Abstract
Background
We examine whether broad factors and specific facets of personality are associated with increased risk of incident Alzheimer’s disease (AD) in a long-run longitudinal study and a meta-analysis of published studies.
Methods
Participants (N=1671) were followed for up to 22 years from a baseline personality assessment. The meta-analysis pooled results from up to 5 prospective studies (N=5054).
Results
Individuals with scores in the top quartile of neuroticism (HR=3.1; 95%CI=1.6–6.0) or the lowest quartile of conscientiousness (HR=3.3; 95%CI=1.4–7.4) had a three-fold increased risk of incident AD. Among the components of these traits, self-discipline and depression had the strongest associations with incident AD. The meta-analysis confirmed the associations of neuroticism (p=2*10−9) and conscientiousness (p=2*10−6), along with weaker effects for openness and agreeableness (p<0.05).
Conclusion
The current study and meta-analysis indicate that personality traits are associated with increased risk of AD, with effect sizes similar to those of well-established clinical and lifestyle risk factors.
Keywords: Alzheimer, dementia, observational prospective study, meta-analysis, neuroticism, anxiety, depression, conscientiousness, order, self-discipline, APOE
1. Introduction
Given the steep rise in individual and societal costs associated with Alzheimer’s disease (AD), there is a need to better understand the etiology of dementia.1 Age is clearly associated with increasing incidence of AD, and there is strong evidence that apolipoprotein E (APOE) ε4 variant is associated with higher risk of AD.1 A number of other genetic, medical, psychological, and social factors have also been linked to AD.2–4 Among the psychological factors, there has been an interest in the role of personality traits. Personality traits are enduring dispositions that underlie individuals’ cognitive, emotional, and behavioral tendencies.5 These traits are related to individuals’ lifestyles and have been implicated in physical and mental health,6–12 including risk of AD.13–17 The present study extends the research on personality traits and risk of incident AD in two ways. First, we examine the association in one of the longest running prospective studies in the United States using a comprehensive and detailed assessment of the domain and facets of the five-factor model of personality. Second, we pooled findings from published studies and performed meta-analyses to summarize evidence for the association of personality traits with incident AD. Based on previous research, we expected that high conscientiousness (those who are organized and have more self-discipline and willpower) and low neuroticism (those who are less likely to be anxious, depressed, and vulnerable to stress) would be associated with a reduced risk of incident AD.
2. Methods
2.1. Participants
The participants (N = 1671) were part of the Baltimore Longitudinal Study of Aging (BLSA), a prospective cohort study of physical and psychological aging.18 At entry, participants are generally in good health; extensive exclusion criteria (see ClinicalTrials.gov Identifier: NCT00233272) ensure that participants have no physical or cognitive impairments when they enter the study. Although they remain a relatively healthy group, participants develop diseases and disabilities as they age. For example, the incidence of metabolic syndrome in the BLSA is in line with data from similar samples 19 and BLSA participants tend to have similar levels of depressive symptoms to other cohorts.20 The incidence of AD in this sample is also consistent with the rate found in other studies.21
At entry in the study, participants agreed to serial follow-ups with systematic physical and psychological examinations. The frequency of follow-up varied with age, with increasing frequency at older ages. Currently, participants 60 to 79 years old are tested every 2 years and participants 80 years old or older are tested approximately every year. Subjects included in the analyses were cognitively normal at time of the baseline personality assessment and had at least one follow-up evaluation. About 7% (n = 119) of participants in this study were lost to follow-up. Prior to each assessment, participants provided written informed consent. The protocol was approved by the local institutional review board.
