Abstract
Obesity has increased at an alarming rate in recent years and is now a worldwide public health problem. Elucidating the mechanisms behind the metabolic dysfunctions associated with obesity is of high priority. The metabolic function of Rho-kinase (ROCK) has been the subject of a great deal of investigation in metabolic-related diseases. It appears that inhibition of ROCK activity is beneficial for the treatment of a wide range of cardiovascular-related diseases. However, recent studies with genetic models of ROCK demonstrate that ROCK plays a positive role in insulin and leptin signaling. Here we discuss the newly identified functions of ROCK in regulating glucose and energy metabolism, with particularly emphasis on metabolic actions of insulin and leptin.
Keywords: Rho-kinase, glucose metabolism, insulin action, energy homeostasis, leptin action
ROCK: key player in energy homeostasis
Obesity has reached epidemic levels in the United States and worldwide, and poses an increasingly severe economic burden [1,2]. Obesity is a major risk factor for developing insulin resistance, impaired glucose tolerance, type 2 diabetes, dyslipidemia and hypertension, all of which are predispose patients to cardiovascular comorbidities [3,4]. This cluster of medical disorders is called the metabolic syndrome [5,6]. Data emerging from several laboratories over the past decade indicate that the Rho-associated coiled-coil-containing kinase (Rho-kinase; ROCK) signaling pathway plays a pivotal role in various metabolic syndrome-related disorders, including cardiovascular diseases CVD) [7,8]. Recent studies demonstrate that ROCK is an important regulator of insulin and leptin action in the context of glucose and energy homeostasis [9-12]. In this article, we will review recent work identifying the physiological roles of ROCK isoforms in controlling glucose homeostasis and insulin sensitivity, underlining the complex and context-dependent nature of this regulation. We will also outline recently-described ROCK-specific functions in hypothalamic neurons in regulating feeding behavior and energy balance, and highlight emerging evidence that ROCK is a molecular mediator underlying the etiopathogenesis of obesity.
ROCK isoforms are increasingly important members of the AGC protein kinase family
ROCK belongs to the protein kinase A/G/C (AGC) subfamily of Ser/Thr protein kinases and is a major downstream effector of small GTPase RhoA [13]. Two isoforms of this enzyme, ROCK1 (also known as ROKβ) and ROCK2 (also known as ROKα), have been identified [14-16]. Each isoform has a kinase domain at its N-terminus, a central coiled-coil domain, and at its C-terminus, a pleckstrin-homology (PH) domain split by a Cys-rich region. The two ROCK isoforms share 65% overall amino acid homology, with their kinase domains exhibiting 92% identity [17]. The Rho binding domain of ROCK lies within the C-terminus of the coiled-coil domain [17]. ROCK isoforms have been implicated in variety of cellular functions, including smooth muscle contraction, actin cytoskeleton organization, cell adhesion and motility, cytokinesis, and gene expression [18].
Due to their important function in regulating numerous cellular activities, several ROCK inhibitors have been developed [19]. The ROCK inhibitor Y-27632 inhibits the kinase activity of both ROCK1 and ROCK2 with equal potency, by competing with ATP for binding to the catalytic site [20]. Fasudil (HA1077), originally developed as a Ca2+ antagonist and vasodilator, also inhibits both ROCK isoforms [21], but it is less selective for ROCK isoforms than Y-27632. Both Y-27632 and fasudil also have limited capacity to inhibit other AGC kinase subfamily members, including protein kinases A, G, and C [22]. Importantly, fasudil has a proven efficacy and safety profile in humans [19,23]. Animal and human studies with ROCK inhibitors have been of fundamental importance to elucidating ROCK physiologic functions [7,8,19]. However interpretation of studies with ROCK inhibitors is limited due to lack of isoform selectivity and incomplete understanding of their respective specificities.
The Rho family of small GTPases are critical regulates of ROCK activity. Interaction of ROCK with RhoA, −B, and −C through ROCK’s the Rho-binding domain increases ROCK catalytic activity [13]. The 22 known Rho family members are classified into four main subfamilies: Rho, Rac, Cdc42 and others that lack GTPase activity [24]. In the Rho subfamilies, RhoA, RhoB and RhoC share 85% amino acid sequence identity [25]. Until now, 11 Rho binding proteins or Rho effectors have been identified, including ROCK1 and ROCK2 [25]. Although RhoA has been thought to be the main upstream mediator of ROCK [18,26], protein interaction studies have revealed that ROCK has a higher affinity for RhoC compared to RhoA and RhoB [15,25,27-29]. In fact, RhoC appears to have a stronger ability to activate ROCK in epithelial cells [30].
Aberrant ROCK activity is associated with diverse aspects of the metabolic syndrome
Abnormalities in RhoA/ROCK expression and/or activity are associated with a number of metabolic syndrome-related disorders, including CVD, obesity, insulin-resistance, and diabetes and its complications [31-34]. In rodents, elevated RhoA/ROCK signaling is observed in heart [35], aorta [31], and kidney [32,33] of insulin-resistant, obese, or diabetic animal models. Moreover, in patients with the metabolic syndrome, ROCK activity is increased in circulating leukocytes [36]. Furthermore, treatment of hypertensive or diabetic nephropathic animal models with ROCK inhibitor fasudil effectively lowers blood pressure and reduces albuminuria, respectively [37,38]. Consistent with this, in patients with angina pectoris, vasospastic angina, pulmonary hypertension, heart failure, or stroke, treatment with ROCK inhibitor fasudil ameliorates CVD risk factors [7,8,19]. By contrast, insulin-stimulated ROCK1 activity is impaired in skeletal muscle of insulin-resistant humans with obesity and type 2 diabetes [39]. Of note, insulin-induced ROCK1 activity is positively correlated with glucose disposal rate in lean subjects but not in obese type 2 diabetic subjects [39]. Collectively, these data suggest that abnormalities in ROCK activation contribute to the development and progression of diverse aspects of the metabolic syndrome.
