Abstract
Current approaches for treating peripheral nerve injury have resulted in promising, yet insufficient functional recovery compared to the clinical standard of care, autologous nerve grafts. In order to design a construct that can match the regenerative potential of the autograft, all facets of nerve tissue must be incorporated in a combinatorial therapy. Engineered biomaterial scaffolds in the future will have to promote enhanced regeneration and appropriate reinnervation by targeting the highly sensitive response of regenerating nerves to their surrounding microenvironment.
Introduction
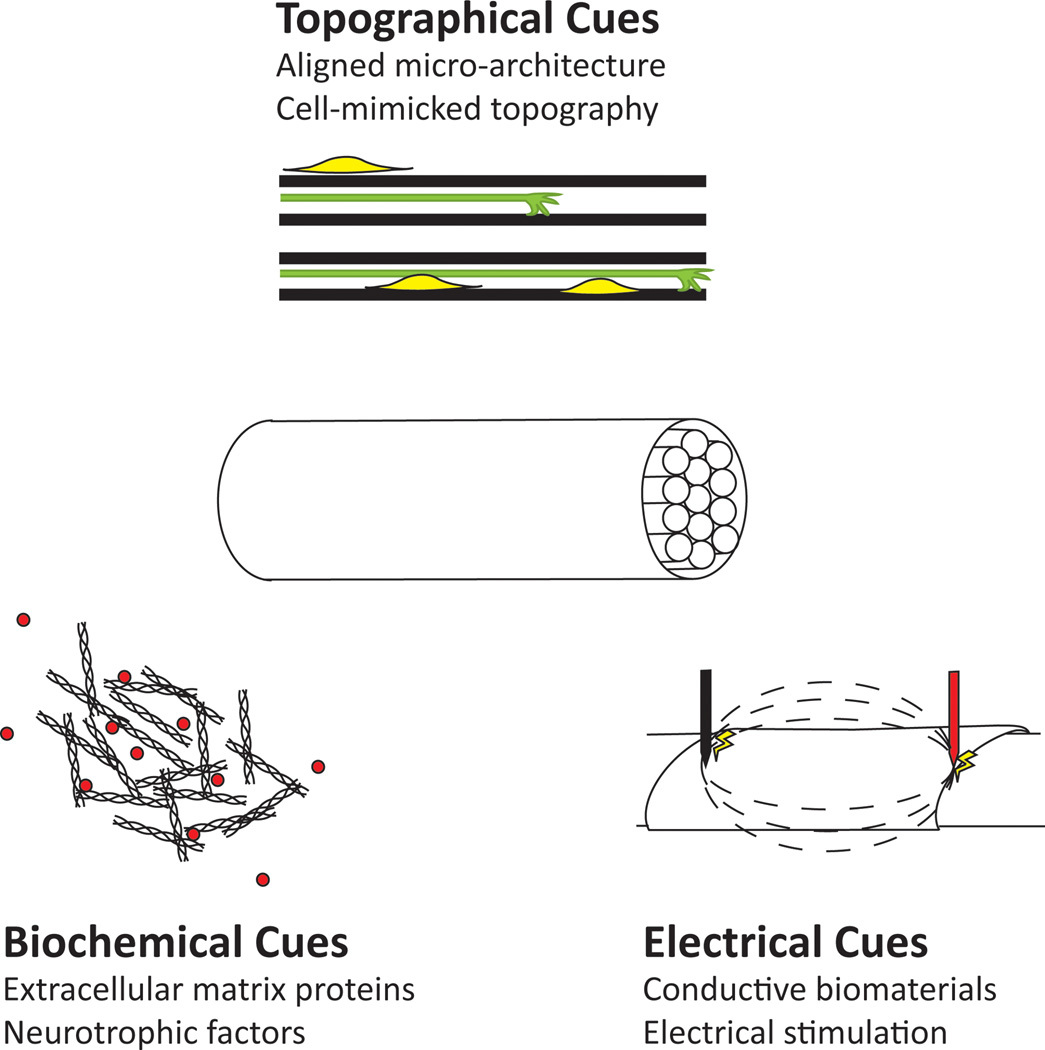
Currently there is a great disparity in functional outcomes between engineered biomaterials for nerve repair and the clinical standard of care, nerve autografts [1]. This disparity has led to a multitude of approaches to target the complexity of nerve regeneration. Biomaterials are currently being tailored to address these issues because currently marketed nerve guidance conduits (NGCs) cannot match the performance of autografts in large nerve defects (greater than 10 mm in rats, or greater than 30 mm in humans) [2]. Yet, an engineered construct capable of promoting neuronal survival, as well as axon extension and guidance is needed to provide equivalent functional outcomes to an autograft. This “off the shelf” alternative is desirable to prevent harvesting tissue that results in donor site morbidity and to improve upon the limitations of autograft recovery, where less than 25% of patients regain proper motor function and less than 3% regain sensation [3]. Current approaches focus on the sensitivity of regenerating axons to the surrounding environment, which includes surface topography, biochemical cues, and electrical activity. Surface topography has been well established as a mediator of axonal guidance and extension [4]. Thus, many groups have focused on incorporating architecture that mimics the native nerve into engineered constructs to better orient regenerating nerves and promote appropriate reinnervation. Neuronal survival and axon extension has been improved by functionalizing biomaterial scaffolds with neurotrophic factors (NFs) and extracellular matrix (ECM) proteins (or peptides derived from these proteins). Research in this field has shown the inclusion of factors found within native nerve tissue, using either natural or synthetic biomaterial scaffolds, yielded enhanced regenerative capacity. The inherent electrical activity of nerve has prompted the development of electrically conductive biomaterials that may promote increased axonal regeneration with electrical stimulation. Alone, each of these strategies may improve regeneration across moderate gaps, yet many studies described in this review have focused on combination therapies that incorporate of two or more of these elements (Figure 1). This review details current research in this field, in which the development of multi-faceted biomaterial scaffolds may improve the functional outcomes to the level of autografts or improve beyond autograft levels.
