Abstract
The papillomavirus E2 proteins are pivotal to the viral life cycle and have well characterized functions in transcriptional regulation, initiation of DNA replication and partitioning the viral genome. The E2 proteins also function in vegetative DNA replication, post-transcriptional processes and possibly packaging. This review describes structural and functional aspects of the E2 proteins and their binding sites on the viral genome. It is intended to be a reference guide to this viral protein.
Keywords: HPV, papillomavirus, E2, replication, transcription, tethering, genomics, structure, mutation
1. Introduction
The full-length E2 protein is an essential regulatory protein encoded by all papillomaviruses. In addition, all viruses have the potential to encode at least one shorter E2 species. All E2 proteins are sequence specific DNA binding proteins that bind to 12bp motifs located mostly within the Upstream Regulatory Region (URR) of the viral genomes. E2 proteins are multifunctional and involved in many viral processes, mostly associated with transcription and replication of the viral genome. They are expressed at early and intermediate stages of the viral life cycle. This review is intended to be an encyclopedic overview of the structure and function of the E2 proteins.
2. E2 Isoforms and Transcripts
2.1 E2 protein isoforms
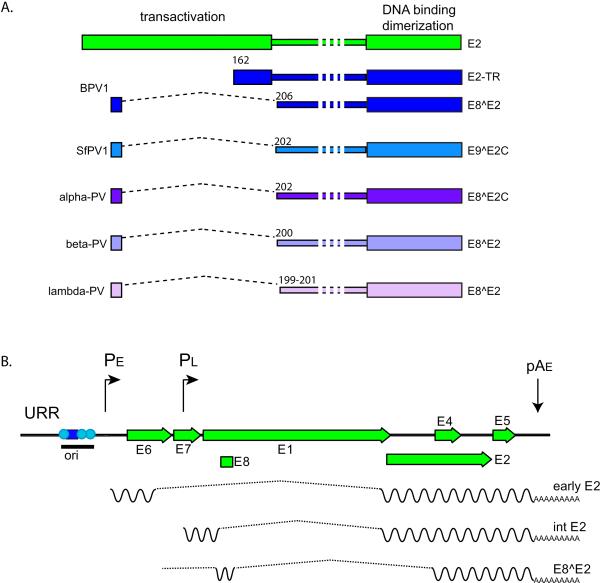
The full-length E2 protein expressed from the entire E2 open reading frame (ORF) consists of a conserved N-terminal terminal “transactivation” domain of about 200 amino acids linked to a C-terminal DNA binding/dimerization domain of about 100 amino acids. The two domains are connected by a flexible linker sequence, often called the “hinge” (Giri and Yaniv, 1988; McBride et al., 1989b; McBride et al., 1988), which varies in length and sequence composition among different genera of papillomaviruses (see Figure 1A).
Figure 1. E2 Isoforms and Transcripts.
A. Isoforms
The two conserved domains of the full-length E2 protein, encoded by all PVs, are shown in green at the top. The region between the domains is of variable length and is named the Hinge. Many PVs have been shown to encode an E8^E2 (or equivalent) repressor form as shown and all PVs encode homologous sequences and splice donor/acceptor sites. BPV1 encodes two repressor forms. Some examples of E2 repressor proteins are shown.
B. E2 transcripts
The URR and early region of an alpha-PV genome is shown. In the lower layers of a papilloma, mRNAs are transcribed from the early promoter, PE, and terminated at the early polyadenylation signal, pAE. At the next stage of differentiation, the late promoter is activated, but messages are still truncated at pAE. Transcripts encoding the full-length E2 protein are transcribed from the early and late promoters (reviewed in (Johansson and Schwartz, 2013)). It is not clear whether both promoters can transcribe the E8^E2 mRNAs, though it seems most likely that they would be transcribed from the early promoter in undifferentiated cells. The origin of replication (ori) is indicates with the E1 binding site shown as a blue square and E2 binding sites as cyan circles.
All PVS have the potential to encode shorter E2 forms that contain the C-terminal domain, the hinge region and a 10-13 residue peptide from an upstream ORF. These E2 fusion proteins are encoded by spliced messages that link sequences from an alternative reading frame in the E1 region of the genome (designated E8) to the C-terminus of E2. These proteins have been alternatively named E8^E2, E8^E2C, E1^E2, E1M^E2 and E9^E2, and are shown in Figure 1A. Invariably, the shorter forms of E2 function as repressors of viral transcription and replication.
The relative abundance of the different E2 isoforms has been determined in BPV-1 infected cells; E2, E2-TR and E8^E2 are expressed in ratios 1:10:3, respectively (Hubbert et al., 1988). This means that most full-length E2 proteins are present as heterodimers. These heterodimers can support transcription and replication initiation, but not partitioning of viral genomes (Kurg et al., 2006; Kurg et al., 2009; Kurg et al., 2010).
2.2 E2 encoding transcripts
In the alpha-PVs, such as HPV16, the full length E2 protein is encoded from spliced messages expressed from either the early or late promoters (Johansson and Schwartz, 2013). These transcripts are shown in Figure 1B. In undifferentiated cells, E2 encoding mRNAs are transcribed from the early promoter and terminate at the early polyadenylation site (pAE). As cells differentiate in the mid-layers of the epithelium, the late promoter becomes activated and transcribes an intermediate class of messages that still use the early polyadenylation signal (Johansson and Schwartz, 2013). These transcripts encode high levels of E2 that are required for vegetative viral DNA replication (Ozbun and Meyers, 1998).
The E8^E2 protein is encoded by a spliced message containing a short exon encoding E8 spliced to the major splice acceptor in the middle of the E2 ORF (Stubenrauch et al., 2000). These mRNAs species use the same splice acceptor as the E1^E4 proteins and a splice donor located in the 5’ region of the E1 ORF (about 400 nucleotides from the E1 start codon). Transcripts encoding shorter E2 forms have been mapped in BPV1 (Choe et al., 1989; Hubbert et al., 1988; Lambert et al., 1989), SfPV1 (Jeckel et al., 2003), HPV11 (Chiang et al., 1991), HPV16 (Lace et al., 2008) and HPV31 (Stubenrauch et al., 2000). However, analysis of sequences in the PaVE database reveals that all PVs have the potential to encode the equivalent of an E8^E2 transcript. A more extensive description of E8 sequences is presented below.
In BPV1, a delta-PV, the E2 proteins can be expressed from spliced messages similar to those encoded by the alpha-PVs, but also from additional promoters located in the early region of the genome (Choe et al., 1989; Lambert et al., 1989; Spalholz et al., 1991; Vaillancourt et al., 1990). BPV1 encodes a second repressor protein, designated E2 TR, that is transcribed from an early region promoter (P3080) and initiated from an internal ATG in the E2 ORF. Three distinct E2 proteins corresponding to full-length E2, E8^E2 and E2-TR have been detected in BPV1 infected cells (Hubbert et al., 1988). Papilllomaviruses from the Delta genus are unique in their ability to cause fibropapillomas. Their unusual transcriptional pattern might reflect this additional tropism for fibroblasts.
Additional E2-derived messages have been described. Two differentiation-dependent transcripts that fuse either 12 or 116 residues from the N-terminus of HPV16 E2 onto E4 have been detected (Tan et al., 2012).
3. E2 Functions
3.1 Transcription
The E2 proteins are the main transcriptional regulators of the papillomaviruses (Chin et al., 1988; Choe et al., 1989; Cripe et al., 1987; Lambert et al., 1987; Phelps and Howley, 1987; Spalholz et al., 1985). The E2 proteins function primarily by recruiting cellular factors to the viral genomes, which activate or repress transcriptional processes. The E2 proteins bind specifically to sequence motifs in the viral genome and can activate or repress transcription, depending on the context of these binding sites and nature of the associated cellular factors. E2 binding sterically hinders the binding of cellular factors such as Sp1 and TBP to proximal promoter elements in the viral genome (Bernard et al., 1989; Dong et al., 1994; Dostatni et al., 1991; Hou et al., 2000; May et al., 1994; Romanczuk et al., 1990; Stubenrauch et al., 1996; Stubenrauch and Pfister, 1994; Tan et al., 1994). In many viruses, the full-length E2 protein either activates or represses viral transcription in a dose dependent manner (Bouvard et al., 1994; Fujii et al., 2001; Steger and Corbach, 1997; Thierry and Yaniv, 1987). Later studies demonstrated that E2 represses transcription by recruiting factors that manipulate cellular chromatin processes (Nishimura et al., 2000; Schweiger et al., 2007; Smith et al., 2010; Wu et al., 2006).
The shorter forms of E2 can act as repressors of E2 function. Repression can be mediated by competition for binding to E2 binding sites (Lambert et al., 1987; Lim et al., 1998; Monini et al., 1993), and by recruitment of repressor complexes by the E8-derived peptide to viral DNA (Ammermann et al., 2008; Fertey et al., 2010; Powell et al., 2010). Expression of shorter forms of E2 can repress transcriptional activation by dimerization with full-length E2 (Barsoum et al., 1992) but heterodimers with just one activation domain can also activate transcription (Kurg et al., 2006).
E2 protein functions are occasionally disrupted by mutation or integration of the viral genome (Schwarz et al., 1985). This inactivation leads to alleviation of E2-mediated repression and increased expression of the E6 and E7 genes (Bernard et al., 1989; Thierry and Howley, 1991; Thierry and Yaniv, 1987), contributing to malignant progression in “high risk” PV infection. Restoration of E2 expression in cervical cancer derived lines leads to senescence (Goodwin et al., 2000) as HPV-associated cancer cells are addicted to, and dependent on, E6 and E7 expression (Desaintes et al., 1997; Dowhanick et al., 1995; Hwang et al., 1993).
3.2 Initiation of Viral DNA Replication
There are three modes of replication in the papillomavirus life cycle. First, limited and unlicensed genome amplification occurs when a viral particle first enters the host cell. In the second phase, the viral genome is maintained at a constant copy number in the proliferating basal cells of a papilloma. This phase requires both genome replication and partitioning. In the third phase, genomes are amplified in differentiated cells to produce progeny virions.
E2 participates in the initiation of viral DNA replication by loading the E1 helicase onto the replication origin (Mohr et al., 1990; Sanders and Stenlund, 2000). The replication origin contains an E1 binding site, an A/T rich region and at least one E2 binding site (Ustav et al., 1993; Ustav et al., 1991). E1 is the primary replication protein but E2 enhances and supports the functions of E1 (Frattini and Laimins, 1994; Mohr et al., 1990). After E1 is loaded it converts to a double-hexameric helicase and E2 is displaced (Sanders and Stenlund, 1998). E2 also displaces nucleosomes from the origin to alleviate repression (Li and Botchan, 1994).
The E8^E2 transcriptional repressor is a potent repressor of viral DNA replication (Lace et al., 2008; Zobel et al., 2003), and this involves the recruitment of cellular corepressor factors (Ammermann et al., 2008). E8^E2 modulates the levels of replication in the maintenance phase of replication in HPV16, HPV18 and HPV31 (Kurg et al., 2010; Lace et al., 2008; Stubenrauch et al., 2000), but only appears to be essential for episomal maintenance in HPV31 (Lace et al., 2008; Stubenrauch et al., 2000). In BPV1, elimination of the E2-TR repressor increases genome copy number (Lambert et al., 1990; Riese et al., 1990) and removal of both repressors results in a replication defect. However, SfPV1 (formerly CRPV) genomes that are defective for E8^E2 (aka E9^E2C) expression can replicate and induce tumors in rabbits at levels comparable to that of wildtype genomes (Jeckel et al., 2003).
Viral DNA replication takes place in nuclear foci and the formation of these foci are dependent on the E2 protein (Fradet-Turcotte et al., 2011; Reinson et al., 2013; Sakakibara et al., 2011; Swindle et al., 1999). Viral DNA replication and viral proteins induce a DNA damage response within these foci that is thought to be important to recruit cellular repair proteins to synthesize viral DNA (Fradet-Turcotte et al., 2011; Gillespie et al., 2012; Moody and Laimins, 2009; Reinson et al., 2013; Sakakibara et al., 2011).
3.3 Genome Maintenance, Partitioning and Tethering
E1 and E2 support transient replication of replicons containing the minimal origin of replication, but these replicons are quickly lost. Persistent and stable maintenance of replicons require additional E2 binding sites in cis to the origin (Piirsoo et al., 1996). For BPV1, at least eight E2 binding sites are required for stable episomal maintenance (Piirsoo et al., 1996), but three E2 binding sites are sufficient for maintenance of HPV18 derived replicons (van Doorslaer et al., 2013) or for episomal maintenance of HPV31 genomes with mutations in individual E2 binding sites (Stubenrauch et al., 1998b). The E2 protein tethers the viral genomes through these sites to the host chromosomes in mitosis to facilitate retention, maintenance and partitioning of the viral genome (Bastien and McBride, 2000; Ilves et al., 1999; Lehman and Botchan, 1998; Skiadopoulos and McBride, 1998). The E2 DNA binding domain binds to binding sites in the viral genome and other regions of E2 (such as the transactivation domain) tether the genome to mitotic chromosomes through protein-protein interactions. Heterodimers formed by long and short forms of E2 can support initiation of replication but they are defective for partitioning (Kurg et al., 2006; Kurg et al., 2009; Kurg et al., 2010).
The E2 proteins from different genera of PVs bind to different regions of mitotic chromosomes (Oliveira et al., 2006). As shown in Table 1, E2 proteins from viruses such as BPV1 and HPV1 bind as punctate foci all over the arms of most mitotic chromosomes (Oliveira et al., 2006; Skiadopoulos and McBride, 1998). On the other hand, E2 proteins from Beta-PVs bind most prominently to pericentromeric regions of chromosomes that overlap the loci for the ribosomal RNA genes (Oliveira et al., 2006; Poddar et al., 2009). The alpha-PVs are difficult to detect by immunofluorescence throughout mitosis on condensed chromosomes, though they are readily detected bound to host chromosomes in telophase (Donaldson et al., 2007; Gammoh et al., 2006; McPhillips et al., 2006). Compared to other E2 proteins, the alpha PV E2s are not tightly bound to host chromatin, even in interphase (McPhillips et al., 2006). When cells are pre-extracted before fixation, most E2 is eluted and the remaining E2 is observed bound to pericentromeric regions of mitotic chromosomes similar to the Beta-PV E2 proteins (Oliveira et al., 2006). It is notable that the three different phenotypes of mitotic chromosome binding correspond well to the phylogeny of papillomaviruses: these groups consist of the Alpha-PVs, the Beta and Gamma PVs, and a diverse group of viruses from the delta (BPV1), mu (HPV1), kappa (OcPV1 and SfPV1) and other genera.
TABLE 1. Tethering Phenotypes and Targets of E2 Proteins from Different PV Genera.
Chromosomal Targets of Viral Tethering Proteins
| PV Genus | Predominant Mitotic Chromosome Binding Phenotype | Proposed targets | Reference |
|---|---|---|---|
| Alpha | • Binding to chromosomes only readily
observed by immunofluorescence at late stages of
mitosis. • Binding to rDNA loci observed in pre-extracted cells |
Brd4, mitotic spindle, ChLRl, TopBPl, | (Donaldson etal., 2007; Gammoh et al., 2006; McPhillips et al., 2006; Parish et al., 2006; Van Tine et al., 2004) |
| Beta | • Prominent binding to pericentromeric
regions and rDNA loci • When E2-rDNAassociation is disrupted, chromosomal foci of Brd4 can be observed. |
Brd4, rDNA, pericentromeric regions | (McBride and van Doorslaer, 2013; Oliveira et al., 2006; Poddar et al., 2009; Sekhar and McBride, 2012; Sekhar et al., 2010) |
| Delta, Mu and others | • E2 and Brd4 form distinct foci on
arms of most host chromosomes • At high levels, some E2 observed at mid-body in late mitosis |
Brd4, ChLRl, Mklp2 | (Baxter et al., 2005; Ilves et al., 2006; Parish et al., 2006; You et al., 2004; Yu et al., 2007) |
One of the best studied tethering targets on the mitotic chromosomes is the chromatin adaptor protein, Brd4 (Baxter et al., 2005; Ilves et al., 2006; You et al., 2004). Many PV E2 proteins colocalize with Brd4 in punctate speckles on mitotic chromatin (McPhillips et al., 2006; Oliveira et al., 2006) and these E2 proteins stabilize the association of Brd4 with chromatin (McPhillips et al., 2006; McPhillips et al., 2005). Disruption of the E2-Brd4 association displaces genomes from chromosomes (Abbate et al., 2006) and results in a loss of BPV1 genomes from infected cells (You et al., 2005). Two highly conserved residues in the E2 transactivation domain, R37 and I73, mediate the interaction with the C-terminus of the Brd4 protein (Abbate et al., 2006; Baxter et al., 2005; Senechal et al., 2007; You et al., 2004). All papillomavirus E2 proteins bind Brd4 to regulate transcription but they are not all readily observed bound to mitotic chromosomes with Brd4 (McPhillips et al., 2006).
The diverse group of E2 proteins described above, best represented by BPV1 and HPV1, bind with high affinity to Brd4 through the transactivation domain and both proteins are easily detected colocalized in punctate speckles on mitotic chromosomes. In contrast, as described above, the beta-PV E2 proteins bind strongly to pericentromeric regions of mitotic chromosomes and do not colocalize with Brd4 at this location (Poddar et al., 2009). The Beta-PV E2 proteins require only the E2 DNA binding domain and a short phosphorylated peptide from the hinge region to bind to mitotic chromosomes (Sekhar and McBride, 2012; Sekhar et al., 2010). However, the beta E2 proteins can bind Brd4 with high affinity and when the chromosomal binding peptide in the E2 hinge is mutated Brd4 is observed on foci on mitotic chromosomes (McBride and van Doorslaer, 2013).
Alpha PV E2s bind to Brd4 relatively weakly and cannot be easily detected on condensed chromosomes throughout mitosis (McPhillips et al., 2006). However, Brd4 localizes to replication foci formed by the E1 and E2 proteins (Sakakibara et al., 2013; Wang et al., 2013), implying a role for Brd4 in replication. But HPV31 genomes that encode a mutated E2 protein (I73L) defective in Brd4 binding can still maintain extrachromosomal viral genomes and undergo amplification in differentiated keratinocytes (Senechal et al., 2007; Stubenrauch et al., 1998b). Therefore the role of Brd4 in Alpha-PV E2 functions is still unclear. Several other cellular targets such as the mitotic spindle (Dao et al., 2006), a mitotic kinesin like protein, MKlp2(Yu et al., 2007), ChlR1 (an ATP-dependent DNA helicase important for sister chromatid cohesion) (Parish et al., 2006) and TopBP1 (Donaldson et al., 2007) have been proposed to be important for tethering of Alpha-E2 proteins. This information is summarized in Table 1.
3.4 Vegetative Viral DNA Replication
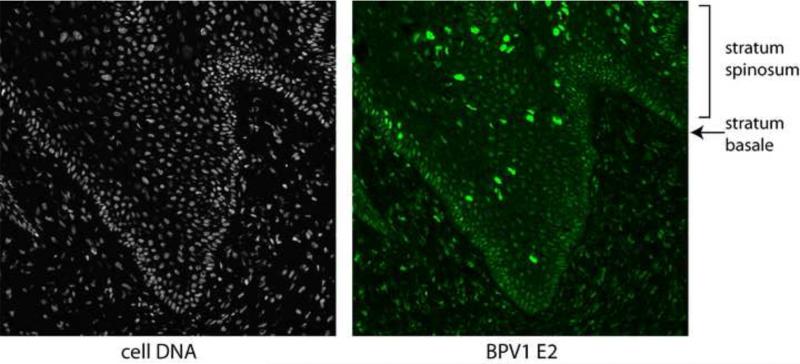
E2 is almost certainly required for vegetative viral DNA amplification. E2-encoding transcripts are abundantly expressed from an intermediate class of transcripts that use the late promoter and early polyadenylation site (reviewed in (Johansson and Schwartz, 2013)) and the E2 protein is highly expressed in cells that are amplifying the viral genome in the stratum spinosum of a bovine wart (Burnett et al., 1990; Penrose and McBride, 2000) and see Figure 8. Vegetative amplification can be induced by growth arrest in mouse cells transformed by BPV1 and this is accompanied by a huge induction in E2 expression (Burnett et al., 1990). Using BPV1 encoding a temperature sensitive mutation in E2 (DiMaio and Settleman, 1988), Alderborn et al. demonstrated that the replication, but not transactivation function of E2 is required for genome amplification (1992). Likewise, Stubenrauch et al. showed that the transactivation function of E2 was not required for HPV31 amplification in differentiated keratinocytes (Stubenrauch et al., 1998a). There is evidence that the mode of replication might change from bidirectional theta mode in maintenance replication to recombination-directed replication in vegetative amplification (Flores and Lambert, 1997; Sakakibara et al., in press 2013).
Figure 8. Expression of E2 in a Bovine FibroPapilloma.
Shown is a section of tissue from a BPV1 infected fibropapilloma. Host cells are visualized in the left panel using DAPI to stain nuclei. The E2 protein is detected in the right panel using a BPV1 E2 specific monoclonal antibody (B201) with an epitope between residues 160 and 200 in the E2 protein. B201 antiserum was a gift from Elliot Androphy (Yao et al., 1998).
3.5 Packaging viral DNA
Early studies indicated that the E2 protein enhanced packaging of viral genomes in virion particles (Zhao et al., 2000). The E2 protein is not absolutely required for this process, but one can envisage several ways in which E2 could augment this process. For example, E2 interacts with the minor capsid protein L2 at ND10 bodies, an association that could be important both for genome establishment early in infection and enhancement of packaging late in infection (Day et al., 2004; Day et al., 1998). The precise role of E2 in these processes requires further investigation.
