Abstract
The transition between wake and sleep states is characterized by rapid and generalized changes in both sensory and motor processing. Sleep is antagonistic to the expression of important behaviors, like feeding, reproduction and learning whose relative importance to an individual will depend on its circumstances at that moment. An understanding of how the decision to sleep is affected by these other drives and how this process is coordinated across the entire brain remains elusive. Neuromodulation is an important regulatory feature of many behavioral circuits and the reconfiguring of these circuits by modulators can have both long-term and short-term consequences. Drosophila melanogaster has become an important model system for understanding the molecular and genetic bases of behaviors and in recent years neuromodulatory systems have been shown to play a major role in regulation of sleep and other behaviors in this organism. The fly, with its increasingly well-defined behavioral circuitry and powerful genetic tools, is a system poised to provide new insight into the complex issue of how neuromodulation can coordinate situation-specific behavioral needs with the brain’s arousal state.
Introduction
Sleep is surprisingly poorly understood given its ubiquitous nature and physiological importance. Sleep has been posited to be essential for life, since prolonged experimental sleep deprivation in rats [1] and in Drosophila [2] is associated with mortality as is sleep deprivation caused by certain neurodegenerative conditions in humans [3]. Even if you put aside the issue of whether it is required, it is clear that even relatively mild sleep deprivation has deleterious effects on performance in the short term and on health in the long term. Consistent with a requirement for optimal well-being, sleep has been shown to be homeostatically regulated in all organisms that have been examined, including flies [4].
Why then do we not just “automatically” sleep for some fraction of each 24 hour day? One major reason is that the day is not uniform in terms of either environmental conditions or probability of interaction with other animals. We have highly tuned circadian processes that schedule our behavioral and metabolic activities to optimal times of day. For humans and flies, sleep occurs preferentially at night in a single highly consolidated episode when our risk of predation is high and opportunities for other activities are low. Most rodents are nocturnally active (perhaps in part since they rely less on vision). A second reason that sleep is not an automatically scheduled activity is that while we need to sleep, sleep does not come for free: there are tradeoffs. If a person or animal is asleep they give up opportunities for foraging, mating, gathering information, etc. Depending on circumstances, survival may depend on prioritizing one of these activities over sleep. The brain has the ability to modify its circadianly programed sleep schedule to accommodate more acute needs.
The mechanisms of sleep control must therefore deal with two unusual demands. First, the sleep/wake transition must be fast and general, reconfiguring the processing of both sensory inputs and motor outputs. Second, the control of the transition has to be addressable by competing drives that are critical to species or individual survival. In this review I will discuss recent work on neuromodulatory systems in Drosophila that may have roles in both levels of sleep regulation.
Properties of neuromodulation
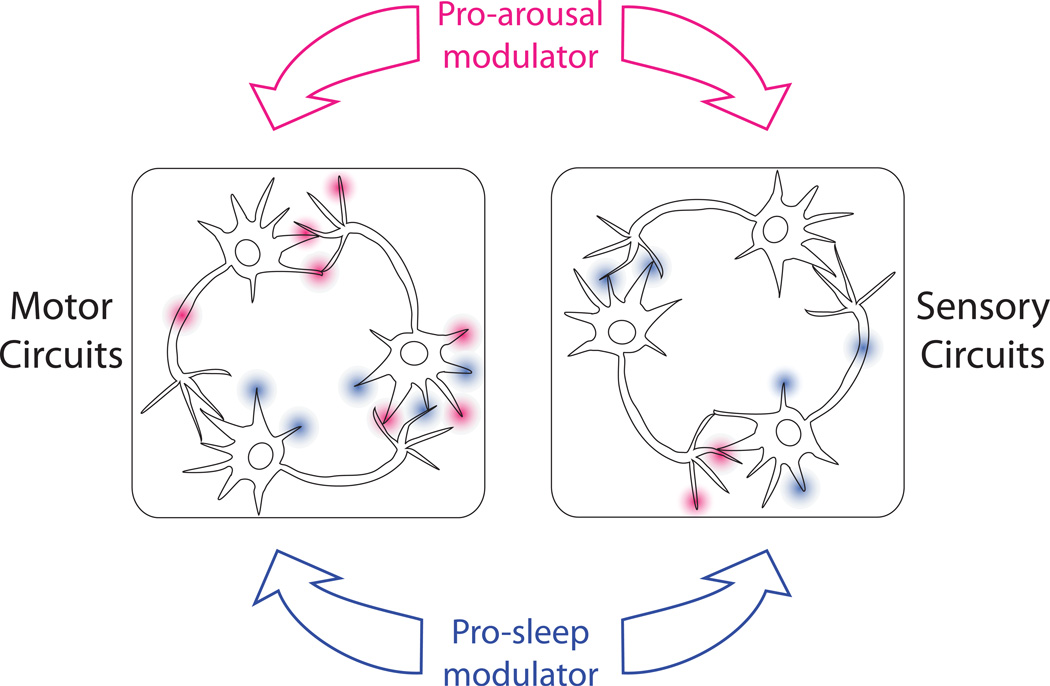