ROCK regulation of glucose metabolism and insulin sensitivity is conditional and complex
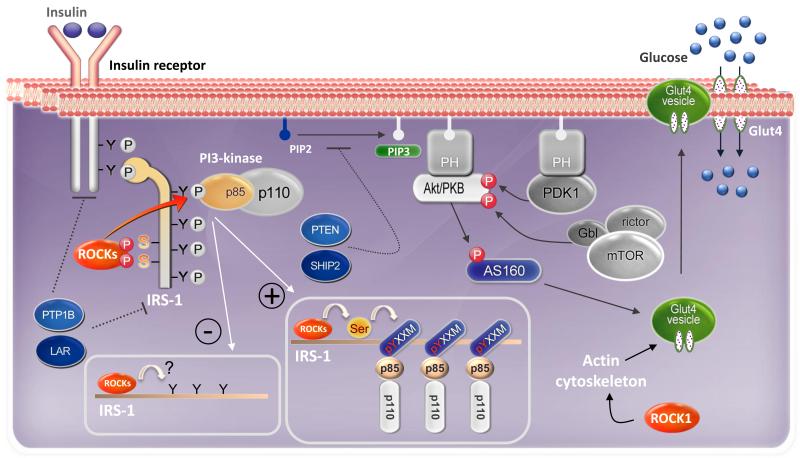
A fundamental mechanism for maintenance of glucose homeostasis is the rapid action of insulin to stimulate glucose uptake in muscle and adipocytes. Insulin action is initiated by binding of insulin to its receptor, which result in tyrosine phosphorylation of insulin receptor substrates (IRSs) including IRS1, IRS2, IRS3, IRS4, and Gab1 [40,41]. Binding of IRSs to the regulatory subunit of phosphoinositide 3-kinase (PI3K) via Src homology 2 (SH2) domains results in activation of PI3K. This then activates Akt/protein kinase B (PKB), which phosphorylates its substrate of 160 kDa (AS160), leading to the translocation of insulin-mediated glucose transporter 4 (Glut4) from intracellular stores to the cell surface (Fig. 1). Defects in insulin-mediated glucose uptake result in insulin resistance, which is a major pathogenic factor in the development of type 2 diabetes [42].
Fig. 1. Divergent roles of ROCK in peripheral tissue insulin signaling and glucose metabolism.
Insulin signaling: Insulin action is initiated by the binding of insulin to its cell surface receptor, which activates the insulin receptor (IR) kinase. Once activated, the IR kinase phosphorylates insulin receptor substrate-1 (IRS-1) on multiple tyrosine residues. Phosphotyrosine residues on IRS-1 act as docking sites for many src homology-2 (SH2) domain-containing proteins, including the p85 regulatory subunit of phosphoinositide 3′ kinase (PI3K). On binding to IRS-1, PI3K is activated and subsequently stimulates Akt/ Akt substrate of 160 kDa (AS160), leading to insulin’s metabolic effects on glucose transport. Akt is fully activated by 3-phosphoinositide-dependent protein kinases (PDK-1) and mammalian target of rapamycin (mTOR)/rictor/Gbl complex. Protein tyrosine phosphatases (PTP1B and LAR) and phosphoinositide phosphatases (PTEN and SHIP2) inhibit insulin action by dephosphorylating IR/IRS-1 or phosphoinositide 3′ phosphate (PIP3), respectively.
Mechanisms of ROCK’s regulation of insulin signaling.
Positive regulation: In adipocytes and muscle cells, insulin stimulates ROCK to bind to and phosphorylate IRS-1 on specific serine residues. ROCK-mediated IRS-1 serine phosphorylation potentially induces conformational change in the adjacent YXXM phosphotyrosine-binding domains, thereby enhancing IRS-1 binding to the p85 regulatory subunit of PI3K. As a result, downstream insulin signaling is activated, leading to enhanced glucose transport activity. In addition, ROCK1’s effect to promote insulin-stimulated glucose transport in these cell types is mediated by changing actin cytoskeleton polymerization. Negative regulation: Currently, the direct molecular mechanisms mediating ROCK’s effects to negative regulate insulin signaling in endothelial and epithelial cells by have yet to be identified.
Unlike the effects of ROCK inhibitors on other aspects of the metabolic syndrome, the reported effects of ROCK inhibitors on glucose homeostasis have been conflicting. Studies with obese Zucker animals have revealed that 4-weeks of treatment with the ROCK inhibitor fasudil (20mg/kg/day for 4wks) decreases blood pressure and improves glucose tolerance [43]. In contrast, acute treatment with ROCK inhibitor Y-27632 (0.25mg/kg/hr for 4hrs) results in insulin resistance in vivo by reducing insulin-mediated glucose uptake in skeletal muscle of normal mice [9]. Moreover, treatment of db/db mice with ROCK fasudil inhibitor (10 mg/kg/day for 16 weeks) has no effects on circulating glucose concentration [33]. Although the mechanisms underlying these conflicting findings remain unclear, the use of different inhibitors, doses, treatment times, and animal models in these in vivo animal studies complicates understanding of the specific roles of ROCK in regulating glucose homeostasis and insulin sensitivity in vivo.
Evidence demonstrates that genetic deletion of ROCK1 globally leads to whole-body insulin resistance and impaired skeletal muscle insulin signaling [10]. These effects are independent of changes in adiposity. In skeletal muscle, insulin action is impaired as evidence by a reduction in insulin-stimulated PI3K activity associated with insulin receptor substrate 1 (IRS-1) though tyrosine phosphorylation of insulin receptor is unaltered [10]. Concurrently, Akt and AS160 phosphorylation, both of which are required for insulin-dependent GLUT4 translocation [44,45], are markedly impaired in skeletal muscle of ROCK1-deficient mice, suggesting impaired glucose transport into muscle. Supporting these studies in animals, in humans, in vivo administration of insulin stimulates ROCK1 activity in skeletal muscle [39]. Moreover, the ability of insulin to activate ROCK1 in vivo in skeletal muscle of type 2 diabetic subjects is significantly impaired compared to lean subjects [39]. Concurrently, defects in insulin-induced IRS-1 associated PI3K activity is also found in skeletal muscle of type 2 diabetic humans [46]. Together, these data identify ROCK1 as a novel regulator of glucose homeostasis and insulin sensitivity in vivo, and the emergence of ROCK1 as a potentially important step in the pathogenesis of insulin resistance could lead to new treatment approaches for obesity and type 2 diabetes. Future studies will need to determine the specific role of ROCK isoforms in the development of insulin resistance and type 2 diabetes.
