Abstract
Orthotopic liver transplantation is the only definitive treatment for end stage liver failure and the shortage of donor organs severely limits the number of patients receiving transplants. Liver tissue engineering aims to address the donor liver shortage by creating functional tissue constructs to replace a damaged or failing liver. Despite decades of work, various bottoms-up, synthetic biomaterials approaches have failed to produce a functional construct suitable for transplantation. Recently, a new strategy has emerged using whole organ scaffolds as a vehicle for tissue engineering. This technique involves preparation of these organ scaffolds via perfusion decellularization with the resulting scaffold retaining the circulatory network of the native organ. This important phenomenon allows for the construct to be repopulated with cells and to be connected to the blood torrent upon transplantation. This opinion paper presents the current advances and discusses the challenges of creating fully functional transplantable liver grafts with this whole liver engineering approach.
Introduction
Orthotopic liver transplantation is the only definitive treatment option for end stage liver failure which causes 27,000 deaths annually in the US. The recent advances in surgical techniques and immunosuppression therapies have resulted in a decreasing trend in mortality rates of patients after receiving a transplant over the last two decades. Unfortunately, the shortage of donor organs remains the primary limiting factor in transplanting more patients on the organ waiting list [1]. There are about 17,000 patients on the waiting list and only about 6,000 patients receive transplants each year [2]. Moreover, the increased incidence of hepatitis C infection and obesity-driven fatty liver disease will likely reduce the number of donor organs suitable for transplantation [3]. Strategies to develop alternative treatment options are continuously being investigated. One approach involves the engineering of liver tissue to fill the gap of insufficient numbers of donor organs for transplantation. This effort which is termed “tissue engineering” is an interdisciplinary field that integrates engineering and life sciences to create functional tissue constructs with the aim of replacing the failing organ or tissue. In its simplest conceptual form, the effort involves seeding and cultivation of cells in a three dimensional structure made of synthetic and/or biological polymer materials that provide physical support and biological cues to support cell growth and function. Despite decades of work, the only tissue engineered products that made the transition to clinic are limited to non-cellular tissues and tissues that function in a mostly mechanical/structural mode such as the skin, cartilage, and bladder [4]. Other organs, like liver, have been difficult to fabricate using traditional tissue engineering approaches, partly due to the lack of a well-defined circulatory network in the scaffold to maintain the cells that are within. A novel technique, whole-organ decellularization, has evolved to address this drawback in current scaffold preparation methods. This technique, first demonstrated for the heart [5] and quickly adopted for the liver [6], retains the circulatory network of the native organ, allowing for the construct to be connected to the blood torrent upon transplantation. Here, we will provide a review of the area of hepatic tissue engineering for creation of a transplantable liver substitute. We will address the key challenges in whole liver tissue engineering such as cell seeding, blood compatibility, source of cells and scaffolds, and immunological issues.
Hepatic tissue engineering
Hepatocyte transplantation has been investigated as a feasible alternative to orthotopic liver transplantation to treat liver-based inborn errors of metabolism where the goal is to replace a single deficient enzyme or its product [7]. In these cases, there is the intact hepatic primary function and architecture, and the transplantation of a hepatocyte mass equivalent to 10% of the patient’s liver is sufficient to normalize liver function [8]. Hepatocyte transplantation involves transfer of cells obtained from a healthy individual into the patient by direct injection into the portal flow or into the spleen [7]. Despite early reports of clinical success [9], progress in the field has been challenged by low cell engraftment and survival post transplantation such that the initial engraftment of transplanted cells is equivalent to less than 1% of the recipient’s liver mass [10].