3.6 Post-transcriptional RNA Processing
There are several indications that E2 regulates processing of viral transcripts, though the exact mechanisms are not yet clear. E2 can promote late gene expression by depleting the polyadenylation complex, thus promoting read through of the early polyadenylation site and transcription of the late (Johansson et al., 2012). This finding correlates well with the increase in E2 expression during differentiation (see below). The E2 proteins of HPV5 and 16 can enhance the expression of, and interact with cellular SR splicing factors (Bodaghi et al., 2009; Lai et al., 1999; McPhillips et al., 2004) and Beta-PV E2 proteins localize to SR splicing speckles (Lai et al., 1999; Sekhar et al., 2010).
3.7 Growth inhibition and E2-mediated Apoptosis
Expression of E2 is growth inhibitory to both HPV positive and HPV negative cells. Only a few cell lines (CHO, CV1, C33-A, U2OS) are able to tolerate low level E2 expression of any PV type. The mechanism by which E2 induces growth arrest and senescence in HPV positive cells is well established: E2 represses the early viral promoter, thus down-regulating E6 and E7 expression. Most HPV positive cancer cells, such as HeLa, are dependent on the viral oncogenes for sustained growth (Desaintes et al., 1997; Dowhanick et al., 1995; Goodwin and DiMaio, 2000; Goodwin et al., 1998; Goodwin et al., 2000). The mechanism by which E2 induces apoptosis in HPV-negative cells is more controversial and involves both p53 dependent and independent pathways (Webster et al., 2000), and the interaction of E2 with caspase 8 (Demeret et al., 2003; Thierry and Demeret, 2008). It is still not clear what role E2-mediated apoptosis plays in the viral life-cycle but Bellanger et al. provide a thorough review of this topic (2011). Keratinocytes that are able to survive E2 expression develop phenotypes typical of terminally differentiated cells, suggesting that E2 may use this to promote viral late functions (Burns et al., 2010).
3.8 Oncogenicity of HPV8 E2 in Transgenic Mice
Expression of the HPV8 E2 protein, but not HPV11 E2, in the skin of transgenic mice results in the development of skin tumors (Leykauf et al., 2008; Pfefferle et al., 2008). Furthermore, the development of these tumors is accelerated by exposure to UV radiation. The mechanism by which this occurs is still not understood but several interesting hypotheses have been raised by McLaughlin and Munger (2008).
3.9 Regulation of Cellular Gene Expression
E2 has been described to regulate the expression of cellular genes in a few cases, but for the most part E2 has very little effect on cellular transcription (Johung et al., 2007). This is probably because while there are many E2 consensus sites in the host genome very few of them are actually bound by E2 (Vosa et al., 2012) Furthermore, E2 does not change the transcription of those that are bound (ibid.). A similar finding was made by Jang et al. who showed that E2 (through a protein-protein interaction with Brd4, not direct DNA binding) bound to most active promoters in the host genome but this did not actually change cellular gene expression (Jang et al., 2009). In contrast, a recent study showed that when HPV16 E2 was expressed from the CMV promoter in an adenovirus vector, hundreds of genes were upregulated or downregulated (Ramirez-Salazar et al., 2011).
However, there are detailed studies examining the role of E2 in regulating the expression of Matrix Metalloproteinase-9 (MMP-9) (Akgul et al., 2006; Behren et al., 2005; Gasparian et al., 2007; Muhlen et al., 2010), IL-10 (Bermudez-Morales et al., 2011), hTERT (Lee et al., 2002b), beta 4-integrin (Oldak et al., 2010; Oldak et al., 2004) and the splicing factor SF2/ASF (Mole et al., 2009).
4. Domain Structure of the E2 Proteins
4.1 Transactivation domain
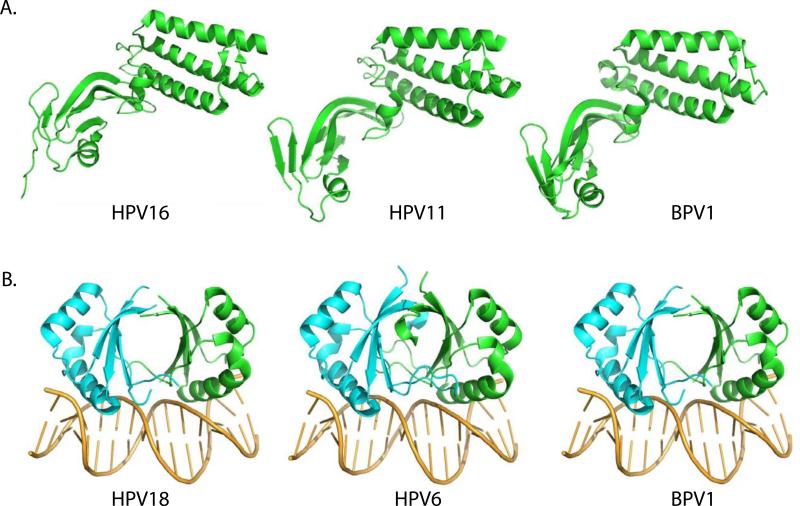
The amino-terminal 200 amino acids of the full-length E2 protein fold into a conserved structural domain. This crystal structure of this domain has been solved for BPV1, HPV11, HPV16 and HPV18 (Abbate et al., 2004; Antson et al., 2000; Harris and Botchan, 1999; Sanders et al., 2007; Wang et al., 2004). Figure 2A shows the structures of this domain for BPV1, HPV11 and HPV16. In all PVs, the transactivation domain forms cashew shaped structure. As described by Antson et al. for HPV16, the N-terminal half of the domain (N1: residues 1-91) is composed of three long alpha helices folded anti-parallel to each other in a bundle. The C-terminal half (N2: residues 110-201) is composed mostly of anti-parallel beta-sheets. The region between N1 and N2 (residues 93-109) has been designated the “fulcrum” and consists of a two-turn helix (Antson et al., 2000). The N-terminal domains of other E2 proteins form almost identical structures (Abbate et al., 2004; Harris and Botchan, 1999; Sanders et al., 2007; Wang et al., 2004). Residues within the E2 “transactivation” domain are important for both transcriptional activation and repression, and replication. Residues important for transcriptional regulation (eg R37 and I73) are found on the outside, convex face of the domain. Conversely, a residue important for replication and interaction with the E1 protein (E39) is found on the concave face of the structure.
Figure 2. Structures of the Transactivation and DNA Binding Domains.
A. Transactivation Domain
The transactivation domain structures for three E2 proteins are shown. Structures PDB: 2JEU (BPV1), PDB: 1R6K (HPV11) and PDB: 1DTO (HPV16) were rendered in Pymol.
B. DNA binding Domain
The DNA binding domain structures for three E2 proteins are shown. Structures PDB: 1JJ4 (HPV18), PDB: 2AYB (HPV6) and PDB: 2BOP (BPV1) were rendered in Pymol.
The HPV16 E2 N-terminal domain can self-interact and in the original structure was observed to dimerize through residues important for transcriptional regulation (R37 and I73) (Antson et al., 2000). This is consistent with several studies that show that the E2 proteins can loop DNA containing widely spaced E2 binding sites and this is mediated by the N-terminal domain (Hernandez-Ramon et al., 2008; Knight et al., 1991; Sim et al., 2008). The crystal structure of the BPV1 transactivation domain was also observed to be dimeric but in this case dimerization was mediated through a cysteine residue (C57) at the end of helix 2 (Sanders et al., 2007). Disulphide formation of residue C57 between two N-terminal domains promoted association of the two concave faces of the structure, sequestering the region required for E1 association (see below). This is the opposite face N-terminal domain found to mediate self-association in the HPV16 structure. A mutational analysis of the BPV1 dimerization interface determined that while it might contribute to replication activity, it was not essential (Gagnon et al., 2013). Lastly, the N-terminal domains of HPV11 E2 and the HPV18 E2 (in complex with E1) were observed to be monomeric (Abbate et al., 2004; Wang et al., 2004). Therefore, more studies are required to determine the significance of the self-association/looping function of the E2 proteins.
4.2 DNA binding and Dimerization
The E2 protein is a sequence specific DNA binding protein that binds to specific consensus motifs (ACCGN4CGGT or ACCN6GGT) located mainly in the URR (Androphy et al., 1987; Hawley-Nelson et al., 1988; Moskaluk and Bastia, 1987). A conserved C-terminal domain (approximately 85-100 amino acids) comprises the DNA binding domain (McBride et al., 1988; Moskaluk and Bastia, 1988). The E2 protein also forms very stable dimers through the DNA binding domain (Dostatni et al., 1988; McBride et al., 1989b; Moskaluk and Bastia, 1989). The structure of the E2 binding domain of BPV1 bound to its DNA target was solved in 1992 and was revealed to form a novel dimeric, eight-stranded, anti-parallel beta-barrel structure (Hegde et al., 1992). Two alpha helices on the surface of the barrel comprise the DNA recognition surface and amino acid residues on these helices are involved in specific base recognition. Since the structure of the BPV DNA binding domain was determined, there have been many additional structures solved for the homologous domains of HPV6, 16, 18 and 31 (Bussiere et al., 1998; Hegde and Androphy, 1998; Hooley et al., 2006; Kim et al., 2000; Nadra et al., 2004) and currently there are 15 different E2 DNA binding domain structures hosted on the PaVE website. Figure 2B shows the structure of the DNA binding domains of HPV18, HPV6 and BPV1 domains. The domains of different PV E2 proteins have a very similar structure overall, although there are differences in the relative orientation of the two subunits (reviewed in (Hegde, 2002).
It should be noted that most structures are of the C-terminal 81 to 87 residues of E2. However, the C-terminal 100 residues are well conserved and form a DNA binding domain that is more stable and binds with up to eight-fold increased affinity (McBride et al., 1988; Pepinsky et al., 1997). A structure of the C-terminal 101 residues of BPV1 E2 (PDB:1DBD) showed that the additional N-terminal sequences form a flap that covers a cavity in the dimer to augment stability and DNA binding (Veeraraghavan et al., 1998).
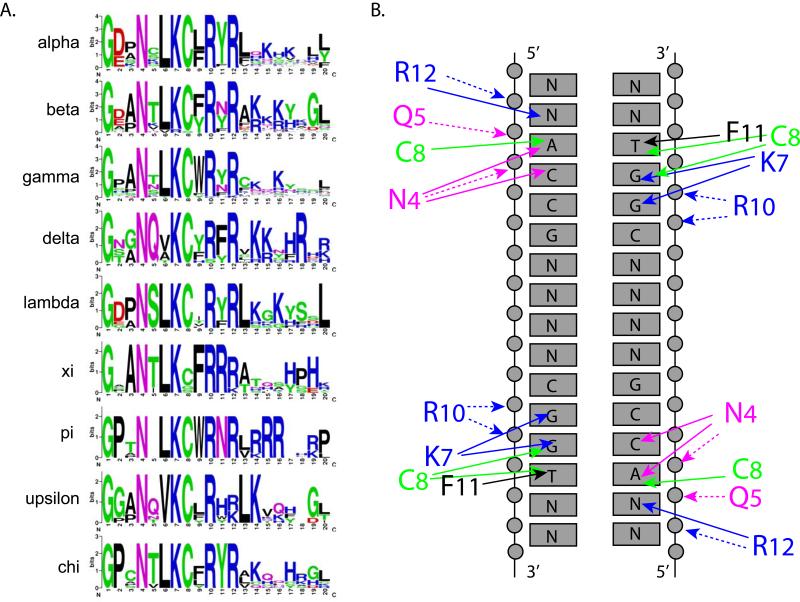
The E2 DNA binding site is bent smoothly around the protein domain to enable successive major grooves to interact with the recognition helices (Hegde et al., 1992). Figure 3A shows sequence conservation logos of the E2 recognition helices from nine different genera of papillomaviruses. There is remarkably high conservation in the specificity determining residues. In the Delta-PV BPV1 E2, the recognition helix residues N4 (BPV N336), K7 (K339), C8 (C340) and F11 (F343) make specific and base discriminatory contacts with nucleotides on each half of the target sequence. R12 (R344) is hydrogen bonded through a water molecule with a nucleotide just outside of the consensus. Figure 3B shows these specific contacts between residues in the recognition helix and the consensus DNA binding site. Hegde predicted that G1 (G333 in BPV) was essential for the sharp turn between beta strand 1 and the recognition helix and that N4 (N336 in BPV) was essential for “capping” the helix. Notably, these residues are absolutely conserved in all PVs sequenced to date. E2 binds to different sequence motifs with varying affinity (Li et al., 1989) but the DNA contacts are similar. Rather, binding affinity correlates with the flexibility of the DNA target sequence (Kim et al., 2000). Furthermore, the preference of different E2 proteins for flexible or non-flexible targets correlates with positive charge on the DNA interaction surface (Kim et al., 2000).
Figure 3. DNA Recognition helices of E2 Proteins from Different Genera.
A. Recognition Helix Sequence Logos
Sequence logos were created for the recognition helix of all genera of PVs with more than six members. The structure of the recognition helix shown at the top is derived from PDB: 2AYB HPV6 E2 bound to DNA (Hooley et al., 2006). Logos were created using Weblogo (http://weblogo.berkeley.edu/logo.cgi) (Crooks et al., 2004).
B. Contacts between the Recognition Helix and DNA Binding Site
This figure was adapted from (Hegde, 2002; Hegde et al., 1998). It shows the contacts between residues in the recognition helix of BPV1 E2 (with residue numbers converted to match those shown in A) and the consensus DNA binding site. Solid arrows signify contacts with specific bases. Dotted arrows represent contacts with the phosphate backbone.
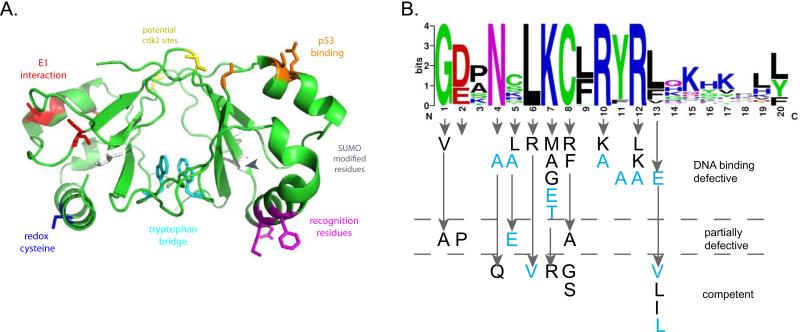
E2 forms a highly stable dimer (McBride et al., 1989b; Mok et al., 1996; Moskaluk and Bastia, 1989) and the first E2 structure revealed why (Hegde et al., 1992). The interface between monomers is extensive and dimerization buries between 1500 Å2 and 2500 Å2 of protein among the different E2 structures (reviewed in (Hegde, 2002). This results in a large hydrophobic beta-barrel containing numerous large and intricately packed side chains. The stable dimer forms upon translation and there is no subsequent mixing of the monomers without the use of strong denaturants (McBride et al., 1989b). The hydrophobic core of the beta-barrel contains a highly conserved tryptophan residue at position 360 (in BPV1) that has been named the tryptophan bridge (Corina et al., 1993; Prakash et al., 1992). The indole rings of the tryptophan from each subunit are in Van der Waals contact which allows them to be crosslinked by UV irradiation (Prakash et al., 1992). Mutated BPV1 E2 proteins containing substitutions of hydrophobic residues at this position are functional but substitution with polar residues disrupts dimerization (see Figure 11a).
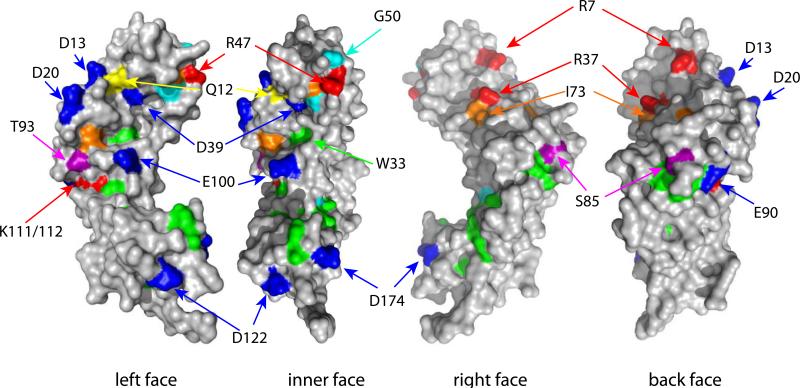
Figure 11. Landmarks and Mutations in the E2 DNA Binding Domain.
A. Key residues on the DNA Binding Domain
The BPV1 E2 DNA binding domain structure PDB: 1DBD was rendered in Pymol. Highlighted on the structure are regions of interest. In red is the region in BPV1 E2 that interacts with E1 when both proteins are bound to adjacent sites (Gillitzer et al., 2000). The same helix interacts with p53, and shown in orange on the other monomer are residues that when mutated (HPV16 D338, E340, W341, D344) reduce p53 binding (Brown et al., 2008). Also indicated are potential SUMO modification sites (grey) and cdk2 phosphorylation sites (yellow) (Johansson et al., 2009; Wu et al., 2008). In cyan are two tryptophan residues that are readily crosslinked in the dimer by UV radiation (Corina et al., 1993). These sites have been mapped in different E2 proteins and are shown on the BPV1 domain for illustrative purposes only.
B. Mutational analysis of the E2 Recognition Helix
The phenotype of the amino acid substitutions shown are divided into three categories according to their ability to specifically bind DNA. Mutations shown in black are from BPV1 (Carruth and McBride, 2001; Grossel et al., 1996; McBride et al., 1992; Prakash et al., 1992; Ustav et al., 1993). Mutations shown in light blue are from HPV11 (Matsumoto et al., 1997).
Remarkably, the E2 DNA binding domain has great structural similarity with that of the EBV EBNA1 protein, despite no sequence similarity (Bochkarev et al., 1995). The DNA binding domain of the KSHV LANA protein likely also has the same fold (Grundhoff and Ganem, 2003).
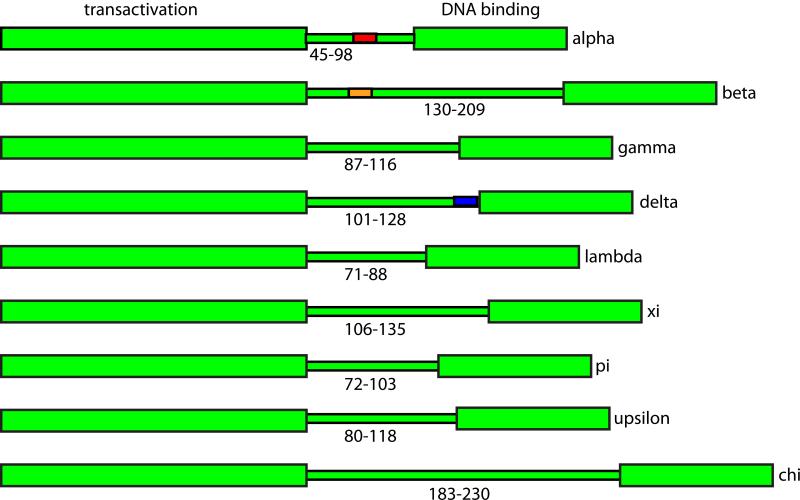
4.3 Hinge region
The hinge region of the E2 protein is encoded by a variable region of the PV genomes that overlaps the E4 ORF. It is not well conserved and is variable both in sequence composition and in length (Giri and Yaniv, 1988; McBride et al., 1989b). As shown in Figure 4, the length of the hinge is very variable between the E2s of different PV genera, but quite similar within each genus. Correspondingly, the length of the E4 proteins also varies considerably between genera. Narechania et al. have shown that purifying selection of the overlapping E4 ORF gives rise to a disproportionate amount of non-synonymous change in the E2 hinge (Narechania et al., 2005).
Figure 4. The Hinge Region.
The variable lengths of the hinge regions from nine genera of papillomaviruses are shown. Functional, conserved regions that have been mapped to the hinge region are shown: Alpha E2s, nuclear localization and nuclear matrix attachment (HPV11, in red); Beta E2s, chromatin attachment and PKA phosphorylation site (HPV8, orange); Delta E2s, conformational switch and CK2 phosphorylation site (BPV1 blue).
The E2 hinge is thought to be unstructured and to form a flexible link between the transactivation and DNA binding domains (Gauthier et al., 1991). Most E2 hinges are rich in proline, serine, threonine, glycine and arginine residues. The hinge is not required for the basic transcriptional and replication functions of E2, though some spacing is required to avoid steric hindrance between the domains (Winokur and McBride, 1992). Nevertheless, the E2 hinge regions from each genus of PVs encode auxiliary functions such as the determinants for intracellular localization, chromatin binding and protein stability. These functions are often regulated by phosphorylation. For example, a short sequence in the BPV1 hinge, next to the DNA binding domain, comprises a phosphorylation-dependent conformational switch (Garcia-Alai et al., 2006; McBride et al., 1989a; Penrose et al., 2004). Phosphorylation of this region by CK2 induces proteasomal degradation of the E2 and modulates genome copy number (McBride and Howley, 1991; Penrose and McBride, 2000). This sequence is conserved among Delta-PVs. In the Beta-PVs, a highly conserved RXXS motif in the E2 hinge is phosphorylated by PKA. This promotes binding to host chromatin and mitotic chromosomes and stabilizes the E2 protein (Sekhar and McBride, 2012; Sekhar et al., 2010). In the alpha-PVs, a highly conserved KRXR motif promotes nuclear localization and association of the E2 proteins with the nuclear matrix (Zou et al., 2000).The Beta-PV E2 hinges are rich in serine-arginine dipeptides and this promotes the localization of these proteins with cellular splicing speckles and their association with splicing proteins (Lai et al., 1999; Sekhar et al., 2010). The hinge region of many E2 proteins also interacts with cellular proteins (see Table 2).
