Abstract
Small molecules have been used since antiquity to regulate our sleep. Despite the explosion of diverse drugs to treat problems of too much or too little sleep, the detailed mechanisms of action and especially the neuronal targets by which these compounds alter human behavioural states are not well understood. Research efforts in model systems such as mouse, zebrafish, and fruit fly are combining conditional genetics and optogenetics with pharmacology to map the effects of sleep-promoting drugs onto neural circuits. Recent studies raise the possibility that many small molecules alter sleep and wake via specific sets of critical neurons rather than through the global modulation of multiple brain targets. These findings also uncover novel brain areas as sleep/wake regulators and indicate that the development of circuit-selective drugs might alleviate sleep disorders with fewer side effects.
Introduction
Sleep potions and arousing elixirs have featured in both legend and practice since ancient times. While dominated for centuries by only a few compounds, the modern medical arsenal features an increasing variety of drugs for the treatment of sleep disorders such as insomnia, hypersomnia, and narcolepsy. These disorders affect millions of people each year, and in the United States alone, more than two billion dollars are spent on sleep aids. Despite the widespread use of sleep- and wake-promoting drugs, much remains unknown about the basic mechanisms and sites of action by which these drugs work [1].
There are many reasons for the gap in understanding. Sleep and arousal are highly complex brain states, involving many different neural circuits and neurotransmitter systems. In some cases, these sleep-promoting drugs have significant affinity for multiple protein targets, each of which may have unique pharmacological properties, anatomical sites of action, and functional outcomes. Furthermore, the molecular targets are expressed in neurons throughout the brain, raising questions about whether specific or global neuronal populations are targeted to modulate sleep. Finally, a large constellation of drugs can cause drowsiness but do not necessarily recapitulate normal sleep [2*].
Recent conceptual and experimental advances in model organisms are providing new insights into the neural and molecular mechanisms for sleep- and wake-promoting drugs. In mice, conditional knockout technology, deliverable by stereotactic injection of viruses in adult brains, provides both spatial and temporal control of sleep gene function. Moreover, there is an increased recognition of the conservation of sleep genetics and pharmacology in less complex model systems including zebrafish [3**, 4] and fruit flies [5]. This has allowed experiments to link sleep- and wake-altering drugs to discrete neural circuits. In this review, we discuss how research in these model systems provides new insights into the molecular and neuronal targets (Figure 1) of major classes of sleep- and wake-regulating drugs (Table 1).
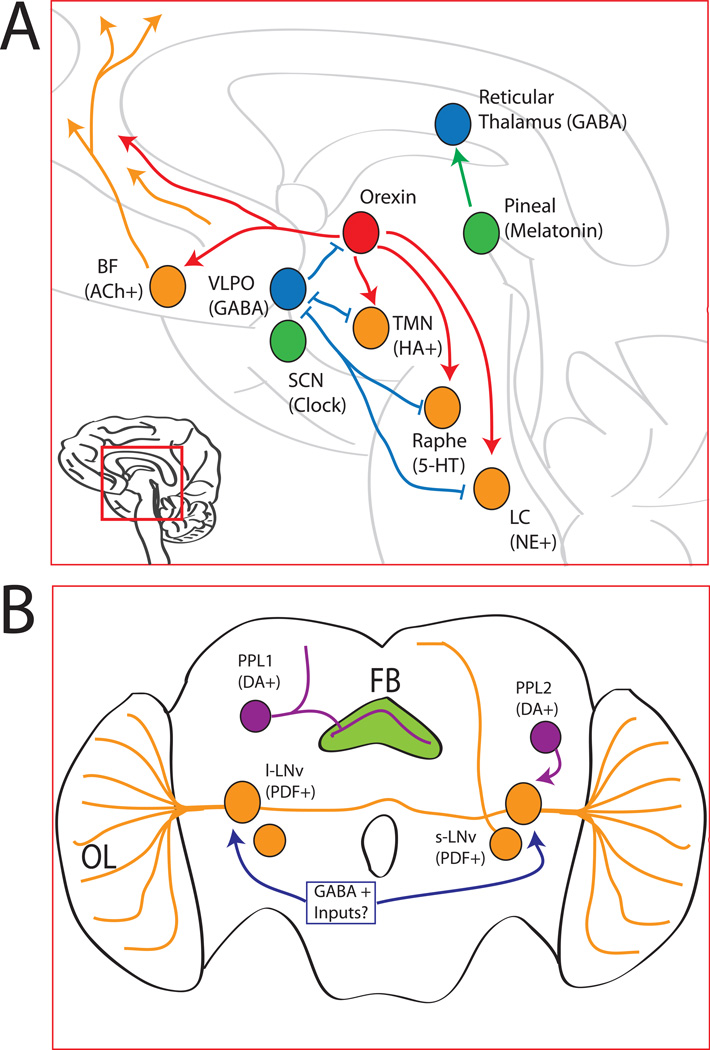
Figure 1. Major Sleep-Wake Pathways in Mammals and Flies.