Figure 1.
In order to engineer a nerve guidance conduit that promotes enhanced functional recovery, many aspects of native nerve architecture and function must be incorporated in the design. Regenerating axons are sensitive to the microenvironment of nerves that includes topographical cues, growth promoting biochemical cues such as ECM proteins and neurotrophic factors, and the excitability of neurons through electrical stimulation.
Engineering physical and topographical cues for neural guidance
Native nerve architecture includes an elongated, fascicular morphology that enables axonal guidance following injury through the formation of the Bands of Büngner. Bands of Büngner are formed by proliferating Schwann cells that help guide regenerating axons to target organs. Commercially available NGCs are often hollow tubes or nerve wraps that lack this native architecture, thus many groups have focused on developing materials that provide guidance within conduits connecting the proximal and distal nerve stumps after injury. Ribeiro-Resende et al. attempted to promote the generation of artificial Bands of Büngner through aligned collagen and poly-ε-caprolactone (PCL) filament constructs. Seeded with Schwann cells, these aligned microfilaments were capable of promoting enhanced, oriented outgrowth of dorsal root ganglia (DRG) neurites in vitro [5]. This study also found through combination of topographical cues, as well as what they termed “polarizing” differentiation factors, nerve growth factor (NGF), neuregulin-1, and transforming growth factor-β (TGF-β), they achieved increased Schwann cell orientation, which in turn provided better axonal guidance. The Hoffman-Kim group has focused on mimicking the native Bands of Büngner architecture through the development of Schwann cell imprinted molds [6]. Cell topographical molds were created from aligned Schwann cell substrates that were also capable of promoting highly aligned neurite outgrowth from DRG neurons in vitro. This group further developed conduits based off of this Schwann cell-mimicking topography that influenced DRG neurite extension, as well as cell migration patterns, which may prove useful in vivo [7].
Many groups have developed highly aligned, porous biomaterial scaffolds of natural [8–11] and synthetic materials [12–14] that aim to provide longitudinally, aligned substrates to guide regenerating axons. In addition to intraluminal porosity and topography, the effect of conduit porosity is important as increased porosity may decrease axonal regeneration toward the distal nerve segment. Oh et al. observed that conduits with nanopores increased longitudinal regeneration, whereas microporous conduits caused regeneration into the pores [15]. In vivo, Daly et al. showed aligned conduits aided regeneration of axons through the use of ultra-structured, grooved collagen fibers. Intraluminal collagen fibers with laser-fabricated, microgrooves reduced axonal mismatch with the distal nerve stumps compared to unstructured collagen fibers or hollow collagen conduits; however, functional recovery has yet to be tested [16].
One of the most popular methods of creating aligned biomaterial substrates is through electrospinning. In vitro, electrospun scaffolds have been shown to promote cell migration and guide neurite extension from DRGs [17]. Electrospun scaffolds are commonly fabricated from synthetic materials, such as PCL [18–20], poly-acrylonitrile (PAN) [21], and poly-L-lactic acid (PLLA) [22], and natural materials, such as silk, collagen, and blends of silk and PLLA [23,24]. In vivo, aligned electrospun fibers promoted significantly enhanced axon regeneration in a sciatic nerve injury model, as assessed by increased nerve fiber number, electrical activity, and motor reinnervation compared to randomly aligned electrospun fiber mats [21,24–26]. These studies show the importance in designing scaffolds that provide structure similar to that of native nerve architecture, as well as topological guidance for regenerating axons to the distal target of innervation.