Table 2. E2 Associated proteins with Functional or Biochemical Validation.
E2 associated proteins validated by functional or biochemical analyses
| Domains | HPV Type | Gene Symbol | Synonyms | UniProt | Protein Name | Reference |
|---|---|---|---|---|---|---|
| E2 | HPV11 16 18 | AR | Uniprot: P10275 | Androgen receptor | (Wu et al., 2007a) | |
| C | HPV18 | BRCA1 | Uniprot: P38398 | Breast cancer type 1 susceptibility protein | (Kim et al., 2003) | |
| N | BPV1 | BRD4 | Uniprot: O60885 | Bromodomain-containing protein 4 | (Baxter et al., 2005) | |
| E2 | BPV1 AaPV1 CPV1 SfPV1 OcPV1 OvPV1 HPV 1 4 11 16 31 57 | BRD4 | Uniprot: O60885 | Bromodomain-containing protein 4 | (McPhillips et al., 2006) | |
| N | HPV16 | BRD4 | Uniprot: O60885 | Bromodomain-containing protein 4 | (Schweiger et al., 2006) | |
| N | SfPV1 HPV 11 31 | BRD4 | Uniprot: O60885 | Bromodomain-containing protein 4 | (Senechal et al., 2007) | |
| E2 | BPV1 HPV16 | BRD4 | Uniprot: O60885 | Bromodomain-containing protein 4 | (Smith et al., 2010) | |
| NC | HPV5 | BRD4 | Uniprot: O60885 | Bromodomain-containing protein 4 | (Wang et al., 2009a) | |
| N | HPV11 | BRD4 | Uniprot: O60885 | Bromodomain-containing protein 4 | (Wu et al., 2006) | |
| N | BPV1 HPV16 | BRD4 | Uniprot: O60885 | Bromodomain-containing protein 4 | (You et al., 2004) | |
| H | HPV5 | C1QBP | Uniprot: Q07021 | Complement component 1 Q subcomponent-binding protein, mitochondrial | (Lai et al., 1999) | |
| N | HPV18 | CASP8 | FLICE | Uniprot: Q14790 | Caspase-8 | (Thierry and Demeret, 2008) |
| N | HPV16 18 | CDC20 | Uniprot: Q12834 | Cell division cycle protein 20 homolog | (Bellanger et al., 2005) | |
| C | BPV1 HPV8 16 18 | CEBPA | Uniprot: P49715 | CCAAT/enhancer-binding protein alpha | (Hadaschik et al., 2003) | |
| C | BPV1 HPV8 16 18 | CEBPB | Uniprot: P17676 | CCAAT/enhancer-binding protein alpha (C/EBP alpha) | (Hadaschik et al., 2003) | |
| E2 | HPV16 | CFLAR | cFLIP | Uniprot: O15519 | CASP8 and FADD-like apoptosis regulator | (Wang et al., 2011) |
| E8^E2 | HPV31 | CHD6 | Uniprot: Q8TD26 | Chromodomain-helicase-DNA-binding protein 6 | (Fertey et al., 2010) | |
| E2 | HPV 9 | CLTA | Uniprot: P09496 | Clathrin light chain A | (Muller et al., 2012) | |
| E2 | HPV16 | CPSF4 | CPSF30 | Uniprot: O95639 | Cleavage and polyadenylation specificity factor subunit 4 | (Johansson et al., 2012) |
| N | HPV18 | CREBBP | CBP | Uniprot: Q92793 | CREB-binding protein | (Lee et al., 2000) |
| E2 | BPV1 | CUL3 | Uniprot: Q13618 | Cullin-3 | (Zheng et al., 2009) | |
| N | BPV1 HPV 11 16 | DDX11 | CHLR1 | Uniprot: Q96FC9 | Probable ATP-dependent RNA helicase DDX11 | (Parish et al., 2006a) |
| N H C | BPV1 HPV 8 18 | EP300 | p300 | Uniprot: Q09472 | Histone acetyltransferase p300 | (Muller et al., 2002) |
| E2 | BPV1 | EP300 | p300 | Uniprot: Q09472 | Histone acetyltransferase p300 | (Peng et al., 2000) |
| E2 | BPV1 HPV16 | EP400 | P400 | Uniprot: Q96L91 | E1A-binding protein p400 | (Smith et al., 2010) |
| N | HPV16 8 | FZR1 | CDH1 | Uniprot: Q9UM11 | Fizzy-related protein homolog | (Bellanger et al., 2005) |
| N | BPV1 | GPS2 | AMF1 | Uniprot: Q13227 | G protein pathway suppressor 2 | (Breiding et al., 1997) |
| E2 | BPV1 | GPS2 | AMF-1 | Uniprot: Q13227 | G protein pathway suppressor 2 | (Peng et al., 2000) |
| E2 | HPV11 16 18 | GRIP1 | Uniprot: Q9Y3R0 | Glutamate receptor-interacting protein 1 | (Wu et al., 2007b) | |
| E2 | HPV 16 | GTF2B | Uniprot: Q00403 | Transcription initiation factor IIB | (Muller et al., 2012) | |
| E8^E2 | HPV31 | HDAC1 | Uniprot: Q13547 | Histone deacetylase 1 | (Ammermann et al., 2008) | |
| E8^E2 | HPV31 | HDAC2 | Uniprot: Q92769 | Histone deacetylase 2 | (Ammermann et al., 2008) | |
| E8^E2 | HPV31 | HDAC3 | Uniprot: O15379 | Histone deacetylase 3 | (Ammermann et al., 2008) | |
| E8^E2 | HPV31 | HDAC3 | Uniprot: O15379 | Histone deacetylase 3 | (Powell et al., 2010) | |
| NH C | HPV18 | KAT2B | PCAF | Uniprot: Q92831 | Histone acetyltransferase KAT2B | (Lee et al., 2002a) |
| E2 | BPV1 HPV16 | KDM5C | JARID1C, SMCX, | Uniprot: P41229 | Lysine-specific demethylase 5C | (Smith et al., 2010) |
| E2 | HPV1 3 5 6 8 9 16 18 | KIF20A | Uniprot: O95235 | Kinesin-like protein KIF20A | (Muller et al., 2012) | |
| N | BPV1 HPV16 11 | KIF20A | Uniprot: O95235 | Kinesin-like protein KIF20A | (Yu et al., 2007) | |
| E2 | BPV1 HPV11 16 | KPNA3 | Uniprot: O00505 | Importin subunit alpha-3 | (Bian and Wilson, 2010) | |
| E2 | BPV1 HPV11 16 | KPNA5 | Uniprot: O15131 | Importin subunit alpha-6 | (Bian and Wilson, 2010) | |
| C | HPV16 | MDM2 | Uniprot: Q00987 | E3 ubiquitin-protein ligase Mdm2 | (Gammoh et al., 2009) | |
| N | BPV1 HPV8 18 | NAP1L1 | NAP1 | Uniprot: P55209 | Nucleosome assembly protein 1-like 1 | (Rehtanz et al., 2004) |
| E8^E2 | HPV31 | NcoR | Uniprot: O75376 | Nuclear receptor corepressor 1 | (Powell et al., 2010) | |
| N | HPV16 | NRIP | Uniprot: P48552 | Nuclear receptor-interacting protein 1 | (Chang et al., 2012) | |
| C | HPV18 | PARP1 | Uniprot: P09874 | Poly [ADP-ribose] polymerase 1 | (Lee et al., 2002b) | |
| E2 | HPV11 16 18 | PLAL1 | ZacI | Uniprot: Q9UM63 | Zinc finger protein PLAGL1 | (Wu et al., 2007b) |
| N | HPV5 | PLK1 | Uniprot: P53350 | Serine/threonine-protein kinase PLK1 | (Wang et al., 2009a) | |
| E2 | BPV1 | RPA1 | RPA-70 | Uniprot: P27694 | Replication protein A 70 kDa DNA-binding subunit | (Li and Botchan, 1993) |
| E8^E2 | HPV31 | SETDB1 | Uniprot: Q15047 | Histone-lysine N-methyltransferase SETDB1 | (Ammermann et al., 2008) | |
| H | HPV5 | SFRS1 | ASF, SF2, SFRS1 | Uniprot: Q07955 | Serine/arginine-rich splicing factor 1 | (Lai et al., 1999) |
| H | HPV5 | SFRS2 | SC35 | Uniprot: Q01130 | Serine/arginine-rich splicing factor 2 | (Lai et al., 1999) |
| H | HPV5 | SFRS7 | Uniprot: Q16629 | Serine/arginine-rich splicing factor 7 | (Lai et al., 1999) | |
| N | HPV18 | SKP2 | Uniprot: Q13309 | S-phase kinase-associated protein 2 | (Bellanger et al., 2010) | |
| N | BPV1 | SMARCA 2 | BRM | Uniprot: P51531 | Probable global transcription activator SNF2L2 | (Kumar et al., 2007) |
| E2 | HPV 18 | SMARCA 4 | Brg1 | Uniprot: P51532 | Transcription activator BRG1 | (Cha and Seo, 2011) |
| N C | HPV6 11 16 18 | SMARCB 1 | hSNF5 | Uniprot: Q12824 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 | (Cha and Seo, 2011) |
| N | HPV11 | SMC5 | Uniprot: Q8IY18 | Structural maintenance of chromosomes protein 5 | (Wu et al., 2006) | |
| N | HPV11 | SMC6 | Uniprot: Q96SB8 | Structural maintenance of chromosomes protein 6 | (Wu et al., 2006) | |
| N | BPV1 HPV11 16 18 | SMN1 | Uniprot: Q16637 | Survival motor neuron protein | (Strasswimmer et al., 1999) | |
| H | HPV5 | SNRNP70 | Uniprot: P08621 | U1 small nuclear ribonucleoprotein 70 kDa | (Lai et al., 1999) | |
| N C | BPV1 HPV18 | SP1 | Uniprot: P08047 | Transcription factor Sp1 | (Steger et al., 2002) | |
| H C | HPV18 8 | SP1 | Uniprot: P08047 | Transcription factor Sp1 | (Steger et al., 2002) | |
| C | HPV16 | SRSF1 | ASF SF2 | Uniprot: Q07955 | Serine/arginine-rich splicing factor 1 | (Bodaghi et al., 2009) |
| C | HPV16 | SRSF4 | Uniprot: Q08170 | Serine/arginine-rich splicing factor 4 | (Bodaghi et al., 2009) | |
| C | HPV16 | SRSF5 | Uniprot: Q13243 | Serine/arginine-rich splicing factor 5 | (Bodaghi et al., 2009) | |
| N C | BPV1 HPV18 | TAF1 | TAFII250 | Uniprot: P21675 | Transcription initiation factor TFIID subunit 1 | (Carrillo et al., 2004) |
| N C | HPV16 | TAF1 | TAFII250 | Uniprot: P21675 | Transcription initiation factor TFIID subunit 1 | (Centeno et al., 2008) |
| N C | BPV1 HPV18 | TAF6 | TAFII80 | Uniprot: P49848 | Transcription initiation factor TFIID subunit 6 | (Carrillo et al., 2004) |
| H C | HPV 8 | TAF7 | TAFII55 | Uniprot: Q15545 | Transcription initiation factor TFIID subunit 7 | (Enzenauer et al., 1998) |
| N | BPV1 HPV16 18 | TAX1BP1 | Uniprot: Q86VP1 | Tax1-binding protein 1 | (Wang et al., 2009b) | |
| N C | BPV1 HPV18 | TBP | Uniprot: P20226 | TATA-box-binding protein | (Carrillo et al., 2004) | |
| C | HPV 8 | TBP | Uniprot: P20226 | TATA-box-binding protein | (Enzenauer et al., 1998) | |
| E2 | HPV11 | TBP | Uniprot: P20226 | TATA-box-binding protein | (Hartley and Alexander, 2002) | |
| C | BPV1 | TBP | Uniprot: P20226 | TATA-box-binding protein | (Rank and Lambert, 1995) | |
| N | HPV16 | TFIIB | Uniprot: Q00403 | Transcription initiation factor IIB | (Benson et al., 1997) | |
| C | BPV1 | TFIIB | Uniprot: Q00403 | Transcription initiation factor IIB | (Rank and Lambert, 1995) | |
| N | BPV1 | TFIIB | Uniprot: Q00403 | Transcription initiation factor IIB | (Yao et al., 1998) | |
| H | HPV5 | TNPO3 | Uniprot: Q9Y5L0 | Transportin-3 | (Lai et al., 1999) | |
| E2 | HPV11 | TOP1 | TopoI | Uniprot: P11387 | DNA topoisomerase 1 | (Clower et al., 2006) |
| N | HPV16 | TOPBP1 | Uniprot: Q92547 | DNA topoisomerase 2-binding protein 1 | (Boner et al., 2002) | |
| C | HPV16 | TP53 | p53 | Uniprot: P04637 | Cellular tumor antigen p53 | (Massimi et al., 1999) |
| C | HPV 6 11 16 | TP53 | p53 | Uniprot: P04637 | Cellular tumor antigen p53 | (Parish et al., 2006b) |
| H | HPV5 | TRA2B | Uniprot: P62995 | Transformer-2 protein homolog beta | (Lai et al., 1999) | |
| E8^E2 | HPV31 | TRIM28 | Uniprot: Q13263 | Transcription intermediary factor 1-beta | (Ammermann et al., 2008) | |
| E2 | BPV1 HPV16 | UBE2I | UBC9 | Uniprot: P63279 | SUMO-conjugating enzyme UBC9 | (Wu et al., 2008) |
| E2 | HPV 1 3 5 8 9 11 18 | VPS39 | Uniprot: Q96JC1 | Vam6/Vps39-like protein | (Muller et al., 2012) |
4.4 E8^E2
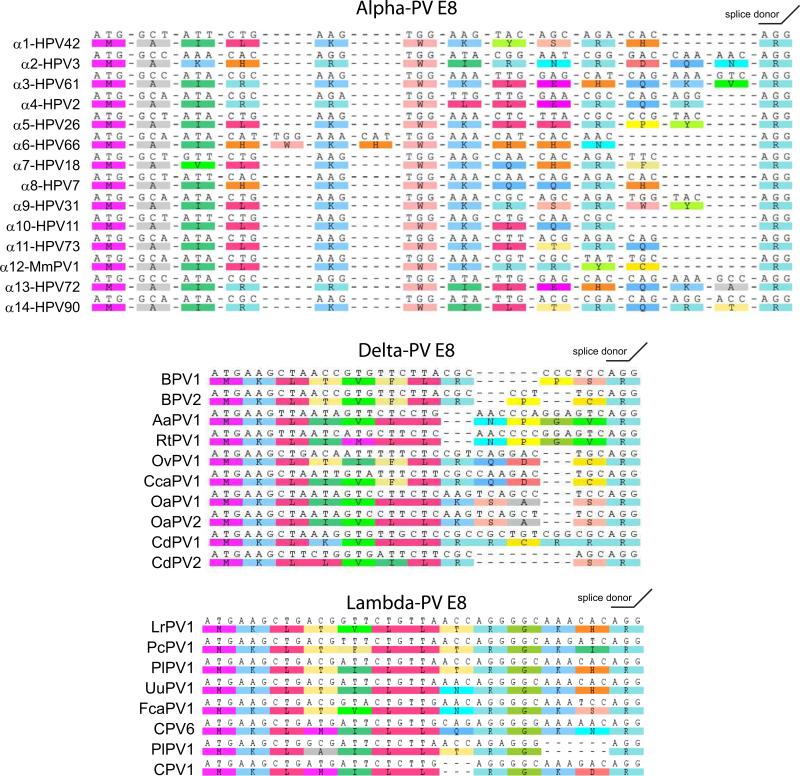
All PVs have the capacity to encode an E8^E2 repressor protein consisting of a short E8 peptide fused to the entire hinge and DNA binding domains of E2. The E8^E2 proteins are powerful repressors of viral transcription and replication (Jeckel et al., 2003; Stubenrauch et al., 2007; Stubenrauch et al., 2001; Zobel et al., 2003). The E8 moiety is just 11-13 amino acids, yet it has powerful repressor properties. In the Alpha-E2 proteins, a KWK motif is important for the repressor function (Stubenrauch et al., 2001; Zobel et al., 2003). Sequence alignments of a selection of Alpha-PV, and all Lambda and Delta-PV E8 domains are shown in Figure 5. Each E8 domain is rich in basic and hydrophobic residues. Table 2 contains cellular proteins that associate with E8^E2.
Figure 5. The E8^E2 Protein.
The E8 moieties from a series of Alpha PVs, all Delta PVs and all Lambda PVs are shown. Sequences were extracted from the PaVE database and aligned using the ClustalW module of Geneious v6 created by Biomatters. Available from http://www.geneious.com/.
5 E2 Binding Sites in Papillomavirus Genomes
5.1 The consensus E2 binding site
The consensus E2 DNA binding motif was first determined for BPV1 E2 using a DNA protein immunoprecipitation assay (Androphy et al., 1987; Hawley-Nelson et al., 1988). The consensus is ACC(N)6GGT, or more stringently ACCG(N)4CGGT. Further studies using DNase I footprinting and mobility shift assays showed that there are actually 17 E2 binding sites in the BPV1 genome, only 12 of which match the consensus. The non-consensus sequences are AACN6GGT or AACN6GTT (Li et al., 1989). Furthermore, the E2 protein binds to these 17 sites with affinities that ranges over 300 fold (Li et al., 1989). The number of spacer (N) nucleotides is always invariant, but Alpha-E2 proteins bind more readily to sites with an AT rich spacer, while there is no apparent preference for BPV1 E2 (Hegde, 2002).
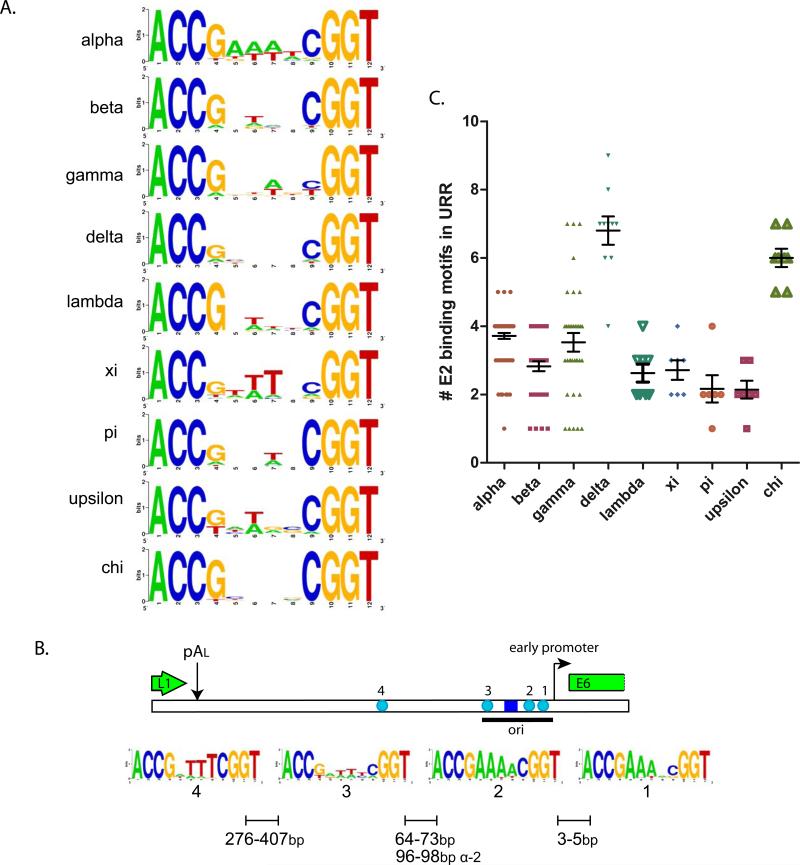
Figure 6A shows sequence conservation logos for the E2 binding consensus sequences (ACC(N)6GGT) from nine different genera of papillomaviruses. The predilection for the complete ACCG(N)4CGGT is apparent in all PVs, and the previously described preference of the alpha-PVs for an A/T rich spacer region is clear.
Figure 6. E2 Binding Sites from Nine Genera of PVs.
A. E2 Binding Site Sequence Logos
E2 binding sites conforming to ACCN6GGT were extracted from the URR sequences in the PaVE database from genera of PVs with more than six members. This amounted to 281 Alpha, 130 Beta, 127 Gamma, 68 Delta, 21 Lambda, 19 Xi, 13 Pi, 15 Epsilon and 48 Chi E2 consensus motifs. Sequence logos were created for the recognition helix of sites from each genera using Weblogo (http://weblogo.berkeley.edu/logo.cgi) (Crooks et al., 2004).
B. E2 Binding Site Sequence Logos for Individual Alpha E2 Sites
Individual E2 binding sites sequences conforming to ACCN6GGT and corresponding to the positions shown (#1, 2, 3, 4) were extracted from the 54 alpha URR sequences in the PaVE database that have the traditional four sites shown. URRs containing non-consensus E2 binding sites were not included. Sequence logos were created for the E2 site at each position using Weblogo (http://weblogo.berkeley.edu/logo.cgi) (Crooks et al., 2004).