2.2 Clinical and Neuropsychological Evaluations
At enrollment, each participant was evaluated for history of cerebrovascular disease, focal neurological abnormalities, and impairment of cognitive or behavioral functioning. Follow-up evaluations included a neuropsychological battery, neurological examination, medication review, and informant/subject structured interview. The latter was based on the Clinical Dementia Rating (CDR)22 scale after 1998 and the Dementia Questionnaire23 before 1998. All subjects were reviewed at a diagnostic consensus conference if their Blessed Information Memory Concentration score24 was 4 or above, if their informant or subject CDR score was 0.5 or above, or if their Dementia Questionnaire was abnormal. All neuropsychological diagnostic tests and clinical data were available for review at the diagnostic conference. Diagnosis of dementia was based on DSM-III-R criteria and diagnosis of AD was based on the National Institute of Neurological and Communication Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria.25
2.3 Personality Assessment
Participants completed the self-report version of the Revised NEO Personality Inventory (NEO-PI-R).5 The NEO-PI-R is a 240-item questionnaire that assesses 30 facets, six for each of the five major dimensions of personality – neuroticism (the tendency to experience negative emotions, such as anxiety, anger, and sadness), extraversion (an inclination toward being sociable, assertive, enthusiastic, and energetic), openness (the tendency to be imaginative, unconventional, curious, emotionally and artistically sensitive), agreeableness (an interpersonal dimension defined by altruism, trust, modesty and cooperativeness), and conscientiousness (the tendency to be organized, strong-willed, persistent, reliable and a follower of rules and ethical principles). Raw scores were standardized using combined-sex norms reported in the manual.5 In the BLSA sample, the NEO-PI-R factor structure shows high congruence with the normative structure (Tucker’s phis = .97 to .99), the internal consistencies for the five dimensions ranged from 0.87 to 0.92, and the test-retest correlations for the five dimensions ranged from 0.78 to 0.85 over an average interval of 10 years.26
2.4 Data Analysis
To test whether personality traits conferred risk of AD, we used proportional hazards regression models, controlling for age of personality assessment, sex, ethnicity (white vs. others), and education (years of schooling). The analyses were conducted separately for each of the five personality domains and each of the facets. Personality scores were standardized so that one unit corresponded to a 1 SD difference. In addition to the continuous scores, domain scores were recoded to provide a statistical and graphical comparison of the top and bottom quartiles of the distribution. We also tested a model that included all five factors simultaneously. The time end point was the year of onset of AD-type clinical dementia. Participants who did not develop AD were censored at the time of their last clinical evaluation. Because of differences in the pathophysiologic processes among dementia subtypes, we excluded 44 participants who developed non-AD dementia (e.g., vascular, Lewy body, Parkinson disease). The results were similar if we included these 44 participants censored at time of onset of non-AD dementia.
We estimated the population attributable risk (PAR) based on the hazards ratio (HR) of incident AD associated with the top or bottom quartile of the distribution on a personality trait vs. the rest of the sample (adjusted for the demographic covariates). We used the formula PAR = PRF*((HR–1)/HR),27 where PRF is the prevalence of the risk factor (i.e., 25%). The PAR estimates are calculated for comparisons with established risk factors. PAR estimates, however, are generally based on clinically recognized cut-points (e.g., blood pressure values that define hypertension), whereas we used statistical thresholds for the personality traits. In addition, in calculating the PAR, we are not necessarily assuming a direct causal link between the risk factor and the outcome.
Secondary analyses with APOE genotype (presence vs. absence of ε4 allele) as a covariate or moderator of the association between personality and incident AD were performed for the subset of 1472 participants with available APOE genotype. We also repeated the analyses excluding individuals younger than 50 years at initial examination, or those who developed AD within two years after the initial personality assessment. Finally, we tested whether sex moderated the association between personality traits and incident AD. The analyses were conducted using SPSS statistical software.
2.5 Meta-analysis
To identify studies for inclusion in the meta-analysis, we searched the PubMed and Scopus databases up to February 2012 and screened the reference lists of relevant articles for additional studies. We focused on prospective cohort studies, with five-factor model personality traits assessed at baseline in cognitively healthy participants who were evaluated at follow-up for incident AD. In cases with multiple publications from the same sample, we considered one effect for each trait from each sample. To reduce variability across studies, we generally chose the risk estimates from the main model, with age, sex, education, and ethnicity as covariates. The logHR and SE were scaled in each study to correspond to the effect associated with 1 SD difference on the trait. We performed random-effect model meta-analyses. Heterogeneity was evaluated using the Q statistic, and publication bias was evaluated statistically with the Kendall’s tau and Egger test. The meta-analyses were conducted using the “Comprehensive Meta-Analysis” software package.
3. Results
3.1 Association results from the BLSA sample
Participants were followed for up to 22 years (M = 12, SD = 6, range 1–22). The onset of clinical AD was diagnosed in 90 individuals within an average of 8 years (SD = 4, range 1–18) from the baseline personality assessment. Table 1 presents the initial demographic characteristics of the overall sample and separately for the group of participants who developed AD and those who did not. The incident dementia group was significantly older and more likely to be white. This effect of ethnicity was due partly to the younger age of the minority group (54.8 vs. 57.2, p = 0.005), because they were recruited later in the study. There were no significant differences between the groups on education or sex.
Table 1.