A) The major mammalian sleep/wake regulatory pathways discussed in this review are shown. The ascending arousal system (ORANGE) is made up of many wake-promoting circuits, including the cholinergic basal forebrain (BF), the histaminergic (HA) tuberomammillary nucleus (TMN), the seroternergic (5-HT) dorsal raphe, and the noradrenaline (NE) producing locus coeruleus (LC). These areas send arousing projections (ORANGE LINES) to the thalamus and neocortex. The orexin neurons (RED) send excitatory projections to the ascending arousal network. The GABAergic neurons of the ventrolateral preoptic nucleus (VLPO; in BLUE), which makes inhibitory connections with both the orexin and ascending arousal systems. The TMN, raphe, and LC make mutual inhibitory connections with the VLPO to form a ‘flip-flop’ circuit. The pineal gland (GREEN) is the major source of melatonin, which can signal to the suprachiasmatic nucleus (SCN), which is the master regulator of circadian rhythms in mammals, and the GABA-positive reticular thalamus (BLUE), which may contribute to melatonin’s hypnotic effects. See text for details.
B) The major Drosophila circuits discussed in this review are shown on this schematic fly brain. Although not shown, all structures are bilaterally symmetric. In ORANGE are the major clock neurons, the large and small ventral lateral neurons (l-LNv and s-LNv). The wake promoting l-LNvs connect to the contralateral optic lobe (OL). Inhibitory GABA inputs (BLUE) are postulated for the l- LNvs. A single dopamine (DA) neuron (PURPLE) in the protocerebral posteriolateral 1 cluster (PPL1) signals to the fan shaped body (FB; GREEN) to increase Drosophila wakefulness. The wakepromoting l-LNvs also receive dopamine inputs, including from, but not limited to, the PPL2 cluster. See text for details.
Table 1.
Molecular and neuronal targets of small molecule sleep regulators.
| Sleep Effects in: |
|||||
|---|---|---|---|---|---|
| Name/Class | Molecular Target/Activity | Putative Neuronal Sleep Target(s) |
Mammals | Zebrash | Drosophila |
| Z-drugs | GABA-A agonist | Ascending Arousal System | |||
| (α1 subunit) | (TMN, LC, dorsal raphe) | increased | increased | increased | |
| Benzodiazepines | GABA-A agonist | Amygdala? | sleep | sleep | sleep |
| (α1,2,3,5) | |||||
| Doxepin | Histamine H1 receptor | Basal Forebrain, Cortex | increased | increased | ? |
| antagonist | sleep | sleep | |||
| Pitolisant | Histamine H3 receptor | TMN | increased | ? | ? |
| antagonist | wake | ||||
| Adenosine | Adenosine Receptor | Basal Forebrain, Cortex | increased | increased | increased |
| A1, A2A Agonist | sleep | sleep | sleep | ||
| Caffeine | Adenosine Receptor | Shell of | increased | increased | increased |
| A2A Antagonist | Nucleus Accumbens | wake | wake | wake | |
| Modafinil | dopamine transporter | ? | increased | increased | increased |
| (DAT)Inhibitor | wake | wake | wake | ||
| Melatonin | Melatonin receptor | SCN, Reticular Thalamus | increased | increased | N/A |
| MT1/MT2 agonist | sleep | sleep | |||
| Almorexant | Orexin receptor | Ascending Arousal System | increased | (orexin/hcrt | N/A |
| Suvorexant | OX1/OX2 antagonist | (TMN, LC, raphe) | sleep | increases wake) | |
GABAA Receptor Agonists
The hypnotic benzodiazepines and Z-drugs (i.e. zolpidem, eszopiclone, and zaleplon, also known in the US by the brand names Ambien, Lunesta, and Sonata, respectively) are popular sleep drugs. They enhance signalling of the brain’s major inhibitory neurotransmitter, γ-aminobutyric acid (GABA), via the type A receptor. GABAA receptors are heteropentameric ligand-gated ion channels typically composed of two α (with six possible isoforms), two β (3 isoforms), and one γ (3 isoforms) subunits [6*]. Benzodiazepines and Z-drugs potentiate GABAA receptor signalling via a modulatory binding site found in α subunits, with Z-drugs selective for the α1 subtype. The diversity of receptor subunit composition coupled with widespread brain expression has raised the questions of how and where the action of these drugs on GABAA receptors induces sleep.