C. Number of E2 Binding Sites in the URRs of PV types in Different Genera
The number of consensus (ACCN6GGT) E2 binding sites in the URR of PV types from genera of PVs with more than six members.
The alpha-PV genomes most often contain four E2 binding sites located at specific positions within the URR. Figure 6B shows the position and sequence conservation of the individual binding sites. Binding sites 1 and 2 are located in the early promoter region. The origin (and E1 binding site) is located just upstream from the promoter and the binding sites flanking the E1 binding site are required for optimal origin activity. Binding site 4 is located about 300-400 nucleotides upstream.
When the conservation of the spacer nucleotides is considered individually for each binding site, it becomes clear that Alpha-PV E2 binding sites 1 and 2 have primarily A nucleotides on the sense strand of the genome while T is preferred for binding site 4. There does not seem to be strong preference for binding site 3. This implies that the orientation of each binding site might be important for function. In several cases the E2 binding sites overlap with the binding site for a cellular factor and this could influence the sequence of individual E2 sites independent from E2 binding (Jackson and Campo, 1995; May et al., 1994; May et al., 1991; Schmidt et al., 1997; Stenlund and Botchan, 1990; Vande Pol and Howley, 1990). More extensive analyses of E2 binding sites can be found in studies by Sanchez et al. and Rogers et al (Rogers et al., 2011; Sanchez et al., 2007).
Notably, most E2 binding sites contain at least one CpG dinucleotide. Methylation of the cytosine in CpG motifs is one of the major epigenetic modifications in mammalian cells and strongly correlates with transcriptional repression. Methylation of the E2 binding site disrupts E2 binding and the ability of E2 to regulate transcription (Kim et al., 2003; Thain et al., 1996). Viral DNA is methylated in clinical isolates and methylation of E2 sites that mediate repression of the early promoter could promote oncogenesis (Kalantari et al., 2004; Kalantari et al., 2008). In general, viral DNA that is actively replicating and transcribing (especially during differentiation) is unmethylated, and integrated DNA or untranscribed regions (such as the late region in cancers) are methylated (Turan et al., 2006). However, there is some controversy about the differential methylation of individual E2 binding sites in different viral states (reviewed in (Chaiwongkot et al., 2013)). It is clear that there is evolutionary pressure to maintain a CpG dinucleotide within an E2 binding site, even although this gives the host cell the ability to regulate viral functions. The presence of the CpG dinucleotide must be advantageous to the viral life cycle for the virus to relinquish this control. For example, DNA methylation is regulated during keratinocyte differentiation and is important for nucleosome positioning and destabilization (reviewed in (Chatterjee and Vinson, 2012), which could add another level of regulation to HPV gene expression.
5.2 Distribution of E2 sites in the URR of different Papillomavirus Genera
The genomes of some papillomaviruses viruses contain only a few E2 binding motifs, while others have many more (see Figure 6C). The number and position of the sites are relatively well conserved among viruses from the same genera. Figure 7 shows the distribution of E2 binding sites in the URR of a selection of papillomaviruses whose genus contains at least six types. For example, most Alpha-PVs contain four consensus E2 binding sites with conserved spacing, as shown in Figure 6B. These sites are important for transcriptional activation and repression, initiation of replication and for viral genome partitioning and maintenance.
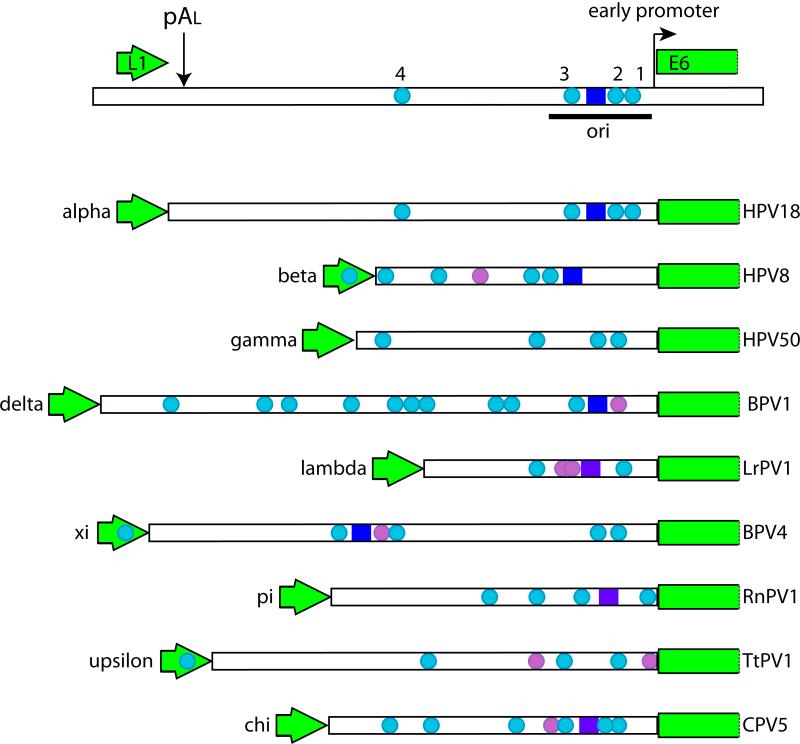
Figure 7. Map of the E1 and E2 binding sites in the URR of Papillomaviruses from several genera.
The URRS of PVs selected from nine different genera are shown. The ends of the L1 and E6 ORFs are indicated. The E1 binding site, when mapped, is indicated by a blue box, while putative sites are indicated by purple boxes. E2 binding sites that match the consensus ACCN6GGT are shown as cyan circles. E2 sites that deviate from this consensus are shown as light purple circles.
The origin of replication is usually located at the 3’ end of the URR and consists of an E1 binding site, an AT rich region and one or more E2 binding sites (Mohr et al., 1990; Ustav et al., 1993; Ustav et al., 1991). E1 binds to an 18bp palindrome that contains multiple overlapping hexanucleotide E1 recognition sequences (Auster and Joshua Tor, 2004), but this sequence is not highly conserved in all PVs (reviewed in (McBride, 2008). E2 binds to adjacent sites to assist in loading the E1 helicase onto the origin (Mohr et al., 1990). E1 and E2 bind cooperatively as dimers to adjacent sites in the origin but in the presence of ATP, E1 converts to a hexamer and E2 is displaced (Sanders and Stenlund, 1998). E2 can load E1 to the origin when the E1 and E2 sites are proximal, but also from a distance. In the latter case, E2 displacement is not required (Sanders and Stenlund, 2001).
Seventeen E2 binding sites have been mapped in the Delta-PV BPV1 and 11 of these are located in the URR (Li et al., 1989). At least eight of these URR E2 binding sites are required for genome maintenance and these have been mapped to an element called the Minichromosome Maintenance Element or MME (Piirsoo et al., 1996). Many papillomaviruses do not have eight E2 binding sites and so there must be differences in the requirements for genome maintenance between different viral types. It is difficult to interpret the phenotype of viruses with mutations in the E2 binding sites in the background of the entire viral genome, because E2 is important for so many key processes in the viral life cycle. However, while the specific arrangement of E2 binding sites is important, at least one site (HPV31 site #3) is dispensable for stable genome maintenance (Stubenrauch et al., 1998b).
Although BPV1 has 17 E2 binding sites, only 12 match the ACCN6GGT consensus. Therefore, it is likely that other PVs contain additional E2 binding sites. Alignment of the URRs from viruses within a specific genus shows that there is very likely to be additional conserved, non-consensus E2 binding sites. Furthermore, many viruses have a few E2 binding sites in the coding regions of the genome, although most are located in the URR. Further studies are required to determine why some viruses have so few apparent E2 binding sites while others have many more and whether the E2 binding sites in the coding regions have any significance. Figure 6C shows the numbers of consensus E2 binding sites found in the URR of nine different genera of PVs.
6 E2 Protein Post-Translational Modifications
6.1 Phosphorylation
The E2 proteins from BPV1, HPV8, HPV16 and SfPV1 have been shown to be phosphorylated and it is likely that all E2 proteins share this modification (Barbosa and Wettstein, 1988; Chang et al., 2011; Johansson et al., 2009; Lehman et al., 1997; McBride et al., 1989a; Sanders et al., 1995; Sekhar and McBride, 2012; Sekhar et al., 2010).
BPV1 E2 contains phosphoserine, plus a small amount of phosphothreonine (McBride et al., 1989a). The major phosphorylation sites in vivo have been mapped by biochemical and genetic analyses to serine residues 298 and 301 (McBride et al., 1989a). An additional minor phosphorylation site was detected at E2 residue 235 by mass spectroscopy (Lehman et al., 1997). It should be noted that in the cloned BPV genome this E2 residue is a serine but in cloned E2 cDNAs (Baker et al., 1987) prepared from wart RNA it is an alanine, indicating that it may be a variant residue (AMcB, unpublished data). The two major phosphorylation sites (298 and 301) are located in the hinge region of E2, just outside the DNA binding domain and they are conserved in all Delta-PV E2 proteins. Phosphorylation of these residues by CK2, induces the proteasomal degradation of E2 and so phosphorylation regulates E2 stability and viral genome copy number (McBride and Howley, 1991; Penrose et al., 2004; Penrose and McBride, 2000).
In HPV16, a putative threonine phosphorylation site at amino acid 286 can be phosphorylated in vitro by Cdk2 (Johansson et al., 2009). This phosphorylation site is conserved in 36 of the 76 Alpha-PVs currently in the PaVE database and examination of this residue in the homologous HPV31 structure 1DHM shows that this residue is on the surface of the DNA binding domain, where the hinge meets the C-terminal domain (see Figure 11A). This is in a similar position to the BPV phosphorylation sites (see Figure 4).
The hinge of the HPV8 E2 protein is highly phosphorylated in vivo (Sekhar and McBride, 2012; Sekhar et al., 2010). Much of this phosphorylation probably occurs on the many SR motifs located in the hinge. Furthermore, we find that the SR protein kinases, SRPK1 and 2, are associated with HPV8 E2 (Jang and McBride, 2013). The hinge of HPV E2 contains a short peptide that mediates tethering to host chromatin and mitotic chromosomes (Sekhar et al., 2010). This peptide contains an RXXS motif (residues 250-253) that is conserved in all Beta-PV E2 proteins. Serine 253 is phosphorylated by PKA in S-phase and this stabilizes E2 and promotes binding of E2 to host chromatin (Sekhar and McBride, 2012).
6.2 Acetylation
Diverse E2 proteins (BPV1, HPV8 and HPV16) interact with the cellular lysine acetyl transferases, p300, CBP and pCAF and this enhances E2-mediated transcriptional activation (Kruppel et al., 2008; Lee et al., 2002a; Lee et al., 2000; Muller et al., 2002; Peng et al., 2000). Quinlan et al. have shown that p300 can acetylate BPV1 E2 in vitro on two highly conserved lysine residues (Quinlan et al., 2013) . These residues (K111 and 112) are highly conserved in all PV E2 proteins and every PV E2 protein has at least one lysine in this position. Lysines 111 and 112 are located in the fulcrum of the transactivation domain, in a region previously shown to modulate nuclear localization of E2 (Abroi et al., 1996; Skiadopoulos and McBride, 1996). Quinlan et al. confirm that these residues are required for nuclear retention and show that p300 and CBP promote nuclear localization of wildtype E2 (Quinlan et al., 2013).
6.3 Sumoylation
The SUMO conjugation enzyme, Ubc9, binds to and sumoylates the BPV1 and HPV16 E2 in vitro (Wu et al., 2008). The consensus motif for sumoylation is the lysine in the consensus motif ΨKx(E/D), where Ψ is a bulky hydrophobic residue. The SUMOylation site in HPV16 E2 is K292, which is just one residue before the highly conserved glycine that makes the turn into the DNA recognition helix (see Figure 11A). This residue is conserved in 46 out of the 76 Alpha-PV E2 proteins currently in the PaVE database. Substitution of K292 with an arginine does not affect the in vitro DNA binding activity of E2 but it does reduce E2-mediated transcriptional regulation (Wu et al., 2008). Increased sumoylation stabilizes E2 proteins from HPV11, HPV16 and HPV18 and this correlates well with the concomitant increase in sumoylation (Deyrieux et al., 2007; Wu et al., 2009) and E2 protein levels that occurs in keratinocyte differentiation (see Section 7.1 and Figure 8).
6.4 Redox regulation
The DNA recognition motif of all E2 proteins contains a highly conserved cysteine residue that makes three direct contacts with each half of the recognition site (see Figure 3A, 3B 11A and B). The basic environment surrounding the cysteine residue makes it highly susceptible to oxidation, most likely to sulfenic acid (McBride et al., 1992). The epithelium is under a redox gradient, becoming more oxidizing at the superficial layers (Korkina and Pastore, 2009), and this is important for the maturation of HPV virions (Conway et al., 2009). Thus, E2 DNA binding could be modulated by the cellular environment during differentiation. Mutation of this cysteine has modest effects on DNA binding (depending on the substitution) and replication, but eliminates E2's transcriptional functions (Grossel et al., 1996; McBride et al., 1992).
7 Localization
7.1 Intracellular localization
Both full-length and shorter E2 proteins localize primarily to the nucleus (Hubbert et al., 1988), where they are observed in a nucleolar excluded pattern (Burnett et al., 1990; Sekine et al., 1989; Skiadopoulos and McBride, 1996; Zou et al., 2000). However, there is one report on nuclear-cytoplasmic shuttling of high risk E2 proteins (Blachon et al., 2005). Nuclear localization signals (NLS) have been mapped in BPV1, HPV11 and HPV16 (Klucevsek et al., 2007; Skiadopoulos and McBride, 1996; Zou et al., 2000) and alpha importins 3 and 5 bind specifically to these proteins (Bian and Wilson, 2010). In BPV1 there are two potential NLSs. One is the recognition helix in the DNA binding domain and this fits the criteria of a classical NLS in that it is required for nuclear localization and is transplantable to another protein (Skiadopoulos and McBride, 1996). In vitro studies confirm that this region is also the NLS for HPV16 E2 (Klucevsek et al., 2007). The second putative BPV1 NLS, PKRCFKKGARV, is in the fulcrum of the transactivation domain and contains two highly conserved lysine residues (111, 112). Proteins deleted of residues 101-110, or 111-120 are retained in the cytoplasm, but this sequence is not transplantable (Skiadopoulos and McBride, 1996). The substitutions K111A or K112A result in a cytoplasmic E2 protein (Abroi et al., 1996). It is possible that this region is structurally sensitive and that deletions here result in an unfolded protein that cannot be transported to the nucleus. In support of this, E2 with a K111R substitution is temperature sensitive for transcription, replication and chromosome binding (Zheng et al., 2005). Further studies showed that K111 and K112 are acetylated and are important for nuclear retention of BPV1 E2 (Quinlan et al., 2013). In HPV11 a conserved patch of basic residues in the hinge region (238-242) is important for both nuclear and nuclear matrix localization (Zou et al., 2000).
The beta-PV E2 proteins have an additional sub-nuclear localization to the SR-rich splicing speckles (Lai et al., 1999; Sekhar et al., 2010). This interaction is mediated by SR rich sequences in the hinge region of the Beta-E2 proteins. When HPV16 or HPV18 E2 are co-expressed with E6 and E6*I proteins, E2 and E6 are found localized to SR rich splicing speckles (Grm et al., 2005). In the absence of E6*I, E2 and E6 colocalize at ND10 domains (Grm et al., 2005).
As discussed above, E2 proteins also bind tightly to host chromatin (Donaldson et al., 2007; Jang et al., 2009; Johansson et al., 2009; Kurg et al., 2005; McPhillips et al., 2005; Sekhar and McBride, 2012), the nuclear matrix (Zou et al., 2000) and mitotic chromosomes (Ilves et al., 1999; Lehman and Botchan, 1998; Oliveira et al., 2006; Skiadopoulos and McBride, 1998).
In the presence of the E1 protein, E1 and E2 form nuclear foci that represent viral replication factories (Fradet-Turcotte et al., 2011; Reinson et al., 2013; Sakakibara et al., 2011; Swindle et al., 1999). In the presence of the L2 minor capsid protein, the E2 protein is recruited to ND10 domains in the nucleus and this is thought to be important for establishment of viral infection (Day et al., 2004; Day et al., 1998). However, ND10 domains are also found adjacent to HPV replication foci (Swindle et al., 1999) implying additional roles of these nuclear bodies in the HPV lifecycle.
7.2 Tissue Expression
The E2 proteins are easily detected in BPV1 infected wart tissue. In this tissue, E2 can be detected at low levels along the stratum basale and then at very high levels in a subset of cells in the stratum spinosum. These cells are also vegetatively amplifying the viral genome, indicating that E2 has an important role at this stage of infection (Burnett et al., 1990; Penrose and McBride, 2000). An example of BPV1 E2 expression in bovine wart tissue is shown in Figure 8. The antibodies used in these studies detect both full-length and repressor forms of E2, so it is difficult to conclude which forms of E2 are present in each cell layer. An antibody against HPV6 E2 stained the nuclei in the middle and upper layers of condyloma acuminata and laryngeal papillomas (Sekine et al., 1989). Antibodies to HPV16 E2 have also detected E2 expression in clinical tissue (Maitland et al., 1998; Xue et al., 2010). These studies found that E2 was expressed in the intermediate and upper layers of cervical CIN and koilocytic lesions. These studies all imply a role for E2 in late stages of infection.
9 E2 Stability and Degradation
The E2 proteins have a short half-life and this is regulated by multiple factors. Full length BPV1 E2 has a half-life of 40 minutes and the shorter repressor proteins E2-TR and E8^E2 have half-lives of 10 minutes and 15 minutes, respectively (Hubbert et al., 1988). The N-terminal domain is a target for proteasomal degradation (Bellanger et al., 2001) and many E2 proteins can be stabilized by association of this domain with a cellular protein. For example, binding of Brd4 to the N-terminal domain of many E2 proteins results in E2 stabilization (Gagnon et al., 2009; Lee and Chiang, 2009; Zheng et al., 2009). Brd4 prevents association of the E3 ubiquitin ligase, cullin-3, with E2 (Zheng et al., 2009). The transactivation domain of HPV18 also binds the Skp1/Cullin1/F-box Skp2 (SCF(Skp2)) ubiquitin ligase in a Brd4-independent manner resulting in the specific degradation of E2 at the end of the G1 phase of the cell cycle (Bellanger et al., 2010). Tax1BP1 also binds to the N-terminus of E2, preventing its proteasomal degradation (Wang et al., 2009).
Interaction of E2 with other viral proteins can also affect stability. The well-studied interaction between E2 and the E1 replication protein can increase E2 stability (King et al., 2011), as can the interaction of E2 with the late protein E1^E4 (Davy et al., 2009).
The stability of E2 proteins can be modulated by phosphorylation. BPV1 E2 is phosphorylated by CK2 on residue 301 and this promotes a conformational switch and proteasomal degradation (McBride et al., 1989a; Penrose et al., 2004; Penrose and McBride, 2000). HPV16 E2 binds the nuclear receptor interaction protein (NRIP), which recruits calcium and calmodulin thus activating the phosphatase calcineurin to dephosphorylate E2, decrease E2 ubiquitination and increase E2 protein stability (Chang et al., 2011). HPV8 E2 is phosphorylated on residue 253 by PKA and this promotes binding to host chromatin and increased protein stability (Sekhar and McBride, 2012). Both HPV8 and HPV16 E2 become phosphorylated and stabilized in S-phase (Johansson et al., 2009; Sekhar and McBride, 2012).
UVB irradiation decreases E2 stability (Taylor et al., 2003) and sumoylation stabilizes E2, though not through direct sumoylation of E2 (Wu et al., 2009) Notably, sumoylation increases with keratinocyte differentiation and could contribute to E2 stabilization at later stages of infection (Deyrieux et al., 2007).
8 E2 Associated Proteins
8.1 Cellular Proteins
E2 is a multifunctional protein that operates by binding to the viral genome and recruiting a multitude of cellular proteins required for each function of E2. Table 2 lists E2 associated proteins that have been validated by either functional or biochemical means. These proteins fall into several categories that include transcriptional regulation, RNA processing, apoptosis, cell cycle, nuclear import and protein degradation (see Figure 9). A discussion on the role of each of these protein interactions is beyond the scope of this chapter and readers are directed to comprehensive reviews on the E2-host protein network (Muller and Demeret, 2012), the role of E2 in tumorigenesis (Bellanger et al., 2011), in viral DNA replication (McBride, 2008), in RNA processing (Johansson and Schwartz, 2013), in genome tethering (McBride et al., 2012) and the role of Brd4 in PV infection (McBride and Jang, 2013). Supplementary Table 1 lists many more E2 associated proteins that have been identified by yeast two hybrid or other screens but have not yet been thoroughly validated.
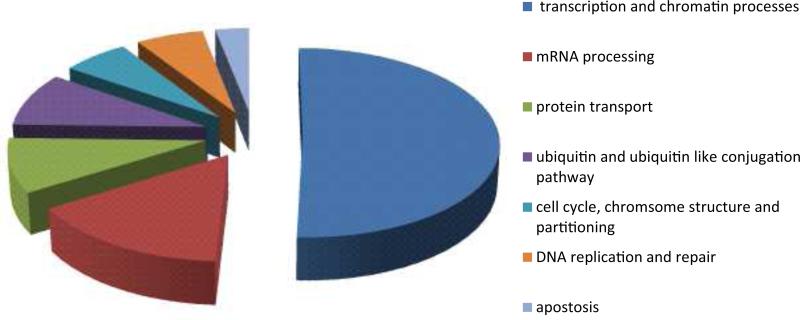
Figure 9. Functional Categories of E2 Associated Proteins.