Enhancing biomaterial scaffolds with axon promoting factors
In addition to topographical cues, many engineered conduits now incorporate important growth factors and adhesion cues, such as NFs and ECM proteins. Laminin mediates cell survival, axon extension and cell adhesion through specific peptide sequences, IKVAV and YIGSR, as well as important integrin signaling so its role in nerve regeneration has been well studied [25,27–30]. Cao et al. developed linear ordered collagen scaffolds that have been modified with laminin by covalent attachment to promote axonal regeneration. In addition, laminin was used as a means for delivery of ciliary neurotrophic factor (CNTF) and brain-derived neurotrophic factor (BDNF) via laminin-binding domains (LBD). Laminin alone improved myelinated axon number in vivo, yet the controlled delivery of CNTF through the LBD, showed an additional improvement in axon regeneration and conduction velocity of the regenerating sciatic nerve [31]. Controlled delivery of BDNF and CNTF also showed improved compound muscle action potential (CMAP) activity of rat facial nerves [32]. The incorporation of biochemical factors, such as laminin, CNTF, and BDNF, indicate that while structural cues from the collagen scaffold are important, additional cues can further enhance functional outcomes.
ECM proteins that are native to nerve architecture have proven useful in enhancing neurite outgrowth in vitro and in vivo. Fibronectin is an ECM protein that is important for cell migration and adhesion via integrin binding to the RGD domain. Fibronectin has shown to promote neurite extension in vitro in combination with various polymer scaffolds, including aligned electrospun PAN-methacrylate, polyethylene glycol, and collagen [17,33]. Engineered elastin-like protein hydrogels, which contain RGD binding sites and mimic native nerve mechanical properties in a controlled manner, significantly increased neurite extension from DRGs in vitro [34]. These tunable hydrogels may prove useful in fabricating tailored biomaterial scaffolds that provide optimal adhesion properties for regenerating axons.
One of the best commercially available options for treating nerve defects, specifically long nerve gaps, are acellular nerve allografts. These are nerve grafts that undergo a decellularization process, either through chemical or thermal processing, that removes immunogenic, cellular components of the tissue [35]. This processing maintains most of the native nerve architecture composed of important ECM proteins, such as laminin and collagen, which can promote enhanced regeneration and functional recovery in combination with the structural cues in long term studies [36]. Acellular nerve allografts are also being used as a platform for delivery of cells and NFs. For example, Wang et al. has optimized acellular grafts to deliver bone mesenchymal stromal cells (BMSCs) to stimulate enhanced axon regeneration, and chondroitinase ABC to remove inhibitory molecules. This combination therapy stimulated secretion of NFs, such as NGF and BDNF, increased Schwann cell markers and angiogenesis markers, vascular endothelial growth factor (VEGF) and CD34, expression and decreased inhibitory chondroitin sulfate proteoglycans in the regenerating nerve. This approach increased myelinated nerve fiber number, myelin thickness, and axon diameter; again suggesting that while the acellular grafts are an excellent platform, combinatorial strategies can further enhance axonal regeneration and functional recovery through acellular grafts [37].
Many scaffolds, from both synthetic and natural polymers, have been functionalized to deliver NFs and ECM proteins through various chemical crosslinking methods. In Shepard, et al., PEG hydrogels were functionalized to locally deliver viral vector constructs for NGF via affinity peptides, which promoted increased neurite outgrowth from DRGs in vitro. These gels also encapsulated protease-secreting HT-1080 cells in order mimic infiltrating cells after injury that may degrade the hydrogel crosslinks and increase viral vector release [38]. Affinity peptides have also proven useful for the controlled delivery of NFs, such as NGF and GDNF, from fibrin matrices, which promoted enhanced motor regeneration, target reinnervation and functional recovery [39–41]. Both affinity-based peptides, as above, and chemical conjugation methods have been used to delivery NFs and ECM proteins in a controlled manner that may prove ideal for in vivo regeneration [42–46].
Stimulating nerve regeneration through conductive biomaterials and electrical stimulation
As previously described, many strategies are focused on mimicking native nerve attributes including architecture, protein composition, and NF delivery. Another strategy has focused on the inherent electrical excitability of neurons. In vitro and in vivo, electrical stimulation has shown to increase neurite extension and axonal regeneration [47,48]. Thus, engineering a biomaterial scaffold that is electrically conductive may improve regeneration and functional recovery following injury.
