Abstract
Background
Gender differences in the relationship between central and peripheral BP are not well described. We sought to investigate gender differences between central systolic blood pressure (cSBP) and peripheral systolic blood pressure (pSBP) in adults in the Bogalusa study population.
Methods
This study enrolled adults in a cross sectional survey conducted in 2007–2010. BP was measured with a standard cuff and Omron applanation tonometer. Data were available from 876 participants.
Results
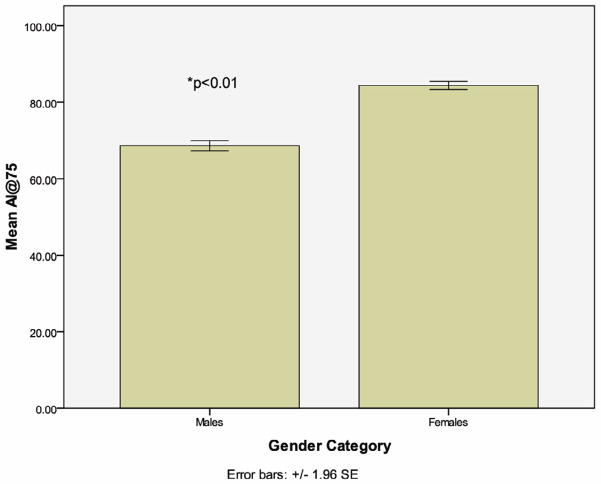
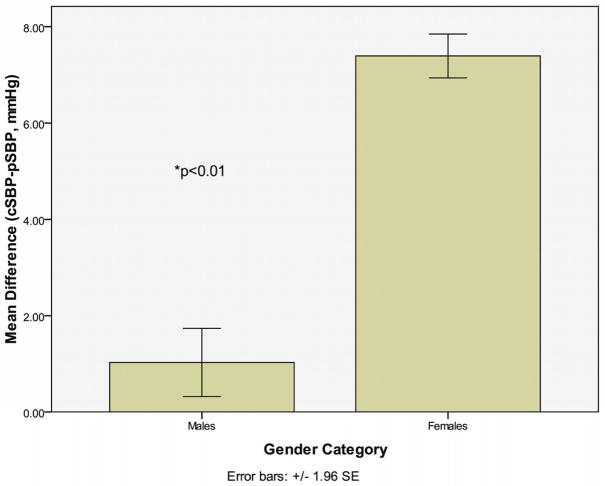
Participants were 57.9% female and 42.1% male (mean age 43.5 years ± 4.4). Mean (SD) for cSBP-pSBP was 1.0 (6.9) for males and 7.4 (5.2) for females (p<0.001). Augmentation index (AI) was higher in women (men: 70.8±14 vs. women: 85.5±13; p<0.01), as well as augmentation index standardized to heart rate (HR) of 75 (AI@75) (men: 68.5±13 vs. women: 84.4±11.8; p<0.01).
Conclusions
Female participants had greater difference between cSBP and pSBP than males. This suggests that given similar peripheral BP females might be at higher risk for developing target organ damage. Women in this study had higher AI, which may contribute to the difference found between cSBP and pSBP. These findings may explain why women have more age-related left ventricular hypertrophy, and poorer prognosis following myocardial infarction compared to males.
Keywords: Blood pressure, Gender differences, Central blood pressure, Augmentation Index
Introduction
Although men have a higher overall prevalence of hypertension than women(1), women have a higher risk of hypertensive target organ damage than age-matched men. Women have more left ventricular hypertrophy (LVH) on echocardiography compared to men (2–4), and female gender is an independent predictor of both microalbuminuria and LVH. Additionally, while both men and women develop LVH with increasing age, women develop more age-related LVH(5,6), and the impact of LVH on adverse cardiovascular events may be greater in women than in men(7). Women have also shown increased risk of death by stroke, especially at younger ages(8), and have higher mortality risk after myocardial infarction (MI) than men(9,10).
The above observations are based on the measurement of systemic arterial blood pressure (BP) in the brachial artery. While peripherally measured BP is a valuable predictor of cardiovascular events, it is reported that non-invasive measurement of central arterial pressure more accurately predicts adverse cardiovascular events and target organ damage(11,12) and that elevations in central arterial pressure are more closely linked to hypertensive target organ damage than peripheral measurements(13).