ROCK targets IRS-1 to regulate insulin signal transduction
Tyrosyl phosphorylation of IRS-1 is a critical step because it permits this docking protein to interact with signaling proteins that promote insulin action [40]. Multiple studies have shown that ROCK isoforms regulate insulin signaling, both positively and negatively, either directly, via IRS-1 seryl phosphorylation or indirectly, by affecting IRS-1 tyrosyl phosphorylation [9,47,48]. Mass spectrometry analysis identified Ser632/635, Ser936, and Ser972 of IRS-1 as substrates of ROCK in vitro [9]. Recent evidence by Chun et al. reveals that siRNA-mediated suppression of each ROCK isoform decreases insulin-dependent IRS-1 Ser632/635 phosphorylation in 3T3-L1 adipocytes [11], indicating these serine sites are regulated via a ROCK-dependent mechanism. Overexpression of the IRS-1 Ser632/635 active mutant significantly increased insulin-stimulated glucose transport in 3T3-L1 adipocytes, whereas overexpression of the IRS-1 Ser632/635 inactive mutant decreased this [11]. Moreover, the ability of insulin to increase IRS-1 tyrosine phosphorylation and PI3K activity was impaired in the IRS-1 Ser632/635 inactive mutant expressing adipocytes compared with WT-IRS-1 expressing adipocytes [11]. Thus, ROCK-mediated IRS-1 serine phosphorylation is necessary for insulin-induced glucose transport and insulin signaling (Fig. 1). Combined with observations from type 2 diabetic humans and ROCK1-deficient mice showing impairments of IRS-1 tyrosine phosphorylation [10,39], these data support a new paradigm for the regulation of glucose metabolism in which ROCK is a key determinant of insulin signaling cascade during insulin stimulation.
In contrast to ROCK’s positive regulation of insulin signaling in adipocytes and skeletal muscle cells in vitro and skeletal muscle in vivo [9-11], ROCK isoforms have been reported to negatively regulate insulin action in cultured vascular smooth muscle cells, endothelial cells, mammary epithelial cells, and fibroblasts (Table 1)[47-50]. Although the mechanisms mediating the negative regulation of insulin action by ROCK isoforms have not been determined, it has been consistently reported that suppression of ROCK activity with ROCK inhibitors fasudil or Y-27632 stimulates insulin signaling at the level of IRS-1 tyrosine phosphorylation in these cell types in vitro. Whether the opposing effects of ROCK isoforms in different cell types or tissues relates to differential relative expression of ROCK isoforms or lack of ROCK inhibitor selectively remains to be clarified. Indeed, experimental evidence suggests that the action of each ROCK isoform on insulin signal transduction differs in Chinese hamster ovary cells expressing insulin receptor and IRS-1, 3T3-L1 adipocyte, and L6 muscle cell lines [11]. An outstanding question for the field is to establish the specific functions of ROCK isoforms in regulating insulin action in different insulin-target tissues in health and disease. Genetic approaches with selective tissue or cell-specific genetic deletion or overexpression of each ROCK isoforms will clarify this important issue.
Table 1. ROCK and the regulation of insulin signal transduction.
| Models | Findings | Action of Rho-kinase | Ref |
|---|---|---|---|
|
Vascular smooth
muscle cells (VSMCs) |
Insulin inhibits ROCK2 | Activate NO/cGMP pathway → MBP activation | [89] [47] |
| RhoA/ROCK2 inhibit insulin signaling | ROCK2 associates with IRS-1 and phosphorylates IRS-1 at serine residue (serine site was not described in details). |
[47] | |
| Increased ROCK2/IRS-1 association in hypertension |
Insulin fails to inhibit ROCK2/IRS-1 association in spontaneously hypertensive rats (SHR). | [47] | |
| Endothelial cells | Rho/ROCK inhibits insulin signaling | ROCK functions as an upstream kinase for LKB1, which phosphorylates PTEN TX A2 receptor pathways inhibit insulin signaling via Rho/ ROCK/LKB1-mediated PTEN up-regulation. |
[49] |
| Xenopus oocytes | ROCK2 inhibits insulin signaling | The non-catalytic carboxyl terminus of ROKa associates with the phosphotyrosine-binding IRS-1 domain, and this association is further increased by active RhoA (MAPK activation). |
[90] |
| ROCK2 enhances insulin-signaling ROCK inhibition reduced insulin signaling |
Microinjection of full-length ROKa or its kinase domain stimulates MAPK activation by insulin and promotes oocyte maturation (No information about IRS-1 tyrosine or serine phosphorylation.) ROCK inhibitor Y-27632 reduces insulin-induced MAPK activation. |
[91] | |
| Mammary epithelial cells (MECs) | RhoA activity is detrimental for insulin signaling |
Active RhoA inhibits IRS-1 tyrosine phosphorylation. Hyperactivation of RhoA/ROCK signaling induces IRS-1 serine phosphorylation, leading to impaired insulin signaling. |
[50] |
| Breast cancer | Rho-A mediates mitogenic insulin signaling |
Insulin signaling to GGTase I appears to be mediated via Shc and MAP kinase and is completely independent of IRS-1. |
[92] |
| Adipocytes | RhoA/ROCK is positive regulator of insulin signaling |
Dominant-negative ROCK suppresses insulin signaling in 3T3-L1 adipocytes. Treatment with C3 exoenzyme of C. botulinum (inactivator of RhoA) or Y27632 inhibits insulin-induced tyrosine phosphorylation of IRS-1. Treatment of 3T3L1 cells with Y27632 inhibits the insulin-induced translocation of GLUT4. |
[9] [93] |
| ROCK is a negative regulator of insulin signaling |
ROCK inhibitors including Y27632 and H89 stimulate adipogenesis and enhance insulin signaling (both PI3K/Akt pathway and the MAPK pathway) |
[94] | |
| Myocytes and Skeletal muscle | ROCK1 is a positive regulator of insulin signaling | Dominant-negative ROCK suppresses insulin signaling in L6 myotubes. ROCK inhibitor suppresses whole body insulin sensitivity and skeletal muscle glucose uptake in mice. Insulin-stimulated ROCK1 activity is decreased in patients with type 2 diabetes. Global ROCK1-deficient mice are insulin resistant; impaired insulin signaling in skeletal muscle. |
[9] [9] [39] [10] |
| ROCK is a negative regulator of insulin signaling |
Treatment with fasudil for 4 weeks improves glucose intolerance in obese Zucker rats. RhoA/ROCK blocks muscle differentiation via serine phosphorylation of IRS-1 and -2. |
[43] [95] |
Roles of hypothalamic ROCK in energy homeostasis
In addition to ROCK’s roles in regulating insulin action, emerging data demonstrate that ROCK1 also plays a fundamental role in controlling energy balance via regulation of leptin action [12]. Leptin, an adipocyte-derived metabolic hormone, regulates body weight homeostasis by controlling hypothalamic neuronal circuits stimulating energy expenditure and inhibiting food intake [51]. Lack of leptin or the leptin receptor (LepR) causes morbid obesity and hyperphagia in rodents and humans [52,53]. However, serum leptin levels are elevated in most cases of human obesity, indicating that impaired leptin action rather than leptin deficiency is associated with the development of obesity [54]. Current evidence indicates that impairments in both leptin transport into the CNS as well as neuronal leptin signal transduction contribute to the pathogenesis of obesity [55,56]. Though several molecular mediators of leptin signal transduction have been identified, the molecular mechanisms underlying leptin signal transduction and its dysregulation in obesity are not fully understood.