Hepatic tissue engineering evolved in an attempt to improve hepatocyte survival and engraftment post transplantation by protecting the cells from the recipient’s immune system and provide cells an extracellular matrix support for survival and function. Cell encapsulation and microcarrier systems were among the first engineered systems to transplant hepatocytes [11]. Early reports demonstrated that the microencapsulated hepatocytes survived for as long as three months after intraperitoneal transplantation in rats due to immunoprotection [12], and they remained functional and compensated for deficient liver function for up to four weeks in animal models of Criggler-Najjar syndrome [13]. Survival of rats undergoing galactosamine induced fulminant hepatic failure increased by 80% after receiving peritoneal injection of microencapsulated hepatocytes [14]. Similarly, a limited number of studies tested the effectiveness of microspheres as hepatocyte microcarriers for transplantation in animal models of metabolic enzyme deficiency [15,16] and acute liver failure [17,18]. Despite early enthusiasm towards microencapsulation and microcarrier systems for hepatocyte transplantation, the approach has not advanced to the clinical setting due to several reasons ranging from technical difficulties in producing uniform microcapsules to limitations in oxygen and nutrient transfer to the center construct leading to a necrotic core [19]. These systems might be best suited for providing temporary hepatic support [11]. More permanent liver support will require a tissue engineered liver substitute able to harbor a large number of hepatocytes within an intact architecture for nutrient delivery and waste removal [20].
The first examples of functional transplantable liver-like structures used prevascularized, non-degradable polyvinyl alcohol sponges to accommodate transplanted hepatocytes [21–23]. The porous sponges provided enough volume to hold 500 million hepatocytes, a number equivalent to a rat liver mass [21]. Prevascularization was achieved by implanting the empty sponges in a reasonably vascularized space such as the mesenteric space for a period of up to a week before hepatocytes were injected into the scaffolds [22]. Decoration of the sponges with a blood vessel network through prevascularization allowed delivery of oxygen and other nutrients to the cells throughout the construct. A similar approach was attempted in later studies where scaffolds were designed for sustained release of growth factors such as vascular endothelial growth factor to induce rapid neovascularization [24,25]. Some of these studies showed success in increasing survival in animal models of acute and chronic liver failure [26–28], and some are in large animal testing. It should be emphasized that it’s been over two decades since the first promising results were reported [21], and the initial engraftment and the loss of cell viability still continues to be a real challenge in hepatic tissue engineering [7]. Additionally, these strategies do not address the fact that liver constructs are best connected to the portal and biliary circulations in order to maintain liver support in homeostasis once transplanted. Although a limited number of studies report “ground-up” approaches to create perfusable liver sinusoidal microarchitecture utilizing microfabrication tools [29,30], these devices are difficult to scale up and thus, are very far from creating clinically transplantable liver grafts [31]. Therefore, the search for an ideal scaffold with the microarchitecture [20] and biological cues [32] to stimulate hepatocyte engraftment, viability and function in vivo for hepatic tissue engineering has persisted.
Whole Liver Engineering
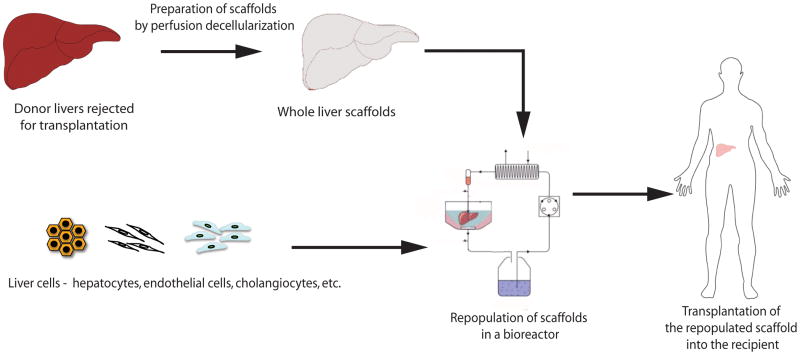
In recent years, a novel technique for preparation of whole organ scaffolds has emerged in tissue engineering (Figure 1). The technique involves perfusion decellularization of cadaveric organs to generate extracellular matrix scaffolds that retain the gross morphology and vascular architecture of the native organ [33]. The scaffold is then repopulated with parenchymal and/or non-parenchymal cells by either direct injection into the parenchyma or perfusion through the vasculature. The construct can be cultured in vitro under perfusion conditions and can be transplanted orthotopically or heterotopically [34]. The technique was first demonstrated for the heart [5] and quickly adopted for liver [6,35–40], lung [41–43], pancreas [44] and kidney [45].
Figure 1. Overview of whole liver engineering.
Cadaveric organs rejected for transplantation are used to prepare decellularized whole liver scaffolds. The scaffolds are repopulated with liver cells ex vivo in a bioreactor system and later transplanted into patients.