It has been suggested that gender differences in target organ damage may be due to differences in pulsatile vascular load(3). Until around age 50 women tend to have lower peripheral BP than men(14,15); however, in this same age group women show greater target organ damage than men. Thus, we speculated that there might be significant differences between men and women in measured central versus peripheral BP. Therefore, we analyzed data from adult males and females enrolled in the Bogalusa Heart Study to describe differences between central and peripheral BP in women compared to men. Further, we sought to determine if other measured parameters would assist in explaining the postulated difference.
Methods
Study Cohort
Data were available for 876 participants in the Bogalusa Heart Study. The Bogalusa Heart Study is a long-term study of cardiovascular disease risk factors in children and adults in the semirural, multi-racial community of Bogalusa, Louisiana. The study began in 1973 by screening children ages 5–17 for cardiovascular risk factors and has 20 cross sectional follow up surveys to date with a total cohort of over 16,000 individuals. Data for the present study were obtained from a cross-sectional survey that enrolled participants in 2007–2010. Participants were excluded if any relevant screening data were missing or if they were currently using any antihypertensive medications. All participants in this study gave informed consent for examination. Study protocols were approved by the Institutional Review Board of the Tulane University Medical Center.
General Examination
Standardized protocols were used by trained examiners. Anthropometric and BP measurements were made in replicate and the mean values were used for analysis. Peripheral BP measurements were obtained using mercury sphygmomanometers on the right arm of participants in a relaxed, sitting position with three replicates each performed by two randomly assigned nurses. The first and fourth Korotkoff phases were used to determine systolic and diastolic BP, respectively. Hypertension was defined as systolic BP of 140 mmHg or higher or diastolic BP of 90 mmHg or higher. Central BP measurements were estimated using an Omron HEM-9000AI device (Omron Healthcare Co., Ltd., Kyoto, Japan). Each participant underwent 4 cuff BP measurements (based on the cuff-oscillometric principle) and 4 radial artery pressure waveform readings acquired using the HEM9000AI radial applanation tonometer. The readings were averaged to calculate each variable listed. Inflection points of the peripheral pulse waveform that corresponded to early (pSBP1) and late systolic BP (pSBP2) were obtained by derivatives of the original waveform. The Omron device then estimates central systolic BP from the pSBP2 value using linear regression(16,17). The Omron device contains software to derive augmentation index (AI), as well as augmentation index standardized to heart rate 75 (AI@75), as described by Richardson, et al (18). Body mass index (BMI) calculated as weight in kilograms divided by the square of height in meters, was used as a measure of overall adiposity. Information on smoking status was obtained from questionnaires. Those who smoked at least one cigarette per week during the past year were considered current smokers.
Laboratory Analyses
Participants were instructed to fast for 12 hours before screening, and compliance with fasting was determined by an interview on the morning of examination. Serum cholesterol and triglycerides (Trig) were determined enzymatically on the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN)(19). Insulin resistance status was assessed as homeostasis model assessment of insulin resistance (HOMA-IR) according to the formula described: insulin (μU/mL) × glucose (mmol/L)/22.5(20). Plasma high sensitivity C-reactive protein (CRP) levels were measured by latex particle-enhanced immunoturbidemetric assay on a Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN, USA).
Statistical Analysis
All analyses were conducted using the SAS software package version 9.2 (SAS Institute, Cary, NC). Continuous variables are expressed as mean +/− standard deviation (SD) unless otherwise indicated. Analysis of covariance (ANCOVA) was used to assess contrasts in race and sex groups for difference in central versus peripheral systolic BP and other cardiovascular (CV) risk factor variables after controlling for age. Post-hoc test with Bonferroni adjustment for multiple comparisons were used where appropriate. Normality of distribution was assessed by the Kolmogorov-Smirnov test. Because Trig, insulin, HOMA-IR and CRP were not normally distributed, log transformation was used to improve normality. All analyses were performed on transformed data where appropriate. Multivariable adjusted linear regression models were used to examine the total and sex-specific independent effects of CV risk factors and hemodynamic variables on measures of difference in central versus peripheral systolic BP and augmentation index standardized to heart rate of 75 bpm. Independent variables for all models included: age, heart rate (HR), height, smoking, HOMA-IR, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), Trig, CRP, race and gender. For gender-specific models, the effect of gender as an independent variable was excluded. All p values were two tailed and adjusted for covariates where appropriate. The level of significance for hypothesis testing was set at 5% (α = 0.05).