In vitro investigation has shown that using electrically conductive materials, such as polypyrrole (PPy) and polyaniline (PANi) in small amounts, combined with other well-characterized, degradable polymers, are capable of promoting enhanced neurite extension with low electrical stimulation. Song et al. demonstrated increased neurite extension area in complex geometries when photoresist patterns were doped with electrically conductive polymers, (PPy) as well as chemically conjugated NGF and poly-L-lysine/laminin [49,50]. The Schmidt group developed scaffolds in which NGF was chemically conjugated to PPy and PPy-PLGA scaffolds where it was found to increase the percentage of neurite expressing cells and the average PC12 cell neurite length in vitro with electrical stimulation [51–54]. PLLA-PANi scaffolds have shown promise for directing neural stem cell (NSC) differentiation, as electrical stimulation of PLLA-PANi scaffolds promoted elongated, neurite morphology of NSCs compared to unstimulated controls [55].
Polycaprolactone fumarate (PCLF)-PPy scaffolds were developed to promote increased neurite extension, where it was observed that only scaffolds formed via specific anions needed for PPy stabilization, naphthalene-2-sulfonic acid sodium salt (NSA) and dodecylbenzenesulfonic acid sodium salt (DBSA), can support cell adhesion, survival, and neurite extension [56]. These scaffolds were then fabricated into NGCs without any disruption of material properties. These scaffolds promoted enhanced neurite length and percent neurite-expressing PC12 cells with electrical stimulation and were capable of promoting aligned neurite extension in the direction of the applied electrical current [57]. In vivo, Huang, et al. demonstrated porous, biodegradable PPy-chitosan conduits increased regeneration and functional recovery following intermittent electrical stimulation. Conductive scaffolds in combination with electrical stimulation increased nerve fiber and myelinated fiber number, enhanced motor and sensory regeneration, functional recovery, and decreased muscle atrophy [58]. These electrically conductive scaffolds have shown promise in increasing nerve regeneration in large gaps with electrical stimulation; thus incorporating electrical conductive materials into chemically and structurally designed constructs may be necessary to promote enhanced functional motor and sensory recovery.
Remaining Challenges
While the current research shows great potential, regeneration across gaps greater than 30 mm remains a major challenge clinically particularly for patients who suffer multiple injuries due to trauma. In addition to providing structural and biochemical cues, future combination therapies should focus on how these cues can be modulated spatially and temporally in response to the speed of regeneration. For example, the growth factor concentration at a given location in the distal nerve may need to increase early during the regeneration process to promote axon growth toward that site, and then decrease after the growth cones have passed to prevent axon trapping at that particular location thus allowing innervation of the target muscle. New methods that allow modulation of cues will be key to improving long-range regeneration and function.
Conclusion
Current strategies, focused on mimicking nerve structure and function, have shown vast improvement over unstructured, commercially available, hollow NGCs. However, to provide a microenvironment similar to a nerve autograft, engineered constructs must incorporate cues from native nerves including surface topography, biochemical signals, and electrically active tissue. Thus, any future development of NGCs of synthetic or natural materials will have to be a combinatorial approach that includes several of these aspects to target functional outcomes that match the clinical standard, autografts.
Highlights.
Commercial nerve guidance conduits are insufficient to promote enhanced regeneration.
Multi-faceted approaches are needed to mimic native nerve architecture and function.
Engineered constructs with topographical cues facilitate aligned axonal regeneration.
Delivery of native nerve biochemical and electrical cues improve functional outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Moore A, Kasukurthi R, Magill C, Farhadi HF, Borschel G, Mackinnon S. Limitations of Conduits in Peripheral Nerve Repairs. HAND. 2009;4:180–186. doi: 10.1007/s11552-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, Moore AM, Tong AY, Mackinnon SE, Borschel GH. Processed Allografts and Type I Collagen Conduits for Repair of Peripheral Nerve Gaps. Muscle & Nerve. 2009;39:787–799. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
- 3.Archibald SJ, Shefner J, Krarup C, Madison RD. Monkey Median Nerve Repaired by Nerve Graft or Collagen Nerve Guide Tube. Journal of Neuroscience. 1995;15:4109–4123. doi: 10.1523/JNEUROSCI.15-05-04109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spivey EC, Khaing ZZ, Shear JB, Schmidt CE. The fundamental role of subcellular topography in peripheral nerve repair therapies. Biomaterials. 2012;33:4264–4276. doi: 10.1016/j.biomaterials.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro-Resende VT, Koenig B, Nichterwitz S, Oberhoffner S, Schlosshauer B. Strategies for inducing the formation of bands of Bungner in peripheral nerve regeneration. Biomaterials. 2009;30:5251–5259. doi: 10.1016/j.biomaterials.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Bruder JM, Lee AP, Hoffman-Kim D. Biomimetic materials replicating Schwann cell topography enhance neuronal adhesion and neurite alignment in vitro. Journal of Biomaterials Science, Polymer Edition. 2007;18:967–982. doi: 10.1163/156856207781494412. [DOI] [PubMed] [Google Scholar]
- 7. Richardson JA, Rementer CW, Bruder JM, Hoffman-Kim D. Guidance of dorsal root ganglion neurites and Schwann cells by isolated Schwann cell topography on poly(dimethyl siloxane) conduits and films. Journal of Neural Engineering. 2011;8 doi: 10.1088/1741-2560/8/4/046015. The authors demonstrated aligned conduits with micropatterned substrates that mimic the natural topography of Schwann cells was capable of supporting highly aligned, directional and increased outgrowth of dorsal root ganglia neurites.