Decellularized tissue matrix in tissue engineering
Biomaterials used in the first generation hepatic tissue engineering have found limited use as they had minimal ability to modulate the repair and regeneration of the host tissue. The ability of biomaterials to interact with the host tissue such as controlling specific cell binding interactions and responding to environmental cues became important design considerations of synthetic biomaterials [46]. Additionally, material microstructure, defined by the pore structure, surface area to volume ratio, texture and surface topography, plays an important role in tissue morphogenesis and have become other important design parameters in tissue engineering scaffolds [47]. Considering these design constraints, decellularized tissue matrix represents an ideal scaffold/material for tissue engineering applications as it retains relevant aspects of complex structure and chemical composition of the extracellular matrix (ECM) [48]. Potential applications of decellularized matrix in tissue engineering have been demonstrated for bladder [49], skin [50], trachea [51] among others. Commercially successful examples include decellularized human dermis (Alloderm®) and small intestinal submucosa [4].
Recellularized liver grafts
Our group was the first to report generation of a transplantable recellularized liver graft using perfusion decellularized whole liver scaffolds [6] followed by others [35–40]. The decellularization process preserved both portal and venous vascular networks which facilitated repopulation of the scaffolds with adult rat primary hepatocytes. The grafts were repopulated with 200 million hepatocytes which is equal to approximately 40% of an entire rat liver hepatocyte mass. The grafts were functional up to 10 days in in vitro perfusion culture as measured by albumin, urea, cytochrome P450 expression at levels that are equivalent to 30% observed from native liver. The grafts were transplanted heterotopically back into the rats for a period of 8 h with minimal ischemic damage. Following our work, there have been other reports of rat liver recellularization with fetal hepatoblasts [35], recellularization of pig livers with human [36] or porcine hepatocytes [40], long term transplantation of recellularized rat livers in a 90% hepatectomy model [37]. Although the process of producing recellularized liver grafts is reproducible and feasibility of the approach has been well established by the preliminary work in small and large animal models, there are still many hurdles to be overcome in the creation of large scale recellularized liver grafts for clinical application. This will not be a 5-year task as many pundits claim.
Challenges of whole liver engineering
Creating the complete liver tissue is a challenging task and requires consideration of several factors such as where the organs to prepare scaffolds will be obtained and the safety issues associated with the source; how to recapture the sinusoidal architecture to correctly mimic the hepatic physiology; and ways to improve the blood compatibility of the grafts for successful transplantation to provide long term liver support.
Source of livers and immunological issues
Cadaveric human livers not used for transplantation constitute an appropriate liver source for preparing scaffolds for whole liver grafts for clinical translation. Donor livers are rejected for transplantation because of excessive steatosis, fibrosis, or level of ischemia is beyond acceptable limits and the rejected organs can be accessible for decellularization. Since the decellularization process successfully removes the human leukocyte antigens (HLA) [51], immunological issues related to their use is expected to be minimal. Another potential source of organs for scaffold preparation is pig livers. Porcine livers are sufficiently large to hold sufficient number of cells to support a failing human liver. Decellularized animal tissue matrices may contain galactose- α-1,3-galactose antigen (α-Gal epitope); a cell membrane antigen that is responsible for hyperacute rejection of xenogeneic grafts [52]. However, there is some evidence that the presence of the alpha-Gal epitope doesn’t contribute to any adverse immunological response [53]. Therefore, porcine livers also constitute a viable source of organs.
Rebuilding the liver microarchitecture
The parenchymal fraction of the liver consists of hepatocytes, which constitute the 80% of the total volume and 60% of the total cell population in the liver [54]. The non-parenchymal fraction consists of bile duct epithelial cells (or cholangiocytes), liver sinusoidal endothelial cells (LSEC), hepatic stellate cells (HSC), Kupffer cells, and pit cells (intrahepatic lymphocytes) [54]. In a tissue engineered transplantable liver, the presence of hepatocytes is essential because they are the primary cell type responsible for major liver functions. Besides hepatocytes, one has to consider including endothelial cells, cholangiocytes and hepatic stellate cells as the non-parenchymal fraction in an engineered transplantable liver construct since these cell types play major roles in regulating hepatocyte functions in vivo [55] and developmentally they arise from common progenitors [56]. In our opinion, other non-parenchymal cells such as Kupffer cells and Pit cells can be omitted in the in vitro repopulation efforts of the whole liver scaffolds because they are usually considered to be a part of the immune system [57] and can be expected to migrate into the engineered liver grafts upon transplantation from the bone marrow of the recipients. So far, tissue engineering efforts for creating a transplantable liver graft employed endothelial cells [6,35] and stellate cells [36] as the non-parenchymal fraction. Although no study has reported the use of adult cholangiocytes for repopulating bile ducts in the whole liver scaffolds, several reports utilized fetal hepatoblasts as a source of hepatocytes [35,38,58] which also have the potential to generate cholangiocytes [59]. The evidence of such a potential has been demonstrated by the formation of CK19 positive ductules as precursors of bile ducts in the engineered liver grafts [35].