Results
Participant characteristics are shown in Table 1. There was no significant difference in age between men and women (men: 43.9±4.3 vs. women: 43.2±4.5). Women had lower peripheral systolic BP (pSBP) (men: 123.3±14.7 mmHg vs. women: 115.9±15.1 mmHg; p<0.01), and diastolic BP (men: 78.4±10.6 mmHg vs. women: 73.6±11.1 mmHg; p<0.01). Despite differences in peripheral BP, there was no significant difference in central systolic BP (cSBP) (men: 124.4±16.3 mmHg vs. women: 123.4±17.5 mmHg; p>0.05). Augmentation index (AI) was higher in women (men: 70.8±14 vs. women: 85.5±13; p<0.01), as well as augmentation index standardized to heart rate (HR) of 75 (AI@75) (men: 68.5±13 vs. women: 84.4±11.8; p<0.01) (Figure 2). Women tended to have more favorable lipid profiles, higher CRP, and lower insulin levels while HOMA-IR levels were similar between men and women (Table 1).
Table 1.
Risk factors and hemodynamic variables by total and gender groups
|
|
||||
|---|---|---|---|---|
| Total (n= 876) | Males (n= 369) | Females (n=507) | p-value* | |
| Age (yrs) | 43.5 (4.4) | 43.9 (4.3) | 43.2 (4.5) | NS |
| SBP (mmHg) | 119.1 (15.4) | 123.3 (14.7) | 115.9 (15.1) | <0.01 |
| DBP (mmHg) | 75.6 (11.1) | 78.4 (10.6) | 73.6 (11.1) | <0.01 |
| cSBP (mmHg) | 123.8 (17) | 124.4 (16.3) | 123.4 (17.5) | NS |
| Heart Rate (bpm) | 71.4 (10.9) | 70 (11.4) | 72.5 (10.4 | NS |
| Weight (Kg) | 88.4 (23.3) | 97.4 (22.4) | 81.4 (21.8) | <0.01 |
| Height (m) | 1.7 (0.1) | 1.8 (0.1) | 1.6 (0.1) | NS |
| BMI (Kg/m2) | 30.7 (7.5) | 30.7 (6.6) | 30.8 (8) | NS |
| AI@75 | 77.8 (14.5) | 68.5 (13) | 84.4 (11.8) | <0.01 |
| AI (%) | 79.3 (15.2) | 70.8 (14) | 85.5 (13) | <0.01 |
| Tot Chol (mg/dL) | 190.7 (39) | 193.5 (41.4) | 188.7 (37.1) | <0.01 |
| HDL-C (mg/dL) | 46.9 (14.4) | 42.6 (14.1) | 50 (13.8) | <0.01 |
| LDL-C (mg/dL) | 124.8 (34.2) | 128.2 (36.5) | 122.4 (32.2) | <0.01 |
| Trig (mg/dL)¥ | 109 (89) | 122 (103) | 99 (74) | <0.01 |
| CRP (mg/L)¥ | 1.3 (2.6) | 0.9 (1.8) | 1.8 (3.4) | <0.01 |
| Insulin (ìU/mL)¥ | 10.1 (11.4) | 10.9 (11.3) | 9.6 (11.3) | <0.01 |
| HOMA-IR¥ | 2.3 (3.1) | 2.5 (3.1) | 2.2 (3) | NS |
Data shown in mean and standard deviation (SD), unless otherwise indicated
Median and interquartile range
Comparison by analysis of covariance, controlling for age.
Fig 2.
Augmentation Index Corrected to Heart Rate of 75bpm by Gender
Overall, women showed a greater difference between cSBP and pSBP than men (women: 7.4±5.2 vs. men: 1.0±6.9; p<0.001) (figure 1). The difference between cSBP-pSBP remained greater in white women compared to white men (women: 7.0±5.1 vs. men: 0.88±6.5; p<0.01), as well as in black women compared to black men (women: 8.3±5.3 vs. men: 1.4±7.8; p<0.01).
Fig 1.