While it is clear in the mammalian brain that there are discrete loci with broad connectivity that promote sleep or arousal, the global nature of the these behavioral states cannot be explained solely by synaptic connections between such loci and the rest of the brain. Many of the changes in the activity of brain areas during sleep or wake appear to occur by regulation of synaptic effectiveness or intrinsic properties of the neurons endogenous to the affected circuits (Figure 1). This type of regulation is called neuromodulation. A recent set of reviews on this topic provides an overview on neuromodulation and in depth discussion of systems in which it is a critical component [5].
Figure 1. Neuromodulation can cause state-dependent changes in neuronal function.
When an animal falls asleep, there is an increase in sensory arousal thresholds that is believed in mammals to occur by alterations in thalamic processing. Motor outputs are also suppressed. Arousal, in contrast, reduces sensory thresholds and facilitates motor output. Neuromodulation may have a role in state changes by acting at multiple sites within these circuits. Pro-arousal and pro-sleep neuromodulators in theory may act in antagonistic manners on the same neurons or may act at distinct points in the circuit.
In brief, neuromodulators are agents (both small molecules and peptides) that alter the ability of cells to respond to the actions of classical excitatory or inhibitory synaptic inputs. Modulators can reach their targets by being co-released with small molecule fast neurotransmitters, being independently released from a different pool of vesicles at synaptic or non-synaptic sites or by being released as humeral factors. Receptors for neuromodulators can be found pre- and postsynaptically as well on almost every other part of the neuron. The spatial scope of action of neuromodulators can in theory be broad since they are not constrained to act at synapses. While much neuromodulation is probably quite local [6], there are also examples of neuromodulators that act far from their site of release [7].
Neuromodulators and sleep in mammals
The number of neuromodulators that affect sleep in mammals is legion. Small molecules such as adenosine and prostaglandin D2 are regulated by the circadian clock and increased by sleep drive, acting to promote sleep [8,9]. Biogenic amines such as dopamine and norepinephrine, while they have many functions, are primary players in arousal and attention [10–12]. Many peptide neuromodulators also affect sleep in mammals. A large number of these peptides also have critical roles in regulating feeding and metabolism. Interestingly, the effect of these peptides on sleep is not uniform. For example Neuropeptide Y, which promotes feeding and has roles in immune function and mood, is sleep-promoting [13]. Orexin/hypocretin, on the other hand, while also increasing food intake, is a potent wake-promoting factor with a clear role in normal arousal [14]. The effects of peptides on sleep therefore cannot be predicted based on their other actions. Whether this reflects completely separate actions on sleep circuits that are unlinked to other behavioral roles or some more subtle integration of internal states is difficult to address in the complicated circuitry of the mammalian brain. As will be detailed below, circuits that regulate sleep and other behaviors in Drosophila can be identified and genetically accessed. The mechanisms by which neuromodulators affect multiple behaviors can therefore be explored at a high level of cellular and molecular detail. Understanding these processes is important obtaining a systems-level appreciation of how animals integrate potentially conflicting needs to optimize survival.