One of the goals of whole liver engineering is to recreate the correct spatial distribution of liver cells within the whole organ scaffold. Uniform distribution of hepatocytes throughout the scaffold, lining of the vascular spaces with endothelial cells and coverage of the bile ducts with cholangiocytes are essential to recapture hepatic physiology. In the recent whole liver engineering reports, dynamic seeding of hepatocytes through vascular perfusion was shown to achieve a more uniform distribution of the cells throughout the scaffold than seeding by injection into the vasculature or by direct injection into the parenchyma [6,39]. In addition, multistep dynamic seeding allowed for seeding high numbers of hepatocytes to achieve about 80% cell density and around 20% of native hepatic mass in the grafts [6,60] which is said to be sufficient to support liver dysfunction due to metabolic deficiencies. When non-parenchymal cells, such as endothelial cells, are introduced into grafts via sequential seeding into the portal circulation [6] or into portal and venous circulation separately [35], they appear to home to their respective niches [60]. The repopulation of the bile ducts with cholangiocytes has not been shown yet, but infusion of cholangiocytes into the bile ducts has been suggested for restricting the seeding of the cells into their niches [60].
Cell source
Choosing a cell source for liver tissue engineering is not a major obstacle but it requires extensive consideration for clinical translation. Advantages and disadvantages of using autologous vs. allogeneic or xenogeneic, primary vs. progenitor-derived, have been well discussed elsewhere [34]. So far, adult primary hepatocytes have been the primary choice for whole liver tissue engineering since they display adult hepatic phenotype. Hepatocytes from different animal sources such as rat [6], mouse [39], pig [40] were used to repopulate whole liver grafts. The limited availability of high quality human livers for cell isolation limited the use of adult primary human hepatocytes in tissue engineering applications. However, current progress on developing strategies to improve the yield of high quality adult human hepatocytes from marginal livers hold the promise to increase their supply [61]. In other studies, human fetal liver cells were used for repopulation [35,36,38]. One advantage of using bipotential cells such as fetal liver cells is that the cells differentiate into both hepatocytes and cholangiocytes and have the capacity to reconstitute the parenchymal and non-parenchymal components in the liver graft [35,36]. However, the use of fetal tissue to obtain the cells creates ethical problems for clinical translation. Pluripotent stem cells are considered as a sustainable cell source for cell based therapies such as tissue engineering. Induced pluripotent stem (iPS) cells are of special interest because they could be patient specific and would reduce the need for immunosuppression upon transplantation. One disadvantage of using iPS cells is that the cells retain their epigenetic memory prior to reprogramming which may alter their potential to differentiate into cells of lineages other than that they were derived from [62,63]. Regardless, it is well established that ECM plays an important role in liver development [64] and only a small number of studies have directly evaluated the effect of the decellularized liver matrix on stem cell fate [65,66]. This effect remains an important direction for research because scalable and efficient methods to differentiate pluripotent cells to hepatocyte-like cells equivalent to adult primary cells remain to be established [67].
Transplantation
The main advantage of using whole liver scaffolds for liver tissue engineering is that the constructs have the intact vascular bed that allows for connection to the recipient’s blood torrent upon transplantation. Although the current reports successfully demonstrated the feasibility of the transplantation in small animal models, the in vivo graft survival times have been only up to 2 h, 8 h, or even less [6,35,36]. The main challenge is the initiation of the blood coagulation cascade once blood contacts the exposed collagen in the scaffold. Theoretically, endothelialization of the vascular bed in the scaffolds should prevent thrombosis but achieving 100% coverage has been challenging. One study has reported 4 d transplantation time [37] by depositing heparin the scaffolds prior to recellularization rendering the construct anti-thrombogenic. Although this allowed for testing the therapeutic efficacy of the tissue-engineered grafts in a 90% hepatectomy model, the success has not been reproduced by others yet.