Difference Between cSBP and pSBP by Gender
Because augmentation index is highly correlated to cSBP, multivariable analysis of independent correlates to cSBP-pSBP was conducted excluding AI@75 and AI (table 2). After adjusting for other variables table 2 shows a gender effect consistent with that in figure 1 in total sample as well as in blacks and whites. Multivariable analysis of independent correlates to AI@75 was also performed and showed a gender effect consistent with that in figure 2 in the total sample as well as in blacks and whites (table 3).
Table 2.
Independent correlates of difference between cSBP and pSBP by mutivariable linear regression analyses.
| Model 1: Total (n= 876)
|
Model 2: Whites (n= 601)
|
Model 3: Blacks (n= 275)
|
||||
|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | |
|
|
|
|
||||
| Age | 0.14 | <0.001 | 0.14 | <0.001 | 0.12 | 0.09 |
| HR | -0.22 | <0.001 | -0.24 | <0.001 | -0.18 | <0.01 |
| Height | -0.22 | <0.001 | -0.23 | <0.001 | -0.20 | 0.04 |
| Smoking (Y/N) | 0.13 | <0.001 | 0.10 | <0.01 | 0.17 | <0.01 |
| HOMA-IR ¥ | -0.04 | 0.35 | -0.03 | 0.51 | -0.04 | 0.57 |
| HDL-C | 0.08 | 0.05 | 0.07 | 0.21 | 0.08 | 0.31 |
| LDL-C | -0.02 | 0.59 | 0.03 | 0.54 | -0.11 | 0.13 |
| Trig¥ | -0.02 | 0.62 | -0.04 | 0.41 | 0.02 | 0.82 |
| CRP¥ | 0.02 | 0.60 | 0.04 | 0.40 | -0.01 | 0.87 |
| Race (W/B) | -0.08 | 0.03 | - | - | - | - |
| Gender (F/M) | 0.18 | <0.001 | 0.17 | 0.01 | 0.24 | 0.02 |
|
| ||||||
| R2 | 0.22 | 0.22 | 0.22 | |||
R2, variance explained
β standardized regression coefficient
Log transformed to improve normality
Table 3.
Independent correlates of AI@75 by mutivariable linear regression analyses
| Model 1: Total (n= 876)
|
Model 2: Whites (n= 601)
|
Model 3: Blacks (n= 275)
|
||||
|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | |
|
|
|
|
||||
| Age | 0.20 | <0.001 | 0.22 | <0.001 | 0.14 | <0.01 |
| HR | -0.06 | 0.04 | -0.10 | <0.01 | 0.02 | 0.638 |
| Height | -0.33 | <0.001 | -0.35 | <0.001 | -0.24 | <0.01 |
| Smoking (Y/N) | 0.14 | <0.001 | 0.14 | <0.001 | 0.13 | <0.01 |
| HOMA-IR ¥ | -0.08 | 0.01 | -0.09 | 0.02 | -0.10 | 0.08 |
| HDL-C | 0.07 | 0.02 | 0.03 | 0.34 | 0.13 | 0.03 |
| LDL-C | 0.01 | 0.62 | 0.02 | 0.54 | 0.01 | 0.90 |
| Trig¥ | 0.04 | 0.20 | 0.02 | 0.62 | 0.08 | 0.16 |
| CRP¥ | 0.06 | 0.03 | 0.12 | <0.001 | -0.05 | 0.40 |
| Race (W/B) | -0.09 | <0.001 | - | - | - | - |
| Gender (F/M) | 0.28 | <0.001 | 0.26 | <0.001 | 0.41 | <0.001 |
|
| ||||||
| R2 | 0.43 | 0.44 | 0.41 | |||
R2, variance explained
β standardized regression coefficient
Log transformed to improve normality
Discussion
In this study women showed significantly greater increases in central systolic BP from peripheral systolic BP when compared to men (figure 1). This was true for both black and white women and was independent of age. Our study also shows that in multivariable analysis of independent predictors of cSBP-pSBP that gender is a strong determining factor in differences between central and peripheral pressures (table 2). Thus, it appears that peripherally measured BP underestimates central systolic BP in women compared to men. Importantly, while the women in this study had a mean pSBP of 115.9±15.1 mmHg, a level which could be characterized as “ideal”, their corresponding cSBP was in a range consistent with “prehypertension” (123.7±16.6 mmHg) (21–23). This contrasts with the males in this study whose pSBP (123.3±14.7 mmHg) was almost identical to their cSBP (124.4±16.3 mmHg), with both values consistent with prehypertension. These findings suggest a scenario wherein peripheral BP underestimates central BP, a more potent predictor of target organ damage, and therefore underestimates risk of hypertensive target organ damage in women but not men.