Baseline descriptive statistics for the overall sample and by dementia status
| Overall (n=1671) | No AD (n=1581) | Incident AD (n=90) | P | |
|---|---|---|---|---|
| Age, years | 56.5 (16.0) | 55.4 (15.7) | 75.7 (7.6) | <0.001 |
| Education, years | 16.7 (2.5) | 16.7 (2.5) | 16.4 (3.0) | 0.29 |
| Female, % | 49.4 | 49.5 | 46.7 | 0.6 |
| Minority, % | 29.3 | 30.5 | 7.8 | <0.001 |
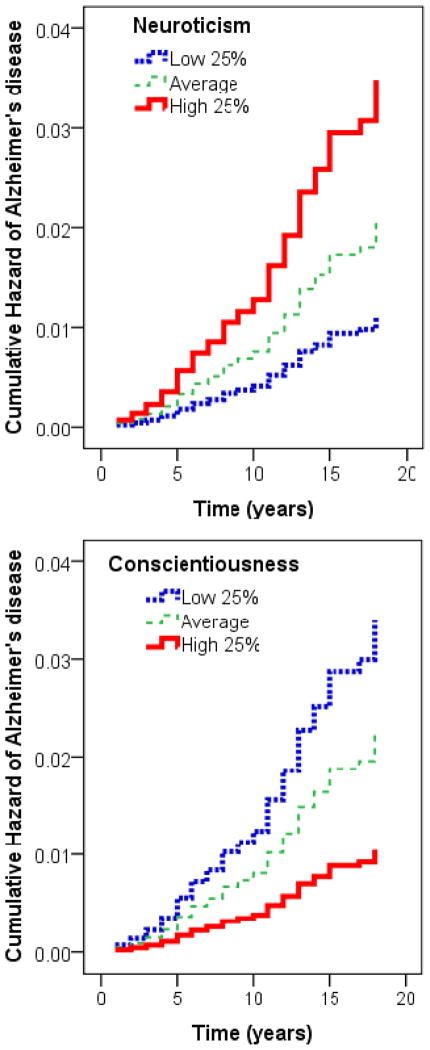
Table 2 presents the results of the survival analyses, with the hazard ratios of AD (adjusted for age, sex, education, and ethnicity) and the 95% confidence intervals associated with each factor and facet. For each SD increase in neuroticism, the risk of incident AD increased by more than 30% (HR = 1.37; 95%CI =1.09–1.73). We contrasted groups with high and low scores on neuroticism and found that the risk of incident AD was threefold higher for the group in the highest quartile compared to the lowest quartile of neuroticism (HR = 3.13; 95%CI = 1.62–6.04; see Figure 1). The absolute risk, which does not account for covariates or censoring, was 7% and 3% for the highest and lowest quartiles of neuroticism. Further, we calculated that over 10% of the AD cases in the population could be attributed to high neuroticism (top quartile vs. others; HR = 2.02; PAR = 13%). A similar effect was observed for conscientiousness (HR = 0.69; 95%CI =0.55–0.87), and as illustrated in Figure 1, the risk of incident AD was threefold higher for the group in the lowest compared to the highest quartile of conscientiousness (HR = 3.26; 95%CI = 1.43–7.40; absolute risk 8% and 2% for the lowest and highest quartiles). Similar to neuroticism, the proportion of AD cases that could be attributed to low conscientiousness (bottom quartile vs. others; HR = 1.74) was about 10% (PAR = 11%).
Table 2.
Results from separate Cox regression with personality factors and facets as predictors of incident AD.
| Hazard Ratios | 95% Confidence Interval | |
|---|---|---|
| Neuroticism | 1.371** | 1.085–1.733 |
| Extraversion | 0.86 | 0.687–1.077 |
| Openness | 0.889 | 0.707–1.116 |
| Agreeableness | 0.858 | 0.679–1.082 |
| Conscientiousness | 0.692** | 0.549–0.873 |
| N1: Anxiety | 1.336** | 1.078–1.657 |
| N2: Angry Hostility | 1.331** | 1.076–1.649 |
| N3: Depression | 1.349* | 1.070–1.701 |
| N4: Self-consciousness | 1.114 | 0.888–1.398 |
| N5: Impulsiveness | 1.065 | 0.833–1.363 |
| N6: Vulnerability | 1.251 | 0.996–1.571 |
| E1: Warmth | 0.864 | 0.685–1.090 |
| E2: Gregariousness | 0.959 | 0.774–1.188 |
| E3: Assertiveness | 0.947 | 0.759–1.182 |
| E4: Activity | 0.834 | 0.665–1.045 |
| E5: Excitement-Seeking | 0.822 | 0.642–1.052 |
| E6: Positive Emotions | 0.968 | 0.770–1.216 |
| O1: Fantasy | 1.039 | 0.831–1.301 |
| O2: Aesthetics | 0.996 | 0.794–1.250 |
| O3: Feelings | 0.901 | 0.722–1.125 |
| O4: Actions | 0.89 | 0.720–1.100 |
| O5: Ideas | 0.764* | 0.605–0.984 |
| O6: Values | 0.968 | 0.784–1.195 |
| A1: Trust | 0.886 | 0.689–1.139 |
| A2: Straightforwardness | 0.942 | 0.743–1.194 |
| A3: Altruism | 0.88 | 0.698–1.110 |
| A4: Compliance | 0.963 | 0.787–1.179 |
| A5: Modesty | 0.86 | 0.689–1.074 |
| A6: Tender-mindedness | 0.91 | 0.725–1.141 |
| C1: Competence | 0.707** | 0.582–0.904 |
| C2: Order | 0.746** | 0.610–0.944 |
| C3: Dutifulness | 0.758* | 0.588–0.978 |
| C4: Achievement Striving | 1.008 | 0.798–1.274 |
| C5: Self-Discipline | 0.681*** | 0.555–0.834 |
| C6: Deliberation | 0.838 | 0.673–1.043 |
Note. Total N = 1671, incident AD cases N = 90. Cox regressions controlling for age, sex, education, and ethnicity.
