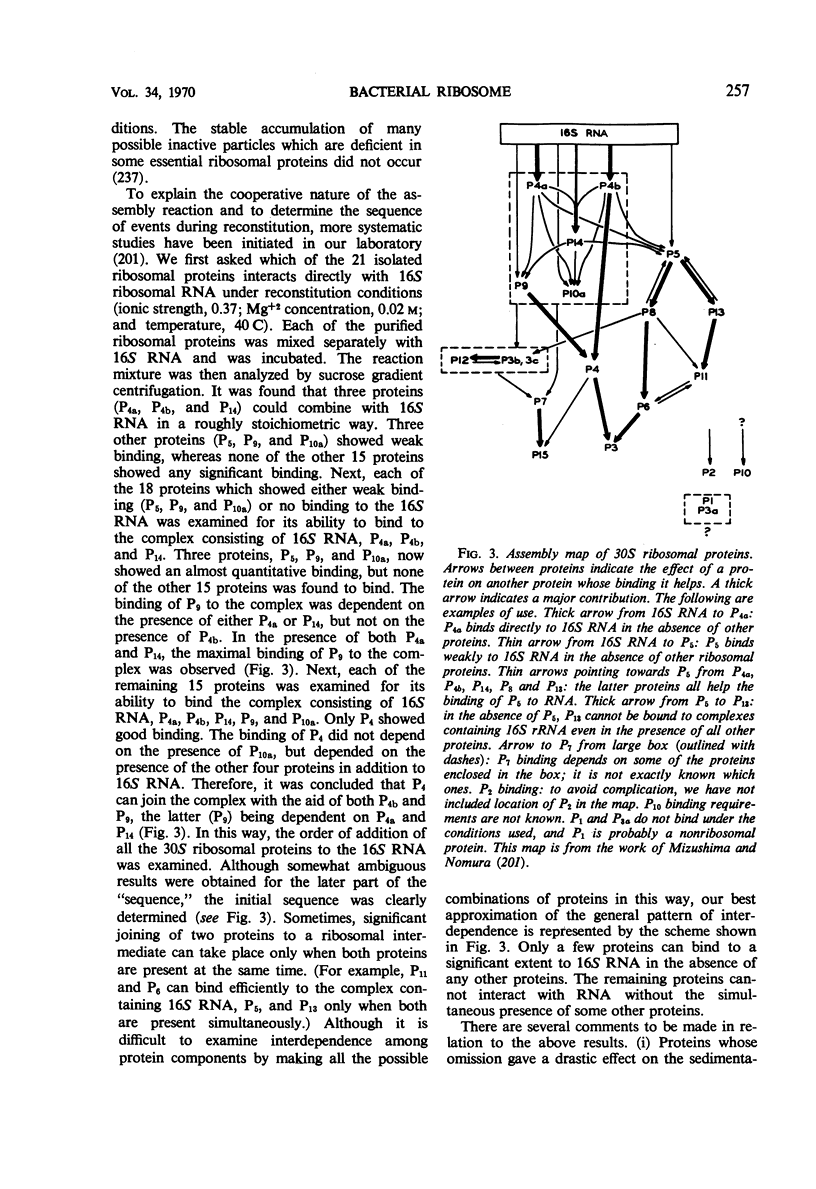
Full text
PDF













































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON A. I., HOLOWCZYK M. A. COMPOSITION OF BACTERIAL RIBOSOMAL RNA. HETEROGENEITY WITHIN A GIVEN ORGANISM. Biochim Biophys Acta. 1965 Feb 8;95:217–231. doi: 10.1016/0005-2787(65)90487-9. [DOI] [PubMed] [Google Scholar]
- Adesnik M., Levinthal C. Synthesis and maturation of ribosomal RNA in Escherichia coli. J Mol Biol. 1969 Dec 14;46(2):281–303. doi: 10.1016/0022-2836(69)90422-7. [DOI] [PubMed] [Google Scholar]
- Algranati I. D., Gonzalez N. S., Bade E. G. Physiological role of 70S ribosomes in bacteria. Proc Natl Acad Sci U S A. 1969 Feb;62(2):574–580. doi: 10.1073/pnas.62.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. S., Bretscher M. S., Clark B. F., Marcker K. A. A GTP requirement for binding initiator tRNA to ribosomes. Nature. 1967 Jul 29;215(5100):490–492. doi: 10.1038/215490a0. [DOI] [PubMed] [Google Scholar]
- Anderson J. S., Dahlberg J. E., Bretscher M. S., Revel M., Clark B. F. GTP-stimulated binding of initiator-tRNA to ribosomes directed by f2 bacteriophage RNA. Nature. 1967 Dec 16;216(5120):1072–1076. doi: 10.1038/2161072a0. [DOI] [PubMed] [Google Scholar]
- Anderson P., Davies J., Davis B. D. Effect of spectinomycin on polypeptide synthesis in extracts of Escherichia coli. J Mol Biol. 1967 Oct 14;29(1):203–215. doi: 10.1016/0022-2836(67)90191-x. [DOI] [PubMed] [Google Scholar]
- Anderson P. Sensitivity and Resistance to Spectinomycin in Escherichia coli. J Bacteriol. 1969 Nov;100(2):939–947. doi: 10.1128/jb.100.2.939-947.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T., Chargaff E. Formation and fate of abnormal ribosomes of E. coli cells treated with 5-fluorouracil. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1181–1189. doi: 10.1073/pnas.54.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. Functional interdependence of ribosomal components of Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):794–799. doi: 10.1073/pnas.63.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D., Phillips S., Sypherd P. Escherichia coli: reversion from streptomycin dependence, a mutation in a specific 30 s ribosomal protein. J Mol Biol. 1969 Jul 28;43(2):327–329. doi: 10.1016/0022-2836(69)90271-x. [DOI] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. The loss of phenotypic suppression in streptomycin-resistant mutants. Proc Natl Acad Sci U S A. 1967 Jul;58(1):206–212. doi: 10.1073/pnas.58.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsmon A., Spitnik-Elson P., Elson D. Detachment of ribosomal proteins by salt. II. Some properties of protein-deficient particles formed by the detachment of ribosomal proteins. J Mol Biol. 1969 Oct 14;45(1):125–135. doi: 10.1016/0022-2836(69)90215-0. [DOI] [PubMed] [Google Scholar]
- Atsmon A., Spitnik-elson P., Elson D. Characterization of the particulate and free proteins obtained after treatment of ribosomes with 2 M-lithium chloride. J Mol Biol. 1967 Apr 14;25(1):161–163. doi: 10.1016/0022-2836(67)90286-0. [DOI] [PubMed] [Google Scholar]
- Attardi G., Huang P. C., Kabat S. Recognition of ribosomal RNA sites in DNA. I. Analysis of the E. coli system. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1490–1498. doi: 10.1073/pnas.53.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti-Tocco G., Brown G. L. Reaction of N-cyclohexyl, N'-beta (4-methylmorpholinium) ethyl carbodiimide iodide with nucleic acids and polynucleotides. Nature. 1965 May 15;206(985):683–685. doi: 10.1038/206683a0. [DOI] [PubMed] [Google Scholar]
- BRAGG P. D., POLGLASE W. J. BIOSYNTHESIS OF VALINE IN STREPTOMYCIN-DEPENDENT ESCHERICHIA COLI. J Bacteriol. 1965 Apr;89:1158–1159. doi: 10.1128/jb.89.4.1158-1159.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITTEN R. J., McCARTHY B. J. The synthesis of ribosomes in E. coli. II. Analysis of the kinetics of tracer incorporation in growing cells. Biophys J. 1962 Jan;2:49–55. doi: 10.1016/s0006-3495(62)86840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach D., Miller I. R. Interaction of deoxyribonucleic acid with poly-4-vinylpyridine. Biochim Biophys Acta. 1966 Feb 21;114(2):311–325. doi: 10.1016/0005-2787(66)90312-1. [DOI] [PubMed] [Google Scholar]
- Bade E. G., González N. S., Algranati I. S. Dissociation of 70S ribosomes: some properties of the dissociating factor from Bacillus stearothermophilus and Escherichia coli. Proc Natl Acad Sci U S A. 1969 Oct;64(2):654–660. doi: 10.1073/pnas.64.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge E. A., Craven G. R., Hardy S. J., Kurland C. G., Voynow P. Structural determinant of a ribosomal protein: K locus. Science. 1969 Jun 13;164(3885):1285–1286. doi: 10.1126/science.164.3885.1285. [DOI] [PubMed] [Google Scholar]
- Birge E. A., Kurland C. G. Altered ribosomal protein in streptomycin-dependent Escherichia coli. Science. 1969 Dec 5;166(3910):1282–1284. doi: 10.1126/science.166.3910.1282. [DOI] [PubMed] [Google Scholar]
- Blundell M. R., Wild D. G. Inhibition of bacterial growth by metal salts. A survey of effects on the synthesis of ribonucleic acid and protein. Biochem J. 1969 Nov;115(2):207–212. doi: 10.1042/bj1150207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell M. R., Wild D. G. Inhibition of bacterial growth by metal salts. The accumulation of ribonucleic acid during inhibition of Escherichia coli by cobalt chloride. Biochem J. 1969 Nov;115(2):213–223. doi: 10.1042/bj1150213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A., Davies J., Ozaki M., Mizushima S. Ribosomal Protein Conferring Sensitivity to the Antibiotic Spectinomycin in Escherichia coli. Science. 1969 Jul 4;165(3888):85–86. doi: 10.1126/science.165.3888.85. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Translocation in protein synthesis: a hybrid structure model. Nature. 1968 May 18;218(5142):675–677. doi: 10.1038/218675a0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Brown D. D. The genes for ribosomal RNA and their transcription during amphibian development. Curr Top Dev Biol. 1967;2:47–73. doi: 10.1016/s0070-2153(08)60283-5. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. Nucleotide sequence of 5S-ribosomal RNA from Escherichia coli. Nature. 1967 Aug 12;215(5102):735–736. doi: 10.1038/215735a0. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Brownstein B. L., Lewandowski L. J. A mutation suppressing streptomycin dependence. I. An effect on ribosome function. J Mol Biol. 1967 Apr 14;25(1):99–109. doi: 10.1016/0022-2836(67)90281-1. [DOI] [PubMed] [Google Scholar]
- Bruening G., Bock R. M. Covalent integrity and molecular weights of yeast ribosomal ribonucleic acid components. Biochim Biophys Acta. 1967 Dec 19;149(2):377–386. doi: 10.1016/0005-2787(67)90166-9. [DOI] [PubMed] [Google Scholar]
- Bruskov V. I., Kiselev N. A. Electron microscope study of the structure of Escherichia coli riboomes and CM-like particles. J Mol Biol. 1968 Nov 14;37(3):367–377. doi: 10.1016/0022-2836(68)90108-3. [DOI] [PubMed] [Google Scholar]
- Bush C. A., Scheraga H. A. Optical rotatory dispersion and RNA base pairing in ribosomes and in tobacco mosaic virus. Biochemistry. 1967 Oct;6(10):3036–3042. doi: 10.1021/bi00862a010. [DOI] [PubMed] [Google Scholar]
- COX E. C., WHITE J. R., FLAKS J. G. STREPTOMYCIN ACTION AND THE RIBOSOME. Proc Natl Acad Sci U S A. 1964 Apr;51:703–709. doi: 10.1073/pnas.51.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAMER F., SEIDEL H. THE SELECTIVE 1-N-OXIDATION OF NUCLEOBASES IN HOMOPOLYNUCLEOTIDES AND IN NUCLEIC ACIDS. Biochim Biophys Acta. 1964 Sep 11;91:14–22. doi: 10.1016/0926-6550(64)90165-3. [DOI] [PubMed] [Google Scholar]
- Cammack K. A., Wade H. E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Klein H. A. Characterization of three proteins involved in polypeptide chain termination. Cold Spring Harb Symp Quant Biol. 1969;34:469–477. doi: 10.1101/sqb.1969.034.01.053. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Polypeptide chain termination in vitro: isolation of a release factor. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1144–1151. doi: 10.1073/pnas.58.3.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Tompkins R., Scolnick E., Caryk T., Nirenberg M. Sequential translation of trinucleotide codons for the initiation and termination of protein synthesis. Science. 1968 Oct 4;162(3849):135–138. doi: 10.1126/science.162.3849.135. [DOI] [PubMed] [Google Scholar]
- Caskey T., Scolnick E., Tompkins R., Goldstein J., Milman G. Peptide chain termination, codon, protein factor, and ribosomal requirements. Cold Spring Harb Symp Quant Biol. 1969;34:479–488. doi: 10.1101/sqb.1969.034.01.054. [DOI] [PubMed] [Google Scholar]