The finding of an increased augmentation index in women compared to men offers a possible explanation for the difference between peripheral and central BP in men compared to women. Augmentation index is a measure of reflected waves and is considered a measure of arterial “stiffness” (24,25). Typically central blood pressure is affected by both a forward pulse wave generated during ventricular contraction and a reflected wave generated at arterial branch points and other areas of impedance mismatch. In normal arteries, the reflected wave arrives centrally during diastole and therefore does not significantly augment central systolic pressure. However, when arteries are stiffened the reflected wave arrives centrally earlier, augmenting the forward wave and increasing the systolic pressure(26–28). In this manner an increased augmentation index, due to increases in vascular stiffness, could be a factor in raising the central systolic BP. Therefore, the higher AI in women in this study may represent decreased arterial elastance than men and may be responsible for the enhancement of central aortic pressure.
Higher AI has been shown to predict adverse cardiovascular events (29–31) and is associated with increases in hypertensive target organ damage (32–34). Consistent with previous reports, women in this study had significantly higher AI (AI in men: 77.8±14 vs. women: 91.3±14.2; p<0.01) and AI@75 than men (men: 68.9±13 vs. women: 84.9±11.5; p<0.01) (6,35,36,37,38). Age is a primary predictor of increasing AI, but heart rate and body height also contribute (39,40). Results of multivariable analysis in our data indicate that these considerations are not sufficient to explain gender differences in AI, and that gender itself is a strong determining factor of AI@75. This suggests gender differences in arterial structure and function may account for differences in peripheral versus central BP in the present study.
The importance of correctly assessing the central BP was shown by the Conduit Artery Functional Evaluation (CAFE) study, a large sub-study of the Anglo- Scandinavian Cardiac Outcomes Trial (ASCOT)(41). CAFE was one of the first studies to monitor central arterial pressures in a major clinical trial because it had previously erroneously been assumed that peripheral BP accurately predicted central BP. However, the CAFE trial demonstrated that anti-hypertensive medications with varying mechanisms of action differentially affected central and peripheral BP. In this study, while participants had similar control of brachial artery BP, there were significant differences in central pressure between treatment groups, and those with lower central pressures saw a significant reduction in adverse outcomes. Investigators in the CAFE trial were the first to link improved central arterial pressures to reduced adverse cardiovascular events, all-cause mortality and evidence of target organ damage such as renal impairment. The CAFE study was closely followed by an analysis of central versus peripheral pressures in the Strong Heart Study (SHS)(11). Investigators of the SHS found that central pulse pressure obtained by radial applanation tonometry more accurately predicted carotid artery hypertrophy, extent of atherosclerosis and adverse cardiovascular events than did brachial pulse pressure.
A strength of our study is the large number of participants, and high participation by females allowing for gender comparisons of BP. Our study was able to compare commonly used peripheral BP measurements to non-invasive pressure measurement to aid in the interpretation of central to peripheral BP relationships. Weaknesses in this study include a relatively narrow age range of participants, which made it difficult to determine whether our parameter of cSBP-pSBP changed with age. Also, because this is a cross-sectional study we were unable to observe changes over time, and were unable to reliably correlate our measure of cSBP-pSBP with changes in organ function consistent with target organ damage. Finally, our use of the Omron HEM-9000AI device, while shown to provide accurate estimation of pressure amplification that is proportional to other similar devices (18,42), could not be correlated with invasive measurements, the gold standard measure of central pressure.
We conclude that the assessment of peripheral versus central arterial BP is related to gender and to indices of arterial structure and function. Recognition of these differences should be incorporated in clinical management and in evaluation of clinical trial data. In the future non-invasive assessment of central blood pressure may be commonly measured and therefore will offer a useful tool in the clinical evaluation of blood pressure (43).
Acknowledgments
Funding:
This study was supported by grants ES-021724 from National Institute of Environmental Health Sciences and AG-16592 from the National Institute on Aging.