Neuromodulators that affect sleep and the circadian clock in Drosophila
The circadian clock is responsible for scheduling behavior and metabolic processes. In Drosophila, the core clock circuit has been defined by the presence of a daily nuclear/cytoplasm shuttle of the period protein, one of the core transcriptional regulators that makes up the molecular machinery of the clock. Not surprisingly, the clock has been shown to be involved in regulation of sleep. In particular, the ventrolateral neurons (LNvs) have been shown to be wake-promoting [15–17]. This small group of cells contains Pigment Dispersing Factor (PDF), a peptide neuromodulator. Signaling by PDF within the clock circuit is critical to maintain activity in constant conditions, but PDF is also an arousal molecule and likely functions to regulate sleep by modulating neurons outside the core clock circuit [16]. The wake-promoting function of LNvs is regulated by GABA [16], an important sleep-promoting neurotransmitter in both mammals and flies. Clock cells also contain many other peptides, and it is likely that these molecules will also have effects on sleep.
Neuromodulators that affect sleep and learning in Drosophila
The mushroom bodies in Drosophila are a critical site for formation of associative memory, and they have also been shown to have complex effects on sleep. Dopamine-containing neurons which innervate the mushroom bodies have been very well studied due to the important role they play in both reward and negative reinforcement in associative learning [18,19]. In the last several years, it has become clear that dopamine is also a major arousal substance in Drosophila. Animals fed methamphetamine sleep less and have difficulty with visual attention [20]. Mutation of the fumin (fmn) gene, which encodes a transporter critical to termination of dopamine signaling, sleep less and have decreased arousal thresholds [21]. The neurons involved in arousal appear to be just a subset of the dopaminergic system: PPL2 neurons which project to the ventrolateral neurons of the circadian clock, cells that are important for light-dependent arousal [15,22], and PPL1/PPL3 neurons that project to the fan-shaped body, a neuropil associated with locomotor control [23,24].
Interestingly, PPL1 neurons that innervate the mushroom body are important for negative reinforcement of associative learning [25]. The fact that dopamine is important for both memory formation and sleep is of potential importance when one considers the role that sleep plays in both learning and memory consolidation. Dopamine appears to be a reinforcing cue for both aversive and appetitive learning and the idea that it could simultaneously act to modulate arousal during a learning experience is appealing. Given the fact that cellular loci of action for both reinforcement and wakefulness have been identified in the fly, understanding their interaction may be possible.
While reinforcement is dependent on dopamine, consolidation of associative memory is believed to require neuromodulatory input to the mushroom bodies from a pair of neurons called the dorsal paired medial (DPM) neurons. These neurons were first identified as being critical for the actions of the amnesiac gene, a predicted PACAP-like neuropeptide [26]. Subsequently these neurons have also been shown to release serotonin [27]. Both the amnesiac gene [28] and serotonin receptors in the mushroom bodies [29] have been shown to contribute to sleep regulation. Whether the actions of DPMs are important to integration of sleep and memory formation is currently unknown.
Neuromodulators that affect sleep and metabolic control in Drosophila
Not surprisingly, starvation in flies has been shown to acutely suppress sleep [30] as flies increase locomotor activity in an attempt to discover new food sources. Starvation also causes neurochemical changes in flies, and as in mammals, there are peptides that regulate feeding and metabolism many of which have similar roles across species. While there are peptidergic neurons throughout the fly brain, the pars intercerebralis is a diverse neuronal group that contains a number of peptides, many of which are key metabolic regulators, including the Drosophila insulin-like peptides (DILPs). The DILP+ cells of the pars have been shown to respond to octopamine to suppress sleep [31], while other pars cells respond to an EGFR ligand [32] to increase sleep. Interestingly, it does not appear that the sleep regulation is occurring via DILPs themselves, suggesting that some other molecule or peptide released from this subset of pars cells is sleep-active [33]. The ability of multiple subpopulations of pars cells to regulate sleep in opposite directions suggests the involvement of multiple metabolic peptides in regulation of arousal.