ROCK isoforms are expressed throughout the brain [12,57]. In the hypothalamus, ROCK1 is expressed in LepRb expressing neurons, as well as other neuronal subtypes [12]. Leptin increases hypothalamic ROCK1 activation in mice and this effect is dependent on the presence of LepRb [12], raising the question of whether defective ROCK1 activation is involved in the pathogenesis of central leptin resistance in obesity. Our recent data indicate that leptin-stimulated hypothalamic ROCK1 activity is reduced in mice made obese by high-fat diet, compared with lean control mice [58]. Taken together, these data suggest that impaired ROCK1 activity in hypothalamus may contribute to the etiopathogenesis of obesity and leptin resistance.
Beyond a potential role of ROCK1 in the pathogenesis of obesity, recent data suggest that ROCK1 activity in the hypothalamus contributes to normal energy homeostasis [12]. Adenoviral-mediated overexpression of ROCK1 in the mediobasal hypothalamus decreases food intake and body weight of mice. Conversely, mediobasal hypothalamic ROCK1 deletion via adenoviral delivery of Cre recombinase in ROCK1loxP/loxP mice causes hyperphagia and a large increase in body weight and adiposity [12]. Moreover, prenatal or postnatal exposure of rats to ROCK inhibitor fasudil increases postnatal weight gain [59]. Consistent with observations in mice with hypothalamic ROCK knockdown, postnatal exposure of rodents to ROCK inhibitor fasudil increases daily food intake, supporting a role of ROCK in the regulation of feeding. Hyperphagia in fasudil treated animals is mediated in part by increased hypothalamus expression of the orexigenic neuropeptide NPY [59]. Collectively, these data demonstrate that ROCK action in hypothalamus is critical for normal body weight homeostasis.
Divergent functions of ROCK1 in POMC and AgRP neurons on energy balance
In the hypothalamic arcuate, two distinct neuron populations, pro-opiomelanocortin (POMC)-or neuropeptide Y/agouti-related peptide (NPY/AgRP)-containing neurons, are key mediators of the metabolic actions of leptin on feeding regulation and energy balance (Fig. 2)[60,61]. Leptin suppresses food intake and promotes energy expenditure by activating anorexigenic POMC neurons and inhibiting orexigenic AgRP neurons directly [62-64]. Mice lacking the long-form of leptin receptor (LepRb) in POMC or AgRP neurons are obese on normal chow diet [4,60,61], suggesting that leptin signaling in these neurons is needed for homeostatic regulation of body weight. POMC and AgRP neurons have opposing physiological functions in the context of leptin action; in mice, disruption of POMC neurons leads to massive weight gain [65], whereas lesioning of AgRP neurons results in starvation [66].
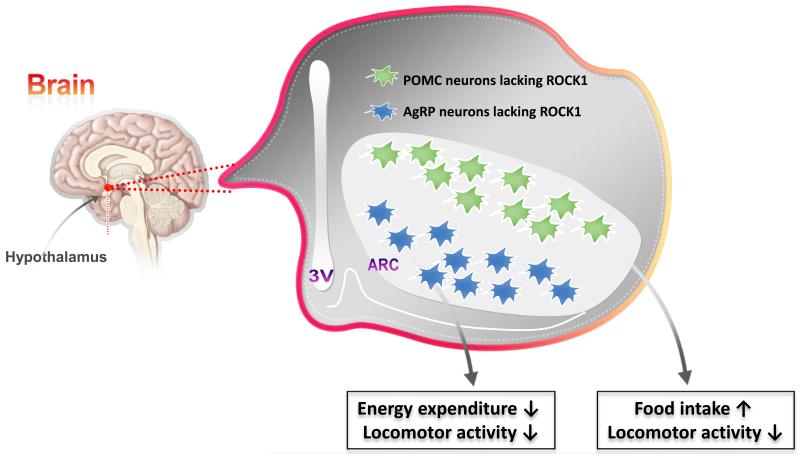
Fig. 2. Roles of ROCK1 in POMC and AgRP neurons in energy balance.
Genetic disruption of ROCK1 in either POMC or AgRP neurons increases body weight and adiposity. Mice lacking ROCK1 in POMC neurons show hyperphagia and hypoactivity. However, AgRP neuron-specific ROCK1-deficient mice display lower oxygen consumption and locomotor activity.