Abbreviations
- LVH
left ventricular hypertrophy
- MI
myocardial infarction
- BP
blood pressure
- pSBP1
early systolic inflection point of radial artery peripheral arterial waveform
- pSBP2
late systolic inflection point of radial artery peripheral arterial waveform
- AI
augmentation index
- AI@75
augmentation index standardized to heart rate of 75 beats per minute
- BMI
body mass index
- Trig
triglycerides
- HDL-C
high density lipoprotein- cholesterol
- LDL-C
low density lipoprotein, cholesterol
- HOMA-IR
homeostasis model assessment of insulin resistance
- CRP
C-reactive protein
- cSBP
central systolic blood pressure
- pSBP
peripheral systolic blood pressure
- DBP
diastolic blood pressure
- HR
heart rate
Footnotes
Disclosure:
Dr. Giles is a consultant and investigator for Forest Laboratories. Dr. Sander is a speaker for Forest Laboratories.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Center for Health Statistics. NCHS Data Brief, Number 107, October 2012. 2012. Sep 24, pp. 1–8. [Google Scholar]
- 2.Liebson PR, Grandits G, Prineas R, Dianzumba S, Flack JM, Cutler JA, et al. Echocardiographic Correlates of Left Ventricular Structure Among 844 Mildly Hypertensive Men and Women in the Treatment of Mild Hypertension Study (TOMHS) Circulation. 1993 Feb 1;87(2):476–86. doi: 10.1161/01.cir.87.2.476. [DOI] [PubMed] [Google Scholar]
- 3.Palatini P, Mos L, Santonastaso M, Saladini F, Benetti E, Mormino P, et al. Premenopausal Women Have Increased Risk of Hypertensive Target Organ Damage Compared with Men of Similar Age. Journal of Women’s Health. 2011 Aug;20(8):1175– 81. doi: 10.1089/jwh.2011.2771. [DOI] [PubMed] [Google Scholar]
- 4.Savage D, Garrison R, Kannel W, Levy D. The Spectrum of Left Ventricular Hypertrophy in a General Population Sample: The Framingham Study. Circulation. 1987 Apr 5;75(suppl I):1–8. [PubMed] [Google Scholar]
- 5.Dannenburg A, Levy D, Garrison R. Impact of Age on Echocardiographic Left Ventricular Mass in a Healthy Population (The Framingham Heart Study) The American Journal of Cardiology. 1989 Nov;64(16):1066–8. doi: 10.1016/0002-9149(89)90816-3. [DOI] [PubMed] [Google Scholar]
- 6.Hayward C, Raymond K. Gender-Related Differences in the Central Arterial Pressure Waveform. Journal of the American College of Cardiology. 1997 Dec 8;30(7):1863–71. doi: 10.1016/s0735-1097(97)00378-1. [DOI] [PubMed] [Google Scholar]
- 7.Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous Relation Between Left Ventricular Mass and Cardiovascular Risk in Essential Hypertension. Hypertension. 2000 Feb 1;35(2):580–6. doi: 10.1161/01.hyp.35.2.580. [DOI] [PubMed] [Google Scholar]
- 8.Prospective Studies Collaboration. Age-specific Relevance of Usual Blood Pressure to Vascular Mortality: a Meta-analysis of Individual Data for One Million Adults in 61 Prospective Studies. Lancet. 2002 Dec 14;360: 1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 9.Vaccarino V, Parsons L, Every N, Barron H, Krumholz H. Sex-Based Differences in Early Mortality after Myocardial Infarction. NEJM. 1999 Jul 22;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 10.Hochman JS, Tamis JE, Thompson TD, Weaver DW, White HD, Van de Werf F, et al. Sex, Clinical Presentation, And Outcome in Patients with Acute Coronary Syndromes. New England Journal of Medicine. 1999 Jul 22;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 11.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central Pressure More Strongly Relates to Vascular Disease and Outcome Than Does Brachial Pressure: The Strong Heart Study. Hypertension. 2007 Apr 30;50(1):197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 12.Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, et al. Central But Not Brachial Blood Pressure Predicts Cardiovascular Events in an Unselected Geriatric Population. Journal of the American College of Cardiology. 2008 Jun;51(25):2432–9. doi: 10.1016/j.jacc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of Central and Brachial Blood Pressure to Left Ventricular Hypertrophy and Geometry: the Strong Heart Study. Journal of Hypertension. 2010 Feb;28(2):384–8. doi: 10.1097/HJH.0b013e328333d228. [DOI] [PubMed] [Google Scholar]