Neuromodulators that affect reproductive behavior and sleep in Drosophila
Mating and sleep are incompatible for flies. Behavioral studies have shown that the consolidated nighttime sleep seen in isolated individuals is disrupted if males and females are housed together [34], suggesting that reproductive drive can suppress or reschedule sleep. The mechanisms underlying this effect are unknown, but it is clear that olfactory cues are important triggers of the acute change in sleep pattern.
Mating can also have longer-term effects on sleep for females. It has been known for many years that substances transferred to the female during copulation alter both her physiology and behavior, increasing egg production and decreasing receptivity to remating. Interestingly, mating also changes female sleep patterns: males and virgin females typically have a prolonged daytime siesta, while mated females sleep much less during the day [35]. This change in sleep pattern, like the aversion to remating, lasts about a week, and both are due to the actions of Sex Peptide (SP), a component of male seminal fluid. The SP receptor has also been shown to alter food choices in mated females, giving this peptide yet another function in retooling mated females for reproduction [36].
Prospects for understanding integration of sleep with other behaviors
There is a major push in the neuroscience community to define the “connectome” of the brain i.e. draw concrete wiring diagrams. While this is a necessary condition for understanding behavior, it is far from sufficient. Decades of work in small circuits have demonstrated the importance of modulation [37]. Neuromodulation allows the brain to generate many gradations and combinations of behavior from a limited number of basic circuits. The most interesting and important behavioral problems involve animals making decisions about complex situations. How inputs are weighted, especially when there is conflicting information or competing needs, is not well understood in any system. This is a particularly salient problem for understanding sleep since it is both necessary for optimal health and antagonistic to many other behaviors.
Genetic model organisms with well understood connectomes offer an excellent platform for studying this important problem in a context where behavioral choice can be assessed. Recent work in C. elegans has shed a lot of light this how this can occur in a simple circuit [38], but the complex behaviors exhibited by Drosophila make it perhaps an even more promising system for providing templates for understanding human behavior. The Drosophila brain contains at least 42 genes which encode peptide precursors [39] and likely also makes many uncharacterized small molecule neuromodulators. Almost all of these substances have roles in regulation of multiple behaviors. Our understanding of the circuits that these molecules modify is growing quickly due to the ability of investigators to bring behavioral, electrophysiological and genetic tools to bear on the problem [40]. In this review I have highlighted known intersections of sleep with other behaviors in which there is evidence for the role of a specific modulator (Figure 2). While at this point we do not have an understanding of how (or if) these particular modulators allow the animal to reach decisions about sleep vs. another action, the system is ripe for investigation.