In our opinion, the solution to blood clotting problem involves a dual approach consisting of endothelialization of blood contacting surfaces in the graft and anti-coagulation therapy of the recipient animals. However, the minimum fraction coverage of the vascular conduits with endothelial cells required for undisturbed blood flow in the graft remains to be determined. We believe that the optimal endothelialization conditions should be determined by exhaustive testing of endothelial cell seeding and culture conditions (such as seeding density, culture duration, shear stress, etc.) in an ex vivo blood perfusion test system that mimics the transplantation conditions.
Conclusion
Since the advent of the field of tissue engineering, the progress in liver tissue engineering has been limited by the challenge of finding the right scaffold material and architecture to facilitate hepatocyte function and survival in vivo. Whole liver scaffolds prepared by perfusion decellularization of cadaveric organs circumvent these issues and present as a promising platform to engineer livers for transplantation. However, the task is complex and challenging and requires approaches from multiple angles to rebuild the liver microarchitecture and successfully transplant to support the functions of a failing liver. The current success in the field so far is exciting and seems to provide the ultimate promise for creating an engineered liver for clinical transplantation.
Highlights.
Engineering liver tissue addresses the shortage of donor organs for transplantation.
Survival of liver constructs largely depends on efficiency of nutrient delivery to the cells.
Whole liver scaffold is a viable platform for engineering liver for permanent support.
Whole liver engineering is a complex and challenging task.
Current efforts are focused on recapturing microarchitecture and transplantation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Basak E Uygun, Email: buygun@partners.org, Center for Engineering in Medicine, Massachusetts General Hospital, Harvard Medical School and Shriners Hospitals for Children in Boston, 51 Blossom Street, Boston, MA 02114 USA, Phone: 1-617-371-4879, Fax: 617-573-9471.
Martin L Yarmush, Email: ireis@sbi.org, Center for Engineering in Medicine, Massachusetts General Hospital, Harvard Medical School and Shriners Hospitals for Children in Boston and the Department of Biomedical Engineering, Rutgers University, 599 Taylor Road, Piscataway, NJ 08854, Phone: 1-617-371-4882, Fax: 617-573-9471.
References
Papers of special interest (*) or outstanding interest (**).
- 1.Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11 :1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim WR, Stock PG, Smith JM, Heimbach K, Skeans MA, Edwards EB, Harper AM, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2011 Annual Data Report: Liver. Am J Transplant. 2013;13:73–102. doi: 10.1111/ajt.12021. [DOI] [PubMed] [Google Scholar]
- 3.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 4.Lysaght MJ, Jaklenec A, Deweerd E. Great expectations: private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng. 2008;14:305–315. doi: 10.1089/tea.2007.0267. [DOI] [PubMed] [Google Scholar]
- *5.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. This article is the first to report preparation of a whole organ scaffold by perfusion decellularization of cadaveric hearts. [DOI] [PubMed] [Google Scholar]
- **6.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis M-L, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. This article is the landmark report of whole liver engineering using perfusion decellularized of rat livers and transplantation of repopulated liver grafts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93:342–347. doi: 10.1097/TP.0b013e31823b72d6. This review paper provides a thorough overview of current clinical status of human hepatocyte transplantation. [DOI] [PubMed] [Google Scholar]
- 8.Asonuma K, Gilbert JC, Stein JE, Takeda T, Vacanti JP. Quantitation of transplanted hepatic mass necessary to cure the Gunn rat model of hyperbilirubinemia. J Pediatr Surg. 1992;27:298–301. doi: 10.1016/0022-3468(92)90850-7. [DOI] [PubMed] [Google Scholar]