- 14.Staessen J, Fagard R, Lijnen P, Thijs L, Van Hoof R, Amery A. Reference Values for Ambulatory Blood Pressure: A Meta-analysis. Journal of Hypertension Supplement. 1990 Dec;8(6):S57–S64. [PubMed] [Google Scholar]
- 15.Burl VL, Whelton P, Roccela EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of Hypertension in the US Adult Population: Results From the Third National Health and Nutrition Examination Survey. Journal of Hypertension; pages. 1995 Mar;25(3):305–13. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 16.Takazawa K, Kobayashi H, Shindo N, Tanaka N, Yamashina A. Relationship Between Radial and Central Arterial Pulse Wave and Evaluation of Central Aortic Pressure Using the Radial Arterial Pulse Wave. Hypertension Research. 2007 Mar 21;30(3):219–28. doi: 10.1291/hypres.30.219. [DOI] [PubMed] [Google Scholar]
- 17.Takazawa K, Kobayashi H, Kojima I, Aizawa A, Kinoh M, Sugo Y, et al. Estimation of Central Aortic Systolic Pressure Using Late Systolic Inflection of Radial Artery Pulse and Its Application to Vasodilator Therapy. Journal of Hypertension. 2012 May;30(5):908–16. doi: 10.1097/HJH.0b013e3283524910. [DOI] [PubMed] [Google Scholar]
- 18.Richardson CJ, Maki-Petaja KM, McDonnell BJ, Hickson SS, Wilkinson IB, McEniery CM. Comparison of Estimates of Central Systolic Blood Pressure and Peripheral Augmentation Index Obtained From the Omron HEM-9000AI and SphygmoCor Systems. Artery Research. 2009 Feb 1;3(1):24–31. [Google Scholar]
- 19.Allain C, Poon L, Chan C, Richmond W, Fu P. Enzymatic Determination of Total Serum Cholesterol. Clinical Chemistry. 2004 Nov 29;20(4):470–5. [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment: Insulin Resistance and Beta-cell Function From Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia. 1985 Jul;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Hsia J, Margolis KL, Eaton CB, Wenger NK, Allison M, Wu L, et al. Prehypertension and Cardiovascular Disease Risk in the Women’s Health Initiative. Circulation. 2007 Feb 20;115(7):855–60. doi: 10.1161/CIRCULATIONAHA.106.656850. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatmentof High Blood Pressure. Journal of the American Medical Association. 2003 May 21;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 23.Vasan R, Larson M, Leip E, Evans J, O’Donnell C, Kannel W, et al. Impact of High-Normal Blood Pressure on the Risk of Cardiovascular Disease. The New England Journal of Medicine. 2001 Nov 1;345(18):1291–7. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu M, Kario K. Review: Role of the Augmentation Index in Hypertension. Therapeutic Advances in Cardiovascular Disease. 2008 Feb 1;2(1):25–35. doi: 10.1177/1753944707086935. [DOI] [PubMed] [Google Scholar]
- 25.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert Consensus Document on Arterial Stiffness: Methodological Issues and Clinical Applications. European Heart Journal. 2006 Sep 25;27(21):2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 26.Levy BI, Ambrosio G, Pries AR, Struijker Boudier HAJ. Microcirculation in Hypertension: A New Target for Treatment? Circulation. 2001 Aug 7;104(6):735–40. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 27.Kelly R, Hayward C, Avolio A, O’Rourke M. Noninvasive Determination of Age-related Changes in the Human Arterial Pulse. Circulation. 1989 Dec 1;80(6):1652–9. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 28.Safar ME, Van Bortel L, Struijker-Boudier H. Resistance and Conduit Arteries Following Converting Enzyme Inhibition in Hypertension. Journal of Vascular Research. 1997 Mar-Apr;34(2):67–81. doi: 10.1159/000159204. [DOI] [PubMed] [Google Scholar]