ROCK1 is expressed in POMC and NPY/AgRP expressing neurons of the hypothalamus (Fig. 2) [12,58]. In mice, ROCK1 loss specifically in either POMC or AgRP neurons increases body weight and adiposity, indicating that ROCK1 action in these critical leptin responsive neuron populations is essential for normal body weight regulation [12,58]. Mice lacking ROCK1 in POMC neurons exhibit hyperphagia and hypoactivity, though energy expenditure as assessed by oxygen consumption is normal [12]. ROCK1 loss in POMC neurons also blocks the effect of leptin to stimulate POMC neuronal activity, but not that of insulin, suggesting that ROCK1 action on POMC neuronal activity is leptin-specific [12]. Deletion of ROCK1 in POMC neurons induces central leptin resistance, as evidenced by impaired anorexigenic action and attenuated Signal transducer and activator of transcription 3 (Stat3) activation in response to leptin in POMC-neuron ROCK1-deficient mice [12]. In contrast to the effects of ROCK1 loss in POMC neurons, weight gain in mice with ROCK1 deficiency in AgRP neurons occurs independently of any changes in food intake. Rather, the AgRP neuron-specific ROCK1-deficient mice show imbalanced energy homeostasis, as indicated by lower oxygen consumption and locomotor activity [58]. Collectively, these observations suggest that ROCK1 plays distinct roles in regulating energy balance in different neuron populations of the arcuate nucleus and further imply that leptin signaling in POMC and AgRP neurons plays major roles in regulating energy balance.
The metabolic phenotypes resulting from ROCK1 deficiency in either POMC or AgRP neurons are consistent with those in mice with deletion of LepRb or STAT3 in these respective neuron populations [60,61,67,68]. Thus, ROCK1 appears to be a key trigger of neuronal leptin signal transduction, like LepRb and STAT3. Indeed, similar magnitudes of effect on energy balance are observed in these models. Of note, the fact that weight gain due to knockdown of ROCK1 in mediobasal hypothalamus is significantly greater than in mice with loss of ROCK1 in either POMC or AGRP neurons suggests that ROCK1 functions in other hypothalamic neuron populations is also critical to normal energy balance [12,58]. Therefore, determining the role of ROCK1 in yet additional neuronal populations that could affect the metabolic effects of leptin will be important. Additionally, as ROCK2 is also expressed in hypothalamus, and ROCK1 and ROCK2 share some common substrates, future studies are needed to address whether ROCK2 plays an important role in energy balance as well.
ROCK1 is a positive regulator of Janus kinase 2 (JAK2) in neuronal leptin signaling
Recent work has revealed that the molecular mechanisms of body weight regulation induced by ROCK1 in hypothalamic arcuate neurons involve the stimulatory effects of ROCK1 on JAK2 activation during leptin stimulation (Fig. 3) [12]. Upon binding to LepRb, leptin activates JAK2 that subsequently phosphorylates and activates STAT3. Leptin also stimulates the direct physical interaction between ROCK1 and JAK2 in hypothalamic cell lines in vitro and murine hypothalamus in vivo. Additionally, leptin-dependent JAK2 tyrosine phosphorylation is impaired when ROCK1 expression/activity is biologically suppressed in hypothalamic cell lines, suggesting that leptin-induced JAK2 activation is ROCK1 dependent. These data identify JAK2 as a new substrate of ROCK1 [12].
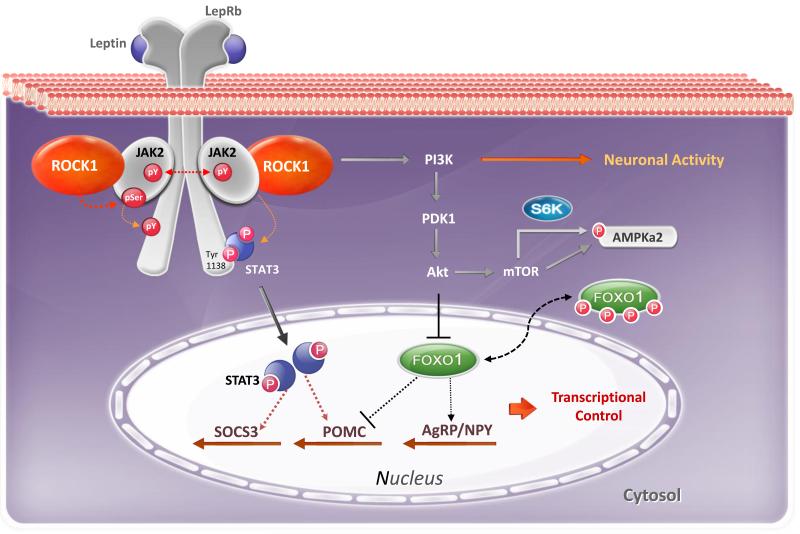
Fig. 3. Central role of ROCK1 in leptin signaling and energy metabolism.
Leptin signaling: Leptin action is triggered by leptin binding to its long-form receptor (LepRb), which activates the receptor-associated janus associated kinase 2 (JAK2). Upon activation, JAK2 phosphorylates Tyr1138 on LepRb, which recruits signal transducer and activator of transcription 3 (STAT3) to the LepRb/JAK2 complex. JAK2 tyrosyl phosphorylation of STAT3 promotes STAT3 nuclear translocation and transactivation of target genes such as suppressor of cytokine signaling 3 (SOCS3) and pro-opiomelanocortin (POMC). JAK2 phosphorylation also activates PI3K signaling involved in the regulation of electrical activity of hypothalamic arcuate neurons. Forkhead box protein O1 (FOXO1), a downstream mediator of PI3K/Akt signaling, inhibits leptin regulation of POMC promoter activity but stimulates transcription of agouti-related protein (AgRP) and neuropeptide Y(NPY).
ROCK-mediated leptin signaling: Upon leptin stimulation, hypothalamic ROCK1 is activated and rapidly phosphorylates JAK2 on serine residues, which in turn promotes downstream signaling pathways of leptin, including STAT3 and PI3K signaling. Specifically, the JAK2/ROCK1→PI3K signaling pathway controls neuronal activity of arcuate neurons and the JAK2/ROCK1→Stat3 signaling pathway is involved in transcriptional regulation of target genes involved in energy metabolism, ultimately leading to control of energy balance.