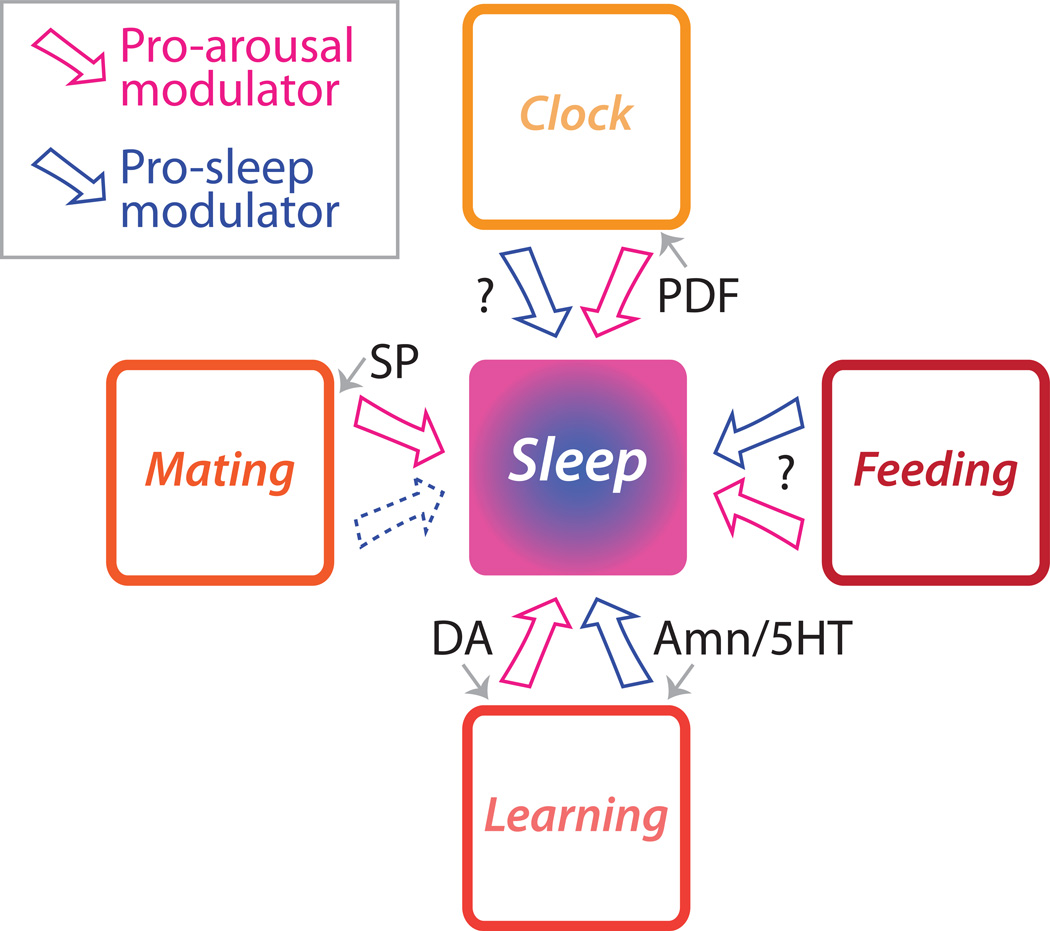
Figure 2. Summary of behaviors and neuromodulators known to regulate sleep in Drosophila.
While only a few of the known neuromodulators in Drosophila have been shown to regulate sleep, these agents affect a diverse array of other systems. All of these modulators have important roles in regulating a non-sleep behavior (indicated by small gray arrows), but they all also have a role in sleep (indicated by large arrows). There are a number of neuromodulators in cells of the core clock circuit. To date, only Pigment Dispersing Factor (PDF) has been shown to directly affect sleep, but there is reason to believe that there are also pro-sleep clock cells which could be neuromodulatory. Feeding and metabolism are regulated by peptidergic cells of the pars intercerebralis. This diverse cell group has been shown to both positively and negatively regulate sleep although the transmitters or modulators underlying these effects are unknown. The circuitry underlying memory formation has been extensively studied, and modulators of this process can regulate both the amount and structure of sleep. Dopamine (DA) is a pro-arousal substance released from multiple neurons in the central brain which is also critical for reinforcement in associative learning. The amnesiac (Amn) gene product and serotonin (5HT) are both found in the dorsal paired medial neurons which are necessary for memory consolidation. Olfactory discovery of opportunities for reproductive behavior can acutely decrease sleep. For females, mating can decrease daytime sleep via actions of SP which is transferred from males in ejaculate. It is unknown if mating or reproductive behaviors can increase sleep.
Conclusions
There are important tradeoffs associated with sleep: inability to feed or find mates are negatives, but the decreased danger of predation, thermal stress and dehydration are positives. The survival of animals in the wild depends on their ability to respond appropriately to new opportunities and dangers. These reactive changes in behavior need to be coordinated with ongoing essential behaviors like feeding and sleeping. Neuromodulators that have roles in other behaviors are often regulators of sleep. Reconfiguration of neuronal circuits by neuromodulators may help animals to set rational priorities for particular situations and still carry out essential baseline functions.
Highlights.
Peptide and small molecule modulators are important regulators of behavior
Multiple neuromodulatory systems regulate sleep in mammals and insects
Neuromodulation may allow coordination of behavioral programs with arousal state
Acknowledgements
This work was supported by National Institutes of Health grant R01 MH067284 to LCG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–184. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 2.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 3.Lugaresi E, Provini F, Cortelli P. Agrypnia excitata. Sleep Med. 2011;12(Suppl 2):S3–S10. doi: 10.1016/j.sleep.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 5. Various. Reviews on neuromodulatory mechanisms. Neuron. 2012;76:1–256. This special issue on neuromodulation covers a lot of ground, from molecular mechanisms to computational treatments of modulation.