Figure 1.
Cumulative hazard of incident AD clinical dementia associated with the low 25% and high 25% on neuroticism and conscientiousness, adjusted for age, sex, ethnicity, and education. The group with average scores (25% to 75%) were included in the analyses but are not shown in the figure. For neuroticism, the low 25% N = 405, the high 25% N = 436. For conscientiousness, the low 25% N = 422, the high 25% N = 393.
Additional analyses supported the robustness of these associations. Because Neuroticism and Conscientiousness are moderately correlated, we tested whether these were independent effects. When we repeated the analyses with all five factors entered in the regression model simultaneously, scoring in the top quartile of neuroticism (HR = 2.53; 95%CI = 1.23–5.23) or the bottom quartile of conscientiousness (HR = 2.63; 95%CI =1.10–6.26) was still associated with increased risk of AD. We further tested whether the combination of high neuroticism and low conscientiousness increased vulnerability to AD beyond the main effects, but we found no significant interaction. The results remained essentially the same when we excluded individuals younger than age 50 at baseline or those who developed AD within 2 years of the baseline personality assessment (p’s < 0.05 for both neuroticism and conscientiousness). In the full sample, we also tested whether the association of the five major factors with incident AD was different for men and women, but we found no significant interactions.
Separate analyses with the APOE ε4 allele as an additional covariate confirmed that an increased risk of incident AD was associated with scoring in the top quartile of neuroticism (HR = 3.82; 95%CI =1.85–7.89) or the bottom quartile of conscientiousness (HR = 3.40; 95%CI =1.39–8.28). We also tested for interactions between each of the five factors and the APOE ε4 risk variant. We found significant interactions between APOE genotype and openness (interaction term: HR = 0.58; 95%CI =0.34–0.98) and agreeableness (interaction term: HR = 1.87; 95%CI =1.10–3.16), such that high openness was protective among the APOE ε4 carriers, whereas high agreeableness was protective among the non-carriers.
At the facet level (Table 2), the anxiety, angry hostility, and depression aspects of neuroticism increased risk of incident AD by more than 30%; there was a trend for vulnerability (p = 0.05). Among the facets of conscientiousness, self-discipline had the strongest association, followed by competence, order, and dutifulness: risk of incident AD was reduced by over 30% for each SD higher score on any of these facets. Finally, one facet of Openness, Openness to Ideas, was associated significantly with an approximately 25% reduced risk of AD for each SD higher score.
3.2 Meta-Analysis
Including the present BLSA sample, we identified five samples with data on neuroticism (total N = 5054; incident AD, N = 607) and three samples with data for each of the other four factors (N = 3342; incident AD, N = 382). We did not conduct meta-analyses for the facets because we found only one study that examined the facets of neuroticism aside from the current BLSA sample. Table 3 presents the characteristics of the studies included in the meta-analyses. All traits were measured with some variation of the NEO-PI-R instrument, and all studies included at least age, sex, and education as covariates. The HRs from each study and the pooled association are presented in Table 4. The meta-analysis confirmed a highly significant effect for neuroticism (p = 2*10−9): for every SD increase in this trait, the risk of AD increased by more than 30% (HR = 1.33; 95%CI =1.21–1.45). The association was highly consistent across studies (Q = 2.6; degrees of freedom (df) = 4; p = 0.63), and in this set of studies we observed no statistical evidence of publication bias (Kendall’s z-tau = 1.22; p = 0.22; and Egger‘s regression intercept test: t = 1.87; df = 3; p = 0.16). The meta-analysis also indicated that lower scores on conscientiousness were associated with higher risk of incident AD (HR = 0.77; 95%CI =0.69–0.86; p = 2*10−6); again there was no evidence of heterogeneity (p = 0.55) or publication bias (p > 0.05). By pooling the results from the three studies, we also found significant associations for Openness (HR = 0.86; 95%CI =0.77–0.96; p = 0.008; heterogeneity: p = 0.91; publication bias p > 0.05) and Agreeableness (HR = 0.88; 95%CI =0.79–0.98; p = 0.019; heterogeneity: p = 0.51; publication bias p > 0.05), such that open and agreeable people were at lower risk of AD. There was no significant association with Extraversion (HR = 0.96; 95%CI =0.86–1.07; p = 0.53).