- 8.Cullen DK, Tang-Schomer MD, Struzyna LA, Patel AR, Johnson VE, Wolf JA, Smith DH. Microtissue Engineered Constructs with Living Axons for Targeted Nervous System Reconstruction. Tissue Engineering Part A. 2012;18:2280–2289. doi: 10.1089/ten.tea.2011.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidenko N, Gibb T, Schuster C, Best SM, Campbell JJ, Watson CJ, Cameron RE. Biomimetic collagen scaffolds with anisotropic pore architecture. Acta Biomaterialia. 2012;8:667–676. doi: 10.1016/j.actbio.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Pawar K, Mueller R, Caioni M, Prang P, Bogdahn U, Kunz W, Weidner N. Increasing capillary diameter and the incorporation of gelatin enhance axon outgrowth in alginate-based anisotropic hydrogels. Acta Biomaterialia. 2011;7:2826–2834. doi: 10.1016/j.actbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Suri S, Han LH, Zhang WD, Singh A, Chen SC, Schmidt CE. Solid freeform fabrication of designer scaffolds of hyaluronic acid for nerve tissue engineering. Biomedical Microdevices. 2011;13:983–993. doi: 10.1007/s10544-011-9568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan JA, Zhang HT, He JH, Xiao ZF, Chen B, Xiaodan JA, Dai JW, Xu RX. Neural regrowth induced by PLGA nerve conduits and neurotrophin-3 in rats with complete spinal cord transection. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2011;97B:271–277. doi: 10.1002/jbm.b.31810. [DOI] [PubMed] [Google Scholar]
- 13.He LM, Zhang YQ, Zeng CG, Ngiam M, Liao S, Quan DP, Zeng YS, Lu J, Ramakrishna S. Manufacture of PLGA Multiple-Channel Conduits with Precise Hierarchical Pore Architectures and In Vitro/Vivo Evaluation for Spinal Cord Injury. Tissue Engineering Part C-Methods. 2009;15:243–255. doi: 10.1089/ten.tec.2008.0255. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, De Laporte L, Zelivyanskaya ML, Whittlesey KJ, Anderson AJ, Cummings BJ, Shea LD. Multiple Channel Bridges for Spinal Cord Injury: Cellular Characterization of Host Response. Tissue Engineering Part A. 2009;15:3283–3295. doi: 10.1089/ten.tea.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh SH, Kim JR, Kwon GB, Namgung U, Song KS, Lee JH. Effect of Surface Pore Structure of Nerve Guide Conduit on Peripheral Nerve Regeneration. Tissue Eng Part C Methods. 2012;19(3):233–243. doi: 10.1089/ten.tec.2012.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly WT, Yao L, Abu-rub MT, O'Connell C, Zeugolis DI, Windebank AJ, Pandit AS. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials. 2012;33:6660–6671. doi: 10.1016/j.biomaterials.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Mukhatyar VJ, Salmeron-Sanchez M, Rudra S, Mukhopadaya S, Barker TH, Garcia AJ, Bellamkonda RV. Role of fibronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials. 2011;32:3958–3968. doi: 10.1016/j.biomaterials.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie JW, MacEwan MR, Li XR, Sakiyama-Elbert SE, Xia YN. Neurite Outgrowth on Nanofiber Scaffolds with Different Orders, Structures, and Surface Properties. Acs Nano. 2009;3:1151–1159. doi: 10.1021/nn900070z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie JW, Willerth SM, Li XR, Macewan MR, Rader A, Sakiyama-Elbert SE, Xia YN. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials. 2009;30:354–362. doi: 10.1016/j.biomaterials.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BK, Ju YM, Cho JG, Jackson JD, Lee SJ, Atala A, Yoo JJ. End-to-side neurorrhaphy using an electrospun PCL/collagen nerve conduit for complex peripheral motor nerve regeneration. Biomaterials. 2012;33:9027–9036. doi: 10.1016/j.biomaterials.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Kim YT, Haftel VK, Kumar S, Bellamkonda RV. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29:3117–3127. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kijenska E, Prabhakaran MP, Swieszkowski W, Kurzydlowski KJ, Ramakrishna S. Electrospun bio-composite P(LLA-CL)/collagen I/collagen III scaffolds for nerve tissue engineering. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2012;100B:1093–1102. doi: 10.1002/jbm.b.32676. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Ki CS, Park YH, Lee KG, Kang SW, Kweon HY, Kim HJ. Functional recovery guided by an electrospun silk fibroin conduit after sciatic nerve injury in rats. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1615. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Hu X, Lin W, Dong C, Wu H. Electrospun PLGA-silk fibroin-collagen nanofibrous scaffolds for nerve tissue engineering. In Vitro Cell Dev Biol Anim. 2011;47:234–240. doi: 10.1007/s11626-010-9381-4. [DOI] [PubMed] [Google Scholar]