Genetic replacement experiments in mice, in which the various alpha subunits are genetically replaced with versions that lack the benzodiazepine binding site, have begun to dissect which subunits are important for the major drug-induced phenotypes, including sedation, anxiety, and addiction. These experiments reveal that the α1 subunit is critical for sedation and the α5 subunit for developing benzodiazepine tolerance [7, 8]. These swap experiments reveal that at least some subsets of the myriad drug effects require different GABA-receptor subunits. Similarly, correlates of addiction were shown to depend on functional α1 binding sites in GABAergic neurons that modulate dopamine signalling in the ventral tegmental area [9]. That both the sedative and addictive properties of benzodiazepines are linked to the α1 subunit highlights that these drugs, including the α1 selective Z-drugs, do not act exclusively and specifically as hypnotics.
Does the hypnotic action of GABAA receptor drugs depend on global modulation or regulation of only a critical set of neurons? According to the flip-flop model of sleep-wake regulation, wake-promoting neurons, including the histaminergic tuberomammillary nucleus (TMN), the noradrenergic locus coeruleus (LC), and the serotonergic neurons of the dorsal raphe sit in a mutually inhibitory switch with sleep-active GABAergic neurons of the ventrolateral preoptic area (VLPO; Figure 1A). During sleep, GABA release from the VLPO is thought to inhibit these wake-promoting centers via GABA-receptors, making these areas prime candidates for the sedative action of GABAergic drugs. However, the importance of these areas in drug activity remains unclear. For example, the genetic elimination of GABA signalling specifically from the wake-inducing TMN histaminergic neurons has no effect on mouse sleep or on sensitivity to GABAergic drugs [10*]. Instead, viral-mediated ablation of the α1 GABAA subunits in the adult mouse amygdala—a brain area understudied in sleep research—abolishes the motor and sedative effects of zolpidem [11]. GABA agonists also do not clearly act upstream to disinhibit the GABA neurons of the VLPO, as Z-drugs such as eszopiclone do not enhance immediate early gene expression in the VLPO [12]. Intriguingly, the sedative effects of a different drug class, the anaesthetic isoflurane, appear due to the direct activation of the VLPO GABAergic neurons in mammals (Figure1A) and sleep-promoting neurons in the Drosophila fan-shaped body (Figure 1B), demonstrating that direct drug modulation of sleep-promoting circuits can in principle account for sedative properties of some small molecules [13**, 14].
While the neuronal specificity of GABA agonists in mammals remains unresolved, insights from Drosophila are framing how GABA-receptor-mediated inhibition of only a few neurons can account for drugs’ hypnotic properties. A Drosophila mutant with a slow-to-desensitize GABAA receptor (Resistant to dieldrein; Rdl) decreases fly sleep latency, while the drug carbamazepine, which enhances channel desensitization, increases latency [15]. These sleep-modulating effects critically depend on functional GABAA receptors in a set of wake-promoting circadian clock neurons, the ventral lateral neurons (Figure 1B) [16**, 17**]. Thus, despite widespread GABA receptor expression in flies, pharmacological actions on select wake-promoting cells underlie the sedative effects of GABA drugs and suggest that these wake-promoting centers lie downstream of sleep-promoting GABAergic circuits, as in mammals. Additional selective replacement studies are needed to determine whether GABA-receptor agonists also act on specific circuits in mammals. Zebrafish larvae are another model suitable for studying the role of GABA signalling in sleep, as GABA-A receptor agonists, including steroidal modulators of GABAA signalling such as alfadolone, enhance zebrafish sleep, while GABAA receptor antagonists inhibit sleep [3**].
Histamine H1 and H3 receptor antagonists
Low dose doxepin (Silenor) is a histamine H1 receptor antagonist approved for insomnia treatment in 2010 [18]. Anti-histamine receptor drugs that cross the blood brain barrier have been used as over-the-counter sleep aids for decades, although the complex pharmacological profiles of first generation anti-histamines such as diphenhydramine and chlorpheniramine has obscured the precise mechanisms of action for causing drowsiness (reviewed in [19*]).