The E2 associated proteins shown in Table 2 were categorized according to Gene Ontology functions using the DAVID v6.7 bioinformatics resource (Vempati, 2012) with some manual curation.
8.2 Viral Proteins
E2 has been shown to bind to several other PV proteins. Best characterized is the interaction between E1 and E2. E1 is the replication initiation helicase and E2 is the helicase loader (Frattini and Laimins, 1994; Mohr et al., 1990). After loading, E2 is displaced from the origin and E1 forms a hexameric helicase that encircles the origin (Sanders and Stenlund, 1998; Schuck and Stenlund, 2005; Sedman and Stenlund, 1998; Titolo et al., 2000). E1 contains both a specific DNA binding domain that contacts the E1 binding sequence in the origin (Chen and Stenlund, 1998; Sarafi and McBride, 1995) and a non-specific DNA binding domain in the helicase region that contacts the flanking A/T rich sequences (Schuck and Stenlund, 2006). The non-specific DNA binding region is masked in the E1-E2 complex and only when E2 is displaced is this region able to associate with DNA (Stenlund, 2003).
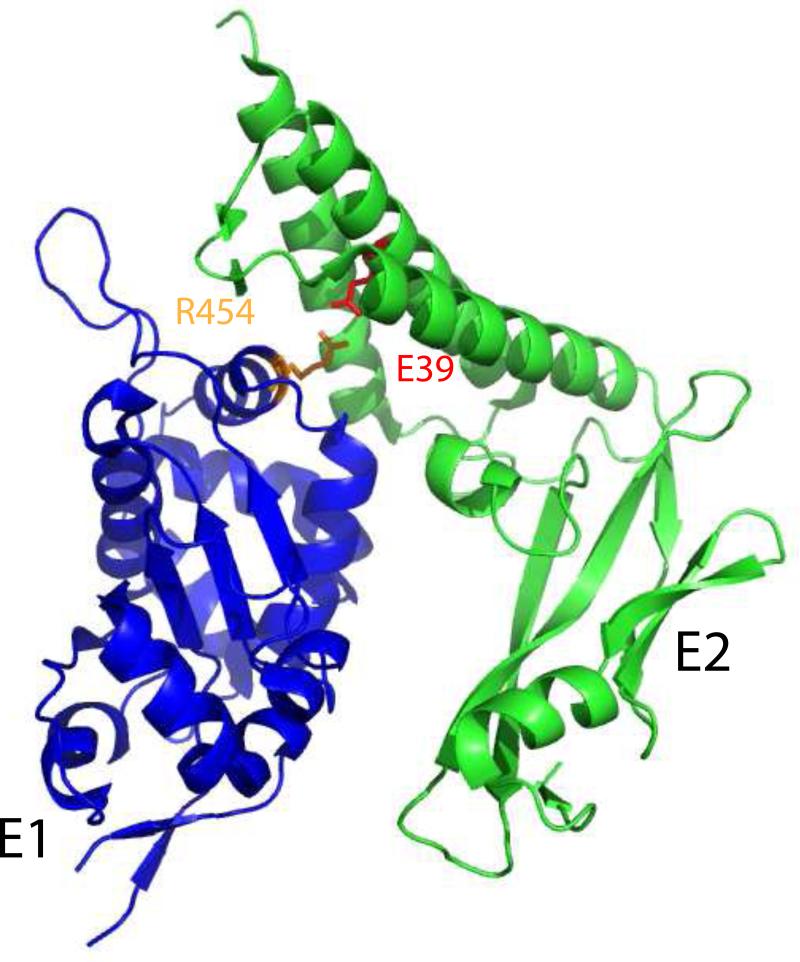
The N-terminal domain of E2 interacts with a large C-terminal region of the E1 protein containing the origin binding region and helicase (Leng et al., 1997; Muller and Sapp, 1996; Sarafi and McBride, 1995; Yasugi et al., 1997). Mutations in the first N-terminal alpha-helix of E2, or antibodies that bind to this region, will disrupt the E1-E2 complex (Baxter and McBride, 2005; Hibma et al., 1995). Probably the best characterized interaction residue is glutamate 39, which (as shown in Table 3) when mutated to alanine abrogates the interaction with E1 in some E2 proteins (Ferguson and Botchan, 1996; Sakai et al., 1996). However, most useful is a structure of the HPV18 E2 transactivation domain in complex with residues 428-631 from the helicase domain of the 657 residue E1 protein (Abbate et al., 2004). As shown in Figure 12, this region of E1 primarily interacts with the bundle of three alpha-helices on the concave surface of the transactivation domain, the fulcrum region and the short beta-sheets between helices 2 and 3. Glutamate 39 (or 43 in HPV18 E2) forms a buried salt bridge with the highly conserved residue arginine 454 in E1. Notably, in BPV1 E1, the equivalent residue is an asparagine (ibid.).
Table 3. Phenotype of Key Mutations in the E2 Transactivation Domain.
The values for each function were estimated from the references listed. (1) Brokaw et al., 1996; (2) Zheng et al., 2005; (3) Abroi et al., 1996; (4) Abroi et al., 2004; (5) Baxter et al., 2005; (6) Senechal et al., 2007; (7) Jeckel et al., 2002; (8) Cooper et al., 1998; (9) Sakai et al., 1996; (10) Schweiger et al., 2006; (11) Stubenrauch et al., 1998; (12) Ferguson and Botchan, 1996; (13) Breiding et al., 1996; (14) DiMaio & Settleman, 1988; (15) Dowhanick et al., 1995; (16) Alderbor et al., 1992; (17) Parish et al., 2006.
| Residue | Virus | Mutation | Transcription | Brd4 binding | Transient replication | E1 binding | Episomal replication | Mitotic chromosome binding | Partitioning | Late functions | Other | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |||
| R37 | BPV 1 | R37K | +++ | +++, +++ | ++ | +++ | transformation:- | (1, 2) | ||||
| R37A | +, −, ++ | + | ++, +, +++ | +++ | ++, ++ | − | (3-5) | |||||
| SfPV 1 | R37K | +++ | ++ | +++ | papillomas:- | (6, 7) | ||||||
| R37A | + | +/− | + | papillomas:- | (6, 7) | |||||||
| HPV 11 | R37K | + | − | (6, 8) | ||||||||
| R37A | + | (8) | ||||||||||
| HPV 16 | R37A | − | +/− | + | +++ | (9, 10) | ||||||
| HPV 31 | R37K | − | + | +++ | + | − | (6, 11) | |||||
| E39 | BPV 1 | E39D | −, − | +/−, ++ ts | ++ | ++ ts | transformation:- | (1, 2) | ||||
| E39A | +, +++, ++, +++ | ++ | +++, ++, +++, + | +++ | +++ . − | − | (3-5, 12) | |||||
| E39Q | +++ | ++ | ++ | +++ | +++ | (5) | ||||||
| E39G | − | +++ | (13) | |||||||||
| SfPV 1 | E39Q | +++ | +/− | papillomas:- | (6, 7) | |||||||
| E39A | ++ | +/− | papillomas:- | (6, 7) | ||||||||
| HPV 11 | E39D | ++ | + | (8) | ||||||||
| E39A | + | +++ | (8) | |||||||||
| HPV 16 | E39A | +++ | +++ | − | − | (9, 10) | ||||||
| HPV 31 | E39Q | +++ | ++ | − | (11) | |||||||
| I73 | BPV 1 | I73N | − | +++ | (12) | |||||||
| I73L | + | +++, +++ | +++ | +++ | transformation:- | (1) | ||||||
| I73A | ++, − | + | ++, +++ | +++ | + | (5) | ||||||
| SfPV 1 | I73L | + | ++ | ++ | papillomas:- | (6, 7) | ||||||
| HPV 16 | I73A | − | − | +++ | +++ | (9, 10) | ||||||
| HPV 31 | I73L | − | − | +++ | +++ | ++ | (6, 11) | |||||
| tsE2 | BPV 1 | ins 180-181 | − | − | +++ | (14-16) | ||||||
| W1 30 | BPV 1 | W130R | ++ | +++ | +++ | − | − | ChLR1: - | (17) |
Figure 12. Conserved Residues on the Surface of the E2 Transactivation Domain.
The transactivation domain structure PDB: 1DTO (HPV16) was rendered in Pymol. Key, conserved residues on the surface of the domain are indicated.
In certain circumstances, the DNA binding domains of E1 and E2 can interact while they are bound to adjacent sites on the origin (Chen and Stenlund, 2000). This interaction might only occur in viruses such as BPV1 where the E1 and E2 sites are only 3bp apart. It is not completely essential as the E2 DNA binding site can be moved further away without detriment (Gillitzer et al., 2000). Furthermore, The E2 DNA binding domain and origin binding site can be replaced with those of Gal4 and maintain a functional origin (Winokur and McBride, 1996). The residues on the DNA binding domain that interact with E1 are shown in Figure 11A.
E2 also interacts with the minor capsid protein L2. The L2 protein colocalizes with ND10 nuclear bodies and recruits the E1 and E2 proteins to this location. Initially it was thought that this was important for viral genome packaging (Day et al., 1998) but further studies indicated that this association is important for establishment of infection (Day et al., 2004). L2 can inhibit the transactivation, but not the replication, function of E2 (Heino et al., 2000; Okoye et al., 2005). The N-terminal 50 amino acids of L2 interact directly with E2 but L2 residues 301-400 are also required to repress E2-mediated transcription (Okoye et al., 2005). The first 50 residues of E2, and sequences spanning the hinge region, interact with the L2 protein (Heino et al., 2000).
E2 interacts with several other PV proteins, though the exact role in the life cycle is not firmly established. Smal and colleagues find that the N-terminus of E7 interacts with the DNA recognition helix of HPV16 E2 whereas Gammoh et al. map an interaction between the hinge of HPV16 E2 and the zinc binding region of E7 (Gammoh et al., 2006; Smal et al., 2009). E2 can recruit E7 to mitotic chromosomes in late mitosis (Gammoh et al., 2006). HPV16 and HPV18 E6 and E6*I proteins directly interact with the C-terminal domain of E2 through E6 residues 28-31 (Grm et al., 2005) and changes the sub-nuclear location of both E2 and E6. The N-terminus of HPV16 E2 also directly binds the E1^E4 protein and relocates E2 from the nucleus to the cytoplasm (Davy et al., 2009).
9. Mutations that Inactivate Specific Functions of the E2 Proteins
9.1 Mutations in the DNA Binding Domain
Figure 11A shows the BPV1 E2 DNA binding domain from PDB:1DBD. This is the only structure to date that contains the entire 100 amino acids of the DNA binding domain (Veeraraghavan et al., 1998). Mapped on this structure are “landmarks” on this domain mapped in E2 proteins from a number of different PV types. In red is the region in BPV1 E2 that interacts with E1 when both proteins are bound to adjacent sites: mutations in BPV1 E2 388, 390 and 401 impaired cooperative E1-E2 binding and replication (Gillitzer et al., 2000). The same helix interacts with p53, and shown in orange are residues that when mutated (HPV16 D338, E340, W341, D344) reduce p53 binding , inhibition of replication by p53 and E2-mediated apoptosis, yet they do not affect replication (Brown et al., 2008). Other residues shown have been mutated and are discussed elsewhere: potential SUMO modification sites (grey, section 6.3); cdk2 phosphorylation sites (yellow, section 6.1); the tryptophan bridge (cyan, section 4.2); and the redox cysteine (blue, section 6.4). Also shown are key residues in the recognition helix (in purple). Figure 11B lists the DNA binding phenotype of amino acid residue substitutions in the recognition helix of BPV1 and HPV11.
9.2 Mutations in the Transactivation Domain
Early studies involved the generation of deletions or amino acid insertions in E2. For the most part, these were not informative because of the precise fold of the conserved domains and most resulting proteins were non-functional. However, there have been several extensive mutational analyses of the E2 transactivation domain (Abroi et al., 1996; Baxter et al., 2005; Breiding et al., 1996; Brokaw et al., 1996; Cooper et al., 1998; Ferguson and Botchan, 1996; Smith et al., 2006). These studies have been reviewed and compared previously (McBride and Myers, 1997) and this analysis is still available on the PaVE website. Most of the studies (except (Baxter et al., 2005)) were undertaken before the structure of the N-terminal domain was solved. The structure revealed that many of the highly conserved residues were internal and/or had a structural role (Antson et al., 2000). Therefore, only mutations in conserved, surface residues are considered here. There have been additional studies further analyzing the phenotypes of these mutations in mitotic chromosomal binding (Abroi et al., 2004; Baxter et al., 2005; Zheng et al., 2005), Brd4 binding (Senechal et al., 2007), episomal maintenance and late transcription and replication (Stubenrauch et al., 1998a), papilloma induction in rabbits (Jeckel et al., 2002), and binding to cellular proteins ChLR1, Mklp2 and AMF1 (Breiding et al., 1997; Parish et al., 2006).
When the 241 E2 proteins currently hosted on the PaVE website are compared, there are 51 residues in the transactivation domain that are identical in 85% or more proteins, or have very conservative alternates. About half of these residues are exposed on the surface and are shown on the structure of the HPV16 transactivation domain shown in Figure 12. Table 3 lists the phenotype of some of the best studied mutations. For example, residues R37 and I73 are involved in transcriptional regulation and Brd4 binding whereas E39 is important for replication and E1 interaction. Mutations in these residues are thought to separate the replication and transcription functions of E2. However, as can be seen in Table 3, caution should be observed as the actual amino acid substitution can result in different phenotypes and varying results with different PVs.
It should be noted that the structural fold of the E2 transactivation domain is quite sensitive to mutation and many amino acid substitutions are temperature sensitive (ts). A ts protein was generated by the insertion of the sequence PRSR between residues 180 and 181 of BPV1 E2 (DiMaio and Settleman, 1988). At the non-permissive temperature, this protein cannot support transactivation, episomal genome replication or transformation (DiMaio and Settleman, 1988), but is competent for transient replication (Alderborn et al., 1992). This ts E2 has been used in several studies to delineate the functions of E2 in specific processes such as repression of E6 and E7 gene expression (Dowhanick et al., 1995) and vegetative viral DNA replication (Alderborn et al., 1992). In fact, many mutations in the E2 transactivation domain are temperature sensitive (Baxter et al., 2005; Ferguson and Botchan, 1996; Zheng et al., 2005). E2 proteins that are ts for interaction with mitotic chromosomes can be induced to bind host chromosomes at lower temperatures or when cells are treated with agents that promote protein folding (Zheng et al., 2005).
9.3 Mutations in the Hinge Region
The hinge region is not required for transcriptional regulation or replication, though a certain amount of linker sequence is required to prevent steric hindrance between the N and C-terminal domains (Winokur and McBride, 1992). The hinge of HPV11 is important for nuclear localization and nuclear matrix association and substitution of residues 238 RKRAR 242 with alanines disrupts this localization. The hinge region of the delta-PVs contains the major phosphorylation sites at residues 298 and 301 and a phosphorylation dependent conformational switch that targets E2 for degradation (Penrose et al., 2004). Penrose and colleagues present a detailed mutational analysis of this region (2004). Likewise, the hinge of HPV8 E2 contains a phosphorylation site at serine 253 that stabilizes E2 and promotes its association with chromatin (Sekhar et al., 2010). Sekhar and colleagues present a detailed mutational analysis of this region (2010).
9.4 Mutations in the E8 Moiety of E8^E2
Stubenrauch et al. conducted an alanine scanning analysis through the entire E8 portion of the E8^E2 protein (Stubenrauch et al., 2001). This showed that tryptophan 6 and lysine 7 are the main determinants of repressor function. A triple mutation KWK (residues 5, 6, 7 mutated to alanine) has been used in many studies of E8^E2 function (Ammermann et al., 2008; Powell et al., 2010; Stubenrauch et al., 2007).
10. Conclusions
The PV E2 proteins are remarkable, multifunctional proteins that are involved in many key viral and cellular processes. The availability of over 240 versions of these proteins, which have slowly evolved for millions of years in specific anatomical niches within each host species, can provide great insight into protein function and evolution.
Supplementary Material
HIGHLIGHTS.
Overview of E2 protein functions
Structural domains of the papillomavirus E2 proteins
Analysis of E2 binding sites in different genera of papillomaviruses
Compilation of E2 associated proteins
Comparison of key mutations in distinct E2 functions
Figure 10. Structure of an E1-E2 Complex.
The structure of the HPV18 E2 transactivation domain in complex with a C-terminal fragment of E1 (residues 428-631) was rendered in Pymol (PDB: 1TUE). Highlighted on the structure in red is E39, which forms a buried salt bridge with the highly conserved residue arginine 454 in E1 (shown in orange).