- Cheng T. Y., Hartman K. A., Jr Properties of ribosomes and ribosomal RNA formed by a relaxed mutant of Escherichia coli during growth with ethionine. J Mol Biol. 1968 Jan 28;31(2):191–207. doi: 10.1016/0022-2836(68)90439-7. [DOI] [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. The role of N-formyl-methionyl-sRNA in protein biosynthesis. J Mol Biol. 1966 Jun;17(2):394–406. doi: 10.1016/s0022-2836(66)80150-x. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Herner A. E., Goldberg I. H. Inhibition by pactamycin of the initiation of protein synthesis. Binding of N-acetylphenylalanyl transfer ribonucleic acid and polyuridylic acid to ribosomes. Biochemistry. 1969 Apr;8(4):1312–1326. doi: 10.1021/bi00832a004. [DOI] [PubMed] [Google Scholar]
- Colli W., Oishi M. Ribosomal RNA genes in bacteria: evidence for the nature of the physical linkage between 16S and 23S RNA genes in Bacillus subtilis. Proc Natl Acad Sci U S A. 1969 Oct;64(2):642–649. doi: 10.1073/pnas.64.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter R. I., Gratzer W. B. Conformation of ribosomal RNA of E. coli: an infrared analysis. Nature. 1969 Jan 11;221(5176):154–156. doi: 10.1038/221154a0. [DOI] [PubMed] [Google Scholar]
- Cotter R. I., McPhie P., Gratzer W. B. Internal organization of the ribosome. Nature. 1967 Dec 2;216(5118):864–868. doi: 10.1038/216864a0. [DOI] [PubMed] [Google Scholar]
- Couturier M., Desmet L., Thomas R. High pleiotropy of streptomycin mutations in Escherichia coli. Biochem Biophys Res Commun. 1964 Jun 15;16(3):244–248. doi: 10.1016/0006-291x(64)90333-x. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Bonanou S. A. A possible structure of the rabbit reticulocyte ribosome. An exercise in model building. Biochem J. 1969 Oct;114(4):769–774. doi: 10.1042/bj1140769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Gould H. J., Kanagalingam K. A study of the alkaline hydrolysis of fractionated reticulocyte ribosomal ribonucleic acid and its relevance to secondary structure. Biochem J. 1968 Feb;106(3):733–741. doi: 10.1042/bj1060733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. The secondary structure of ribosomal ribonucleic acid in solution. Biochem J. 1966 Mar;98(3):841–857. doi: 10.1042/bj0980841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer F., Erdmann V. A. Amount of adenine and uracil base pairs in E. coli 23S, 16S and 5S ribosomal RNA. Nature. 1968 Apr 6;218(5136):92–93. doi: 10.1038/218092a0. [DOI] [PubMed] [Google Scholar]
- Craven G. R., Gavin R., Fanning T. The transfer RNA binding site of the 30 S ribosome and the site of tetracycline inhibition. Cold Spring Harb Symp Quant Biol. 1969;34:129–137. doi: 10.1101/sqb.1969.034.01.019. [DOI] [PubMed] [Google Scholar]
- Craven G. R., Voynow P., Hardy S. J., Kurland C. G. The ribosomal proteins of Escherichia coli. II. Chemical and physical characterization of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2906–2915. doi: 10.1021/bi00835a032. [DOI] [PubMed] [Google Scholar]
- Cundliffe E. Polyribosomes and ribosomal sub-units of bacterial protoplasts. Biochem Biophys Res Commun. 1968 Oct 24;33(2):247–252. doi: 10.1016/0006-291x(68)90776-6. [DOI] [PubMed] [Google Scholar]
- Cutler R. G., Evans J. E. Relative transcription activity of different segments of the genome throughout the cell division cycle of Escherichia coli. The mapping of ribosomal and transfer RNA and the determination of the direction of replication. J Mol Biol. 1967 May 28;26(1):91–105. doi: 10.1016/0022-2836(67)90263-x. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., WHITE A. E., WILD D. G., SYKES J. Synthesis of protein and ribosomes by bacteria. Nature. 1962 Apr 7;194:25–27. doi: 10.1038/194025a0. [DOI] [PubMed] [Google Scholar]
- DAVIES J. E. STUDIES ON THE RIBOSOMES OF STREPTOMYCIN-SENSITIVE AND RESISTANT STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Apr;51:659–664. doi: 10.1073/pnas.51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES J., GILBERT W., GORINI L. STREPTOMYCIN, SUPPRESSION, AND THE CODE. Proc Natl Acad Sci U S A. 1964 May;51:883–890. doi: 10.1073/pnas.51.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN D. T., ELKORT A. T. SOME ABNORMAL PROPERTIES OF CHLORAMPHENICOL RNA. J Mol Biol. 1964 Dec;10:508–518. doi: 10.1016/s0022-2836(64)80069-3. [DOI] [PubMed] [Google Scholar]
- DUBIN D. T. SOME EFFECT OF STREPTOMYCIN ON RNA METABOLISM IN ESCHERICHIA COLI. J Mol Biol. 1964 May;8:749–767. doi: 10.1016/s0022-2836(64)80122-4. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F. C., Jr, Sells B. H. Synthesis and assembly of ribosomal protein into 50 s subunits during recovery from chloramphenicol treatment. J Mol Biol. 1969 Feb 14;39(3):503–521. doi: 10.1016/0022-2836(69)90141-7. [DOI] [PubMed] [Google Scholar]
- Dingman C. W., Peacock A. C. Analytical studies on nuclear ribonucleic acid using polyacrylamide gel electrophoresis. Biochemistry. 1968 Feb;7(2):659–668. doi: 10.1021/bi00842a022. [DOI] [PubMed] [Google Scholar]
- Doty P., Boedtker H., Fresco J. R., Haselkorn R., Litt M. SECONDARY STRUCTURE IN RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1959 Apr;45(4):482–499. doi: 10.1073/pnas.45.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Marmur J. Gene conservation in Bacillus species. II. The location of genes concerned with the synthesis of ribosomal components and soluble RNA. Proc Natl Acad Sci U S A. 1965 Sep;54(3):724–730. doi: 10.1073/pnas.54.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSON D. Latent enzymic activity of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:372–386. doi: 10.1016/0006-3002(59)90179-9. [DOI] [PubMed] [Google Scholar]
- ELSON D. Latent ribonuclease activity in a ribonucleoprotein. Biochim Biophys Acta. 1958 Jan;27(1):216–217. doi: 10.1016/0006-3002(58)90320-2. [DOI] [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt J. M., Brawerman G. A factor from Escherichia coli concerned with the stimulation of cell-free polypeptide synthesis by exogenous ribonucleic acid. I. Evidence for the occurrence of a stimulation factor. Biochemistry. 1966 Sep;5(9):2777–2783. doi: 10.1021/bi00873a001. [DOI] [PubMed] [Google Scholar]
- Eisenstadt J. M., Brawerman G. The role of the native subribosomal particles of Escherichia coli in polypeptide chain initiation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1560–1565. doi: 10.1073/pnas.58.4.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbe R. W., Nau M. M., Leder P. Translation and translocation of defined RNA messengers. J Mol Biol. 1969 Feb 14;39(3):441–460. doi: 10.1016/0022-2836(69)90137-5. [DOI] [PubMed] [Google Scholar]
- Ertel R., Brot N., Redfield B., Allende J. E., Weissbach H. Binding of guanosine 5'-triphosphate by soluble factors required for polypeptide synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):861–868. doi: 10.1073/pnas.59.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel D. H., Valulis B. Control of ribonucleic acid synthesis in Escherichia coli cells with altered transfer ribonucleic acid concentration. Biochim Biophys Acta. 1966 Oct 24;129(1):123–139. doi: 10.1016/0005-2787(66)90014-1. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WHITE J. R. Inhibition of polypeptide synthesis by streptomycin. Biochem Biophys Res Commun. 1962 May 11;7:385–389. doi: 10.1016/0006-291x(62)90320-0. [DOI] [PubMed] [Google Scholar]
- Fellner P., Ehresmann C., Ebel J. P. Nucleotide sequences present within the 16S ribosomal RNA of Escherichia coli. Nature. 1970 Jan 3;225(5227):26–29. doi: 10.1038/225026a0. [DOI] [PubMed] [Google Scholar]
- Fellner P. Nucleotide sequences from specific areas of the 16S and 23S ribosomal RNAs of E. coli. Eur J Biochem. 1969 Nov;11(1):12–27. doi: 10.1111/j.1432-1033.1969.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Fellner P., Sanger F. Sequence analysis of specific areas of the 16S and 23S ribosomal RNAs. Nature. 1968 Jul 20;219(5151):236–238. doi: 10.1038/219236a0. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L. The effect of ribonuclease on polysomes and ribosomes of bacteria and animal cells. Biochem J. 1968 Apr;107(4):481–489. doi: 10.1042/bj1070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaks J. G., Leboy P. S., Birge E. A., Kurland C. G. Mutations and genetics concerned with the ribosome. Cold Spring Harb Symp Quant Biol. 1966;31:623–631. doi: 10.1101/sqb.1966.031.01.081. [DOI] [PubMed] [Google Scholar]
- Fogel S., Sypherd P. S. Chemical basis for heterogeneity of ribosomal proteins. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1329–1336. doi: 10.1073/pnas.59.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S., Sypherd P. S. Extraction and isolation of individual ribosomal proteins from Escherichia coli. J Bacteriol. 1968 Aug;96(2):358–364. doi: 10.1128/jb.96.2.358-364.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Jordan B. 5S RNA synthesized by Escherichia coli in presence of chloramphenicol: different 5'-terminal sequences. Science. 1970 Jan 23;167(3917):382–384. doi: 10.1126/science.167.3917.382. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Varricchio F. A naturally occurring 43 s ribosomal precursor particle in Escherichia coli: nature and ribonucleic acid composition. J Mol Biol. 1970 Mar;48(3):409–419. doi: 10.1016/0022-2836(70)90054-9. [DOI] [PubMed] [Google Scholar]