- 25.Neal RA, Tholpady SS, Foley PL, Swami N, Ogle RC, Botchwey EA. Alignment and composition of laminin-polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J Biomed Mater Res A. 2011;100A(2):406–423. doi: 10.1002/jbm.a.33204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu YQ, Wang AJ, Patel S, Kurpinski K, Diao E, Bao X, Kwong G, Young WL, Li S. Engineering Bi-Layer Nanofibrous Conduits for Peripheral Nerve Regeneration. Tissue Engineering Part C-Methods. 2011;17:705–715. doi: 10.1089/ten.tec.2010.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurga M, Dainiak MB, Sarnowska A, Jablonska A, Tripathi A, Plieva FM, Savina IN, Strojek L, Jungvid H, Kumar A, et al. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials. 2011;32:3423–3434. doi: 10.1016/j.biomaterials.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 28.Marquardt L, Willits RK. Neurite growth in PEG gels: Effect of mechanical stiffness and laminin concentration. Journal of Biomedical Materials Research Part A. 2011;98A:1–6. doi: 10.1002/jbm.a.33044. [DOI] [PubMed] [Google Scholar]
- 29.Swindle-Reilly KE, Papke JB, Kutosky HP, Throm A, Hammer JA, Harkins AB, Willits RK. The impact of laminin on 3D neurite extension in collagen gels. Journal of Neural Engineering. 2012;9 doi: 10.1088/1741-2560/9/4/046007. [DOI] [PubMed] [Google Scholar]
- 30.Zustiak SP, Durbal R, Leach JB. Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties. Acta Biomaterialia. 2010;6:3404–3414. doi: 10.1016/j.actbio.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao JI, Sun CK, Zhao H, Xiao ZF, Chen B, Gao J, Zheng TZ, Wu W, Wu S, Wang JY, et al. The use of laminin modified linear ordered collagen scaffolds loaded with laminin-binding ciliary neurotrophic factor for sciatic nerve regeneration in rats. Biomaterials. 2011;32:3939–3948. doi: 10.1016/j.biomaterials.2011.02.020. Linear ordered collagen scaffolds were functionalized with laminin, which served both as a growth-promoting element, as well as a method of controlled delivery for CNTF. CNTF with a laminin-binding domain significantly prolonged the release of CNTF from the scaffold, and enhanced functional recovery following sciatic nerve repair was seen.
- 32. Cao J, Xiao Z, Jin W, Chen B, Meng D, Ding W, Han S, Hou X, Zhu T, Yuan B, et al. Induction of rat facial nerve regeneration by functional collagen scaffolds. Biomaterials. 2012;34(4):1302–1310. doi: 10.1016/j.biomaterials.2012.10.031. CNTF and BDNF were delivered from linear ordered collagen scaffolds through collagen and laminin-binding domains allowing for sustained delivery that increased regeneration of rat facial nerves and compound muscle action potential activity compared to controls.
- 33.Zhou W, Blewitt M, Hobgood A, Willits RK. Comparison of neurite growth in three dimensional natural and synthetic hydrogels. Journal of Biomaterials Science, Polymer Edition. 2012:1–14. doi: 10.1080/09205063.2012.690277. [DOI] [PubMed] [Google Scholar]
- 34. Lampe KJ, Antaris AL, Heilshorn SC. Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2012;9(3):5590–5599. doi: 10.1016/j.actbio.2012.10.033. In vitro studies here demonstrated the ability to design elastin-like protein hydrogels with highly tailored ligand binding sites of RGD peptide as well as mechanical properties. The stiffness of the hydrogel, in addition to the number of RGD ligands, significantly influenced DRG outgrowth further demonstrating the sensitivity of growing neurites to their microenvironment. These hydrogels can easily be fabricated into matrices for nerve conduits.