These data raise the possibility that all LepRb signaling pathways downstream of JAK2 are controlled by ROCK1, including STAT3 and PI3K signaling. Indeed, leptin-induced STAT3 and PI3K signaling are both impaired in hypothalamic cells lacking ROCK1 [12]. Further supporting this, recent data indicate that the ROCK1/JAK2→ STAT3 signaling in cultured hypothalamic cell lines controls expression of genes involved in energy homeostasis, such as POMC and suppression of cytokine signaling 3 (SOCS3), a STAT3 target gene. Additionally, inhibition of ROCK1 in hypothalamic cell lines abolishes the leptin-induced JAK2/PI3K-mediated nuclear export of the transcription factor Forkhead box protein O1 (FOXO1) [12]. Moreover, deletion of ROCK1 in POMC neurons of mice abolishes leptin-dependent POMC neuronal activity, demonstrating that the ROCK1/JAK2 → PI3K signaling cascade contributes to the hypothalamic regulation of energy balance [12]. Along with this, experimental evidence reveals that ROCK1 regulates PI3K signaling by phosphorylating phosphatase and tensin homolog (PTEN) to enhance protein synthesis and cell growth [69]. Collectively, these data support the notion that ROCK1 is a key regulator of proximal leptin signaling events in vivo.
In addition to the effect of ROCK1 on JAK2 in LepRb signaling, the ROCK1 signaling cascade could be involved in various types of cytokine receptors signaling that are triggered by JAK2 activation, including the growth hormone receptor, erythropoietin receptor, and interleukin-6 (IL-6) or gp130 family of receptors [70-72]. For example, growth hormone has been shown to activate RhoA and ROCK in NIH-3T3 cells via a JAK2 dependent mechanism [73]. Activation of RhoA and ROCK signaling is also required for JAK/STAT5-mediated transcription [73]. Impairments in these cytokine signaling pathways contributes to the etiopathogenesis of autoimmune diseases, inflammation, thrombosis, stroke, growth retardation, and cancers such as prostate cancer and multiple myeloma [70-72]. Future studies are needed to determine whether ROCK is a common component of JAK2-mediated signaling pathways and whether it contributes to pathogenesis these diseases.
ROCK and Alzheimer’s disease
Alzheimer Disease (AD) is a progressive neurodegenerative disorder and the most common cause of memory impairment and cognitive dysfunction [74]. Accumulation of amyloid β peptide (Aβ) in the brain is a key feature of AD and could derive from overproduction or impaired clearance [74,75]. It has been reported that dysfunction of insulin receptor signaling in the brain impairs clearance of Aβ [76], suggesting involvement of insulin signaling for AD. Indeed, insulin resistance and hyperinsulinemia reportedly increase the risk for developing AD and memory impairment [77-81]. Epidemiological studies also demonstrate that circulating leptin level inversely correlates with AD development risk, and lower levels of circulating leptin are seen in patients with AD [82]. Consistent with ROCK’s role as a key regulator of insulin and leptin action, a role for ROCK signaling in the pathogenesis of AD has been hypothesized. Supporting this, evidence demonstrates that Rho and ROCK are involved in the regulation of the 42 residue amyloid-β peptide (Aβ42), which is a hallmark peptide of AD [74]. ROCK induces the processing of amyloid precursor protein to the toxic Aβ42, whereas inhibition of ROCK by statins or certain nonsteroidal anti-inflammatory drugs block this effect [83]. Furthermore, intracerebroventricular injection of the ROCK inhibitor Y-27632 into a transgenic AD mouse model leads to a significant reduction in brain Aβ42 [84]. The molecular mechanisms by which ROCK controls Aβ42 accumulation, aggregation, and deposition in the brain remain to be elucidated. Combined with the reported up-regulation of ROCK signaling in various neurological disorders [85,86], these data raise the possibility that drugs that inhibit ROCK may have utility in the treatment of neurodegenerative and neurological disorders.
Concluding remarks and future perspectives
The identification of ROCK1 as a key regulator of insulin and leptin action provides new insight into the pathogenesis of type 2 diabetes and obesity. During the past several years, a number of studies have shed light on the roles of ROCK in diverse physiologic functions, facilitated by the introduction of ROCK chemical inhibitors and in vivo gene targeting approaches [10,19]. Animal and patient studies with the ROCK inhibitor fasudil consistently demonstrate that inhibitor pharmacotherapy is beneficial for a wide range of cardiovascular-related diseases [8,38,87,88]. On the other hand, accumulating evidence demonstrates that insulin resistance and obesity are observed when ROCK1 expression or activity is suppressed in skeletal muscle [39], adipocytes [9], or hypothalamus [12], suggesting the development of a tissue-targeted ROCK1 activator as a novel treatment for metabolic-related disorders such as type 2 diabetes and obesity. Based on what we now know, it is clear that ROCK is emerging as a pivotal regulator of diverse aspects of metabolic-related diseases. Genetic engineering approaches to achieve selective tissue- or cell-specific deletion, activation, or overexpression of each ROCK isoform are still needed to clarify the important roles of these proteins in metabolic physiology. Finally, establishing the endogenous physiologic activators and inhibitors of ROCK1 and ROCK2 will clarify the potential of these kinases as metabolic drug targets.
Box 1. Outstanding Questions.
How do insulin and leptin levels and/or diet impact ROCK activity and the development of insulin resistance and obesity?
What are the molecular mechanisms by which insulin and leptin stimulate or suppress ROCK activity?
Does the ROCK2 isoform also regulate glucose homeostasis, body weight, or CVD? What are the specific physiological roles of each ROCK isoform in individual metabolic tissues?
What are the endogenous activators and inhibitors of ROCK?
Do ROCK isoforms contribute to the pathogenesis of other neurological diseases?
Highlights.