Table 3.
. Baseline characteristics of the samples included in the meta-analyses
| Sample | N | AD-N | Max Follow-up | Average follow-up | Age | Females% | Education | Minority |
|---|---|---|---|---|---|---|---|---|
| Rush Biracial13 | 1064 | 170 | 6 | † | 74 (9.6) | 62% | 12.9(6.3) | 50% |
| Rush MAP14 | 648 | 55 | 7 | 3 | 81 (6.9) | 74% | 14.6(2.9) | 2% |
| ROS15 | 904 | 176 | 12 | † | 74–80 (6.5) | 68%-71% | 18 (3.3) | 8% |
| GEM16 | 767 | 116 | 7 | 6 | 79 (3.1) | 42% | † | 8% |
| BLSA | 1671 | 90 | 22 | 12 | 57 (16.0) | 49% | 16.7 (2.5) | 29% |
Rush MAP = Rush Memory and Aging Project; ROS= Religious Order Study; GEM = Ginkgo Evaluation of Memory study; BLSA=Baltimore Longitudinal Study of Aging.
See original study
Table 4.
Risk of incident AD associated with personality traits reported in each study, meta-analytic effect sizes and heterogeneity.
| Sample | Neuroticism | Extraversion | Openness | Agreeableness | Conscientiousness |
|---|---|---|---|---|---|
| Rush Biracial13 | 1.338 (1.065–1.682) | ||||
| Rush MAP14 | 1.480 (1.143–1.918) | ||||
| ROS15 | 1.191 (1.008–1.408) | 1.093 (0.911–1.311) | 0.906 (0.760–1.080) | 0.900 (0.765–1.059) | 0.809 (0.692–0.947) |
| GEM16 | 1.390 (1.158–1.668) | 0.900 (0.745–1.087) | 0.780 (0.640–0.950) | 0.860 (0.711–1.041) | 0.760 (0.626–0.923) |
| BLSA | 1.371 (1.085–1.733) | 0.86 (0.687–1.077) | 0.889 (0.708–1.117) | 0.858 (0.680–1.083) | 0.692 (0.549–0.873) |
| Meta-Analyses (random model) | 1.326 (1.210–1.454) | 0.954 (0.824–1.105) | 0.858 (0.766–0.961) | 0.877 (0.786–0.979) | 0.767 (0.689–0.855) |
| Heterogeneity Q (p) | 2.6 (0.63) | 3.3 (0.19) | 0.2 (0.91) | 1.4 (0.51) | 1.2 (0.55) |
Rush MAP = Rush Memory and Aging Project; ROS= Religious Order Study; GEM = Ginkgo Evaluation of Memory study; BLSA=Baltimore Longitudinal Study of Aging
All studies controlled for age, sex, and education. Ethnicity was an additional covariate in the Rush Biracial, GEM and BLSA. The Rush Biracial further adjusted for the presence of APOE-ε4 allele. The HRs from the ROS are from a model that included all five factors. The logHR and SE were scaled in the ROS, Rush Biracial, and Rush MAP study to correspond to the effect associated with 1 SD difference on the trait. For neuroticism (k=5, total N = 5054; incident AD, N = 607) and for each of the other 4 factors (k = 3, total N = 3342; incident AD, N = 382).
4. Discussion
In a large sample followed for up to 22 years, we found those in the highest quartile of neuroticism (vs. the lowest quartile) or those in the lowest quartile of conscientiousness (vs. the highest quartile) were at a three-fold increased risk of incident AD. We further estimated that neuroticism and conscientiousness could account for 13% and 11% of the AD cases in the population, respectively. These effect sizes and PAR estimates are comparable to those reported for recognized clinical and lifestyle risk factors for AD, such as diabetes (RR = 1.39, 1.17–1.66; PAR = 3%), lower education (RR = 1.59, 1.35–1.86; PAR = 7%), smoking (RR = 1.59, 1.15–2.20; PAR = 11%), midlife obesity (RR = 1.60; 1.34–1.92; PAR = 7%), midlife hypertension (RR = 1.61; 1.16–2.24; PAR = 8%), physical inactivity (RR = 1.82; 1.19–2.78; PAR = 21%), or depression (RR = 1.90; 1.55–2.33; PAR = 15%).2 These findings indicate that personality traits may help identify individuals at greater risk of AD, and potentially aid in the early detection of AD.