- Friedman H., Lu P., Rich A. Ribosomal subunits produced by cold sensitive initiation of protein synthesis. Nature. 1969 Aug 30;223(5209):909–913. doi: 10.1038/223909a0. [DOI] [PubMed] [Google Scholar]
- Friesen J. D. A study of the relationship between polyribosomes and messenger RNA in Escherichia coli. J Mol Biol. 1968 Mar 14;32(2):183–200. doi: 10.1016/0022-2836(68)90003-x. [DOI] [PubMed] [Google Scholar]
- Fry M., Artman M. Studies of newly synthesized ribosomal ribonucleic acid of Escherichia coli. Biochem J. 1969 Nov;115(2):295–305. doi: 10.1042/bj1150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller W., Hutchinson F., Spencer M., Wilkins M. H. Molecular and crystal structures of double-helical RNA. I. An x-ray diffraction study of fragmented yeast RNA and a preliminary double-helical RNA model. J Mol Biol. 1967 Aug 14;27(3):507–524. doi: 10.1016/0022-2836(67)90055-1. [DOI] [PubMed] [Google Scholar]
- Furano A. V., Bradley D. F., Childers L. G. The conformation of the ribonucleic acid in ribosomes. Dye stacking studies. Biochemistry. 1966 Sep;5(9):3044–3056. doi: 10.1021/bi00873a038. [DOI] [PubMed] [Google Scholar]
- Furano A. V. Chromatography of Escherichia coli ribosomes on diethylaminoethyl cellulose. J Biol Chem. 1966 May 25;241(10):2237–2244. [PubMed] [Google Scholar]
- GORDON J., BOMAN H. G., ISAKSSON L. A. IN VIVO INHIBITION OF RNA METHYLATION IN THE PRESENCE OF CHLORAMPHENICOL. J Mol Biol. 1964 Sep;9:831–833. doi: 10.1016/s0022-2836(64)80190-x. [DOI] [PubMed] [Google Scholar]
- GORINI L., KATAJA E. PHENOTYPIC REPAIR BY STREPTOMYCIN OF DEFECTIVE GENOTYPES IN E. COLI. Proc Natl Acad Sci U S A. 1964 Mar;51:487–493. doi: 10.1073/pnas.51.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M. H., HALL B. D. A comparison of the native and derived 30S and 50S ribosomes of Escherichia coli. Biophys J. 1961 Jul;1:517–523. doi: 10.1016/s0006-3495(61)86905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner T. K., Orias E. Effects of mutations to streptomycin resistance on the rate of translation of mutant genetic information. J Bacteriol. 1966 Mar;91(3):1021–1028. doi: 10.1128/jb.91.3.1021-1028.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin R. T., Julian G. R., Rogers S. J. Dye-sensitized photooxidation of the Escherichia coli ribosome. Science. 1969 May 2;164(3879):583–584. doi: 10.1126/science.164.3879.583. [DOI] [PubMed] [Google Scholar]
- Gavrilova L. P., Ivanov D. A., Spirin A. S. Studies on the structure of ribosomes. 3. Stepwise unfolding of the 50 s particles without loss of ribosomal protein. J Mol Biol. 1966 Apr;16(2):473–489. doi: 10.1016/s0022-2836(66)80186-9. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Staehelin T. Electrophoretic analysis of proteins from normal and cesium chloride-treated Escherichia coli ribosomes. J Mol Biol. 1967 Mar 14;24(2):149–155. doi: 10.1016/0022-2836(67)90323-3. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Unfolding of Escherichia coli ribosomes by removal of magnesium. J Mol Biol. 1966 Jul;18(2):356–371. doi: 10.1016/s0022-2836(66)80253-x. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Khorana H. G. Studies on polynucleotides, LXXXIV. On the role of ribosomal subunits in protein synthesis. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2455–2461. doi: 10.1073/pnas.58.6.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierer L., Gierer A. Synthesis of ribosomal proteins and formation of ribosomes in Escherichia coli. J Mol Biol. 1968 Jul 14;34(2):293–303. doi: 10.1016/0022-2836(68)90254-4. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. The replication of bacteriophage MS2. VI. Interaction between bacteriophage RNA and cellular components in MS2-infected Escherichia coli. J Mol Biol. 1967 Feb 14;23(3):495–521. doi: 10.1016/s0022-2836(67)80121-9. [DOI] [PubMed] [Google Scholar]
- Goldthwaite C., Dubnau D., Smith I. Genetic mapping of antibiotic resistance in markers Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):96–103. doi: 10.1073/pnas.65.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D., Manor H., Rombauts W. Ribosomal protein synthesis during and after amino acid starvation in relaxed and stringent bacteria. J Mol Biol. 1969 Mar 14;40(2):247–260. doi: 10.1016/0022-2836(69)90473-2. [DOI] [PubMed] [Google Scholar]
- Goodman R. E., Spotts C. R. Effects of streptomycin deprivation on enzyme synthesis in streptomycin-dependent Escherichia coli. J Bacteriol. 1967 Oct;94(4):1154–1161. doi: 10.1128/jb.94.4.1154-1161.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini L., Jacoby G. A., Breckenridge L. Ribosomal ambiguity. Cold Spring Harb Symp Quant Biol. 1966;31:657–664. doi: 10.1101/sqb.1966.031.01.084. [DOI] [PubMed] [Google Scholar]
- Gorini L. The contrasting role of strA and ram gene products in ribosomal functioning. Cold Spring Harb Symp Quant Biol. 1969;34:101–109. doi: 10.1101/sqb.1969.034.01.016. [DOI] [PubMed] [Google Scholar]
- Greenshpan H., Revel M. Initiator protein dependent binding of messenger RNA to ribosomes. Nature. 1969 Oct 25;224(5217):331–335. doi: 10.1038/224331a0. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Nakada D. Ribosomal subunits and MS2 phage RNA-directed protein synthesis. Nature. 1969 Sep 20;223(5212):1242–1247. doi: 10.1038/2231242a0. [DOI] [PubMed] [Google Scholar]
- Grunberg-Manago M., Clark B. F., Revel M., Rudland P. S., Dondon J. Stability of different ribosomal complexes with initiator transfer RNA and synthetic messenger RNA. J Mol Biol. 1969 Feb 28;40(1):33–44. doi: 10.1016/0022-2836(69)90294-0. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Nashimoto H., Nomura M. Structure and function of E. coli ribosomes. 8. Cold-sensitive mutants defective in ribosome assembly. Proc Natl Acad Sci U S A. 1969 Jun;63(2):384–391. doi: 10.1073/pnas.63.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C., Nashimoto H., Nomura M. Studies on the assembly of ribosomes in vivo. Cold Spring Harb Symp Quant Biol. 1969;34:69–75. doi: 10.1101/sqb.1969.034.01.011. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Nomura M. Initiation of protein synthesis: a critical test of the 30S subunit model. Nature. 1968 Jul 20;219(5151):232–235. doi: 10.1038/219232a0. [DOI] [PubMed] [Google Scholar]
- HIEROWSKI M. INHIBITION OF PROTEIN SYNTHESIS BY CHLORTETRACYCLINE IN THE E. COLI IN VITRO SYSTEM. Proc Natl Acad Sci U S A. 1965 Mar;53:594–599. doi: 10.1073/pnas.53.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSOKAWA K., NOMURA M. INCOMPLETE RIBOSOMES PRODUCED IN CHLORAMPHENICOL- AND PUROMYCIN-INHIBITED ESCHERICHIA COLI. J Mol Biol. 1965 May;12:225–241. doi: 10.1016/s0022-2836(65)80296-0. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Lucas-Lenard J. Stepwise synthesis of a tripeptide. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1363–1369. doi: 10.1073/pnas.61.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Hart R. G. Surface features of the 50S ribosomal component of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1415–1420. doi: 10.1073/pnas.53.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Streptomycin Resistance in Escherichia Coli Analyzed by Transduction. Genetics. 1960 Jan;45(1):49–62. doi: 10.1093/genetics/45.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht N. B., Bleyman M., Woese C. R. The formation of 5S ribosomal ribonucleic acid in Bacillus subtilis by posttranscriptional modification. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1278–1283. doi: 10.1073/pnas.59.4.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht N. B., Woese C. R. Separation of bacterial ribosomal ribonucleic acid from its macromolecular precursors by polyacrylamide gel electrophoresis. J Bacteriol. 1968 Mar;95(3):986–990. doi: 10.1128/jb.95.3.986-990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey J. W., Dewey K. F., Thach R. E. Purification and properties of initiation factor f-1. Nature. 1969 Jun 7;222(5197):944–947. doi: 10.1038/222944a0. [DOI] [PubMed] [Google Scholar]
- Herzberg M., Lelong J. C., Revel M. Purification of initiator C from Escherichia coli: a protein which binds messenger RNA and initiator tRNA to the 30 s ribosome. J Mol Biol. 1969 Sep 14;44(2):297–308. doi: 10.1016/0022-2836(69)90176-4. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Rossetti G. P., Van Holde K. E. Physical studies of ribosomes from Escherichia coli. J Mol Biol. 1969 Sep 14;44(2):263–277. doi: 10.1016/0022-2836(69)90174-0. [DOI] [PubMed] [Google Scholar]