ROCK inhibitor is beneficial for cardiovascular-related diseases
ROCK positively regulates metabolic action of insulin on glucose transport
Loss of ROCK1 in hypothalamic neurons of mice promotes adiposity
ROCK1 regulates energy balance by targeting hypothalamic leptin receptor signaling
Acknowledgement
This work was supported by grants from the National Institutes of Health (1R01DK083567 to Y.B.K) and the American Heart Association (12GRNT12040170 to Y.B.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahima RS. Digging deeper into obesity. J Clin Invest. 2011;121(6):2076–2079. doi: 10.1172/JCI58719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. Jama. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3 Suppl 1):S12–18. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am. 2008;37(4):841–856. doi: 10.1016/j.ecl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romeo GR, et al. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2013;32(8):1771–1776. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mommersteeg PM, Pouwer F. Personality as a risk factor for the metabolic syndrome: a systematic review. J Psychosom Res. 2013;73(5):326–333. doi: 10.1016/j.jpsychores.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Hirooka Y, Shimokawa H. Therapeutic potential of rho-kinase inhibitors in cardiovascular diseases. Am J Cardiovasc Drugs. 2005;5(1):31–39. doi: 10.2165/00129784-200505010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hu E, Lee D. Rho kinase as potential therapeutic target for cardiovascular diseases: opportunities and challenges. Expert Opin Ther Targets. 2005;9(4):715–736. doi: 10.1517/14728222.9.4.715. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa N, et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005;2(2):119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Lee DH, et al. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem. 2009;284(18):11776–11780. doi: 10.1074/jbc.C900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun KH, et al. Regulation of glucose transport by ROCK1 differs from that of ROCK2 and is controlled by actin polymerization. Endocrinology. 2012;153(4):1649–1662. doi: 10.1210/en.2011-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H, et al. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nature Neuroscience. 2012;(15):1391–1398. doi: 10.1038/nn.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui T, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. Embo J. 1996;15(9):2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa O, et al. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392(2):189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 15.Ishizaki T, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. Embo J. 1996;15(8):1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 16.Leung T, et al. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270(49):29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- 17.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 18.Amano M, et al. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261(1):44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 19.Liao JK, et al. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50(1):17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narumiya S, et al. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000;325:273–284. doi: 10.1016/s0076-6879(00)25449-9. [DOI] [PubMed] [Google Scholar]
- 21.Ono-Saito N, et al. H-series protein kinase inhibitors and potential clinical applications. Pharmacol Ther. 1999;82(2-3):123–131. doi: 10.1016/s0163-7258(98)00070-9. [DOI] [PubMed] [Google Scholar]
- 22.Tamura M, et al. Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta. 2005;1754(1-2):245–252. doi: 10.1016/j.bbapap.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 23.RM V, et al. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;(46):1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 24.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2(2):133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301(1):43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med (Berl) 2002;80(10):629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- 27.Mukai H, et al. PKN associates and phosphorylates the head-rod domain of neurofilament protein. J Biol Chem. 1996;271(16):9816–9822. doi: 10.1074/jbc.271.16.9816. [DOI] [PubMed] [Google Scholar]
- 28.Madaule P, et al. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394(6692):491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- 29.Reid T, et al. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996;271(23):13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- 30.Feng J, et al. Rho-associated kinase of chicken gizzard smooth muscle. J Biol Chem. 1999;274(6):3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- 31.Wingard C, et al. Altered penile vascular reactivity and erection in the Zucker obese-diabetic rat. J Sex Med. 2007;4(2):348–362. doi: 10.1111/j.1743-6109.2007.00439.x. discussion 362-343. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi Y, et al. A Rho-kinase inhibitor, fasudil, prevents development of diabetes and nephropathy in insulin-resistant diabetic rats. J Endocrinol. 2007;192(3):595–603. doi: 10.1677/JOE-06-0045. [DOI] [PubMed] [Google Scholar]
- 33.Kolavennu V, et al. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes. 2008;57(3):714–723. doi: 10.2337/db07-1241. [DOI] [PubMed] [Google Scholar]
- 34.Mishra RK, et al. Potential Role of Rho Kinase Inhibitors in Combating Diabetes-Related Complications Including Diabetic Neuropathy-A Review. Curr Diabetes Rev. 2013 doi: 10.2174/1573399811309030006. [DOI] [PubMed] [Google Scholar]
- 35.Lin G, et al. Acute inhibition of Rho-kinase improves cardiac contractile function in streptozotocin-diabetic rats. Cardiovasc Res. 2007;75(1):51–58. doi: 10.1016/j.cardiores.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Liu PY, et al. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol. 2007;49(15):1619–1624. doi: 10.1016/j.jacc.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukai Y, et al. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. Faseb J. 2001;15(6):1062–1064. doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- 38.Uehata M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389(6654):990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 39.Chun KH, et al. In vivo activation of ROCK1 by insulin is impaired in skeletal muscle of humans with type 2 diabetes. Am J Physiol Endocrinol Metab. 2012;300(3):E536–542. doi: 10.1152/ajpendo.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283(3):E413–422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi CM, et al. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 42.Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998;92(5):593–596. doi: 10.1016/s0092-8674(00)81125-3. [DOI] [PubMed] [Google Scholar]
- 43.Kanda T, et al. Rho-kinase as a molecular target for insulin resistance and hypertension. Faseb J. 2006;20(1):169–171. doi: 10.1096/fj.05-4197fje. [DOI] [PubMed] [Google Scholar]
- 44.Sano H, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278(17):14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 45.Jiang ZY, et al. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci U S A. 2003;100(13):7569–7574. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YB, et al. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest. 1999;104(6):733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begum N, et al. Active Rho kinase (ROK-alpha) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem. 2002;277(8):6214–6222. doi: 10.1074/jbc.M110508200. [DOI] [PubMed] [Google Scholar]
- 48.Sordella R, et al. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev Cell. 2002;2(5):553–565. doi: 10.1016/s1534-5807(02)00162-4. [DOI] [PubMed] [Google Scholar]