- 29.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial Wave Reflections and Survival in End-Stage Renal Failure. Hypertension. 2001 Sep 1;38(3):434–8. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 30.Weber T, Auer J, O’Rourke M, Kvas E, Lassnig E, Lamm G, et al. Increased Arterial Wave Reflections Predict Severe Cardiovascular Events in Patients Undergoing Percutaneous Coronary Interventions. European Heart Journal. 2005 Oct 25;26(24):2657–63. doi: 10.1093/eurheartj/ehi504. [DOI] [PubMed] [Google Scholar]
- 31.Chirinos J, Zambrano J, Chakko S, Veerani A, Schob A, Willens H, et al. Aortic Pressure Augmentation Predicts Adverse Cardiovascular Events in Patients With Established Coronary Artery Disease. Hypertension. 2005 Apr 11;45(5):980–5. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 32.Saba PS, Roman MJ, Pini R, Spitzer M, Ganau A, Devereux RB. Relation of Arterial Pressure Waveform to Left Ventricular and Carotid Anatomy in Normotensive Subjects. Journal of the American College of Cardiology. 1993 Dec 18;22(7):1873–80. doi: 10.1016/0735-1097(93)90772-s. [DOI] [PubMed] [Google Scholar]
- 33.Marchais SJ, Guerin AP, Pannier BM, Levy BI, Safar ME, London GM. Wave Reflections and Cardiac Hypertrophy in Chronic Uremia. Influence of body size. Hypertension. 1993 Dec 1;22(6):876–83. doi: 10.1161/01.hyp.22.6.876. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto J, Watabe D, Hatanaka R, Hanasawa T, Metoki H, Asayama K, et al. Enhanced Radial Late Systolic Pressure Augmentation in Hypertensive Patients With Left Ventricular Hypertrophy. American Journal of Hypertension. 2006 Jan;19(1):27–32. doi: 10.1016/j.amjhyper.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF, Parise H, Benjamin EJ, Larson M, Keyes MJ, Vita JA, et al. Changes in Arterial Stiffness and Wave Reflection With Advancing Age in Healthy Men and Women: The Framingham Heart Study. Hypertension. 2004 May 10;43(6):1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 36.Gatzka C, Kingwell B, Cameron J, Berry K, Liang Y-L, Dewar E, et al. Gender Differences in the Timing of Arterial Wave Reflection Beyond Differences in Body Height. Journal of Hypertension. 2001 Nov 12;19(12):2197–203. doi: 10.1097/00004872-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in Arterial Stiffness and Wave Reflection With Advancing Age in Healthy Men and Women: The Framingham Heart Study. Hypertension. 2004 May 3;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 38.Brown Y, Brown MJ. Similarities and Differences Between Augmentation Index and Pulse Wave Velocity in the Assessment of Arterial Stiffness. Q J Med. 1999 Jul 1;92:595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson IB, MacCallum H, Flint L, Cockcroft J, Newby D, Webb D. The Influence of Heart Rate on Augmentation Index and Central Arterial Pressure in Humans. Journal of Physiology. 2000 Feb 23;525(1):263–70. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGrath BP, Liang Y-L, Kotsopoulos D, JDC Impact of Physical and Physiological Factors on Arterial Function. Clinical and Experimental Pharmacology and Physiology. 2001 Dec 13;28(12):1104–7. doi: 10.1046/j.1440-1681.2001.03591.x. [DOI] [PubMed] [Google Scholar]
- 41.The CAFE Investigators. Differential Impact of Blood Pressure-Lowering Drugs on Central Aortic Pressure and Clinical Outcomes: Principal Results of the Conduit Artery Function Evaluation (CAFE) Study. Circulation. 2006 Mar 7;113(9):1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 42.Ding F-H, Li Y, Zhang R-Y, Zhang Q, Wang J-G. Comparison of the SphygmoCor and Omron Devices in the Estimation of Pressure Amplification Against the Invasive Catheter Measurement. Journal of Hypertension. 2013 Jan;31(1):86–93. doi: 10.1097/HJH.0b013e32835a8eca. [DOI] [PubMed] [Google Scholar]
- 43.Sharman JE, Laurent S. Central Blood Pressure in the Management of Hypertension: Soon Reaching the Goal? J Human Hypertens. 2013 Mar 28; doi: 10.1038/jhh.2013.23. advanced online publication. [DOI] [PubMed] [Google Scholar]