- Hille M. B., Miller M. J., Iwasaki K., Wahba A. J. Translation of the genetic message. VI. The role of ribosomal subunits in binding of formylmethionyl-tRNA and its reaction with puromycin. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1652–1654. doi: 10.1073/pnas.58.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills D. C., Horowitz J. Ribosome synthesis in Escherichia coli treated with 5-fluorouracil. Biochemistry. 1966 May;5(5):1625–1632. doi: 10.1021/bi00869a025. [DOI] [PubMed] [Google Scholar]
- Hindley J., Staples D. H. Sequence of a ribosome binding site in bacteriophage Q-beta-RNA. Nature. 1969 Dec 6;224(5223):964–967. doi: 10.1038/224964a0. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Buck C. A., McCarthy B. J. Stimulation of protein synthesis in vitro by partially degraded ribosomal ribonucleic acid and transfer ribonucleic acid. Biochemistry. 1966 Jan;5(1):358–365. doi: 10.1021/bi00865a046. [DOI] [PubMed] [Google Scholar]
- Horowitz J., Hills D. C. Evidence for the direct conversion of chloramphenicol particles into ribosomes in Escherichia coli. Biochim Biophys Acta. 1966 Aug 17;123(2):416–419. doi: 10.1016/0005-2787(66)90293-0. [DOI] [PubMed] [Google Scholar]
- Hosokawa K., Fujimura R. K., Nomura M. Reconstitution of functionally active ribosomes from inactive subparticles and proteins. Proc Natl Acad Sci U S A. 1966 Jan;55(1):198–204. doi: 10.1073/pnas.55.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Otaka E., Osawa S. Release of ribosomal proteins from Escherichia coli ribosomes with high concentrations of lithium chloride. J Mol Biol. 1968 Apr 14;33(1):109–122. doi: 10.1016/0022-2836(68)90284-2. [DOI] [PubMed] [Google Scholar]
- Iwabuchi M., Kono M., Oumi T., Osawa S. The RNA components in ribonucleoprotein particles occurring during the course of ribosome formation in Escherichia coli. Biochim Biophys Acta. 1965 Oct 11;108(2):211–219. doi: 10.1016/0005-2787(65)90005-5. [DOI] [PubMed] [Google Scholar]
- KENNELL D., MAGASANIK B. The relation of ribosome content to the rate of enzyme synthesis in Aerobacter aerogenes. Biochim Biophys Acta. 1962 Jan 22;55:139–151. doi: 10.1016/0006-3002(62)90940-x. [DOI] [PubMed] [Google Scholar]
- KJELDGAARD N. O., MAALOE O., SCHAECHTER M. The transition between different physiological states during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):607–616. doi: 10.1099/00221287-19-3-607. [DOI] [PubMed] [Google Scholar]
- KJELDGAARD N. O. The kinetics of ribonucleic acid- and protein formation in Salmonella typhimurium during the transition between different states of balance growth. Biochim Biophys Acta. 1961 Apr 29;49:64–76. doi: 10.1016/0006-3002(61)90870-8. [DOI] [PubMed] [Google Scholar]
- KLUG A., HOLMES K. C., FINCH J. T. X-ray diffraction studies on ribosomes from various sources. J Mol Biol. 1961 Feb;3:87–100. doi: 10.1016/s0022-2836(61)80010-7. [DOI] [PubMed] [Google Scholar]
- KONO M., OSAWA S. INTERMEDIARY STEPS OF RIBOSOME FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Jun 22;87:326–334. doi: 10.1016/0926-6550(64)90228-2. [DOI] [PubMed] [Google Scholar]
- KONO M., OTAKA E., OSAWA S. CHANGES IN SEDIMENTATION PROPERTIES OF RIBOSOMAL RIBONUCLEIC ACIDS DURING THE COURSE OF RIBOSOME FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Dec 16;91:612–618. doi: 10.1016/0926-6550(64)90009-x. [DOI] [PubMed] [Google Scholar]
- KURLAND C. G., MAALOE O. Regulation of ribosomal and transfer RNA synthesis. J Mol Biol. 1962 Mar;4:193–210. doi: 10.1016/s0022-2836(62)80051-5. [DOI] [PubMed] [Google Scholar]
- KURLAND C. G., NOMURA M., WATSON J. D. The physical properties of the chloromycetin particles. J Mol Biol. 1962 May;4:388–394. doi: 10.1016/s0022-2836(62)80019-9. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Meselson M., Raskas H. J. Cyclic dissociation into stable subunits and re-formation of ribosomes during bacterial growth. J Mol Biol. 1968 Jan 28;31(2):277–289. doi: 10.1016/0022-2836(68)90444-0. [DOI] [PubMed] [Google Scholar]
- Kaempfer R., Meselson M. Studies of ribosomal subunit exchange. Cold Spring Harb Symp Quant Biol. 1969;34:209–220. doi: 10.1101/sqb.1969.034.01.027. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Ribosomal subunit exchange during protein synthesis. Proc Natl Acad Sci U S A. 1968 Sep;61(1):106–113. doi: 10.1073/pnas.61.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H., Fukutome H., Kawade Y. Inactivation of Escherichia coli ribosomes by ultraviolet irradiation. I. Activity of poly U-directed polyphenylalanine synthesis. J Mol Biol. 1967 Jun 14;26(2):249–265. doi: 10.1016/0022-2836(67)90295-1. [DOI] [PubMed] [Google Scholar]
- Kaji H., Tanaka Y. Binding of dihydrostreptomycin to ribosomal subunits. J Mol Biol. 1968 Mar 14;32(2):221–230. doi: 10.1016/0022-2836(68)90006-5. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Dzionara M., Donner D., Wittmann H. G. Ribosomal proteins. I. Isolation, amino acid composition, molecular weights and peptide mapping of proteins from E. coli ribosomes. Mol Gen Genet. 1967;100(4):364–373. doi: 10.1007/BF00334063. [DOI] [PubMed] [Google Scholar]
- Kelley W. S., Schaechter M. Magnesium ion-dependent dissociation of polysomes and free 70 s ribosomes in Bacillus megaterium. J Mol Biol. 1969 Jun 28;42(3):599–602. doi: 10.1016/0022-2836(69)90248-4. [DOI] [PubMed] [Google Scholar]
- Kohler R. E., Ron E. Z., Davis B. D. Significance of the free 70 s ribosomes in Escherichia coli extracts. J Mol Biol. 1968 Aug 28;36(1):71–82. doi: 10.1016/0022-2836(68)90220-9. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Ohta T., Thach R. E. Junction of the 50S ribosomal subunit with the 30S initiation complex. Nature. 1968 Oct 19;220(5164):244–247. doi: 10.1038/220244a0. [DOI] [PubMed] [Google Scholar]
- Kondo M., Eggerston G., Eisenstadt J., Lengyel P. Ribosome formation from subunits: dependence on formylmethionyl-transfer RNA in extracts from E. coli. Nature. 1968 Oct 26;220(5165):368–371. doi: 10.1038/220368a0. [DOI] [PubMed] [Google Scholar]
- Kuriki Y., Kaji A. Factor- and guanosine 5'-triphosphate-dependent release of deacylated transfer RNA from 70S ribosomes. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1399–1405. doi: 10.1073/pnas.61.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland C. G. The requirements for specific sRNA binding by ribosomes. J Mol Biol. 1966 Jun;18(1):90–108. doi: 10.1016/s0022-2836(66)80079-7. [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Voynow P., Hardy S. J., Randall L., Lutter L. Physical and functional heterogeneity of E. coli ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:17–24. doi: 10.1101/sqb.1969.034.01.006. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Endo H., Ohnishi Y. Mutations to spectinomycin resistance which alleviate the restriction of an amber suppressor by streptomycin resistance. J Bacteriol. 1969 Feb;97(2):940–943. doi: 10.1128/jb.97.2.940-943.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Ishizawa M., Endo H. Su-II-specific restriction of amber suppression by mutation to streptomycin resistance. J Mol Biol. 1968 Apr 28;33(2):513–516. doi: 10.1016/0022-2836(68)90209-x. [DOI] [PubMed] [Google Scholar]
- LANDMAN O. E., BURCHARD W. The mechanism of action of streptomycin as revealed by normal and abnormal division in streptomycin-dependent Salmonellae. Proc Natl Acad Sci U S A. 1962 Feb;48:219–228. doi: 10.1073/pnas.48.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGRIDGE R., HOLMES K. C. X-ray diffraction studies of concentrated gels of ribosomes from E. coli. J Mol Biol. 1962 Dec;5:611–617. doi: 10.1016/s0022-2836(62)80089-8. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkovits I., Di Girolamo M. Properties of ribonucleoprotein particles in chloramphenicol-treated cells of Escherichia coli B. Biochim Biophys Acta. 1969 Feb 18;174(2):561–565. doi: 10.1016/0005-2787(69)90285-8. [DOI] [PubMed] [Google Scholar]
- Lefkovits I., Di Girolamo M. Reutilization of ribosomal proteins in vivo for the formation of new ribosomal particles in Escherichia coli B. Biochim Biophys Acta. 1969 Feb 18;174(2):566–573. doi: 10.1016/0005-2787(69)90286-x. [DOI] [PubMed] [Google Scholar]
- Lekover T. E., Kurland C. G. Ribosomes from a streptomycin-dependent strain of Escherichia coli. J Mol Biol. 1967 May 14;25(3):497–504. doi: 10.1016/0022-2836(67)90201-x. [DOI] [PubMed] [Google Scholar]
- Lengyel P., Söll D. Mechanism of protein biosynthesis. Bacteriol Rev. 1969 Jun;33(2):264–301. doi: 10.1128/br.33.2.264-301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman M. I., Spirin A. S., Gavrilova L. P., Golov V. F. Studies on the structure of ribosomes. II. Stepwise dissociation of protein from ribosomes by caesium chloride and the re-assembly of ribosome-like particles. J Mol Biol. 1966 Jan;15(1):268–281. doi: 10.1016/s0022-2836(66)80226-7. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Brownstein B. L. Characterization of a 43 s ribonucleoprotein component of a mutant of Escherichia coli. J Mol Biol. 1969 Apr;41(2):277–290. doi: 10.1016/0022-2836(69)90392-1. [DOI] [PubMed] [Google Scholar]