- 49.Song P, et al. Thromboxane A2 receptor activates a Rho-associated kinase/LKB1/PTEN pathway to attenuate endothelium insulin signaling. J Biol Chem. 2009;284(25):17120–17128. doi: 10.1074/jbc.M109.012583. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Lee YJ, et al. Extracellular matrix controls insulin signaling in mammary epithelial cells through the RhoA/Rok pathway. J Cell Physiol. 2009;220(2):476–484. doi: 10.1002/jcp.21793. [DOI] [PubMed] [Google Scholar]
- 51.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;(21):263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 52.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 53.Montague CT, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;(387):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 54.Frederich RC, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 55.Banks WA, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53(5):1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 56.Banks WA. Role of the blood-brain barrier in the evolution of feeding and cognition. Ann N Y Acad Sci. 2012;1264(1):13–19. doi: 10.1111/j.1749-6632.2012.06568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iizuka M, et al. Distinct distribution and localization of Rho-kinase in mouse epithelial, muscle and neural tissues. Cell Struct Funct. 2012;37(2):155–175. doi: 10.1247/csf.12018. [DOI] [PubMed] [Google Scholar]
- 58.Huang H, et al. ROCK1 in AgRP neurons regulates energy expenditure and locomotor activity. 2013. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butruille L, et al. Prenatal Fasudil exposure alleviates fetal growth but programs hyperphagia and overweight in the adult male rat. Eur J Pharmacol. 2012;689(1-3):278–284. doi: 10.1016/j.ejphar.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 60.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;(42):983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Van de Wall E, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;(149):1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elias CF, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23(4):775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 63.van den Top M, et al. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7(5):493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 64.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 65.Yaswen L, et al. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5(9):1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 66.Luquet S, et al. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 67.Xu AW, et al. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148(1):72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 68.Gong L, Y F, Hockman K, Heng HH, Morton GJ, Takeda K, Akira S, Low MJ, Rubinstein M, MacKenzie RG. Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide Y neurons for normal energy homeostasis. Endocrinology. 2008;(149):3346–3354. doi: 10.1210/en.2007-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vemula S, S J, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood. 2010;(4):1785–1796. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narazaki M, et al. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc Natl Acad Sci U S A. 1994;91(6):2285–2289. doi: 10.1073/pnas.91.6.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brooks AJ, et al. Growth hormone receptor; mechanism of action. Int J Biochem Cell Biol. 2008;40(10):1984–1989. doi: 10.1016/j.biocel.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 72.Cohen J, et al. Erythropoietin and its receptor: signaling and clinical manifestations. Isr Med Assoc J. 2002;4(11):1072–1076. [PubMed] [Google Scholar]
- 73.Ling L, L P. RhoA/ROCK activation by growth hormone abrogates p300/histone deacetylase 6 repression of Stat5-mediated transcription. J Biol Chem. 2004;(279):32737–32750. doi: 10.1074/jbc.M400601200. [DOI] [PubMed] [Google Scholar]
- 74.Iqbal K, Grundke-Iqbal I. Opportunities and challenges in developing Alzheimer disease therapeutics. Acta Neuropathol. 2011;122(5):543–549. doi: 10.1007/s00401-011-0878-z. [DOI] [PubMed] [Google Scholar]
- 75.Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115(5):1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao WQ, et al. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric a{beta} J Biol Chem. 2009;284(28):18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.A O, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;(53):1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 78.Peila R, R B, Launer LJ, Honolulu-Asia Aging Study Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;(51):1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 79.Luchsinger JA, T M, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;(63):1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 80.K T, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;(122):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao WQ, et al. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490(1-3):71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 82.Lee EB. Obesity, leptin, and Alzheimer’s disease. Ann N Y Acad Sci. 2011;1243:15–29. doi: 10.1111/j.1749-6632.2011.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liou B.L.T.a.Y.C. Novel modulators of amyloid-b precursor protein processing. Journal of Neurochemistry. 2007;(100):314–323. doi: 10.1111/j.1471-4159.2006.04215.x. [DOI] [PubMed] [Google Scholar]
- 84.Y Z, et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;(302):1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- 85.Wang QM, Liao JK. ROCKs as immunomodulators of stroke. Expert Opin Ther Targets. 2012;16(10):1013–1025. doi: 10.1517/14728222.2012.715149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kubo T, Yamashita T. Rho-ROCK inhibitors for the treatment of CNS injury. Recent Pat CNS Drug Discov. 2007;2(3):173–179. doi: 10.2174/157488907782411738. [DOI] [PubMed] [Google Scholar]
- 87.Shibuya M, H S, Seto M, Satoh S, Ohtomo E, Fasudil Ischemic Stroke Study Group Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;(238):31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 88.A M, et al. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;(105):1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 89.Sandu OA, et al. Selected contribution: insulin utilizes NO/cGMP pathway to activate myosin phosphatase via Rho inhibition in vascular smooth muscle. J Appl Physiol. 2001;91(3):1475–1482. doi: 10.1152/jappl.2001.91.3.1475. [DOI] [PubMed] [Google Scholar]
- 90.Farah S, et al. A rho-associated protein kinase, ROKalpha, binds insulin receptor substrate-1 and modulates insulin signaling. J Biol Chem. 1998;273(8):4740–4746. doi: 10.1074/jbc.273.8.4740. [DOI] [PubMed] [Google Scholar]
- 91.Ohan N, et al. RHO-associated protein kinase alpha potentiates insulin-induced MAP kinase activation in Xenopus oocytes. J Cell Sci. 1999;112(Pt 13):2177–2184. doi: 10.1242/jcs.112.13.2177. [DOI] [PubMed] [Google Scholar]
- 92.Finlayson CA, et al. Enhanced insulin signaling via Shc in human breast cancer. Metabolism. 2003;52(12):1606–1611. doi: 10.1016/s0026-0495(03)00311-1. [DOI] [PubMed] [Google Scholar]
- 93.Takaguri A, et al. Effects of atorvastatin and pravastatin on signal transduction related to glucose uptake in 3T3L1 adipocytes. J Pharmacol Sci. 2008;107(1):80–89. doi: 10.1254/jphs.fp0072403. [DOI] [PubMed] [Google Scholar]
- 94.Kato Y, et al. H-89 potentiates adipogenesis in 3T3-L1 cells by activating insulin signaling independently of protein kinase A. Life Sci. 2007;80(5):476–483. doi: 10.1016/j.lfs.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 95.Batterham RL, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]