In addition to effect sizes that are similar to other major risk factors for AD, the meta-analyses indicated a high degree of consistency across studies; all strongly supported the association of neuroticism and conscientiousness with risk of AD. This lack of heterogeneity is a notable finding, given the differences in demographic characteristics of the samples, study design, and length of the follow-up. Furthermore, the greater statistical power afforded by combining multiple samples suggested a significant association for the openness factor, which was significant in only one of the primary studies,16 and for agreeableness. There was no significant association for extraversion.
These associations might be explained, in part, by the links between personality and health-related behaviors, lifestyle factors, and clinical conditions. Low conscientiousness and high neuroticism are associated with cigarette smoking,7 physical inactivity,28 and obesity,9 which in turn are risk factors for dementia.2 Neuroticism is a strong vulnerability factor for major depression,6 which is also associated with AD.2, 29, 30 Personality traits are related to coping skills, and chronic stress over the lifespan might contribute to the inability to cope with the neurodegenerative process underlying AD. Direct physiological pathways are another plausible mechanism, given that low conscientiousness and high neuroticism are related to inflammatory markers such as IL6, CRP, and WBC counts.8, 31 Neuroticism is also related to level of brain derived neurotrophic factors (BDNF),32 which is thought to play an important role in neurodegenerative disorders. Another possibility is shared genetic liability. For example, in a large founder population33 but not in other samples,34 variants in the DYRK1A gene were associated with conscientiousness. The gene DYRK1A maps in the Down syndrome critical region on chromosome 21, its activity is up-regulated by Aβ, and it is involved in tau phosphorylation.35 We also found suggestive evidence that personality traits may interact with the APOE genotype, such that openness and agreeableness were protective factors depending on the ε4 carrier status in the BLSA sample.
The meta-analysis supported an inverse association between openness and risk of AD. Open individuals prefer variety, are attentive to inner feelings, are sensitive to art and beauty, and are intellectually engaged, curious, and imaginative. Open individuals tend to perform well on cognitive measures, such as executive functions and working memory tasks,36–38 and on measures of academic achievement.39 The higher intellectual engagement of these individuals is consistent with other evidence that relates cognitive activity with risk of AD.40, 41 Openness, especially its Ideas facet, is correlated with aspects of intelligence and education, which are associated with greater cognitive reserve and decreased risk for AD.40 It should be noted that openness was associated with risk of AD even with education as a covariate, suggesting that the effects of openness on AD might thus have been underestimated in our analyses.
A novel finding from our meta-analysis was that agreeable people have a reduced risk of AD. We interpret this finding with caution, since none of the individual studies show a significant association, and therefore replication in additional samples is particularly warranted. Still, it is worth considering that agreeableness measures interpersonal tendencies, and an inclination to be altruistic and cooperative might facilitate the formation of interpersonal connections and the stability of social networks.42 Extraversion, however, the other major personality dimension that shapes interpersonal tendencies, was not associated with risk of AD. Another possibility is that individuals who score low on Agreeableness tend to be aggressive, competitive, and antagonistic, which increase risk of cardiovascular disease.43, 44 Thus, the greater cardiovascular burden carried by antagonistic individuals may contribute to their risk of AD.
Among the strengths of this study was the use of a psychometrically robust and detailed personality assessment administered over a relatively long follow-up interval. The facet-level analyses allowed us to examine which aspects of the heterogeneous domains were most strongly related to AD. For neuroticism, we found the strongest effects for the depression, anxiety, and angry-hostility facets, and a trend for vulnerability. Given the large literature documenting the role of depression in dementia2, 30, 45, 46 it was not surprising to find an association between this facet and risk of AD. Comparatively less is known about the role of anxiety as a risk factor of AD, but some studies have found similar associations.47, 48 The association of angry-hostility supports the above hypothesis for agreeableness (i.e., hostility increases risk), and the effect of vulnerability supports the role of stress vulnerability in dementia.48, 49 Turning to conscientiousness, most of the facets were associated with reduced risk of AD. The strongest effect was observed for self-discipline, a measure of motivation, persistence, and ability to achieve a goal despite distractions, boredom, or difficulties. This impulsivity-related trait plays an important role in health-risk behaviors and outcomes,8, 9 and might be related to AD through a number of the direct and indirect pathways discussed above.