Acknowledgments
I am grateful to Koenraad van Doorslaer for critical reading of the manuscript. I apologize to authors whose work I have omitted because of space constraints. This work was funded by the Intramural Research Program of the NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbate EA, Berger JM, Botchan MR. The X-ray structure of the papillomavirus helicase in complex with its molecular matchmaker E2. Genes Dev. 2004;18:1981–1996. doi: 10.1101/gad.1220104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbate EA, Voitenleitner C, Botchan MR. Structure of the papillomavirus DNA-tethering complex E2:Brd4 and a peptide that ablates HPV chromosomal association. Mol.Cell. 2006;24:877–889. doi: 10.1016/j.molcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Abroi A, Ilves I, Kivi S, Ustav M. Analysis of chromatin attachment and partitioning functions of bovine papillomavirus type 1 E2 protein. J.Virol. 2004;78:2100–2113. doi: 10.1128/JVI.78.4.2100-2113.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abroi A, Kurg R, Ustav M. Transcriptional and replicational activation functions in the BPV1 E2 protein are encoded by different structural determinants. J.Virol. 1996;70:6169–6179. doi: 10.1128/jvi.70.9.6169-6179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgul B, Pfefferle R, Marcuzzi GP, Zigrino P, Krieg T, Pfister H, Mauch C. Expression of matrix metalloproteinase (MMP)-2, MMP-9, MMP-13, and MT1-MMP in skin tumors of human papillomavirus type 8 transgenic mice. Exp.Dermatol. 2006;15:35–42. doi: 10.1111/j.0906-6705.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- Alderborn A, Jareborg N, Burnett S. Evidence that the transcriptional trans activating function of the bovine papillomavirus type 1 E2 gene is not required for viral DNA amplification in division arrested cells. J.Gen.Virol. 1992;73:2639–2651. doi: 10.1099/0022-1317-73-10-2639. [DOI] [PubMed] [Google Scholar]
- Ammermann I, Bruckner M, Matthes F, Iftner T, Stubenrauch F. Inhibition of transcription and DNA replication by the papillomavirus E8-E2C protein is mediated by interaction with corepressor molecules. J.Virol. 2008;82:5127–5136. doi: 10.1128/JVI.02647-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androphy EJ, Lowy DR, Schiller JT. Bovine papillomavirus E2 trans-activating gene product binds to specific sites in papillomavirus DNA. Nature. 1987;325:70–73. doi: 10.1038/325070a0. [DOI] [PubMed] [Google Scholar]
- Antson AA, Burns JE, Moroz OV, Scott DJ, Sanders CM, Bronstein IB, Dodson GG, Wilson KS, Maitland NJ. Structure of the intact transactivation domain of the human papillomavirus E2 protein. Nature. 2000;403:805–809. doi: 10.1038/35001638. [DOI] [PubMed] [Google Scholar]
- Auster AS, Joshua-Tor L. The DNA-binding domain of human papillomavirus type 18 E1. Crystal structure, dimerization, and DNA binding. J.Biol.Chem. 2004;279:3733–3742. doi: 10.1074/jbc.M311681200. [DOI] [PubMed] [Google Scholar]
- Baker CC, Phelps WC, Lindgren V, Braun MJ, Gonda MA, Howley PM. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J.Virol. 1987;61:962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MS, Wettstein FO. E2 of cottontail rabbit papillomavirus is a nuclear phosphoprotein translated from an mRNA encoding multiple open reading frames. J.Virol. 1988;62:3242–3249. doi: 10.1128/jvi.62.9.3242-3249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum J, Prakash SS, Han P, Androphy EJ. Mechanism of action of the papillomavirus E2 repressor: repression in the absence of DNA binding. J.Virol. 1992;66:3941–3945. doi: 10.1128/jvi.66.6.3941-3945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N, McBride AA. Interaction of the papillomavirus E2 protein with mitotic chromosomes. Virology. 2000;270:124–134. doi: 10.1006/viro.2000.0265. [DOI] [PubMed] [Google Scholar]
- Baxter MK, McBride AA. An acidic amphipathic helix in the Bovine Papillomavirus E2 protein is critical for DNA replication and interaction with the E1 protein. Virology. 2005;332:78–88. doi: 10.1016/j.virol.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Baxter MK, McPhillips MG, Ozato K, McBride AA. The mitotic chromosome binding activity of the papillomavirus E2 protein correlates with interaction with the cellular chromosomal protein, Brd4. J.Virol. 2005;79:4806–4818. doi: 10.1128/JVI.79.8.4806-4818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behren A, Simon C, Schwab RM, Loetzsch E, Brodbeck S, Huber E, Stubenrauch F, Zenner HP, Iftner T. Papillomavirus E2 protein induces expression of the matrix metalloproteinase-9 via the extracellular signal-regulated kinase/activator protein-1 signaling pathway. Cancer Res. 2005;65:11613–11621. doi: 10.1158/0008-5472.CAN-05-2672. [DOI] [PubMed] [Google Scholar]
- Bellanger S, Demeret C, Goyat S, Thierry F. Stability of the human papillomavirus type 18 E2 protein is regulated by a proteasome degradation pathway through its amino terminal transactivation domain. J.Virol. 2001;75:7244–7251. doi: 10.1128/JVI.75.16.7244-7251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger S, Tan CL, Nei W, He PP, Thierry F. The human papillomavirus type 18 E2 protein is a cell cycle-dependent target of the SCFSkp2 ubiquitin ligase. J Virol. 2010;84:437–444. doi: 10.1128/JVI.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger S, Tan CL, Xue YZ, Teissier S, Thierry F. Tumor suppressor or oncogene? A critical role of the human papillomavirus (HPV) E2 protein in cervical cancer progression. American journal of cancer research. 2011;1:373–389. [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Morales VH, Peralta-Zaragoza O, Alcocer-Gonzalez JM, Moreno J, Madrid-Marina V. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Molecular medicine reports. 2011;4:369–375. doi: 10.3892/mmr.2011.429. [DOI] [PubMed] [Google Scholar]
- Bernard BA, Bailly C, Lenoir MC, Darmon M, Thierry F, Yaniv M. The human papillomavirus type 18 (HPV18) E2 gene product is a repressor of the HPV18 regulatory region in human keratinocytes. J.Virol. 1989;63:4317–4324. doi: 10.1128/jvi.63.10.4317-4324.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian XL, Wilson VG. Common importin alpha specificity for papillomavirus E2 proteins. Virus Res. 2010;150:135–137. doi: 10.1016/j.virusres.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S, Bellanger S, Demeret C, Thierry F. Nucleo cytoplasmic shuttling of high risk human Papillomavirus E2 proteins induces apoptosis. J Biol Chem. 2005;280:36088–36098. doi: 10.1074/jbc.M505138200. [DOI] [PubMed] [Google Scholar]
- Bochkarev A, Barwell JA, Pfuetzner RA, Furey W, Jr., Edwards AM, Frappier L. Crystal structure of the DNA-binding domain of the Epstein Barr virus origin-binding protein EBNA 1. Cell. 1995;83:39–46. doi: 10.1016/0092-8674(95)90232-5. [DOI] [PubMed] [Google Scholar]
- Bodaghi S, Jia R, Zheng ZM. Human papillomavirus type 16 E2 and E6 are RNA-binding proteins and inhibit in vitro splicing of pre-mRNAs with suboptimal splice sites. Virology. 2009;386:32–43. doi: 10.1016/j.virol.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard V, Storey A, Pim D, Banks L. Characterization of the human papillomavirus E2 protein: evidence of trans-activation and trans-repression in cervical keratinocytes. EMBO J. 1994;13:5451–5459. doi: 10.1002/j.1460-2075.1994.tb06880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiding DE, Grossel MJ, Androphy EJ. Genetic analysis of the bovine papillomavirus E2 transcriptional activation domain. Virology. 1996;221:34–43. doi: 10.1006/viro.1996.0350. [DOI] [PubMed] [Google Scholar]
- Breiding DE, Sverdrup F, Grossel MJ, Moscufo N, Boonchai W, Androphy EJ. Functional interaction of a novel cellular protein with the papillomavirus E2 transactivation domain. Mol Cell Biol. 1997;17:7208–7219. doi: 10.1128/mcb.17.12.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw JL, Blanco M, McBride AA. Amino acids critical for the functions of the bovine papillomavirus type 1 E2 transactivator. J.Virol. 1996;70:23–29. doi: 10.1128/jvi.70.1.23-29.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Kowalczyk AM, Taylor ER, Morgan IM, Gaston K. p53 represses human papillomavirus type 16 DNA replication via the viral E2 protein. Virol.J. 2008;5:5. doi: 10.1186/1743-422X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Strom AC, Jareborg N, Alderborn A, Dillner J, Moreno L,J, Pettersson U, Kiessling U. Induction of bovine papillomavirus E2 gene expression and early region transcription by cell growth arrest: correlation with viral DNA amplification and evidence for differential promoter induction. J.Virol. 1990;64:5529–5541. doi: 10.1128/jvi.64.11.5529-5541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JE, Walker HF, Schmitz C, Maitland NJ. Phenotypic effects of HPV-16 E2 protein expression in human keratinocytes. Virology. 2010;401:314–321. doi: 10.1016/j.virol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Bussiere DE, Kong X, Egan DA, Walter K, Holzman TF, Lindh F, Robins T, Giranda VL. Structure of the E2 DNA-binding domain from human papillomavirus serotype 31 at 2.4 A. Acta crystallographica. Section D, Biological crystallography. 1998;54:1367–1376. doi: 10.1107/s0907444998005587. [DOI] [PubMed] [Google Scholar]
- Carruth M, McBride AA. 2001.
- Chaiwongkot A, Vinokurova S, Pientong C, Ekalaksananan T, Kongyingyoes B, Kleebkaow P, Chumworathayi B, Patarapadungkit N, Reuschenbach M, von Knebel Doeberitz M. Differential methylation of E2 binding sites in episomal and integrated HPV 16 genomes in preinvasive and invasive cervical lesions. Int J Cancer. 2013;132:2087–2094. doi: 10.1002/ijc.27906. [DOI] [PubMed] [Google Scholar]
- Chang SW, Tsao YP, Lin CY, Chen SL. NRIP, a novel calmodulin binding protein, activates calcineurin to dephosphorylate human papillomavirus E2 protein. J.Virol. 2011;85:6750–6763. doi: 10.1128/JVI.02453-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R, Vinson C. CpG methylation recruits sequence specific transcription factors essential for tissue specific gene expression. Biochim Biophys Acta. 2012;1819:763–770. doi: 10.1016/j.bbagrm.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Stenlund A. Characterization of the DNA-binding domain of the bovine papillomavirus replication initiator E1. J.Virol. 1998;72:2567–2576. doi: 10.1128/jvi.72.4.2567-2576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Stenlund A. Two patches of amino acids on the E2 DNA binding domain define the surface for interaction with E1. J.Virol. 2000;74:1506–1512. doi: 10.1128/jvi.74.3.1506-1512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CM, Broker TR, Chow LT. An E1M^E2C fusion protein encoded by human papillomavirus type 11 is a sequence-specific transcription repressor. J.Virol. 1991;65:3317–3329. doi: 10.1128/jvi.65.6.3317-3329.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MT, Hirochika R, Hirochika H, Broker TR, Chow LT. Regulation of human papillomavirus type 11 enhancer and E6 promoter by activating and repressing proteins from the E2 open reading frame: functional and biochemical studies. J.Virol. 1988;62:2994–3002. doi: 10.1128/jvi.62.8.2994-3002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J, Vaillancourt P, Stenlund A, Botchan M. Bovine papillomavirus type 1 encodes two forms of a transcriptional repressor: Structural and functional analysis of new viral cDNAs. J.Virol. 1989;63:1743–1755. doi: 10.1128/jvi.63.4.1743-1755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MJ, Alam S, Ryndock EJ, Cruz L, Christensen ND, Roden RB, Meyers C. Tissue-spanning redox gradient dependent assembly of native human papillomavirus type 16 virions. J Virol. 2009;83:10515–10526. doi: 10.1128/JVI.00731-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CS, Upmeyer SN, Winokur PL. Identification of single amino acids in the human papillomavirus 11 E2 protein critical for the transactivation or replication functions. Virology. 1998;241:312–322. doi: 10.1006/viro.1997.8941. [DOI] [PubMed] [Google Scholar]
- Corina K, Grossman SR, Barsoum J, Prakash SS, Androphy EJ, Pepinsky RB. The tryptophan bridge is a critical feature of the papillomavirus E2 DNA binding domain. Virology. 1993:391–396. doi: 10.1006/viro.1993.1600. [DOI] [PubMed] [Google Scholar]
- Cripe TP, Haugen TH, Turk JP, Tabatabai F, Schmid P.G.d., Dürst M, Gissmann L, Roman A, Turek LP. Transcriptional regulation of the human papillomavirus- 16 E6 E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987;6:3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao LD, Duffy A, Van Tine BA, Wu SY, Chiang CM, Broker TR, Chow LT. Dynamic localization of the human papillomavirus type 11 origin binding protein E2 through mitosis while in association with the spindle apparatus. J.Virol. 2006;80:4792–4800. doi: 10.1128/JVI.80.10.4792-4800.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy C, McIntosh P, Jackson DJ, Sorathia R, Miell M, Wang Q, Khan J, Soneji Y, Doorbar J. A novel interaction between the human papillomavirus type 16 E2 and E1--E4 proteins leads to stabilization of E2. Virology. 2009;394:266–275. doi: 10.1016/j.virol.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Day PM, Baker CC, Lowy DR, Schiller JT. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc.Natl.Acad.Sci.U.S.A. 2004;101:14252–14257. doi: 10.1073/pnas.0404229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Roden RB, Lowy DR, Schiller JT. The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains. J.Virol. 1998;72:142–150. doi: 10.1128/jvi.72.1.142-150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeret C, Garcia-Carranca A, Thierry F. Transcription-independent triggering of the extrinsic pathway of apoptosis by human papillomavirus 18 E2 protein. Oncogene. 2003;22:168–175. doi: 10.1038/sj.onc.1206108. [DOI] [PubMed] [Google Scholar]
- Desaintes C, Demeret C, Goyat S, Yaniv M, Thierry F. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 1997;16:504–514. doi: 10.1093/emboj/16.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyrieux AF, Rosas-Acosta G, Ozbun MA, Wilson VG. Sumoylation dynamics during keratinocyte differentiation. J Cell Sci. 2007;120:125–136. doi: 10.1242/jcs.03317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D, Settleman J. Bovine papillomavirus mutant temperature sensitive for transformation, replication and transactivation. EMBO J. 1988;7:1197–1204. doi: 10.1002/j.1460-2075.1988.tb02931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson MM, Boner W, Morgan IM. TopBP1 regulates human papillomavirus type 16 E2 interaction with chromatin. J.Virol. 2007;81:4338–4342. doi: 10.1128/JVI.02353-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Broker TR, Chow LT. Human papillomavirus type 11 E2 proteins repress the homologous E6 promoter by interfering with the binding of host transcription factors to adjacent elements. J.Virol. 1994;68:1115–1127. doi: 10.1128/jvi.68.2.1115-1127.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostatni N, Lambert P, Sousa R, Ham J, Howley P, Yaniv M. The functional BPV-1 E2 transactivating protein can act as a repressor by preventing formation of the initiation complex. Genes Dev. 1991 doi: 10.1101/gad.5.9.1657. [DOI] [PubMed] [Google Scholar]
- Dostatni N, Thierry F, Yaniv M. A dimer of BPV-1 E2 containing a protease resistant core interacts with its DNA target. EMBO J. 1988;7:3807–3816. doi: 10.1002/j.1460-2075.1988.tb03265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowhanick JJ, McBride AA, Howley PM. Suppression of cellular proliferation by the papillomavirus E2 protein. J.Virol. 1995;69:7791–7799. doi: 10.1128/jvi.69.12.7791-7799.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MF, Botchan MR. Genetic analysis of the activation domain of bovine papillomavirus protein E2:its role in transcription and replication. J.Virol. 1996;70:4193–4199. doi: 10.1128/jvi.70.7.4193-4199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertey J, Ammermann I, Winkler M, Stoger R, Iftner T, Stubenrauch F. Interaction of the papillomavirus E8--E2C protein with the cellular CHD6 protein contributes to transcriptional repression. J.Virol. 2010;84:9505–9515. doi: 10.1128/JVI.00678-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores ER, Lambert PF. Evidence for a switch in the mode of human papillomavirus type 16 DNA replication during the viral life cycle. J.Virol. 1997;71:7167–7179. doi: 10.1128/jvi.71.10.7167-7179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradet-Turcotte A, Bergeron-Labrecque F, Moody CA, Lehoux M, Laimins LA, Archambault J. Nuclear accumulation of the papillomavirus E1 helicase blocks S-phase progression and triggers an ATM-dependent DNA damage response. J.Virol. 2011;85:8996–9012. doi: 10.1128/JVI.00542-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattini MG, Laimins LA. Binding of the human papillomavirus E1 origin-recognition protein is regulated through complex formation with the E2 enhancer-binding protein. PNAS temp. 1994;91:12398–12402. doi: 10.1073/pnas.91.26.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Brandsma JL, Peng X, Srimatkandada S, Li L, Canaan A, Deisseroth AB. High and low levels of cottontail rabbit papillomavirus E2 protein generate opposite effects on gene expression. J.Biol.Chem. 2001;276:867–874. doi: 10.1074/jbc.M007120200. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Joubert S, Senechal H, Fradet-Turcotte A, Torre S, Archambault J. Proteasomal degradation of the papillomavirus E2 protein is inhibited by overexpression of bromodomain-containing protein 4. J.Virol. 2009;83:4127–4139. doi: 10.1128/JVI.02468-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon D, Senechal H, D'Abramo CM, Alvarez J, McBride AA, Archambault J. Genetic analysis of the E2 transactivation domain dimerization interface from bovine papillomavirus type 1. Virology. 2013;439:132–139. doi: 10.1016/j.virol.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammoh N, Grm HS, Massimi P, Banks L. Regulation of human papillomavirus type 16 E7 activity through direct protein interaction with the E2 transcriptional activator. J.Virol. 2006;80:1787–1797. doi: 10.1128/JVI.80.4.1787-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alai MM, Gallo M, Salame M, Wetzler DE, McBride AA, Paci M, Cicero DO, de Prat Gay G. Molecular basis for phosphorylation-dependent, PEST-mediated protein turnover. Structure. 2006;14:309–319. doi: 10.1016/j.str.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Gasparian AV, Fedorova MD, Kisselev FL. Regulation of matrix metalloproteinase-9 transcription in squamous cell carcinoma of uterine cervix: the role of human papillomavirus gene E2 expression and activation of transcription factor NF-kappaB. Biochemistry. Biokhimiia. 2007;72:848–853. doi: 10.1134/s0006297907080068. [DOI] [PubMed] [Google Scholar]
- Gauthier JM, Dillner J, Yaniv M. Structural analysis of the human papillomavirus type 16-E2 transactivator with antipeptide antibodies reveals a high mobility region linking the transactivation and the DNA-binding domains. Nucleic.Acids.Res. 1991;19:7073–7079. doi: 10.1093/nar/19.25.7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie KA, Mehta KP, Laimins LA, Moody CA. Human papillomaviruses recruit cellular DNA repair and homologous recombination factors to viral replication centers. J Virol. 2012;86:9520–9526. doi: 10.1128/JVI.00247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillitzer E, Chen G, Stenlund A. Separate domains in E1 and E2 proteins serve architectural and productive roles for cooperative DNA binding. EMBO J. 2000;19:3069–3079. doi: 10.1093/emboj/19.12.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri I, Yaniv M. Structural and mutational analysis of E2 trans- activating proteins of papillomaviruses reveals three distinct functional domains. EMBO J. 1988;7:2823–2829. doi: 10.1002/j.1460-2075.1988.tb03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc.Natl.Acad.Sci.U.S.A. 2000;97:12513–12518. doi: 10.1073/pnas.97.23.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin EC, Naeger LK, Breiding DE, Androphy EJ, DiMaio D. Transactivation competent bovine papillomavirus E2 protein is specifically required for efficient repression of human papillomavirus oncogene expression and for acute growth inhibition of cervical carcinoma cell lines. J.Virol. 1998;72:3925–3934. doi: 10.1128/jvi.72.5.3925-3934.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin EC, Yang E, Lee CJ, Lee HW, DiMaio D, Hwang ES. Rapid induction of senescence in human cervical carcinoma cells. Proc.Natl.Acad.Sci.U.S.A. 2000;97:10978–10983. doi: 10.1073/pnas.97.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grm HS, Massimi P, Gammoh N, Banks L. Crosstalk between the human papillomavirus E2 transcriptional activator and the E6 oncoprotein. Oncogene. 2005;24:5149–5164. doi: 10.1038/sj.onc.1208701. [DOI] [PubMed] [Google Scholar]
- Grossel MJ, Barsoum J, Prakash SS, Androphy EJ. The BPV-1 E2 DNA-contact helix cysteine is required for transcriptional activation but not replication in mammalian cells. Virology. 1996;217:301–310. doi: 10.1006/viro.1996.0117. [DOI] [PubMed] [Google Scholar]
- Grundhoff A, Ganem D. The latency-associated nuclear antigen of Kaposi's sarcoma associated herpesvirus permits replication of terminal repeat-containing plasmids. J.Virol. 2003;77:2779–2783. doi: 10.1128/JVI.77.4.2779-2783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SF, Botchan MR. Crystal structure of the human papillomavirus type 18 E2 activation domain. Science. 1999;284:1673–1677. doi: 10.1126/science.284.5420.1673. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P, Androphy EJ, Lowy DR, Schiller JT. The specific DNA recognition sequence of the bovine papillomavirus E2 protein is an E2-dependent enhancer. EMBO J. 1988;7:525–531. doi: 10.1002/j.1460-2075.1988.tb02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde RS. The papillomavirus E2 proteins: structure, function, and biology. Annual review of biophysics and biomolecular structure. 2002;31:343–360. doi: 10.1146/annurev.biophys.31.100901.142129. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Androphy EJ. Crystal structure of the E2 DNA-binding domain from human papillomavirus type 16: implications for its DNA binding-site selection mechanism. Journal of molecular biology. 1998;284:1479–1489. doi: 10.1006/jmbi.1998.2260. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Grossman SR, Laimins LA, Sigler PB. Crystal structure at 1.7 A of the bovine papillomavirus-1 E2 DNA-binding domain bound to its DNA target. Nature. 1992;359:505–512. doi: 10.1038/359505a0. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Wang AF, Kim SS, Schapira M. Subunit rearrangement accompanies sequence specific DNA binding by the bovine papillomavirus-1 E2 protein. Journal of molecular biology. 1998;276:797–808. doi: 10.1006/jmbi.1997.1587. [DOI] [PubMed] [Google Scholar]
- Heino P, Zhou J, Lambert PF. Interaction of the papillomavirus Transcription/Replication factor, E2, and the viral capsid protein, L2. Virology. 2000;276:304–314. doi: 10.1006/viro.2000.0342. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ramon EE, Burns JE, Zhang W, Walker HF, Allen S, Antson AA, Maitland NJ. Dimerization of the human papillomavirus type 16 E2 N terminus results in DNA looping within the upstream regulatory region. J.Virol. 2008;82:4853–4861. doi: 10.1128/JVI.02388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibma MH, Raj K, Ely SJ, Stanley M, Crawford L. The interaction between human papillomavirus type 16 E1 and E2 proteins is blocked by an antibody to the N-terminal region of E2. Eur.J.Biochem. 1995;229:517–525. doi: 10.1111/j.1432-1033.1995.0517k.x. [DOI] [PubMed] [Google Scholar]
- Hooley E, Fairweather V, Clarke AR, Gaston K, Brady RL. The recognition of local DNA conformation by the human papillomavirus type 6 E2 protein. Nucleic Acids Research. 2006;34:3897–3908. doi: 10.1093/nar/gkl466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou SY, Wu SY, Zhou T, Thomas MC, Chiang CM. Alleviation of human papillomavirus E2-mediated transcriptional repression via formation of a TATA binding protein (or TFIID)-TFIIB-RNA polymerase II-TFIIF preinitiation complex. Mol.Cell Biol. 2000;20:113–125. doi: 10.1128/mcb.20.1.113-125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert NL, Schiller JT, Lowy DR, Androphy EJ. Bovine papilloma virus-transformed cells contain multiple E2 proteins. Proc.Natl.Acad.Sci.USA. 1988;85:5864–5868. doi: 10.1073/pnas.85.16.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Riese D.J.d., Settleman J, Nilson LA, Honig J, Flynn S, DiMaio D. Inhibition of cervical carcinoma cell line proliferation by the introduction of a bovine papillomavirus regulatory gene. J.Virol. 1993;67:3720–3729. doi: 10.1128/jvi.67.7.3720-3729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilves I, Kivi S, Ustav M. Long-term episomal maintenance of bovine papillomavirus type 1 plasmids is determined by attachment to host chromosomes, which Is mediated by the viral E2 protein and its binding sites. J.Virol. 1999;73:4404–4412. doi: 10.1128/jvi.73.5.4404-4412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilves I, Maemets K, Silla T, Janikson K, Ustav M. Brd4 is involved in multiple processes of the bovine papillomavirus type 1 life cycle. J.Virol. 2006;80:3660–3665. doi: 10.1128/JVI.80.7.3660-3665.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Campo MS. Both viral E2 protein and the cellular factor PEBP2 regulate transcription via E2 consensus sites within the bovine papillomavirus type 4 long control region. J Virol. 1995;69:6038–6046. doi: 10.1128/jvi.69.10.6038-6046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MK, Kwon D, McBride AA. Papillomavirus E2 proteins and the host BRD4 protein associate with transcriptionally active cellular chromatin. J.Virol. 2009;83:2592–2600. doi: 10.1128/JVI.02275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MK, McBride AA. 2013. unpublished data.