- Lipmann F. Polypeptide chain elongation in protein biosynthesis. Science. 1969 May 30;164(3883):1024–1031. doi: 10.1126/science.164.3883.1024. [DOI] [PubMed] [Google Scholar]
- Lubin M. Observations on the shape of the 50S ribosomal subunit. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1454–1461. doi: 10.1073/pnas.61.4.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J., Haenni A. L. Release of transfer RNA during peptide chain elongation. Proc Natl Acad Sci U S A. 1969 May;63(1):93–97. doi: 10.1073/pnas.63.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J., Haenni A. L. Requirement of granosine 5'-triphosphate for ribosomal binding of aminoacyl-SRNA. Proc Natl Acad Sci U S A. 1968 Feb;59(2):554–560. doi: 10.1073/pnas.59.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Initiation of polyphenylalanine synthesis by N-acetylphenylalanyl-SRNA. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1050–1057. doi: 10.1073/pnas.57.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L., Schlessinger D., Apirion D. Escherichia coli: high resistance or dependence on streptomycin produced by the same allele. Science. 1968 Aug 2;161(3840):478–479. doi: 10.1126/science.161.3840.478. [DOI] [PubMed] [Google Scholar]
- MESELSON M., NOMURA M., BRENNER S., DAVERN C., SCHLESSINGER D. CONSERVATION OF RIBOSOMES DURING BACTERIAL GROWTH. J Mol Biol. 1964 Sep;9:696–711. doi: 10.1016/s0022-2836(64)80176-5. [DOI] [PubMed] [Google Scholar]
- MILES H. T. Quantitative infrared spectra in D20 of some nucleosides, nucleotides, and polynucleotides; a new measure of polynucleotide interaction. Biochim Biophys Acta. 1958 Nov;30(2):324–328. doi: 10.1016/0006-3002(58)90057-x. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E. Technique for the isolation of ribonucleic acid-rich mutants from Escherichia coli. J Bacteriol. 1969 Nov;100(2):974–976. doi: 10.1128/jb.100.2.974-976.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Turnock G., Forchhammer J. The synthesis and function of ribosomes in a new mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Jan;57(1):141–147. doi: 10.1073/pnas.57.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D., Silengo L. Biosynthetic precursors of 30S and 50S ribosomal particles in Escherichia coli. Biochemistry. 1968 Jan;7(1):456–472. doi: 10.1021/bi00841a058. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Schlessinger D. Polyribosome metabolism in Escherichia coli. I. Extraction of polyribosomes and ribosomal subunits from fragile, growing Escherichia coli. J Mol Biol. 1966 Sep;20(1):123–143. doi: 10.1016/0022-2836(66)90122-7. [DOI] [PubMed] [Google Scholar]
- Manor H., Haselkorn R. Properties of ribonucleic acid components in ribonucleoprotein particle preparations obtained from an Escherichia coli RC-relaxed strain. J Mol Biol. 1967 Mar 14;24(2):269–288. doi: 10.1016/0022-2836(67)90332-4. [DOI] [PubMed] [Google Scholar]
- Manor H., Haselkorn R. Properties of ribonucleoprotein particles produced by an Escherichia coli RC-relaxed strain starved for histidine. J Mol Biol. 1967 Mar 14;24(2):323–328. doi: 10.1016/0022-2836(67)90337-3. [DOI] [PubMed] [Google Scholar]
- Marcot-Queiroz J., Monier R. Les ribosomes d'Escherichia coli. II. Préparation de particules 18S et 25S par traitement des ribosomes au chlorure de lithium. Bull Soc Chim Biol (Paris) 1967;49(5):477–494. [PubMed] [Google Scholar]
- Marrs B., Kaplan S. 23 s precursor ribosomal RNA of Rhodopseudomonas spheroides. J Mol Biol. 1970 Apr 28;49(2):297–317. doi: 10.1016/0022-2836(70)90247-0. [DOI] [PubMed] [Google Scholar]
- Marushige K., Bonner J. Template properties of liver chromatin. J Mol Biol. 1966 Jan;15(1):160–174. doi: 10.1016/s0022-2836(66)80218-8. [DOI] [PubMed] [Google Scholar]
- Mayuga C., Meier D., Wang T. Escherichia coli: the K 12 ribosomal protein and the streptomycin region of the chromosome. Biochem Biophys Res Commun. 1968 Oct 24;33(2):203–206. doi: 10.1016/0006-291x(68)90768-7. [DOI] [PubMed] [Google Scholar]
- McIlreavy D. J., Midgley J. E. The chemical structure of bacterial ribosomal RNA. I. Terminal nucleotide sequences of Escherichia coli ribosomal RNA. Biochim Biophys Acta. 1967 Jun 20;142(1):47–64. doi: 10.1016/0005-2787(67)90514-x. [DOI] [PubMed] [Google Scholar]
- McPhie P., Gratzer W. B. The optical rotatory dispersion of ribosomes and their constituents. Biochemistry. 1966 Apr;5(4):1310–1315. doi: 10.1021/bi00868a026. [DOI] [PubMed] [Google Scholar]
- Miall S. H., Walker I. O. Structural studies on ribosomes. II. Denaturation and sedimentation of ribosomal subunits unfolded in EDTA. Biochim Biophys Acta. 1969 Feb 18;174(2):551–560. doi: 10.1016/0005-2787(69)90284-6. [DOI] [PubMed] [Google Scholar]
- Michelson E. L., Sutama Y. Evidence for two types of 18-S ribosomal RNA in Neurospora crassa. Biochim Biophys Acta. 1968 Mar 18;157(1):200–203. doi: 10.1016/0005-2787(68)90280-3. [DOI] [PubMed] [Google Scholar]
- Midgley J. E. Studies on the structure of 23-S ribosomal ribonucleic acid from Escherichia coli. Biochim Biophys Acta. 1965 Nov 8;108(3):348–354. doi: 10.1016/0005-2787(65)90027-4. [DOI] [PubMed] [Google Scholar]
- Midgley J. E. The estimation of polynucleotide chain length by a chemical method. Biochim Biophys Acta. 1965 Nov 8;108(3):340–347. doi: 10.1016/0005-2787(65)90026-2. [DOI] [PubMed] [Google Scholar]
- Miles H. T., Frazier J. Infrared study of helix strandedness in the poly A-poly U system. Biochem Biophys Res Commun. 1964;14:21–28. doi: 10.1016/0006-291x(63)90204-3. [DOI] [PubMed] [Google Scholar]
- Milman G., Goldstein J., Scolnick E., Caskey T. Peptide chain termination. 3. Stimulation of in vitro termination. Proc Natl Acad Sci U S A. 1969 May;63(1):183–190. doi: 10.1073/pnas.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Modolell J., Davis B. D. A unitary mechanism for the several effects of streptomycin on the ribosome. Cold Spring Harb Symp Quant Biol. 1969;34:113–116. doi: 10.1101/sqb.1969.034.01.017. [DOI] [PubMed] [Google Scholar]
- Modolell J., Davis B. D. Mechanism of inhibition of ribosomes by streptomycin. Nature. 1969 Oct 25;224(5217):345–348. doi: 10.1038/224345a0. [DOI] [PubMed] [Google Scholar]
- Monier R., Feunteun J., Forget B., Jordan B., Reynier M., Varricchio F. 5 S RNA and the assembly of bacterial ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:139–148. doi: 10.1101/sqb.1969.034.01.020. [DOI] [PubMed] [Google Scholar]
- Monro R. E. Catalysis of peptide bond formation by 50 S ribosomal subunits from Escherichia coli. J Mol Biol. 1967 May 28;26(1):147–151. doi: 10.1016/0022-2836(67)90271-9. [DOI] [PubMed] [Google Scholar]
- Monro R. E., Marcker K. A. Ribosome-catalysed reaction of puromycin with a formylmethionine-containing oligonucleotide. J Mol Biol. 1967 Apr 28;25(2):347–350. doi: 10.1016/0022-2836(67)90146-5. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Traut R. R., Noller H., Pearson P., Delius H. Ribosomal proteins of Escherichia coli. II. Proteins from the 30 s subunit. J Mol Biol. 1968 Feb 14;31(3):441–461. doi: 10.1016/0022-2836(68)90420-8. [DOI] [PubMed] [Google Scholar]
- Morell P., Marmur J. Association of 5S ribonucleic acid to 50S ribosomal subunits of Escherichia coli and Bacillus subtilis. Biochemistry. 1968 Mar;7(3):1141–1152. doi: 10.1021/bi00843a035. [DOI] [PubMed] [Google Scholar]
- Morris D. W., DeMoss J. A. Polysome transitions and the regulation of ribonucleic acid synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):262–268. doi: 10.1073/pnas.56.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan M. A., Hershey J. W., Dewey K. F., Thach R. E. Binding of formylmethionyl-tRNA to 30S ribosomal sub-units. Nature. 1968 Mar 16;217(5133):1013–1016. doi: 10.1038/2171013a0. [DOI] [PubMed] [Google Scholar]
- Muto A. Messenger activity of nascent ribosomal RNA. J Mol Biol. 1968 Aug 28;36(1):1–14. doi: 10.1016/0022-2836(68)90214-3. [DOI] [PubMed] [Google Scholar]
- Muto A., Otaka E., Osawa S. Protein synthesis in a relaxed-control mutant of Escherichia coli upon recovery from methionine starvation. J Mol Biol. 1966 Aug;19(1):60–73. doi: 10.1016/s0022-2836(66)80050-5. [DOI] [PubMed] [Google Scholar]
- Möller W., Amons R., Groene J. C., Garrett R. A., Terhorst C. P. Protein-ribonucleic acid interactions in ribosomes. Biochim Biophys Acta. 1969 Oct 22;190(2):381–390. doi: 10.1016/0005-2787(69)90088-4. [DOI] [PubMed] [Google Scholar]
- Möller W., Chrambach A. Physical heterogeneity of the ribosomal proteins from Escherichia coli. J Mol Biol. 1967 Feb 14;23(3):377–390. doi: 10.1016/s0022-2836(67)80112-8. [DOI] [PubMed] [Google Scholar]