- 9.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288–298. doi: 10.1038/nrgastro.2010.44. [DOI] [PubMed] [Google Scholar]
- 11.Davis MW, Vacanti JP. Toward development of an implantable tissue engineered liver. Biomaterials. 1996;17:365–372. doi: 10.1016/0142-9612(96)85575-x. [DOI] [PubMed] [Google Scholar]
- 12.Balladur P, Crema E, Honiger J, Calmus Y, Baudrimont M, Delelo R, Capeau J, Nordlinger B. Transplantation of allogeneic hepatocytes without immunosuppression: Long-term survival. Surgery. 1995;117:189–194. [PubMed] [Google Scholar]
- 13.Dixit V, Darvasi R, Arthur M, Brezina M, Lewin K, Gitnick G. Restoration of liver function in Gunn rats without immunosuppression using transplanted microencapsulated hepatocytes. Hepatology. 1990;12:1342–1349. doi: 10.1002/hep.1840120615. [DOI] [PubMed] [Google Scholar]
- 14.Wong H, Chang TM. Bioartificial liver: implanted artificial cells microencapsulated living hepatocytes increases survival of liver failure rats. Int J Artif Org. 1986;9:335–336. [PubMed] [Google Scholar]
- 15.Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, Kram M, Chowdhury JR. Replacement of liver function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986;233:1190–1192. doi: 10.1126/science.2426782. [DOI] [PubMed] [Google Scholar]
- 16.Demetriou AA, Levenson SM, Novikoff PM, Novikoff AB, Chowdhury NR, Whiting J, Reisner A, Chowdhury JR. Survival, organization, and function of microcarrier-attached hepatocytes transplanted in rats. Proc Natl Acad Sci USA. 1986;83:7475–7479. doi: 10.1073/pnas.83.19.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosman DK, de Haan JG, Smit J, Jorning GG, Maas MA, Chamuleau RA. Metabolic activity of microcarrier attached liver cells after intraperitoneal transplantation during severe liver insufficiency in the rat. J Hepatol. 1989;9:49–58. doi: 10.1016/0168-8278(89)90075-5. [DOI] [PubMed] [Google Scholar]
- 18.Nagaki M, Kano T, Muto Y, Yamada T, Ohnishi H, Moriwaki H. Effects of intraperitoneal transplantation of microcarrier-attached hepatocytes on D-galactosamine-induced acute liver failure in rats. Gastroenterol Jpn. 1990;25:78–87. doi: 10.1007/BF02785333. [DOI] [PubMed] [Google Scholar]
- 19.Murua A, Portero A, Orive G, Hernández RM, de Castro M, Pedraz JL. Cell microencapsulation technology: Towards clinical application. J Control Release. 2008;132:76–83. doi: 10.1016/j.jconrel.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Kulig KM. Hepatic tissue engineering. Transpl Immunol. 2004;12:303–310. doi: 10.1016/j.trim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Uyama S, Kaufmann PM, Takeda T, Vacanti JP. Delivery of whole liver-equivalent hepatocyte mass using polymer devices and hepatotrophic stimulation. Transplantation. 1993;55:932. doi: 10.1097/00007890-199304000-00044. [DOI] [PubMed] [Google Scholar]
- 22.Takeda T, Murphy S, Uyama S, Organ GM, Schloo BL, Vacanti JP. Hepatocyte transplantation in swine using prevascularized polyvinyl alcohol sponges. Tissue Eng. 1995;1:253–262. doi: 10.1089/ten.1995.1.253. [DOI] [PubMed] [Google Scholar]
- 23.Kneser U, Kaufmann PM, Fiegel HC, Pollok JM, Kluth D, Herbst H, Rogiers X. Long-term differentiated function of heterotopically transplanted hepatocytes on three-dimensional polymer matrices. J Biomed Mater Res A. 1999;47:494–503. doi: 10.1002/(sici)1097-4636(19991215)47:4<494::aid-jbm5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Kedem A, Perets A, Gamlieli-Bonshtein I, Dvir-Ginzberg M, Mizrahi S, Cohen S. Vascular endothelial growth factor-releasing scaffolds enhance vascularization and engraftment of hepatocytes transplanted on liver lobes. Tissue Eng. 2005;11 :715–722. doi: 10.1089/ten.2005.11.715. [DOI] [PubMed] [Google Scholar]