- Jeckel S, Huber E, Stubenrauch F, Iftner T. A transactivator function of cottontail rabbit papillomavirus e2 is essential for tumor induction in rabbits. J.Virol. 2002;76:11209–11215. doi: 10.1128/JVI.76.22.11209-11215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel S, Loetzsch E, Huber E, Stubenrauch F, Iftner T. Identification of the E9/E2C cDNA and functional characterization of the gene product reveal a new repressor of transcription and replication in cottontail rabbit papillomavirus. J.Virol. 2003;77:8736–8744. doi: 10.1128/JVI.77.16.8736-8744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Graham SV, Dornan ES, Morgan IM. The human papillomavirus 16 E2 protein is stabilised in S phase. Virology. 2009;394:194–199. doi: 10.1016/j.virol.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Johansson C, Schwartz S. Regulation of human papillomavirus gene expression by splicing and polyadenylation. Nature reviews. Microbiology. 2013;11:239–251. doi: 10.1038/nrmicro2984. [DOI] [PubMed] [Google Scholar]
- Johansson C, Somberg M, Li X, Backstrom Winquist E, Fay J, Ryan F, Pim D, Banks L, Schwartz S. HPV-16 E2 contributes to induction of HPV 16 late gene expression by inhibiting early polyadenylation. EMBO J. 2012;31:3212–3227. doi: 10.1038/emboj.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johung K, Goodwin EC, DiMaio D. Human papillomavirus E7 repression in cervical carcinoma cells initiates a transcriptional cascade driven by the retinoblastoma family, resulting in senescence. Journal of Virology. 2007;81:2102–2116. doi: 10.1128/JVI.02348-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantari M, Calleja-Macias IE, Tewari D, Hagmar B, Lie K, Barrera-Saldana HA, Wiley DJ, Bernard HU. Conserved methylation patterns of human papillomavirus type 16 DNA in asymptomatic infection and cervical neoplasia. J Virol. 2004;78:12762–12772. doi: 10.1128/JVI.78.23.12762-12772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantari M, Lee D, Calleja-Macias IE, Lambert PF, Bernard HU. Effects of cellular differentiation, chromosomal integration and 5-aza 2'-deoxycytidine treatment on human papillomavirus-16 DNA methylation in cultured cell lines. Virology. 2008;374:292–303. doi: 10.1016/j.virol.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Garner-Hamrick PA, Fisher C, Lee D, Lambert PF. Methylation patterns of papillomavirus DNA, its influence on E2 function, and implications in viral infection. J.Virol. 2003;77:12450–12459. doi: 10.1128/JVI.77.23.12450-12459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Tam JK, Wang AF, Hegde RS. The structural basis of DNA target discrimination by papillomavirus E2 proteins. J Biol Chem. 2000;275:31245–31254. doi: 10.1074/jbc.M004541200. [DOI] [PubMed] [Google Scholar]
- King LE, Dornan ES, Donaldson MM, Morgan IM. Human papillomavirus 16 E2 stability and transcriptional activation is enhanced by E1 via a direct protein-protein interaction. Virology. 2011;414:26–33. doi: 10.1016/j.virol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Klucevsek K, Wertz M, Lucchi J, Leszczynski A, Moroianu J. Characterization of the nuclear localization signal of high risk HPV16 E2 protein. Virology. 2007;360:191–198. doi: 10.1016/j.virol.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JD, Li R, Botchan M. The activation domain of the bovine papillomavirus E2 protein mediates association of DNA-bound dimers to form DNA loops. Proc.Natl.Acad.Sci.USA. 1991;88:3204–3208. doi: 10.1073/pnas.88.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkina L, Pastore S. The role of redox regulation in the normal physiology and inflammatory diseases of skin. Frontiers in bioscience (Elite edition) 2009;1:123. doi: 10.2741/E13. [DOI] [PubMed] [Google Scholar]
- Kruppel U, Muller-Schiffmann A, Baldus SE, Smola-Hess S, Steger G. E2 and the coactivator p300 can cooperate in activation of the human papillomavirus type 16 early promoter. Virology. 2008;377:151–159. doi: 10.1016/j.virol.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Kurg R, Sild K, Ilves A, Sepp M, Ustav M. Association of bovine papillomavirus E2 protein with nuclear structures in vivo. J.Virol. 2005;79:10528–10539. doi: 10.1128/JVI.79.16.10528-10539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurg R, Tekkel H, Abroi A, Ustav M. Characterization of the functional activities of the bovine papillomavirus type 1 E2 protein single-chain heterodimers. J.Virol. 2006;80:11218–11225. doi: 10.1128/JVI.01127-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurg R, Uusen P, Sepp T, Sepp M, Abroi A, Ustav M. Bovine papillomavirus type 1 E2 protein heterodimer is functional in papillomavirus DNA replication in vivo. Virology. 2009;386:353–359. doi: 10.1016/j.virol.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Kurg R, Uusen P, Vosa L, Ustav M. Human papillomavirus E2 protein with single activation domain initiates HPV18 genome replication, but is not sufficient for long term maintenance of virus genome. Virology. 2010;408:159–166. doi: 10.1016/j.virol.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Lace MJ, Anson JR, Thomas GS, Turek LP, Haugen TH. The E8--E2 gene product of human papillomavirus type 16 represses early transcription and replication but is dispensable for viral plasmid persistence in keratinocytes. J Virol. 2008;82:10841–10853. doi: 10.1128/JVI.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Teh BH, Tarn WY. A human papillomavirus E2 transcriptional activator. The interactions with cellular splicing factors and potential function in pre-mRNA processing. J Biol Chem. 1999;274:11832–11841. doi: 10.1074/jbc.274.17.11832. [DOI] [PubMed] [Google Scholar]
- Lambert PF, Hubbert NL, Howley PM, Schiller JT. Genetic assignment of multiple E2 gene products in bovine papillomavirus-transformed cells. J.Virol. 1989;63:3151–3154. doi: 10.1128/jvi.63.7.3151-3154.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert PF, Monk BC, Howley PM. Phenotypic analysis of bovine papillomavirus type 1 E2 repressor mutants. J Virol. 1990;64:950–956. doi: 10.1128/jvi.64.2.950-956.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert PF, Spalholz BA, Howley PM. A transcriptional repressor encoded by BPV 1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987;50:69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Lee AY, Chiang CM. Chromatin adaptor Brd4 modulates E2 transcription activity and protein stability. J Biol Chem. 2009;284:2778–2786. doi: 10.1074/jbc.M805835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Hwang SG, Kim J, Choe J. Functional interaction between p/CAF and human papillomavirus E2 protein. J Biol Chem. 2002a;277:6483–6489. doi: 10.1074/jbc.M105085200. [DOI] [PubMed] [Google Scholar]
- Lee D, Kim HZ, Jeong KW, Shim YS, Horikawa I, Barrett JC, Choe J. Human papillomavirus E2 down-regulates the human telomerase reverse transcriptase promoter. J Biol Chem. 2002b;277:27748–27756. doi: 10.1074/jbc.M203706200. [DOI] [PubMed] [Google Scholar]
- Lee D, Lee B, Kim J, Kim DW, Choe J. cAMP response element-binding protein binding protein binds to human papillomavirus E2 protein and activates E2-dependent transcription. J Biol Chem. 2000;275:7045–7051. doi: 10.1074/jbc.275.10.7045. [DOI] [PubMed] [Google Scholar]
- Lehman CW, Botchan MR. Segregation of viral plasmids depends on tethering to chromosomes and is regulated by phosphorylation. Proc.Natl.Acad.Sci U.S.A. 1998;95:4338–4343. doi: 10.1073/pnas.95.8.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman CW, King DS, Botchan MR. A papillomavirus E2 phosphorylation mutant exhibits normal transient replication and transcription but is defective in transformation and plasmid retention. J.Virol. 1997;71:3652–3665. doi: 10.1128/jvi.71.5.3652-3665.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X, Ludes-Meyers JH, Wilson VG. Isolation of an amino terminal region of bovine papillomavirus type 1 E1 protein that retains origin binding and E2 interaction capacity. J.Virol. 1997;71:848–852. doi: 10.1128/jvi.71.1.848-852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leykauf K, Kabsch K, Gassler N, Gissmann L, Alonso A, Schenkel J. Expression of the HPV11 E2 gene in transgenic mice does not result in alterations of the phenotypic pattern. Transgenic research. 2008;17:1–8. doi: 10.1007/s11248-007-9130-y. [DOI] [PubMed] [Google Scholar]
- Li R, Botchan MR. Acidic transcription factors alleviate nucleosome-mediated repression of DNA replication of bovine papillomavirus type 1. Proc.Natl.Acad.Sci.USA. 1994;91:7051–7055. doi: 10.1073/pnas.91.15.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Knight J, Bream G, Stenlund A, Botchan M. Specific recognition nucleotides and their DNA context determine the affinity of E2 protein for 17 binding sites in the BPV-1 genome. Genes Dev. 1989;3:510–526. doi: 10.1101/gad.3.4.510. [DOI] [PubMed] [Google Scholar]
- Lim DA, Gossen M, Lehman CW, Botchan MR. Competition for DNA binding sites between the short and long forms of E2 dimers underlies repression in bovine papillomavirus type 1 DNA replication control. J.Virol. 1998;72:1931–1940. doi: 10.1128/jvi.72.3.1931-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland NJ, Conway S, Wilkinson NS, Ramsdale J, Morris JR, Sanders CM, Burns JE, Stern PL, Wells M. Expression patterns of the human papillomavirus type 16 transcription factor E2 in low- and high-grade cervical intraepithelial neoplasia. J.Pathol. 1998;186:275–280. doi: 10.1002/(SICI)1096-9896(1998110)186:3<275::AID-PATH159>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Nakashima N, Takase K, Hirochika H, Mizuno H. A mutation study of the DNA binding domain of human papillomavirus type11 E2 protein. J.Biochem.(Tokyo.) 1997;121:138–144. doi: 10.1093/oxfordjournals.jbchem.a021556. [DOI] [PubMed] [Google Scholar]
- May M, Grassmann K, Pfister H, Fuchs PG. Transcriptional silencer of the human papillomavirus type 8 late promoter interacts alternatively with the viral trans activator E2 or with a cellular factor. J Virol. 1994;68:3612–3619. doi: 10.1128/jvi.68.6.3612-3619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M, Helbl V, Pfister H, Fuchs PG. Unique topography of DNA-protein interactions in the non-coding region of epidermodysplasia verruciformis-associated human papillomaviruses. J Gen Virol. 1991;72(Pt 12):2989–2997. doi: 10.1099/0022-1317-72-12-2989. [DOI] [PubMed] [Google Scholar]
- McBride AA. Replication and partitioning of papillomavirus genomes. Advances in virus research. 2008;72:155–205. doi: 10.1016/S0065-3527(08)00404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, Bolen JB, Howley PM. Phosphorylation sites of the E2 transcriptional regulatory proteins of bovine papillomavirus type 1. J.Virol. 1989a;63:5076–5085. doi: 10.1128/jvi.63.12.5076-5085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, Byrne JC, Howley PM. E2 polypeptides encoded by bovine papillomavirus type 1 form dimers through the common carboxyl-terminal domain: transactivation is mediated by the conserved amino-terminal domain. Proc.Natl.Acad.Sci.U.S.A. 1989b;86:510–514. doi: 10.1073/pnas.86.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, Howley PM. Bovine papillomavirus with a mutation in the E2 serine 301 phosphorylation site replicates at a high copy number. J.Virol. 1991;65:6528–6534. doi: 10.1128/jvi.65.12.6528-6534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, Jang MK. Current Understanding of the Role of the Brd4 protein in the Papillomavirus Lifecycle. Viruses. 2013;5 doi: 10.3390/v5061374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, Klausner RD, Howley PM. Conserved cysteine residue in the DNA-binding domain of the bovine papillomavirus type 1 E2 protein confers redox regulation of the DNA-binding activity in vitro. Proc.Natl.Acad.Sci.U.S.A. 1992;89:7531–7535. doi: 10.1073/pnas.89.16.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, Myers G. The E2 proteins: an update. In: Myers G, Baker C, Munger K, Sverdrup F, McBride A, Bernard HU, editors. Human Papillomaviruses 1997. Los Alamos National Laboratory; Los Alamos: 1997. pp. III54–III99. [Google Scholar]
- McBride AA, Sakakibara N, Stepp WH, Jang MK. Hitchhiking on host chromatin: how papillomaviruses persist. Biochim Biophys Acta. 2012;1819:820–825. doi: 10.1016/j.bbagrm.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, Schlegel R, Howley PM. The carboxy-terminal domain shared by the bovine papillomavirus E2 transactivator and repressor proteins contains a specific DNA binding activity. EMBO J. 1988;7:533–539. doi: 10.1002/j.1460-2075.1988.tb02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, van Doorslaer K. 2013. unpublished data.
- McLaughlin-Drubin M, Munger K. The human papillomavirus type 8 E2 gene encodes a transforming activity sufficient for skin tumor formation in transgenic mice. J Invest Dermatol. 2008;128:2142–2144. doi: 10.1038/jid.2008.217. [DOI] [PubMed] [Google Scholar]
- McPhillips MG, Oliveira JG, Spindler JE, Mitra R, McBride AA. Brd4 is required for e2-mediated transcriptional activation but not genome partitioning of all papillomaviruses. J.Virol. 2006;80:9530–9543. doi: 10.1128/JVI.01105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhillips MG, Ozato K, McBride AA. Interaction of bovine papillomavirus E2 protein with Brd4 stabilizes its association with chromatin. J.Virol. 2005;79:8920–8932. doi: 10.1128/JVI.79.14.8920-8932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhillips MG, Veerapraditsin T, Cumming SA, Karali D, Milligan SG, Boner W, Morgan IM, Graham SV. SF2/ASF binds the human papillomavirus type 16 late RNA control element and is regulated during differentiation of virus-infected epithelial cells. J Virol. 2004;78:10598–10605. doi: 10.1128/JVI.78.19.10598-10605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr IJ, Clark R, Sun S, Androphy EJ, MacPherson P, Botchan MR. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990;250:1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- Mok YK, de Prat GG, Butler PJ, Bycroft M. Equilibrium dissociation and unfolding of the dimeric human papillomavirus strain-16 E2 DNA-binding domain. Protein Sci. 1996;5:310–319. doi: 10.1002/pro.5560050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole S, Milligan SG, Graham SV. Human papillomavirus type 16 E2 protein transcriptionally activates the promoter of a key cellular splicing factor, SF2/ASF. J Virol. 2009;83:357–367. doi: 10.1128/JVI.01414-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monini P, Blitz IL, Cassai E. Cooperative DNA binding of the bovine papillomavirus E2 transcriptional activator is antagonized by truncated E2 polypeptides. J Virol. 1993;67:5668–5676. doi: 10.1128/jvi.67.9.5668-5676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody CA, Laimins LA. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS.Pathog. 2009;5:e1000605. doi: 10.1371/journal.ppat.1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaluk C, Bastia D. The E2 “gene: of bovine papillomavirus encodes an enhancer-binding protein. Proc.Natl.Acad.Sci.USA. 1987;84:1215–1218. doi: 10.1073/pnas.84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaluk CA, Bastia D. Interaction of the bovine papillomavirus type 1 E2 transcriptional control protein with the viral enhancer: purification of the DNA-binding domain and analysis of its contact points with DNA. J Virol. 1988;62:1925–1931. doi: 10.1128/jvi.62.6.1925-1931.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaluk CA, Bastia D. The bovine papillomavirus type 1 transcriptional activator E2 protein binds to its DNA recognition sequence as a dimer. Virology. 1989;169:236–238. doi: 10.1016/0042-6822(89)90062-7. [DOI] [PubMed] [Google Scholar]
- Muhlen S, Behren A, Iftner T, Simon C. Influence of HPV16 E2 and its localisation on the expression of matrix metalloproteinase-9. International journal of oncology. 2010;37:337–345. doi: 10.3892/ijo_00000682. [DOI] [PubMed] [Google Scholar]
- Muller A, Ritzkowsky A, Steger G. Cooperative activation of human papillomavirus type 8 gene expression by the E2 protein and the cellular coactivator p300. J.Virol. 2002;76:11042–11053. doi: 10.1128/JVI.76.21.11042-11053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, Sapp M. Domains of the E1 protein of human papillomavirus type 33 involved in binding to the E2 protein. Virology. 1996;219:247–256. doi: 10.1006/viro.1996.0242. [DOI] [PubMed] [Google Scholar]
- Muller M, Demeret C. The HPV E2-Host Protein-Protein Interactions: A Complex Hijacking of the Cellular Network. Open Virol J. 2012;6:173–189. doi: 10.2174/1874357901206010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadra AD, Eliseo T, Mok YK, Almeida CL, Bycroft M, Paci M, de Prat-Gay G, Cicero DO. Solution structure of the HPV-16 E2 DNA binding domain, a transcriptional regulator with a dimeric beta-barrel fold. J.Biomol.NMR. 2004;30:211–214. doi: 10.1023/b:jnmr.0000048942.96866.76. [DOI] [PubMed] [Google Scholar]
- Narechania A, Terai M, Burk RD. Overlapping reading frames in closely related human papillomaviruses result in modular rates of selection within E2. J Gen Virol. 2005;86:1307–1313. doi: 10.1099/vir.0.80747-0. [DOI] [PubMed] [Google Scholar]
- Nishimura A, Ono T, Ishimoto A, Dowhanick JJ, Frizzell MA, Howley PM, Sakai H. Mechanisms of human papillomavirus E2-mediated repression of viral oncogene expression and cervical cancer cell growth inhibition. J.Virol. 2000;74:3752–3760. doi: 10.1128/jvi.74.8.3752-3760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye A, Cordano P, Taylor ER, Morgan IM, Everett R, Campo MS. Human papillomavirus 16 L2 inhibits the transcriptional activation function, but not the DNA replication function, of HPV-16 E2. Virus Res. 2005;108:1–14. doi: 10.1016/j.virusres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Oldak M, Maksym RB, Sperling T, Yaniv M, Smola H, Pfister HJ, Malejczyk J, Smola S. Human papillomavirus type 8 E2 protein unravels JunB/Fra 1 as an activator of the beta4-integrin gene in human keratinocytes. J Virol. 2010;84:1376–1386. doi: 10.1128/JVI.01220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldak M, Smola H, Aumailley M, Rivero F, Pfister H, Smola-Hess S. The human papillomavirus type 8 E2 protein suppresses beta4-integrin expression in primary human keratinocytes. J Virol. 2004;78:10738–10746. doi: 10.1128/JVI.78.19.10738-10746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JG, Colf LA, McBride AA. Variations in the association of papillomavirus E2 proteins with mitotic chromosomes. Proc.Natl.Acad.Sci.U.S.A. 2006;103:1047–1052. doi: 10.1073/pnas.0507624103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun MA, Meyers C. Human papillomavirus type 31b E1 and E2 transcript expression correlates with vegetative viral genome amplification. Virology. 1998;248:218–230. doi: 10.1006/viro.1998.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish JL, Bean AM, Park RB, Androphy EJ. ChlR1 is required for loading papillomavirus E2 onto mitotic chromosomes and viral genome maintenance. Mol.Cell. 2006;24:867–876. doi: 10.1016/j.molcel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Peng YC, Breiding DE, Sverdrup F, Richard J, Androphy EJ. AMF-1/Gps2 binds p300 and enhances its interaction with papillomavirus E2 proteins. J Virol. 2000;74:5872–5879. doi: 10.1128/jvi.74.13.5872-5879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose KJ, Garcia-Alai M, Prat-Gay G, McBride AA. CK2 phosphorylation-induced conformational switch triggers degradation of the papillomavirus E2 protein. J.Biol.Chem. 2004;279:22430–22439. doi: 10.1074/jbc.M314340200. [DOI] [PubMed] [Google Scholar]
- Penrose KJ, McBride AA. Proteasome-mediated degradation of the papillomavirus E2-TA protein is regulated by phosphorylation and can modulate viral genome copy number. J.Virol. 2000;74:6031–6038. doi: 10.1128/jvi.74.13.6031-6038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky RB, Prakash SS, Corina K, Grossel MJ, Barsoum J, Androphy EJ. Sequences flanking the core DNA-binding domain of bovine papillomavirus type 1 E2 contribute to DNA binding function. J.Virol. 1997;71:828–831. doi: 10.1128/jvi.71.1.828-831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle R, Marcuzzi GP, Akgul B, Kasper HU, Schulze F, Haase I, Wickenhauser C, Pfister H. The human papillomavirus type 8 E2 protein induces skin tumors in transgenic mice. J.Invest Dermatol. 2008;128:2310–2315. doi: 10.1038/jid.2008.73. [DOI] [PubMed] [Google Scholar]
- Phelps WC, Howley PM. Transcriptional trans activation by the human papillomavirus type 16 E2 gene product. J.Virol. 1987;61:1630–1638. doi: 10.1128/jvi.61.5.1630-1638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piirsoo M, Ustav E, Mandel T, Stenlund A, Ustav M. Cis and trans requirements for stable episomal maintenance of the BPV-1 replicator. EMBO J. 1996;15:1–11. [PMC free article] [PubMed] [Google Scholar]
- Poddar A, Reed SC, McPhillips MG, Spindler JE, McBride AA. The human papillomavirus type 8 E2 tethering protein targets the ribosomal DNA loci of host mitotic chromosomes. J.Virol. 2009;83:640–650. doi: 10.1128/JVI.01936-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ML, Smith JA, Sowa ME, Harper JW, Iftner T, Stubenrauch F, Howley PM. NCoR1 mediates papillomavirus E8;E2C transcriptional repression. J.Virol. 2010;84:4451–4460. doi: 10.1128/JVI.02390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash SS, Grossman SR, Pepinsky RB, Laimins LA, Androphy EJ. Amino acids necessary for DNA contact and dimerization imply novel motifs in the papillomavirus E2 transactivator. Genes Dev. 1992;6:105–116. doi: 10.1101/gad.6.1.105. [DOI] [PubMed] [Google Scholar]