- Möller W. Substructures in ribosomes of E. coli? A hypothesis for the attachment of transfer RNAs. Nature. 1969 Jun 7;222(5197):979–981. doi: 10.1038/222979a0. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C. Properties of a bacterial mutant lacking amino acid control of RNA synthesis. Biochim Biophys Acta. 1963 Mar 26;68:365–379. doi: 10.1016/0006-3002(63)90158-6. [DOI] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA M., HOSOKAWA K. BIOSYNTHESIS OF RIBOSOMES: FATE OF CHLORAMPHENICOL PARTICLES AND OF PULSE-LABELED RNA IN ESCHERICHIA COLI. J Mol Biol. 1965 May;12:242–265. doi: 10.1016/s0022-2836(65)80297-2. [DOI] [PubMed] [Google Scholar]
- Nakada D. Formation of ribosomes by a "relaxed" mutant of Escherichia coli. J Mol Biol. 1965 Jul;12(3):695–725. doi: 10.1016/s0022-2836(65)80322-9. [DOI] [PubMed] [Google Scholar]
- Nakada D., Marquisee M. J. Relaxed synthesis of ribosomal RNA by a stringent strain of Escherichia coli. J Mol Biol. 1965 Sep;13(2):351–361. doi: 10.1016/s0022-2836(65)80102-4. [DOI] [PubMed] [Google Scholar]
- Nanninga N. The conformation of the 50S ribosomal subunit of Bacillus subtilis. Proc Natl Acad Sci U S A. 1968 Oct;61(2):614–620. doi: 10.1073/pnas.61.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori S., Maruta H., Mizuno D. Unfolding of Escherichia coli ribosomes by phosphate ion in the presence of oligonucleotides. J Mol Biol. 1968 Nov 28;38(1):109–119. doi: 10.1016/0022-2836(68)90131-9. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. The regulation RNA synthesis in bacteria. Prog Nucleic Acid Res Mol Biol. 1964;3:145–181. doi: 10.1016/s0079-6603(08)60741-2. [DOI] [PubMed] [Google Scholar]
- Nichols J. L., Lane B. G. The terminal groups of ribonucleate chains in each of the 16S and 23S components of Escherichia coli ribosomal RNA. Can J Biochem. 1967 Jun;45(6):937–948. doi: 10.1139/o67-104. [DOI] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V., Guthrie C. The initiation of protein synthesis: joining of the 50S ribosomal subunit to the initiation complex. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1487–1493. doi: 10.1073/pnas.58.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V. PHAGE f2 RNA-DIRECTED BINDING OF FORMYLMETHIONYL-TRNA TO RIBOSOMES AND THE ROLE OF 30S RIBOSOMAL SUBUNITS IN INITIATION OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1967 Sep;58(3):946–953. doi: 10.1073/pnas.58.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Mizushima S., Ozaki M., Traub P., Lowry C. V. Structure and function of ribosomes and their molecular components. Cold Spring Harb Symp Quant Biol. 1969;34:49–61. doi: 10.1101/sqb.1969.034.01.009. [DOI] [PubMed] [Google Scholar]
- Nomura M., Traub P., Bechmann H. Hybrid 30S ribosomal particles reconstituted from components of different bacterial origins. Nature. 1968 Aug 24;219(5156):793–799. doi: 10.1038/219793b0. [DOI] [PubMed] [Google Scholar]
- Nomura M., Traub P., Guthrie C., Nashimoto H. The assembly of ribosomes. J Cell Physiol. 1969 Oct;74(2 Suppl):241+–241+. doi: 10.1002/jcp.1040740428. [DOI] [PubMed] [Google Scholar]
- Nomura M., Traub P. Structure and function of Escherichia coli ribosomes. 3. Stoichiometry and rate of the reconstitution of ribosomes from subribosomal particles and split proteins. J Mol Biol. 1968 Jun 28;34(3):609–619. doi: 10.1016/0022-2836(68)90184-8. [DOI] [PubMed] [Google Scholar]
- O'Neil D. M., Baron L. S., Sypherd P. S. Chromosomal location of ribosomal protein cistrons determined by intergeneric bacterial mating. J Bacteriol. 1969 Jul;99(1):242–247. doi: 10.1128/jb.99.1.242-247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M., Sueoka N. Location of genetic loci of ribosomal RNA on Bacillus subtilis chromosome. Proc Natl Acad Sci U S A. 1965 Aug;54(2):483–491. doi: 10.1073/pnas.54.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. The transcribing strands of bacillus subtilis DNA for ribosomal and transfer RNA. Proc Natl Acad Sci U S A. 1969 Jan;62(1):256–262. doi: 10.1073/pnas.62.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Skoultchi A., Klein A., Lengyel P. Peptide chain elongation: discrimination against the initiator transfer RNA by microbial amino-acid polymerization factors. Nature. 1968 Dec 28;220(5174):1304–1307. doi: 10.1038/2201304a0. [DOI] [PubMed] [Google Scholar]
- Ono Y., Skoultchi A., Waterson J., Lengyel P. Peptide chain elongation: GTP cleavage catalysed by factors binding aminoacyl-transfer RNA to the ribosome. Nature. 1969 May 17;222(5194):645–648. doi: 10.1038/222645a0. [DOI] [PubMed] [Google Scholar]
- Osawa S. Biosynthesis of ribosomes in bacterial cells. Prog Nucleic Acid Res Mol Biol. 1965;4:161–188. doi: 10.1016/s0079-6603(08)60787-4. [DOI] [PubMed] [Google Scholar]
- Osawa S., Otaka E., Itoh T., Fukui T. Biosynthesis of 50 s ribosomal subunit in Escherichia coli. J Mol Biol. 1969 Mar 28;40(3):321–351. doi: 10.1016/0022-2836(69)90158-2. [DOI] [PubMed] [Google Scholar]
- Osawa S. Ribosome formation and structure. Annu Rev Biochem. 1968;37:109–130. doi: 10.1146/annurev.bi.37.070168.000545. [DOI] [PubMed] [Google Scholar]
- Otaka E., Itoh T., Osawa S. Ribosomal proteins of bacterial cells: strain- and species-specificity. J Mol Biol. 1968 Apr 14;33(1):93–107. doi: 10.1016/0022-2836(68)90283-0. [DOI] [PubMed] [Google Scholar]
- Otsuji N., Aono H. Effect of mutation to streptomycin resistance on amber suppressor genes. J Bacteriol. 1968 Jul;96(1):43–50. doi: 10.1128/jb.96.1.43-50.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Parenti-Rosina R., Eisenstadt A., Eisenstadt J. M. Isolation of protein initiation factors from 30S ribosomal subunits. Nature. 1969 Jan 25;221(5178):363–365. doi: 10.1038/221363a0. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E. Transcription on an RNA polymerase-DNA complex from E. coli: evidence for in vitro synthesis of ribosomal RNA. Biochem Biophys Res Commun. 1969 Feb 21;34(4):541–547. doi: 10.1016/0006-291x(69)90416-1. [DOI] [PubMed] [Google Scholar]
- Phillips L. A., Franklin R. M. The in vivo distribution of bacterial polysomes, ribosomes, and ribosomal subunits. Cold Spring Harb Symp Quant Biol. 1969;34:243–253. doi: 10.1101/sqb.1969.034.01.030. [DOI] [PubMed] [Google Scholar]
- Phillips L. A., Hotham-Iglewski B., Franklin R. M. Polyribosomes of Escherichia coli. I. Effects of monovalent cations on the distribution of polysomes, ribosomes and ribosomal subunits. J Mol Biol. 1969 Mar 14;40(2):279–288. doi: 10.1016/0022-2836(69)90475-6. [DOI] [PubMed] [Google Scholar]
- ROSENKRANZ H. S. Unusual alkaline phosphatase levels in streptomycin-dependent strains of E. coli. Biochemistry. 1963 Jan-Feb;2:122–125. doi: 10.1021/bi00901a021. [DOI] [PubMed] [Google Scholar]
- ROSSET R., MONIER R., JULIEN J. LES RIBOSOMES D'ESCHERICHIA COLI. I. MISE EN 'EVIDENCE D'UN RNA RIBOSOMIQUE DE FAIBLE POIDS MOL'ECULAIRE. Bull Soc Chim Biol (Paris) 1964;46:87–109. [PubMed] [Google Scholar]
- Ravel J. M. Demonstration of a guanosine triphosphate-dependent enzymatic binding of aminoacyl-ribonucleic acid to Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1811–1816. doi: 10.1073/pnas.57.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsema J. A., Conway T. W. Reversible dissociation of Escherichia coli ribosomes by N-ethylmaleimide. Biochim Biophys Acta. 1969 Apr 22;179(2):369–380. doi: 10.1016/0005-2787(69)90045-8. [DOI] [PubMed] [Google Scholar]
- Revel M., Gros F. A factor from E. coli required for the translation of natural messenger RNA. Biochem Biophys Res Commun. 1966 Oct 5;25(1):124–132. doi: 10.1016/0006-291x(66)90649-8. [DOI] [PubMed] [Google Scholar]
- Revel M., Lelong J. C., Brawerman G., Gros F. Function of three protein factors and ribosomal subunits in the initiation of protein synthesis in E. coli. Nature. 1968 Sep 7;219(5158):1016–1021. doi: 10.1038/2191016a0. [DOI] [PubMed] [Google Scholar]
- Reynier M., Monier R. Les ribosomes d'Escherichia coli. 3. Etude de la dissociation réversible du RNA 5S par le chlorure de lithium. Bull Soc Chim Biol (Paris) 1969 Jan 30;50(10):1583–1600. [PubMed] [Google Scholar]
- Ron E. Z., Kohler R. E., Davis B. D. Increased stability of polysomes in an Escherichia coli mutant with relaxed control of RNA synthesis. Proc Natl Acad Sci U S A. 1966 Aug;56(2):471–475. doi: 10.1073/pnas.56.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron E. Z., Kohler R. E., Davis B. D. Magnesium ion dependence of free and polysomal ribosomes from Escherichia coli. J Mol Biol. 1968 Aug 28;36(1):83–89. doi: 10.1016/0022-2836(68)90221-0. [DOI] [PubMed] [Google Scholar]
- Rosset R., Gorini L. A ribosomal ambiguity mutation. J Mol Biol. 1969 Jan 14;39(1):95–112. doi: 10.1016/0022-2836(69)90336-2. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Dube S. K. Specific interaction of an initiator tRNA fragment with 30 s ribosomal subunits. J Mol Biol. 1969 Jul 28;43(2):273–280. doi: 10.1016/0022-2836(69)90267-8. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Whybrow W. A., Marcker K. A., Clark B. F. Recognition of bacterial initiator tRNA by initiation factors. Nature. 1969 May 24;222(5195):750–753. doi: 10.1038/222750a0. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- SHAKULOV R. S., AITKHOZHIN M. A., SPIRIN A. S. [On latent degradation of ribosomes]. Biokhimiia. 1962 Jul-Aug;27:744–751. [PubMed] [Google Scholar]