- 6.van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987;23:1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- 8.Blutstein T, Haydon PG. The Importance of astrocyte-derived purines in the modulation of sleep. Glia. 2013;61:129–139. doi: 10.1002/glia.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urade Y, Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med Rev. 2011;15:411–418. doi: 10.1016/j.smrv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Noudoost B, Moore T. The role of neuromodulators in selective attention. Trends Cogn Sci. 2011;15:585–591. doi: 10.1016/j.tics.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmiter RD. Dopamine signaling as a neural correlate of consciousness. Neuroscience. 2011;198:213–220. doi: 10.1016/j.neuroscience.2011.06.089. [DOI] [PubMed] [Google Scholar]
- 12.Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16:187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyzma M, Boudjeltia KZ, Faraut B, Kerkhofs M. Neuropeptide Y and sleep. Sleep Med Rev. 2010;14:161–165. doi: 10.1016/j.smrv.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Girault EM, Yi CX, Fliers E, Kalsbeek A. Orexins, feeding, and energy balance. Prog Brain Res. 2012;198:47–64. doi: 10.1016/B978-0-444-59489-1.00005-7. [DOI] [PubMed] [Google Scholar]
- 15.Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aso Y, Herb A, Ogueta M, Siwanowicz I, Templier T, Friedrich AB, Ito K, Scholz H, Tanimoto H. Three dopamine pathways induce aversive odor memories with different stability. PLoS Genet. 2012;8:e1002768. doi: 10.1371/journal.pgen.1002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 20.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebestky T, Chang JS, Dankert H, Zelnik L, Kim YC, Han KA, Wolf FW, Perona P, Anderson DJ. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol. 2012;22:2114–2123. doi: 10.1016/j.cub.2012.09.008. With the Ueno paper, a beautiful dissection of the ciruitry underlying dopaminergic regulation of arousal.
- 24. Ueno T, Tomita J, Tanimoto H, Endo K, Ito K, Kume S, Kume K. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci. 2012;15:1516–1523. doi: 10.1038/nn.3238. With the Liu paper, a beautiful dissection of the ciruitry underlying dopaminergic regulation of arousal.
- 25.Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- 27.Lee PT, Lin HW, Chang YH, Fu TF, Dubnau J, Hirsh J, Lee T, Chiang AS. Serotoninmushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc Natl Acad Sci U S A. 2011;108:13794–13799. doi: 10.1073/pnas.1019483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Guo F, Lu B, Guo A. amnesiac regulates sleep onset and maintenance in Drosophila melanogaster. Biochem Biophys Res Commun. 2008;372:798–803. doi: 10.1016/j.bbrc.2008.05.119. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Keene AC, Duboue ER, McDonald DM, Dus M, Suh GS, Waddell S, Blau J. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol. 2010;20:1209–1215. doi: 10.1016/j.cub.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65:670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10:1160–1167. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- 33. Erion R, DiAngelo JR, Crocker A, Sehgal A. Interaction between sleep and metabolism in Drosophila with altered octopamine signaling. J Biol Chem. 2012;287:32406–32414. doi: 10.1074/jbc.M112.360875. This study takes on the complexity of the interactions between feeding and sleep.
- 34.Fujii S, Krishnan P, Hardin P, Amrein H. Nocturnal male sex drive in Drosophila. Curr Biol. 2007;17:244–251. doi: 10.1016/j.cub.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaac RE, Li C, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc Biol Sci. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol. 2010;20:1000–1005. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang H, Kim K, Neal SJ, Macosko E, Kim D, Butcher RA, Zeiger DM, Bargmann CI, Sengupta P. Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron. 2012;75:585–592. doi: 10.1016/j.neuron.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nassel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. A comprehensive review of peptidergic signaling in Drosophila
- 40.Griffith LC. Identifying behavioral circuits in Drosophila melanogaster: moving targets in a flying insect. Curr Opin Neurobiol. 2012;22:609–614. doi: 10.1016/j.conb.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]