- Quinlan EJ, Culleton SP, Wu SY, Chiang CM, Androphy EJ. Acetylation of conserved lysines in bovine papillomavirus E2 by p300. J Virol. 2013;87:1497–1507. doi: 10.1128/JVI.02771-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Salazar E, Centeno F, Nieto K, Valencia-Hernandez A, Salcedo M, Garrido E. HPV16 E2 could act as down-regulator in cellular genes implicated in apoptosis, proliferation and cell differentiation. Virol J. 2011;8:247. doi: 10.1186/1743-422X-8-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinson T, Toots M, Kadaja M, Pipitch R, Allik M, Ustav E, Ustav M. Engagement of the ATR-Dependent DNA Damage Response at the Human Papillomavirus 18 Replication Centers during the Initial Amplification. J Virol. 2013;87:951–964. doi: 10.1128/JVI.01943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese DJ, Settleman J, Neary K, DiMaio D. Bovine papillomavirus E2 repressor mutant displays a high-copy-number phenotype and enhanced transforming activity. J.Virol. 1990;64:944–949. doi: 10.1128/jvi.64.2.944-949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Waltke M, Angeletti PC. Evolutionary variation of papillomavirus E2 protein and E2 binding sites. Virol J. 2011;8:379. doi: 10.1186/1743-422X-8-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanczuk H, Thierry F, Howley PM. Mutational analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P 97 and type 18 P 105 promoters. J.Virol. 1990;64:2849–2859. doi: 10.1128/jvi.64.6.2849-2859.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Yasugi T, Benson JD, Dowhanick JJ, Howley PM. Targeted mutagenesis of the human papillomavirus type 16 E2 transactivation domain reveals separable transcriptional activation and DNA replication functions. J.Virol. 1996;70:1602–1611. doi: 10.1128/jvi.70.3.1602-1611.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara N, Chen D, Jang MK, McBride AA. The Brd4 Chromatin Adaptor Protein is displaced from nuclear foci as the HPV E2 protein switches from Transcriptional to Replicational Modes. 2013 manuscript in submission. [Google Scholar]
- Sakakibara N, Chen D, McBride AA. Papillomaviruses use Recombination Dependent Replication to Vegetatively Amplify their Genomes in Differentiated Cells. PLOS Pathogens. 2013 doi: 10.1371/journal.ppat.1003321. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara N, Mitra R, McBride AA. The papillomavirus E1 helicase activates a cellular DNA damage response in viral replication foci. J.Virol. 2011;85:8981–8995. doi: 10.1128/JVI.00541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez IE, Dellarole M, Gaston K, Gay GD. Comprehensive comparison of the interaction of the E2 master regulator with its cognate target DNA sites in 73 human papillomavirus types by sequence statistics. Nucleic Acids Research. 2007 doi: 10.1093/nar/gkm1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CM, Sizov D, Seavers PR, Ortiz-Lombardia M, Antson AA. Transcription activator structure reveals redox control of a replication initiation reaction. Nucleic Acids Research. 2007;35:3504–3515. doi: 10.1093/nar/gkm166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CM, Stenlund A. Recruitment and loading of the E1 initiator protein: an ATP-dependent process catalysed by a transcription factor. EMBO J. 1998;17:7044–7055. doi: 10.1093/emboj/17.23.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CM, Stenlund A. Transcription factor dependent loading of the E1 initiator reveals modular assembly of the papillomavirus origin melting complex. J.Biol.Chem. 2000;275:3522–3534. doi: 10.1074/jbc.275.5.3522. [DOI] [PubMed] [Google Scholar]
- Sanders CM, Stenlund A. Mechanism and requirements for bovine papillomavirus, type 1, E1 initiator complex assembly promoted by the E2 transcription factor bound to distal sites. J.Biol.Chem. 2001;276:23689–23699. doi: 10.1074/jbc.M101861200. [DOI] [PubMed] [Google Scholar]
- Sanders CM, Stern PL, Maitland NJ. Characterization of human papillomavirus type 16 E2 protein and subdomains expressed in insect cells. Virology. 1995;211:418–433. doi: 10.1006/viro.1995.1424. [DOI] [PubMed] [Google Scholar]
- Sarafi TR, McBride AA. Domains of the BPV 1 E1 replication protein required for origin specific DNA binding and interaction with the E2 transactivator. Virology. 1995;211:385–396. doi: 10.1006/viro.1995.1421. [DOI] [PubMed] [Google Scholar]
- Schmidt HM, Steger G, Pfister H. Competitive binding of viral E2 protein and mammalian core binding factor to transcriptional control sequences of human papillomavirus type 8 and bovine papillomavirus type 1. J Virol. 1997;71:8029–8034. doi: 10.1128/jvi.71.10.8029-8034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Stenlund A. Assembly of a double hexameric helicase. Mol.Cell. 2005;20:377–389. doi: 10.1016/j.molcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Schuck S, Stenlund A. Surface mutagenesis of the bovine papillomavirus E1 DNA binding domain reveals residues required for multiple functions related to DNA replication. J.Virol. 2006;80:7491–7499. doi: 10.1128/JVI.00435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Schweiger MR, Ottinger M, You J, Howley PM. Brd4 independent transcriptional repression function of the papillomavirus E2 proteins. J.Virol. 2007;81:9612–9622. doi: 10.1128/JVI.00447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedman J, Stenlund A. The papillomavirus E1 protein forms a DNA-dependent hexameric complex with ATPase and DNA helicase activities. J.Virol. 1998;72:6893–6897. doi: 10.1128/jvi.72.8.6893-6897.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhar V, McBride AA. Phosphorylation regulates binding of the human papillomavirus type 8 E2 protein to host chromosomes. J Virol. 2012;86:10047–10058. doi: 10.1128/JVI.01140-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhar V, Reed SC, McBride AA. Interaction of the betapapillomavirus E2 tethering protein with mitotic chromosomes. J Virol. 2010;84:543–557. doi: 10.1128/JVI.01908-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine H, Fuse A, Inaba N, Takamizawa H, Simizu B. Detection of the human papillomavirus 6b E2 gene product in genital condyloma and laryngeal papilloma tissues. Virology. 1989;170:92–98. doi: 10.1016/0042-6822(89)90355-3. [DOI] [PubMed] [Google Scholar]
- Senechal H, Poirier GG, Coulombe B, Laimins LA, Archambault J. Amino acid substitutions that specifically impair the transcriptional activity of papillomavirus E2 affect binding to the long isoform of Brd4. Virology. 2007;358:10–17. doi: 10.1016/j.virol.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Sim J, Ozgur S, Lin BY, Yu JH, Broker TR, Chow LT, Griffith J. Remodeling of the human papillomavirus type 11 replication origin into discrete nucleoprotein particles and looped structures by the E2 protein. J.Mol.Biol. 2008;375:1165–1177. doi: 10.1016/j.jmb.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiadopoulos MH, McBride AA. The BPV 1 E2 transactivator and E2-TR repressor use different nuclear localization signals. J.Virol. 1996;70:1117–1124. doi: 10.1128/jvi.70.2.1117-1124.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiadopoulos MH, McBride AA. Bovine papillomavirus type 1 genomes and the E2 transactivator protein are closely associated with mitotic chromatin. J.Virol. 1998;72:2079–2088. doi: 10.1128/jvi.72.3.2079-2088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smal C, Wetzler DE, Dantur KI, Chemes LB, Garcia Alai MM, Dellarole M, Alonso LG, Gaston K, de Prat-Gay G. The human papillomavirus E7-E2 interaction mechanism in vitro reveals a finely tuned system for modulating available E7 and E2 proteins. Biochemistry. 2009;48:11939–11949. doi: 10.1021/bi901415k. [DOI] [PubMed] [Google Scholar]
- Smith DI, Zhu Y, McAvoy S, Kuhn R. Common fragile sites, extremely large genes, neural development and cancer. Cancer letters. 2006;232:48–57. doi: 10.1016/j.canlet.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Smith JA, White EA, Sowa ME, Powell ML, Ottinger M, Harper JW, Howley PM. Genome-wide siRNA screen identifies SMCX, EP400, and Brd4 as E2 dependent regulators of human papillomavirus oncogene expression. Proc Natl Acad Sci U S A. 2010;107:3752–3757. doi: 10.1073/pnas.0914818107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz BA, Vande Pol SB, Howley PM. Characterization of the cis elements involved in basal and E2-transactivated expression of the bovine papillomavirus P 2443 promoter. J.Virol. 1991;65:743–753. doi: 10.1128/jvi.65.2.743-753.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz BA, Yang YC, Howley PM. Transactivation of a bovine papilloma virus transcriptional regulatory element by the E2 gene product. Cell. 1985;42:183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- Steger G, Corbach S. Dose-dependent regulation of the early promoter of human papillomavirus type 18 by the viral E2 protein. J.Virol. 1997;71:50–58. doi: 10.1128/jvi.71.1.50-58.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlund A. E1 initiator DNA binding specificity is unmasked by selective inhibition of non specific DNA binding. EMBO J. 2003;22:954–963. doi: 10.1093/emboj/cdg091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlund A, Botchan MR. The E2 trans-activator can act as a repressor by interfering with a cellular transcription factor. Genes Dev. 1990;4:123–136. doi: 10.1101/gad.4.1.123. [DOI] [PubMed] [Google Scholar]
- Stubenrauch F, Colbert AM, Laimins LA. Transactivation by the E2 protein of oncogenic human papillomavirus type 31 is not essential for early and late viral functions. J.Virol. 1998a;72:8115–8123. doi: 10.1128/jvi.72.10.8115-8123.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch F, Hummel M, Iftner T, Laimins LA. The E8E2C protein, a negative regulator of viral transcription and replication, is required for extrachromosomal maintenance of human papillomavirus type 31 in keratinocytes. J.Virol. 2000;74:1178–1186. doi: 10.1128/jvi.74.3.1178-1186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch F, Leigh IM, Pfister H. E2 represses the late gene promoter of human papillomavirus type 8 at high concentrations by interfering with cellular factors. J.Virol. 1996;70:119–126. doi: 10.1128/jvi.70.1.119-126.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch F, Lim HB, Laimins LA. Differential requirements for conserved E2 binding sites in the life cycle of oncogenic human papillomavirus type 31. J.Virol. 1998b;72:1071–1077. doi: 10.1128/jvi.72.2.1071-1077.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch F, Pfister H. Low-affinity E2-binding site mediates downmodulation of E2 transactivation of the human papillomavirus type 8 late promoter. J.Virol. 1994;68:6959–6966. doi: 10.1128/jvi.68.11.6959-6966.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch F, Straub E, Fertey J, Iftner T. The E8 repression domain can replace the E2 transactivation domain for growth inhibition of HeLa cells by papillomavirus E2 proteins. Int.J.Cancer. 2007;121:2284–2292. doi: 10.1002/ijc.22907. [DOI] [PubMed] [Google Scholar]
- Stubenrauch F, Zobel T, Iftner T. The E8 domain confers a novel long-distance transcriptional repression activity on the E8E2C protein of high-risk human papillomavirus type 31. J.Virol. 2001;75:4139–4149. doi: 10.1128/JVI.75.9.4139-4149.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle CS, Zou N, Van Tine BA, Shaw GM, Engler JA, Chow LT. Human papillomavirus DNA replication compartments in a transient DNA replication system. J.Virol. 1999;73:1001–1009. doi: 10.1128/jvi.73.2.1001-1009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Gunaratne J, Lai D, Carthagena L, Wang Q, Xue YZ, Quek LS, Doorbar J, Bachelerie F, Thierry F, Bellanger S. HPV-18 E2^E4 chimera: 2 new spliced transcripts and proteins induced by keratinocyte differentiation. Virology. 2012;429:47–56. doi: 10.1016/j.virol.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Tan SH, Leong LE, Walker PA, Bernard HU. The human papillomavirus type 16 E2 transcription factor binds with low cooperativity to two flanking sites and represses the E6 promoter through displacement of Sp1 and TFIID. J.Virol. 1994;68:6411–6420. doi: 10.1128/jvi.68.10.6411-6420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ER, Boner W, Dornan ES, Corr EM, Morgan IM. UVB irradiation reduces the half-life and transactivation potential of the human papillomavirus 16 E2 protein. Oncogene. 2003;22:4469–4477. doi: 10.1038/sj.onc.1206746. [DOI] [PubMed] [Google Scholar]
- Thain A, Jenkins O, Clarke AR, Gaston K. CpG methylation directly inhibits binding of the human papillomavirus type 16 E2 protein to specific DNA sequences. J.Virol. 1996;70:7233–7235. doi: 10.1128/jvi.70.10.7233-7235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F, Demeret C. Direct activation of caspase 8 by the proapoptotic E2 protein of HPV18 independent of adaptor proteins. Cell death and differentiation. 2008;15:1356–1363. doi: 10.1038/cdd.2008.53. [DOI] [PubMed] [Google Scholar]
- Thierry F, Howley PM. Functional analysis of E2-mediated repression of the HPV18 P105 promoter. New Biol. 1991;3:90–100. [PubMed] [Google Scholar]
- Thierry F, Yaniv M. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987;6:3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titolo S, Pelletier A, Pulichino AM, Brault K, Wardrop E, White PW, Cordingley MG, Archambault J. Identification of domains of the human papillomavirus type 11 E1 helicase involved in oligomerization and binding to the viral origin. J.Virol. 2000;74:7349–7361. doi: 10.1128/jvi.74.16.7349-7361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan T, Kalantari M, Calleja Macias IE, Cubie HA, Cuschieri K, Villa LL, Skomedal H, Barrera Saldana HA, Bernard HU. Methylation of the human papillomavirus-18 L1 gene: a biomarker of neoplastic progression? Virology. 2006;349:175–183. doi: 10.1016/j.virol.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Ustav E, Ustav M, Szymanski P, Stenlund A. The bovine papillomavirus origin of replication requires a binding site for the E2 transcriptional activator. Proc.Natl.Acad.Sci.U.S.A. 1993;90:898–902. doi: 10.1073/pnas.90.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustav M, Ustav E, Szymanski P, Stenlund A. Identification of the origin of replication of bovine papillomavirus and characterization of the viral origin recognition factor E1. EMBO J. 1991;10:4321–4329. doi: 10.1002/j.1460-2075.1991.tb05010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt P, Nottoli T, Choe J, Botchan MR. The E2 transactivator of bovine papillomavirus type 1 is expressed from multiple promoters. J.Virol. 1990;64:3927–3937. doi: 10.1128/jvi.64.8.3927-3937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorslaer K, Khan J, Chapman S, McBride AA. Three E2 binding sites are sufficient for stable episomal maintenance of HPV18. 2013 Manuscript in preparation. [Google Scholar]
- Vande Pol SB, Howley PM. A bovine papillomavirus constitutive enhancer is negatively regulated by the E2 repressor through competitive binding for a cellular factor. J.Virol. 1990;64:5420–5429. doi: 10.1128/jvi.64.11.5420-5429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan S, Mello CC, Lee KM, Androphy EJ, Baleja JD. 1H, 15N, and 13C NMR resonance assignments for the DNA-binding domain of the BPV 1 E2 protein. J.Biomol.NMR. 1998;11:457–458. doi: 10.1023/a:1008237029912. [DOI] [PubMed] [Google Scholar]
- Vempati RK. DNA damage in the presence of chemical genotoxic agents induce acetylation of H3K56 and H4K16 but not H3K9 in mammalian cells. Molecular biology reports. 2012;39:303–308. doi: 10.1007/s11033-011-0739-9. [DOI] [PubMed] [Google Scholar]
- Vosa L, Sudakov A, Remm M, Ustav M, Kurg R. Identification and analysis of papillomavirus E2 protein binding sites in the human genome. J Virol. 2012;86:348–357. doi: 10.1128/JVI.05606-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Helfer CM, Pancholi N, Bradner JE, You J. Recruitment of brd4 to the human papillomavirus type 16 DNA replication complex is essential for replication of viral DNA. J Virol. 2013;87:3871–3884. doi: 10.1128/JVI.03068-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Naidu SR, Sverdrup F, Androphy EJ. Tax1BP1 interacts with papillomavirus E2 and regulates E2-dependent transcription and stability. J Virol. 2009;83:2274–2284. doi: 10.1128/JVI.01791-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Coulombe R, Cameron DR, Thauvette L, Massariol MJ, Amon LM, Fink D, Titolo S, Welchner E, Yoakim C, Archambault J, White PW. Crystal structure of the E2 transactivation domain of human papillomavirus type 11 bound to a protein interaction inhibitor. J.Biol.Chem. 2004;279:6976–6985. doi: 10.1074/jbc.M311376200. [DOI] [PubMed] [Google Scholar]
- Webster K, Parish J, Pandya M, Stern PL, Clarke AR, Gaston K. The human papillomavirus (HPV) 16 E2 protein induces apoptosis in the absence of other HPV proteins and via a p53 dependent pathway. J.Biol.Chem. 2000;275:87–94. doi: 10.1074/jbc.275.1.87. [DOI] [PubMed] [Google Scholar]
- Winokur PL, McBride AA. Separation of the transcriptional activation and replication functions of the bovine papillomavirus-1 E2 protein. EMBO J. 1992;11:4111–4118. doi: 10.1002/j.1460-2075.1992.tb05504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winokur PL, McBride AA. The transactivation and DNA binding domains of the BPV-1 E2 protein have different roles in cooperative origin binding with the E1 protein. Virology. 1996;221:44–53. doi: 10.1006/viro.1996.0351. [DOI] [PubMed] [Google Scholar]
- Wu SY, Lee AY, Hou SY, Kemper JK, Erdjument Bromage H, Tempst P, Chiang CM. Brd4 links chromatin targeting to HPV transcriptional silencing. Genes Dev. 2006;20:2383–2396. doi: 10.1101/gad.1448206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Bian XL, Heaton PR, Deyrieux AF, Wilson VG. Host cell sumoylation level influences papillomavirus E2 protein stability. Virology. 2009;387:176–183. doi: 10.1016/j.virol.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Roark AA, Bian XL, Wilson VG. Modification of papillomavirus E2 proteins by the small ubiquitin-like modifier family members (SUMOs). Virology. 2008;378:329–338. doi: 10.1016/j.virol.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Bellanger S, Zhang W, Lim D, Low J, Lunny D, Thierry F. HPV16 E2 is an immediate early marker of viral infection, preceding E7 expression in precursor structures of cervical carcinoma. Cancer Res. 2010;70:5316–5325. doi: 10.1158/0008-5472.CAN-09-3789. [DOI] [PubMed] [Google Scholar]
- Yao JM, Breiding DE, Androphy EJ. Functional interaction of the bovine papillomavirus E2 transactivation domain with TFIIB. J Virol. 1998;72:1013–1019. doi: 10.1128/jvi.72.2.1013-1019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasugi T, Benson JD, Sakai H, Vidal M, Howley PM. Mapping and characterization of the interaction domains of human papillomavirus type 16 E1 and E2 proteins. J.Virol. 1997;71:891–899. doi: 10.1128/jvi.71.2.891-899.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Croyle JL, Nishimura A, Ozato K, Howley PM. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell. 2004;117:349–360. doi: 10.1016/s0092-8674(04)00402-7. [DOI] [PubMed] [Google Scholar]
- You J, Schweiger MR, Howley PM. Inhibition of E2 binding to Brd4 enhances viral genome loss and phenotypic reversion of bovine papillomavirus transformed cells. J.Virol. 2005;79:14956–14961. doi: 10.1128/JVI.79.23.14956-14961.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Peng YC, Androphy EJ. Mitotic kinesin like protein 2 binds and colocalizes with papillomavirus E2 during mitosis. J.Virol. 2007;81:1736–1745. doi: 10.1128/JVI.01638-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao KN, Hengst K, Liu WJ, Liu YH, Liu XS, McMillan NA, Frazer IH. BPV1 E2 Protein Enhances Packaging of Full-Length Plasmid DNA in BPV1 Pseudovirions. Virology. 2000;272:382–393. doi: 10.1006/viro.2000.0348. [DOI] [PubMed] [Google Scholar]
- Zheng G, Schweiger MR, Martinez-Noel G, Zheng L, Smith JA, Harper JW, Howley PM. Brd4 regulation of papillomavirus protein E2 stability. J Virol. 2009;83:8683–8692. doi: 10.1128/JVI.00674-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng PS, Brokaw J, McBride AA. Conditional mutations in the mitotic chromosome binding function of the bovine papillomavirus type 1 E2 protein. J.Virol. 2005;79:1500–1509. doi: 10.1128/JVI.79.3.1500-1509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel T, Iftner T, Stubenrauch F. The papillomavirus E8-E2C protein represses DNA replication from extrachromosomal origins. Mol.Cell Biol. 2003;23:8352–8362. doi: 10.1128/MCB.23.22.8352-8362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou N, Lin BY, Duan F, Lee KY, Jin G, Guan R, Yao G, Lefkowitz EJ, Broker TR, Chow LT. The hinge of the human papillomavirus type 11 E2 protein contains major determinants for nuclear localization and nuclear matrix association. J.Virol. 2000;74:3761–3770. doi: 10.1128/jvi.74.8.3761-3770.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.