- SPAHR P. F. Amino acid composition of ribosomes from Escherichia coli. J Mol Biol. 1962 May;4:395–406. doi: 10.1016/s0022-2836(62)80020-5. [DOI] [PubMed] [Google Scholar]
- SPENCER M., POOLE J. ON THE ORIGIN OF CRYSTALLIZABLE RNA FROM YEAST. J Mol Biol. 1965 Feb;11:314–326. doi: 10.1016/s0022-2836(65)80060-2. [DOI] [PubMed] [Google Scholar]
- SPEYER J. F., LENGYEL P., BASILIO C. Ribosomal localization of streptomycin sensitivity. Proc Natl Acad Sci U S A. 1962 Apr 15;48:684–686. doi: 10.1073/pnas.48.4.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIRIN A. S., KISELEV N. A., SHAKULOV R. S., BOGDANOV A. A. IZUCHENIE STRUKTURY RIBOSOM; OBRATIMOE RAZVORACHIVANIE RIBOSOMNYKH CHASTITS V RIBONUKLEOPROTEIDNYE TIAZHI I MODEL' UKLADKI. Biokhimiia. 1963 Sep-Oct;28:920–930. [PubMed] [Google Scholar]
- SPITNIK-ELSON P. THE PREPARATION OF RIBOSOMAL PROTEIN FROM ESCHERICHIA COLI WITH LITHIUM CHLORIDE AND UREA. Biochem Biophys Res Commun. 1965 Feb 17;18:557–562. doi: 10.1016/0006-291x(65)90790-4. [DOI] [PubMed] [Google Scholar]
- SPITNIK-ELSON P. The solubilization of ribosomal protein from Escherichia coli. Biochim Biophys Acta. 1962 May 14;55:741–747. doi: 10.1016/0006-3002(62)90852-1. [DOI] [PubMed] [Google Scholar]
- SPOTTS C. R. Physiological and biochemical studies on streptomycin dependence in Escherichia coli. J Gen Microbiol. 1962 Jun;28:347–365. doi: 10.1099/00221287-28-2-347. [DOI] [PubMed] [Google Scholar]
- SPOTTS C. R., STANIER R. Y. Mechanism of streptomycin action on bacteria: a unitary hypothesis. Nature. 1961 Nov 18;192:633–637. doi: 10.1038/192633a0. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENT G. S. THE OPERON: ON ITS THIRD ANNIVERSARY. MODULATION OF TRANSFER RNA SPECIES CAN PROVIDE A WORKABLE MODEL OF AN OPERATOR-LESS OPERON. Science. 1964 May 15;144(3620):816–820. doi: 10.1126/science.144.3620.816. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Santer M., Ruebush T. K., Van Brunt J., Oldmixon E., Hess R., Primakoff P., Palade P. Identification of a precursor pool of ribosome protein in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1355–1367. doi: 10.1128/jb.95.4.1355-1367.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer M., Smith J. R. Ribonuclease sensitivity of Escherichia coli ribosomes. J Bacteriol. 1966 Oct;92(4):1099–1110. doi: 10.1128/jb.92.4.1099-1110.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P. K., Yang J. T., Doty P. Optical rotatory dispersion of E. coli ribosomes and their constituents. Biopolymers. 1967 Jan;5(1):1–4. doi: 10.1002/bip.1967.360050102. [DOI] [PubMed] [Google Scholar]
- Schaup H. W., Best J. B., Goodman A. B. Evidence for heterogeneity of 23S RNA in E. coli. Nature. 1969 Mar 1;221(5183):864–865. doi: 10.1038/221864a0. [DOI] [PubMed] [Google Scholar]
- Schleif R. F. Origin of chloramphenicol particle protein. J Mol Biol. 1968 Oct 14;37(1):119–129. doi: 10.1016/0022-2836(68)90077-6. [DOI] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Apirion D. Escherichia coli ribosomes: recent developments. Annu Rev Microbiol. 1969;23:387–426. doi: 10.1146/annurev.mi.23.100169.002131. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Mangiarotti G., Apirion D. The formation and stabilization of 30S and 50S ribosome couples in Escherichia coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1782–1789. doi: 10.1073/pnas.58.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Caskey C. T. Peptide chain termination. V. The role of release factors in mRNA terminator codon recognition. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1235–1241. doi: 10.1073/pnas.64.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A. Y., Eisenstadt J., Lengyel P. On the relation between ribonucleic acid synthesis and peptide chain initiation in E. coli. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1599–1605. doi: 10.1073/pnas.56.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Dubnau D., Morrell P., Marmur J. Chromosomal location of DNA base sequences complementary to transfer RNA and to 5 s, 16 s and 23 s ribosomal RNA in Bacillus subtilis. J Mol Biol. 1968 Apr 14;33(1):123–140. doi: 10.1016/0022-2836(68)90285-4. [DOI] [PubMed] [Google Scholar]
- Smith I., Goldthwaite C., Dubnau D. The genetics of ribosomes in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1969;34:85–89. doi: 10.1101/sqb.1969.034.01.013. [DOI] [PubMed] [Google Scholar]
- Sober H. A., Schlossman S. F., Yaron A., Latt SA RUSHIZKY G. W. Protein-nucleic acid interaction. I. Nuclease-resistant polylysine-ribonculeic acid complexes. Biochemistry. 1966 Nov;5(11):3608–3616. doi: 10.1021/bi00875a033. [DOI] [PubMed] [Google Scholar]
- Sparling P. F. Kasugamycin resistance: 30S ribosomal mutation with an unusual location on the Escherichia coli chromosome. Science. 1970 Jan 2;167(3914):56–58. doi: 10.1126/science.167.3914.56. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Modolell J., Takeda Y., Davis B. D. Ribosomes from Escherichia coli merodiplods heterozygous for resistance to streptomycin and to spectinomycin. J Mol Biol. 1968 Nov 14;37(3):407–421. doi: 10.1016/0022-2836(68)90111-3. [DOI] [PubMed] [Google Scholar]
- Spencer M., Pigram W. J., Littlechild J. Studies on ribosomal RNA structure. I. The isolation of crystallizable fragments. Biochim Biophys Acta. 1969 Apr 22;179(2):348–359. [PubMed] [Google Scholar]
- Spirin A. S. How does the ribosome work? A hypothesis based on the two subunit construction of the ribosome. Curr Mod Biol. 1968 Jul-Aug;2(3):115–127. doi: 10.1016/0303-2647(68)90017-8. [DOI] [PubMed] [Google Scholar]
- Spitnik-Elson P., Atsmon A. Detachment of ribosomal proteins by salt. I. Effect of conditions on the amount of protein detached. J Mol Biol. 1969 Oct 14;45(1):113–124. doi: 10.1016/0022-2836(69)90214-9. [DOI] [PubMed] [Google Scholar]
- Staehelin T., Maglott D. M., Monro R. E. On the catalytic center of peptidyl transfer: a part of the 50 S ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:39–48. doi: 10.1101/sqb.1969.034.01.008. [DOI] [PubMed] [Google Scholar]
- Staehelin T., Meselson M. In vitro recovery o ribosomes and of synthetic activity from synthetically inactive ribosomal subunits. J Mol Biol. 1966 Mar;16(1):245–249. doi: 10.1016/s0022-2836(66)80277-2. [DOI] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Bock R. M. Isolation and physical properties of the ribosomal ribonucleic acid of Escherichia coli. Biochemistry. 1965 Jul;4(7):1302–1311. doi: 10.1021/bi00883a014. [DOI] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Salas M., Wahba A. J., Ochoa S. Translation of the genetic message: factors involved in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jul;56(1):290–295. doi: 10.1073/pnas.56.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Stent G. S. Genetic transcription. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):181–197. doi: 10.1098/rspb.1966.0022. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Sypherd P. S. Unique protein moieties for 30S and 50S ribosomes of Escherichia coli. J Bacteriol. 1969 Jun;98(3):1080–1086. doi: 10.1128/jb.98.3.1080-1086.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D., Beller R. J. The ribosome dissociation factor and the ribosome-polysome cycle. Cold Spring Harb Symp Quant Biol. 1969;34:223–230. doi: 10.1101/sqb.1969.034.01.028. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Ron E. Z., Davis B. D. A factor required for ribosome dissociation in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Oct;61(2):761–767. doi: 10.1073/pnas.61.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M., Takanami M. Analysis of the 5'-terminal nucleotide sequences of ribonucleic acids. II. Comparison of the 5'-terminal nucleotide sequences of ribosomal RNA's from different organisms. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1595–1602. doi: 10.1073/pnas.58.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypherd P. S. Amino acid differences in a 30S ribosomal protein from two strains of Escherichia coli. J Bacteriol. 1969 Aug;99(2):379–382. doi: 10.1128/jb.99.2.379-382.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypherd P. S., Fansler B. S. Structural transitions in ribonucleic acid during ribosome development. J Bacteriol. 1967 Mar;93(3):920–929. doi: 10.1128/jb.93.3.920-929.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypherd P. S. Message activity of RNA derived from immature ribosomes. J Mol Biol. 1967 Mar 14;24(2):329–332. doi: 10.1016/0022-2836(67)90338-5. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S., O'Neil D. M., Taylor M. M. The chemical and genetic structure of bacterial ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:77–84. doi: 10.1101/sqb.1969.034.01.012. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S. Ribosome development and the methylation of ribosomal ribonucleic acid. J Bacteriol. 1968 May;95(5):1844–1850. doi: 10.1128/jb.95.5.1844-1850.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANFORD C. COHESIVE FORCES AND DISRUPTIVE REAGENTS. Brookhaven Symp Biol. 1964 Dec;17:154–183. [PubMed] [Google Scholar]