In mammals, histamine is predominately made by the TMN (Figure 1A) and signals through four histamine receptors (H1–H4), which are widely expressed in many neuronal types in the brain, including the cerebral cortex and many wake-promoting areas such as the LC and the basal forebrain [19*]. Numerous pharmacological and genetic studies implicate the H1 receptor as the major wake-promoting histamine target. For example, H1 knockout mice have increased (but modest) non-REM sleep [20] ,as do animals injected with more selective second generation H1-antagonists, such as ketotifen [21]. At low doses, the first generation doxepin is highly selective for the H1-histamine receptor [22] and also increases sleep. Whether specific populations of H1-receptor expressing neurons are required for doxepin’s sleep-promoting effects is unknown. However, direct injection of histamine into the wake-promoting basal forebrain in rat [23] or into the cat mesopontine tegmentum increases wakefulness, an effect that is strongly inhibited by H1 receptor antagonists [24]. Thus, histamine signalling in specific circuits is sufficient to drive wakefulness and may therefore be key targets of sleep-promoting H1 antagonists.
The histamine system also regulates wake-fulnesss in zebrafish. Morpholino knockdown of histamine decarboxylase reduces wakefulness [25], as do histamine receptor H1 antagonists, such as pyrilamine, chloropyramine, and loratadine [3**]. Given the molecular and anatomical conservation of the histamine system between fish and mammals, the zebrafish system is a good model for elucidating how histamine H1 antagonists regulate wakefulness [26].
Pitolisant (tiprolisant; BF2.649) is a histamine H3 inverse agonist/antagonist currently in Phase III clinical trials for the treatment of excessive daytime sleepiness and narcolepsy [27]. Histamine H3 receptors act as autoreceptors on histaminergic neurons, creating a negative feedback loop thought to reduce the synthesis and release of histamine [28, 29]. H3 autoreceptors on the wake-promoting TMN neurons (Figure 1A) are presumed to underlie H3 receptor antagonist’s effects on waking, based on pharmacological and genetic evidence that show increased histaminergic tone and wakefulness [27–29]. However, histamine H3 receptors are expressed by most monoamine neurons and also can form heterocomplexes that regulate the function of many other neurotransmitter systems, including cholinergic, GABAergic, glutamatergic, and peptiderigic transmission [29]. Whether blockade of these other heteroreceptors contributes to the wake-promoting effects of H3 antagonists will require additional study. For both the histamine H1 receptor and H3 autoreceptors, locus-specific knockout and replacement studies are promising strategies to dissect these sites of action.
Adenosine Receptor Antagonists
A host of other sleep-regulatory substances, or somnogens, have been identified, including the cytokines interleukin-1 and tumor-necrosis factor alpha (TNF), prostaglandins, and adenosine [30, 31]. Although the proposed cascading interactions among these somnogens are complex, adenosine signalling is thought to be a major downstream effector for all of these signals [30–32].
Adenosine can signal through four adenosine receptors, of which the A1 and A2A receptors have been most strongly implicated in sleep regulation (reviewed in [32]). Likely produced in part by glia [33], endogenous adenosine levels in the cortex and especially the basal forebrain rise during wakefulness [34], where it is thought to inhibit wake-promoting cholinergic neurons via the inhibitory A1 receptor (Figure 1A)[32, 35]. Direct infusion of A1 receptor agonists into the BF increases sleep while both small molecule antagonists and locally delivered siRNA-mediated A1 receptor knockdown increase wakefulness [36, 37]. Similar pharmacological experiments have implicated other A1 receptor-expressing neurons, including the orexin neurons in the lateral hypothalamus [38] and the histaminergic neurons in the TMN as direct targets of adenosine’s sleep signals [39]; however, the rise of adenosine levels during waking in the basal forebrain makes it a prime candidate for adenosine-mediated sleep homeostasis. Stimulatory A2A receptors are also thought to be important for adenosine’s sleep effects in areas including the sleep-active VLPO and the basal ganglia, as perfusion of selective A2A receptor agonists into these areas promotes sleep and increases the activity of VLPO neurons [40–42].