- 25.Hou YT, Ijima H, Takei T, Kawakami K. Growth factor/heparin-immobilized collagen gel system enhances viability of transplanted hepatocytes and induces angiogenesis. J Biosci Bioeng. 2011;112:265–272. doi: 10.1016/j.jbiosc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Soto-Gutierrez A, Navarro-Alvarez N, Rivas-Carrillo JD, Tanaka K, Chen Y, Misawa H, Okitsu T, Noguchi H, Tanaka N, Kobayashi N. Construction and transplantation of an engineered hepatic tissue using a polyaminourethane-coated nonwoven polytetrafluoroethylene fabric. Transplantation. 2007;83:129–137. doi: 10.1097/01.tp.0000250561.14108.03. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-Alvarez N, Soto-Gutierrez A, Chen Y, Caballero-Corbalan J, Hassan WARA, Kobayashi S, Kondo Y, Iwamuro M, Yamamoto K, Kondo E, et al. Intramuscular transplantation of engineered hepatic tissue constructs corrects acute and chronic liver failure in mice. J Hepatol. 2010;52:211–219. doi: 10.1016/j.jhep.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Katsuda T, Teratani T, Ochiya T, Sakai Y. Transplantation of a fetal liver cell-loaded hyaluronic acid sponge onto the mesentery recovers a Wilson’s disease model rat. J Biochem. 2010;148:281–288. doi: 10.1093/jb/mvq063. [DOI] [PubMed] [Google Scholar]
- 29.Tsang VL, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21:790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 30.Hsu W-M, Carraro A, Kulig KM, Miller ML, Kaazempur-Mofrad MR, Weinberg E, Entabi F, Albadawi H, Watkins MT, Borenstein JT, et al. Liver-Assist Device With a Microfluidics-Based Vascular Bed in an Animal Model. Ann Surg. 2010;252:351–357. doi: 10.1097/SLA.0b013e3181e982ba. [DOI] [PubMed] [Google Scholar]
- 31.Borenstein JT, Weinberg EJ, Orrick BK, Sundback C, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication of three-dimensional engineered scaffolds. Tissue Eng. 2007;13 :1837–1844. doi: 10.1089/ten.2006.0156. [DOI] [PubMed] [Google Scholar]
- 32.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–213. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- **33.Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ. Decellularization for whole organ bioengineering. Biomed Mater. 2013;8:014106. doi: 10.1088/1748-6041/8/1/014106. This is a thorough review of perfusion decellularization techniques used for preparation of whole organ scaffolds for various organs to date. [DOI] [PubMed] [Google Scholar]
- **34.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. This review provides the insight to different factors for consideration in whole organ tissue engineering such as decellularization techniques, different cell sources for repopulation and bioreactor systems for culture of recellularized scaffolds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- **36.Barakat S, Abbasi S, Rodriguez G, Rios J, Wood P, Ozaki C, Holley L, Gauthier P. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173:e11–e25. doi: 10.1016/j.jss.2011.09.033. This research article is the first paper to report human size whole liver engineering for transplantation. Porcine liver scaffolds were repopulated with human fetal liver cells and proof-of-principle transplantation into pigs were reported. [DOI] [PubMed] [Google Scholar]
- **37.Bao J, Shi Y, Sun H, Yin X, Yang R, Li L, Chen X, Bu H. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant. 2011;20:753–766. doi: 10.3727/096368910X536572. This research article reports increased survival of rats undergoing 90% hepatectomy after transplantation of recellularized whole liver grafts. This remains to be the only report to date for transplantation whole liver scaffolds beyond a couple of hours. [DOI] [PubMed] [Google Scholar]
- 38.Zhou P, Lessa N, Estrada DC, Severson EB, Lingala S, Zern MA, Nolta JA, Wu J. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl. 2011;17:418–427. doi: 10.1002/lt.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk DM, Jiang H, Reing JE, Gramignoli R, Komori J, Ross M, et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Meth. 2011;17:677–686. doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yagi H, Fukumitsu K, Fukuda K, Kitago M, Shinoda M, Obara H, Itano O, Kawachi S, Tanabe M, Coudriet GM, et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant. 2013;22:231–242. doi: 10.3727/096368912X654939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton DN, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 43.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Orlando G, Farney AC, Iskandar SS, Mirmalek-Sani S-H, Sullivan DC, Moran E, Aboushwareb T, De Coppi P, Wood KJ, Stratta RJ, et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg. 2012;256:363–370. doi: 10.1097/SLA.0b013e31825a02ab. [DOI] [PubMed] [Google Scholar]