- 35.Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Engineering. 2004;10:1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 36. Nagao RJ, Lundy S, Khaing ZZ, Schmidt CE. Functional characterization of optimized acellular peripheral nerve graft in a rat sciatic nerve injury model. Neurological Research. 2011;33:600–608. doi: 10.1179/1743132810Y.0000000023. Acellular nerve allografts, developed though a chemical decellularization process, showed significant regeneration and functional recovery similar to that of isografts in long-term studies. They showed comparable immune response to isografts and significantly less response compared to thermal and other chemical decellularization methods.
- 37. Wang Y, Jia H, Li WY, Tong XJ, Liu GB, Kang SW. Synergistic Effects of Bone Mesenchymal Stem Cells and Chondroitinase ABC on Nerve Regeneration After Acellular Nerve Allograft in Rats. Cellular and Molecular Neurobiology. 2012;32:361–371. doi: 10.1007/s10571-011-9764-4. Acellular nerve allografts were utilized to deliver BMSCs for NF secretion and chondroitinase ABC to remove any inhibitory CSPG molecules. This combinatorial strategy resulted in enhanced regeneration and nerve conduction velocity, as well as increased NGF, BDNF, S-100b, VEGF, and CD34 expression.
- 38. Shepard JA, Wesson PJ, Wang CE, Stevans AC, Holland SJ, Shikanov A, Grzybowski BA, Shea LD. Gene therapy vectors with enhanced transfection based on hydrogels modified with affinity peptides. Biomaterials. 2011;32:5092–5099. doi: 10.1016/j.biomaterials.2011.03.083. To enhance local delivery of viral vectors, the authors developed a PEG hydrogel that uses different affinity peptides (plasmin-sensitive and lysine-based peptides) that bind and release lipoplexes. The viral vectors encoding for NGF released from functionalized hydrogels were able to support significant neurite extension from DRG neurons. These peptides were also able to bind and release NGF, indicating dual usage of these gels to deliver both vector and growth factor.
- 39.Moore AM, Wood MD, Chenard K, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE, Borschel GH. Controlled Delivery of Glial Cell Line-Derived Neurotrophic Factor Enhances Motor Nerve Regeneration. Journal of Hand Surgery-American Volume. 2010;35A:2008–2017. doi: 10.1016/j.jhsa.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Wood MD, Borschel GH, Sakiyama-Elbert SE. Controlled release of glial-derived neurotrophic factor from fibrin matrices containing an affinity-based delivery system. Journal of Biomedical Materials Research Part A. 2009;89A:909–918. doi: 10.1002/jbm.a.32043. [DOI] [PubMed] [Google Scholar]
- 41. Wood MD, MacEwan MR, French AR, Moore AM, Hunter DA, Mackinnon SE, Moran DW, Borschel GH, Sakiyama-Elbert SE. Fibrin Matrices With Affinity-Based Delivery Systems and Neurotrophic Factors Promote Functional Nerve Regeneration. Biotechnology and Bioengineering. 2010;106:970–979. doi: 10.1002/bit.22766. This study showed the effect of controlled NF release, specifically GDNF and NGF, on motor nerve regeneration. GDNF and NGF conduits with a heparin binding delivery system demonstrated similar behavioral results to that of isografts. Delivery of GDNF resulted in the most significant motor neuron regeneration through retrograde labeling, though both GDNF and NGF were greater than no growth factor conduits.
- 42.Cho YI, Choi JS, Jeong SY, Yoo HS. Nerve growth factor (NGF)-conjugated electrospun nanostructures with topographical cues for neuronal differentiation of mesenchymal stem cells. Acta Biomaterialia. 2010;6:4725–4733. doi: 10.1016/j.actbio.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 43. Hsieh SC, Tang CM, Huang WT, Hsieh LL, Lu CM, Chang CJ, Hsu SH. Comparison between two different methods of immobilizing NGF in poly(DL-lactic acid-co-glycolic acid) conduit for peripheral nerve regeneration by EDC/NHS/MES and genipin. Journal of Biomedical Materials Research Part A. 2011;99A:576–585. doi: 10.1002/jbm.a.33157. The authors developed two methods of immobilizing NGF within PLGA conduits: through EDC chemistry and genipin (GP) chitosan-crosslinking. Through various combinations of EDC and GP chemistry, they found GP crosslinking of chitosan and NGF greatly improved in vitro PC12 survival and morphology. A GP/EDCs combination demonstrated the highest NGF release, which was characterized by a 5 day burst release, then 10–40 day controlled release, and had the highest number of midline and distal, myelinated nerve fibers in vivo.