- TISSIERES A., WATSON J. D. Ribonucleoprotein particles from Escherichia coli. Nature. 1958 Sep 20;182(4638):778–780. doi: 10.1038/182778b0. [DOI] [PubMed] [Google Scholar]
- TRAUT R. R., MONRO R. E. THE PUROMYCIN REACTION AND ITS RELATION TO PROTEIN SYNTHESIS. J Mol Biol. 1964 Oct;10:63–72. doi: 10.1016/s0022-2836(64)80028-0. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Kessler D. P., Ingraham J. Cold-sensitive mutations in Salmonella typhimurium which affect ribosome synthesis. J Bacteriol. 1969 Mar;97(3):1298–1304. doi: 10.1128/jb.97.3.1298-1304.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami M. Analysis of the 5'-terminal nucleotide sequences of ribonucleic acids 1. the 5'-termini of Excherichia coli ribosomal RNA. J Mol Biol. 1967 Jan 28;23(2):135–148. doi: 10.1016/s0022-2836(67)80022-6. [DOI] [PubMed] [Google Scholar]
- Tal M. Thermal denaturation of ribosomes. Biochemistry. 1969 Jan;8(1):424–435. doi: 10.1021/bi00829a058. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Spencer M. Studies on ribosomal RNA structure. II. Secondary structures in solution of rRNA and crystallizable fragments. Biochim Biophys Acta. 1969 Apr 22;179(2):360–368. [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Marino P., Colvill A. J. Mutant of E. coli containing an altered DNA-dependent RNA polymerase. Nature. 1968 Oct 19;220(5164):275–276. doi: 10.1038/220275a0. [DOI] [PubMed] [Google Scholar]
- Traub P., Hosokawa K., Craven G. R., Nomura M. Structure and function of E. coli ribosomes, IV. Isolation and characterization of functionally active ribosomal proteins. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2430–2436. doi: 10.1073/pnas.58.6.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Streptomycin resistance mutation in Escherichia coli: altered ribosomal protein. Science. 1968 Apr 12;160(3824):198–199. doi: 10.1126/science.160.3824.198. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. I. Partial fractionation of the functionally active ribosomal proteins and reconstitution of artificial subribosomal particles. J Mol Biol. 1968 Jun 28;34(3):575–593. doi: 10.1016/0022-2836(68)90182-4. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. VI. Mechanism of assembly of 30 s ribosomes studied in vitro. J Mol Biol. 1969 Mar 28;40(3):391–413. doi: 10.1016/0022-2836(69)90161-2. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M., Tu L. Physical and functional heterogeneity of ribosomal proteins. J Mol Biol. 1966 Aug;19(1):215–218. doi: 10.1016/s0022-2836(66)80064-5. [DOI] [PubMed] [Google Scholar]
- Traub P., Söll D., Nomura M. Structure and function of Escherichia coli ribosomes. II. Translational fidelity and efficiency in protein synthesis of a protein-deficient subribosomal particle. J Mol Biol. 1968 Jun 28;34(3):595–608. doi: 10.1016/0022-2836(68)90183-6. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Delius H., Ahmad-Zadeh C., Bickle T. A., Pearson P., Tissières A. Ribosomal proteins of E. Coli: stoichiometry and implications for ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:25–38. doi: 10.1101/sqb.1969.034.01.007. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Haenni A. L. The effect of sulfhydryl reagents on ribosome activity. Eur J Biochem. 1967 Jul;2(1):4–73. doi: 10.1111/j.1432-1033.1967.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Moore P. B., Delius H., Noller H., Tissières A. Ribosomal proteins of Escherichia coli. I. Demonstration of different primary structures. Proc Natl Acad Sci U S A. 1967 May;57(5):1294–1301. doi: 10.1073/pnas.57.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi M., Matsuo K., Ts'o P. O. Interaction of poly-L-lysine and nucleic acids. J Mol Biol. 1966 Jan;15(1):256–267. doi: 10.1016/s0022-2836(66)80225-5. [DOI] [PubMed] [Google Scholar]
- Turnock G. A genetic analysis of a mutant of Escherichia coli with a defect in the assembly of ribosomes. Mol Gen Genet. 1969 Aug 15;104(4):295–312. doi: 10.1007/BF00334229. [DOI] [PubMed] [Google Scholar]
- WALLER J. P. FRACTIONATION OF THE RIBOSOMAL PROTEIN FROM ESCHERICHIA COLI. J Mol Biol. 1964 Nov;10:319–336. doi: 10.1016/s0022-2836(64)80050-4. [DOI] [PubMed] [Google Scholar]
- WALLER J. P., HARRIS J. I. Studies on the composition of the protein from Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1961 Jan 15;47:18–23. doi: 10.1073/pnas.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON J. D. THE SYNTHESIS OF PROTEINS UPON RIBOSOMES. Bull Soc Chim Biol (Paris) 1964;46:1399–1425. [PubMed] [Google Scholar]
- WELLER D. L., HOROWITZ J. A 30-S RIBONUCLEOPROTEIN PARTICLE DERIVED FROM 50-S RIBOSOMES OF ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Jun 22;87:361–364. doi: 10.1016/0926-6550(64)90239-7. [DOI] [PubMed] [Google Scholar]
- WHITNEY P. L., TANFORD C. Solubility of amino acids in aqueous urea solutions and its implications for the denaturation of proteins by urea. J Biol Chem. 1962 May;237:1735–1737. [PubMed] [Google Scholar]
- Wahba A. J., Chae Y. B., Iwasaki K., Mazumder R., Miller M. J., Sabol S., Sillero M. A. Initiation of protein synthesis in Escherichia coli. I. Purification and properties of the initiation factors. Cold Spring Harb Symp Quant Biol. 1969;34:285–290. doi: 10.1101/sqb.1969.034.01.034. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Iwasaki K., Miller M. J., Sabol S., Sillero M. A., Vasquez C. Initiation of protein synthesis in Escherichia coli. II. Role of the initiation factors in polypeptide synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:291–299. doi: 10.1101/sqb.1969.034.01.035. [DOI] [PubMed] [Google Scholar]
- Walker I. O. Electrometric and spectrophotometric titration of histone and deoxyribonucleohistone. J Mol Biol. 1965 Dec;14(2):381–398. doi: 10.1016/s0022-2836(65)80189-9. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]
- Weller D. L., Morgan R. S. A new method for preparing ribosomal ribonucleic acid. Biochemistry. 1967 Apr;6(4):983–988. doi: 10.1021/bi00856a005. [DOI] [PubMed] [Google Scholar]
- Weller D. L., Morgan R. S. Extraction of high molecular weight complexes of protein from 50-S ribosomes. Biochim Biophys Acta. 1967 Dec 19;149(2):553–561. doi: 10.1016/0005-2787(67)90183-9. [DOI] [PubMed] [Google Scholar]
- Weller D. L., Schechter Y., Musgrave D., Rougvie M., Horowitz J. Conformational changes in Escherichia coli ribosomes at low magnesium ion concentrations. Biochemistry. 1968 Oct;7(10):3668–3675. doi: 10.1021/bi00850a046. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Primary structure homology within the 23S ribosomal RNA. Nature. 1968 Nov 30;220(5170):923–923. doi: 10.1038/220923a0. [DOI] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. Distinct cistrons for the two ribosomal RNA components. Proc Natl Acad Sci U S A. 1963 Apr;49:538–544. doi: 10.1073/pnas.49.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. The identification of the ribosomal RNA cistron by sequence complementarity. I. Specificity of complex formation. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1069–1078. doi: 10.1073/pnas.48.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. The identification of the ribosomal RNA cistron by sequence complementarity. II. Saturation of and competitive interaction at the RNA cistron. Proc Natl Acad Sci U S A. 1962 Aug;48:1466–1472. doi: 10.1073/pnas.48.8.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Osawa S. Origin of the protein component of chlormaphenicaol particles in Escherichia coli. J Mol Biol. 1968 May 14;33(3):559–569. doi: 10.1016/0022-2836(68)90306-9. [DOI] [PubMed] [Google Scholar]
- Young R. J. The fractionation of quaternary ammonium complexes of nucleic acids. Evidence for heterogeneity of ribosomal ribonucleic acid. Biochemistry. 1968 Jun;7(6):2263–2272. doi: 10.1021/bi00846a032. [DOI] [PubMed] [Google Scholar]
- Yura T., Igarashi K. RNA polymerase mutants of Escherichia coli. I. Mutants resistant to streptovaricin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1313–1319. doi: 10.1073/pnas.61.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehavi-Willner T., Comb D. G. Studies on the relationship between transfer RNA and transfer-like RNA. J Mol Biol. 1966 Mar;16(1):250–254. doi: 10.1016/s0022-2836(66)80278-4. [DOI] [PubMed] [Google Scholar]