- 46.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23 :47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 47.Chan G, Mooney DJ. New materials for tissue engineering: towards greater control over the biological response. Trends Biotechnol. 2008;26:382–392. doi: 10.1016/j.tibtech.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 49.Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. 1998;51:221–225. doi: 10.1016/s0090-4295(97)00644-4. [DOI] [PubMed] [Google Scholar]
- 50.Schechner JS. Engraftment of a vascularized human skin equivalent. FASEB J. 2003;17 :2250–2256. doi: 10.1096/fj.03-0257com. [DOI] [PubMed] [Google Scholar]
- 51.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- **52.Soto-Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. J Clin Invest. 2012;122:3817–3823. doi: 10.1172/JCI61974. This review provides the clinical perspective for whole organ engineering and discusses the hurdles for translating the preliminary successes to the clinics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daly KA, Stewart-Akers AM, Hara H, Ezzelarab M, Long C, Cordero K, Johnson SA, Ayares D, Cooper DKC, Badylak SF. Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng. 2009;15:3877–3888. doi: 10.1089/ten.TEA.2009.0089. [DOI] [PubMed] [Google Scholar]
- 54.LeCluyse EL, Witek RP, Andersen ME, Powers MJ. Organotypic liver culture models: meeting current challenges in toxicity testing. Critical Reviews in Toxicology. 2012;42 :501–548. doi: 10.3109/10408444.2012.682115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodes J, Benhamou J-P, Blei A, Reichen J, Rizzetto M, editors. Textbook of Hepatology. Blackwell Publishing Ltd; 2007. [Google Scholar]
- 56.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naito M, Hasegawa G, Takahashi K. Development, differentiation, and maturation of Kupffer cells. Microsc Res Tech. 1997;39:350–364. doi: 10.1002/(SICI)1097-0029(19971115)39:4<350::AID-JEMT5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Cui J, Zhang B-Q, Zhang H, Bi Y, Kang Q, Wang N, Bie P, Yang Z, Wang H, et al. Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors. J Biomed Mater Res. 2013 doi: 10.1002/jbm.a.34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oertel M, Menthena A, Chen YQ, Teisner B, Jensen CH, Shafritz DA. Purification of Fetal Liver Stem/Progenitor Cells Containing all the Repopulation Potential for Normal Adult Rat Liver. Gastroenterol. 2008;134:823–832. doi: 10.1053/j.gastro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- **60.Uygun BE, Yarmush ML, Uygun K. Application of whole-organ tissue engineering in hepatology. Nat Rev Gastroenterol Hepatol. 2012;9:738–744. doi: 10.1038/nrgastro.2012.140. This recent review article provides an overview of current status of whole liver engineering, and discusses the major challenges for clinical translation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Izamis M-L, Calhoun C, Uygun BE, Guzzardi MA, Luitje M, Price G, Saeidi N, Yarmush ML, Uygun K. Simple isolated liver perfusion significantly improves hepatocyte yields from non-pretreated donor organs. Cell Med. 2013:1–24. doi: 10.3727/215517912X658927. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LIR, et al. Epigenetic memory in induced pluripotent stem cells. Nat. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amenta PS, Harrison D. Expression and potential role of the extracellular matrix in hepatic ontogenesis: A review. Microsc Res Tech. 1997;39:372–386. doi: 10.1002/(SICI)1097-0029(19971115)39:4<372::AID-JEMT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Cui C-B, Yamauchi M, Miguez P, Roach M, Malavarca R, Costello MJ, Cardinale V, Wauthier E, Barbier C, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53:293–305. doi: 10.1002/hep.24012. [DOI] [PubMed] [Google Scholar]
- 66.Marongiu F, Gramignoli R, Dorko K, Miki T, Ranade AR, Serra MP, Doratiotto S, Sini M, Sharma S, Mitamura K, et al. Hepatic differentiation of amniotic epithelial cells. Hepatology. 2011;53:1719–1729. doi: 10.1002/hep.24255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kakinuma S, Nakauchi H, Watanabe M. Hepatic stem/progenitor cells and stem-cell transplantation for the treatment of liver disease. J Gastroenterol. 2009;44:167–172. doi: 10.1007/s00535-008-2297-z. [DOI] [PubMed] [Google Scholar]