- 44.Scott RA, Elbert DL, Willits RK. Modular poly(ethylene glycol) scaffolds provide the ability to decouple the effects of stiffness and protein concentration on PC12 cells. Acta Biomaterialia. 2011;7:3841–3849. doi: 10.1016/j.actbio.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vulic K, Shoichet MS. Tunable growth factor delivery from injectable hydrogels for tissue engineering. J Am Chem Soc. 2012;134:882–885. doi: 10.1021/ja210638x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu HX, Yan YH, Li SP. PDLLA/chondroitin sulfate/chitosan/NGF conduits for peripheral nerve regeneration. Biomaterials. 2011;32:4506–4516. doi: 10.1016/j.biomaterials.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Alrashdan MS, Park JC, Sung MA, Yoo SB, Jahng JW, Lee TH, Kim SJ, Lee JH. Thirty minutes of low intensity electrical stimulation promotes nerve regeneration after sciatic nerve crush injury in a rat model. Acta Neurol Belg. 2010;110:168–179. [PubMed] [Google Scholar]
- 48.Wood MD, Willits RK. Applied electric field enhances DRG neurite growth: influence of stimulation media, surface coating and growth supplements. Journal of Neural Engineering. 2009;6 doi: 10.1088/1741-2560/6/4/046003. [DOI] [PubMed] [Google Scholar]
- 49.Kim SY, Kim KM, Hoffman-Kim D, Song HK, Palmore GT. Quantitative control of neuron adhesion at a neural interface using a conducting polymer composite with low electrical impedance. ACS Appl Mater Interfaces. 2011;3:16–21. doi: 10.1021/am1008369. [DOI] [PubMed] [Google Scholar]
- 50.Song HK, Toste B, Ahmann K, Hoffman-Kim D, Palmore GTR. Micropatterns of positive guidance cues anchored to polypyrrole doped with polyglutamic acid: A new platform for characterizing neurite extension in complex environments. Biomaterials. 2006;27:473–484. doi: 10.1016/j.biomaterials.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 51.Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: Bioactive electrically conducting polymer for enhanced neurite extension. Journal of Biomedical Materials Research Part A. 2007;81A:135–149. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JY, Bashur CA, Goldstein AS, Schmidt CE. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–4335. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee JY, Bashur CA, Milroy CA, Forciniti L, Goldstein AS, Schmidt CE. Nerve Growth Factor-Immobilized Electrically Conducting Fibrous Scaffolds for Potential Use in Neural Engineering Applications. Ieee Transactions on Nanobioscience. 2012;11:15–21. doi: 10.1109/TNB.2011.2159621. This group fabricated PPy-coated PLGA electrospun scaffolds that were chemically conjugated to NGF to become neurite growth-promoting, conductive meshes. Immobilized NGF scaffolds promoted neurite extension similar to that of soluble NGF cultures. Furthermore, with electrical stimulation, both length and percentage of cells expressing neurites increased.
- 54.Lee JY, Lee JW, Schmidt CE. Neuroactive conducting scaffolds: nerve growth factor conjugation on active ester-functionalized polypyrrole. Journal of the Royal Society Interface. 2009;6:801–810. doi: 10.1098/rsif.2008.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prabhakaran MP, Ghasemi-Mobarakeh L, Jin GR, Ramakrishna S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. Journal of Bioscience and Bioengineering. 2011;112:501–507. doi: 10.1016/j.jbiosc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Runge MB, Dadsetan M, Baltrusaitis J, Knight AM, Ruesink T, Lazcano EA, Lu LC, Windebank AJ, Yaszemski MJ. The development of electrically conductive polycaprolactone fumaratepolypyrrole composite materials for nerve regeneration. Biomaterials. 2010;31:5916–5926. doi: 10.1016/j.biomaterials.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moroder P, Runge MB, Wang HA, Ruesink T, Lu LC, Spinner RJ, Windebank AJ, Yaszemski MJ. Material properties and electrical stimulation regimens of polycaprolactone fumarate-polypyrrole scaffolds as potential conductive nerve conduits. Acta Biomaterialia. 2011;7:944–953. doi: 10.1016/j.actbio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang JH, Lu L, Zhang JB, Hu XY, Zhang YG, Liang W, Wu SY, Luo ZJ. Electrical Stimulation to Conductive Scaffold Promotes Axonal Regeneration and Remyelination in a Rat Model of Large Nerve Defect. Plos One. 2012;7 doi: 10.1371/journal.pone.0039526. Porous, PPy-chitosan scaffolds were used in 15 mm sciatic nerve defects and electrically stimulated with 3V at 20 Hz intermittently. Conductive scaffolds with electrical stimulation produced significantly increased CMAP amplitude, nerve conduction velocity, and number of regenerated motor and sensory axons. In addition, markers of regeneration S100, P0, P3, and BDNF were significantly upregulated compared to unstimulated and non-conductive scaffolds.