Among the limitations of this study is the observational design of the BLSA and most studies included in the meta-analyses. The BLSA is also not a representative sample of the US population. However, as the meta-analysis supports, the BLSA results were similar to those obtained in other samples, and we found no evidence of heterogeneity in the published studies. The number of studies that measured the five factors is still relatively small, but for neuroticism the meta-analysis included a total 5054 individuals, with 607 cases of incident AD. Additional studies are needed to increase these numbers and to test the potential mechanisms underlying the association between personality traits and incident AD. Given the strength of the association between personality traits and risk of AD, the consistency of these associations across the published studies, and the clinical value of identifying factors that may promote resilience to AD neuropathology,50 personality traits should be further evaluated as important risk factors of AD.
Research in Context
Systematic review – We reviewed the literature using PubMed and Scopus, and examined the reference list of identified articles. Numerous studies have examined personality traits in Alzheimer’s disease (AD) patients, but few have prospective measures of personality traits to test as predictors of incident dementia, and most used a brief personality measure within 3 to 6 years from the onset of dementia.
Interpretation – We found consistent evidence that personality traits predict incident AD; effect sizes were comparable to those of more established risk factors. The BLSA findings further contribute to current knowledge by (a) testing this association over a significantly longer follow-up period (M = 12 years), and (b) examining the association with a more comprehensive and detailed measure of personality.
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
The authors have no financial or other conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131–68. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer’s Disease International. World Alzheimer Report 2011: The benefits of early diagnosis and intervention. 2011. [Google Scholar]
- 4.Daviglus ML, Plassman BL, Pirzada A, Bell CC, Bowen PE, Burke JR, et al. Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol. 2011;68(9):1185–90. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 5.Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 6.Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depression: a Swedish longitudinal, population-based twin study. Arch Gen Psychiatry. 2006;63(10):1113–20. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- 7.Terracciano A, Lockenhoff CE, Crum RM, Bienvenu OJ, Costa PT., Jr Five-Factor Model personality profiles of drug users. BMC Psychiatry. 2008;8:22. doi: 10.1186/1471-244X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutin AR, Terracciano A, Deiana B, Naitza S, Ferrucci L, Uda M, et al. High Neuroticism and low Conscientiousness are associated with interleukin-6. Psychol Med. 2010;40:1485–93. doi: 10.1017/S0033291709992029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. J Pers Soc Psychol. 2011;101(3):579–92. doi: 10.1037/a0024286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman BP, Roberts B, Duberstein P. Personality and longevity: knowns, unknowns, and implications for public health and personalized medicine. J Aging Res. 2011;2011:759170. doi: 10.4061/2011/759170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death: How researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychological Science in the Public Interest. 2010;11:53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- 12.Roberts BW, Kuncel NR, Shiner R, Caspi A, Goldberg LR. The Power of Personality: The Comparative Validity of Personality Traits, Socioeconomic Status, and Cognitive Ability for Predicting Important Life Outcomes. Perspectives on Psychological Science. 2007;2(4):313–345. doi: 10.1111/j.1745-6916.2007.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, et al. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64(2):380–2. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143–53. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry. 2007;64(10):1204–12. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- 16.Duberstein PR, Chapman BP, Tindle HA, Sink KM, Bamonti P, Robbins J, et al. Personality and risk for Alzheimer’s disease in adults 72 years of age and older: a 6-year follow-up. Psychol Aging. 2011;26(2):351–62. doi: 10.1037/a0021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terracciano A, Iacono D, O’Brien RJ, Troncoso JC, An Y, Sutin AR, Ferrucci L, Zonderman AB, Resnick SM. Personality and resilience to Alzheimer's disease neuropathology: a prospective autopsy study. Neurobiol Aging. 2013;34(4):1045–50. doi: 10.1016/j.neurobiolaging.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Jr, Lakatta EG, et al. NIH Publication No 84–2450. Bethesda, MD: National Institutes of Health; 1984. Normal human aging: The Baltimore Longitudinal Study of Aging. [Google Scholar]
- 19.Scuteri A, Morrell CH, Najjar SS, Muller D, Andres R, Ferrucci L, et al. Longitudinal paths to the metabolic syndrome: can the incidence of the metabolic syndrome be predicted? The Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2009;64(5):590–8. doi: 10.1093/gerona/glp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hek K, Demirkan A, Lahti J, Terracciano A, Teumer A, Cornelis MC, et al. A Genome-Wide Association Study of Depressive Symptoms. Biological Psychiatry. 2013;73(7):667–78. doi: 10.1016/j.biopsych.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54(11):2072–7. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–6. doi: 10.1017/s1041610297004870. discussion 177–8. [DOI] [PubMed] [Google Scholar]
- 23.Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51(9):901–6. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 24.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Terracciano A, Costa PT, Jr, McCrae RR. Personality plasticity after age 30. Personality and Social Psychology Bulletin. 2006;32:999–1009. doi: 10.1177/0146167206288599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes RE, Smith NE. Personality correlates of physical activity: a review and meta-analysis. Br J Sports Med. 2006;40(12):958–65. doi: 10.1136/bjsm.2006.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer’s disease. Annals of Neurology. 2005;57(3):381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 30.Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532–9. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutin AR, Milaneschi Y, Cannas A, Ferrucci L, Uda M, Schlessinger D, et al. Impulsivity-related traits are associated with higher white blood cell counts. J Behav Med. 2012;35:616–623. doi: 10.1007/s10865-011-9390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terracciano A, Lobina M, Piras MG, Mulas A, Cannas A, Meirelles O, et al. Neuroticism, depressive symptoms, and serum BDNF. Psychosom Med. 2011;73(8):638–42. doi: 10.1097/PSY.0b013e3182306a4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terracciano A, Sanna S, Uda M, Deiana B, Usala G, Busonero F, et al. Genome-wide association scan for five major dimensions of personality. Mol Psychiatry. 2010;15:647–656. doi: 10.1038/mp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Moor MH, Costa PT, Terracciano A, Krueger RF, de Geus EJ, Toshiko T, et al. Meta-analysis of genome-wide association studies for personality. Mol Psychiatry. 2012;17(3):337–49. doi: 10.1038/mp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu F, Liang Z, Wegiel J, Hwang YW, Iqbal K, Grundke-Iqbal I, et al. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J. 2008;22(9):3224–33. doi: 10.1096/fj.07-104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharp ES, Reynolds CA, Pedersen NL, Gatz M. Cognitive engagement and cognitive aging: is openness protective? Psychol Aging. 2010;25(1):60–73. doi: 10.1037/a0018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutin AR, Terracciano A, Kitner-Triolo MH, Uda M, Schlessinger D, Zonderman AB. Personality traits prospectively predict verbal fluency in a lifespan sample. Psychol Aging. 2011;26(4):994–9. doi: 10.1037/a0024276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soubelet A, Salthouse TA. Personality-cognition relations across adulthood. Dev Psychol. 2011;47(2):303–10. doi: 10.1037/a0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noftle EE, Robins RW. Personality predictors of academic outcomes: big five correlates of GPA and SAT scores. J Pers Soc Psychol. 2007;93(1):116–30. doi: 10.1037/0022-3514.93.1.116. [DOI] [PubMed] [Google Scholar]
- 40.Scarmeas N, Stern Y. Cognitive reserve: implications for diagnosis and prevention of Alzheimer’s disease. Curr Neurol Neurosci Rep. 2004;4(5):374–80. doi: 10.1007/s11910-004-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911–20. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 42.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–12. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 43.Dembroski TM, MacDougall JM, Costa PT, Jr, Grandits G. Components of hostility as predictors of sudden death and myocardial infarction in the Multiple Risk Factor Intervention Trial Psychosomatic Medicine. 1989;51:514–522. doi: 10.1097/00006842-198909000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Sutin AR, Scuteri A, Lakatta EG, Tarasov KV, Ferrucci L, Costa PT, Jr, et al. Trait antagonism and the progression of arterial thickening: women with antagonistic traits have similar carotid arterial thickness as men. Hypertension. 2010;56(4):617–22. doi: 10.1161/HYPERTENSIONAHA.110.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75(1):27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry. 2008;65(4):439–45. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- 47.Gallacher J, Bayer A, Fish M, Pickering J, Pedro S, Dunstan F, et al. Does anxiety affect risk of dementia? Findings from the Caerphilly Prospective Study. Psychosom Med. 2009;71(6):659–66. doi: 10.1097/PSY.0b013e3181a6177c. [DOI] [PubMed] [Google Scholar]
- 48.Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry. 2011;19(4):327–34. doi: 10.1097/JGP.0b013e31820119da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crowe M, Andel R, Pedersen NL, Gatz M. Do work-related stress and reactivity to stress predict dementia more than 30 years later? Alzheimer Dis Assoc Disord. 2007;21(3):205–9. doi: 10.1097/WAD.0b013e31811ec10a. [DOI] [PubMed] [Google Scholar]
- 50.Alzheimer’s Association Expert Advisory Workgroup on NAPA. Workgroup on NAPA’s scientific agenda for a national initiative on Alzheimer’s disease. Alzheimers Dement. 2012;8(4):357–71. doi: 10.1016/j.jalz.2012.04.003. [DOI] [PubMed] [Google Scholar]