Caffeine is an adenosine A1 and A2A receptor antagonist whose wake promoting effects are thought to be mediated by blocking the adenosine A2A receptor. A1 knockout mice are still aroused by caffeine whereas A2A knockouts are insensitive [43]. Does caffeine act on the wake- and sleep-promoting neurons proposed as loci for endogenous adenosine signalling, such as the basal forebrain? Remarkably, when adenosine A2A receptors in the shell of the nucleus accumbens (but not other areas) are deleted genetically in mice or by RNA interference in rats, the arousing effects of caffeine are blocked [44**]. This directly implicates classical motivational systems, including dopamine circuits (see below) in caffeine’s wake-promoting effects and raises intriguing questions about how this system interacts with other sleep- and wake- promoting centers [45]. Because elimination of adenosine A2A receptors in the nucleus accumbens has no effect on baseline wakefulness, the extent to which adenosine signalling in this area regulates normal sleep/wake behavior is unclear. It is possible that small molecules like caffeine alter behaviour through nonphysiological activation/inhibition of auxiliary arousal systems and not by direct modulation of core sleep circuitry per se.
Adenosine agonists increase sleep, while adenosine antagonists decrease sleep in other model species, including zebrafish [3**] and Drosophila [46]. Intriguingly, Drosophila mutants lacking the adenosine receptor have normal sleep and caffeine responses, and caffeine may act instead through inhibition of cAMP phosphodiesterase, which is also a strong caffeine binding target in mammals [46]. Future work will clarify in these systems where and how adenosine regulates evolutionarily conserved sleep processes, such as homeostasis.
Dopamine transporter antagonists
Modafinil and the related armodafinil are small molecules that promote wakefulness in many species and are used clinically in the treatment of excessive daytime sleepiness and narcolepsy. Although the only significant binding affinities demonstrated for modafinil are to the dopamine transporter (DAT) and, more weakly, the norepinephrine transporter (NET), a mechanism of action involving inhibition of DAT remains controversial, mainly because modafinil appears to lack the addiction potential of other DAT inhibitors, such as amphetamine, methylphenidate, and cocaine. Nevertheless, experimental evidence continues to support dopamine modulation as modafinil’s mechanism of action. DAT knockout mice are unresponsive to modafinil [47], as are mutant mice lacking dopamine D1 and D2 receptor signalling [48], and some aspects of modafinil-induced arousal depend on the dopamine D4 receptor [49]. In vivo binding studies using positron emission tomography (PET) in monkeys [50] and humans [51], as well as direct dopamine measurements in monkey brain [52] are consistent with modafinil inhibition of DAT. Why modafinil lacks the potent addiction potential of other psychostimulants remains a mystery, but structure/binding studies on wild type and mutant DATs show that modafinil binding to the transporter is mechanistically distinct from cocaine [53], possibly altering functional outcomes.
Which dopamine circuits participate in mammalian sleep/wake regulation is unknown, but experiments in Drosophila demonstrate that a discrete dopamine circuit controls wakefulness independently from other behaviours, such as situational arousal. DAT mutant flies are short sleepers [54, 55], while D1 dopamine receptor loss-of-function mutants (DopR) are long sleepers [56]. The short-sleeping mutant, insomniac, in which the adaptor for the E3 ubiquitin ligase, Cullin-3, is disrupted, also has upregulated dopamine signalling [57, 58]. Dopamine also increases the activity of the wake-promoting l-LNvs, an effect dampened by light [59]. Remarkably, studies using genetics and neural activation/imaging [60**, 61**] to map the critical dopamine sleep circuit converged onto only two dopamine neurons that signal to the fan shaped body, a brain area implicated in fly sleep/wake regulation and isoflurane-induced anaesthesia (Figure 1B) [62, 14]. This exquisite neuronal specialization underscores the notion that drug-induced behavioural changes may require activity at only a fraction of all drug-affected neurons.
Zebrafish is another potential model system for studying the role of modafinil and dopamine in sleep/wake regulation. Modafinil increases wakefulness of larval zebrafish [63], and dopamine receptor D1–D4 agonists and antagonists alter sleep/wake behaviour, albeit in a complex manner; for example, D2 receptor agonists such as pergolide increased sleep, while D2-receptor antagonists like droperidol paradoxically increased both sleep and locomotor activity during waking [3**]. Future studies in zebrafish will need to map which neurons are activated by modafinil and more carefully address the structure-activity relationship of dopamine receptor drugs to better understand how dopamine is involved in zebrafish sleep [4].
Melatonin Receptor Agonists
Melatonin is a hormone produced in a circadian (about 24-hour) rhythm by the pineal gland (Figure 1A) and signals predominantly through two G-protein coupled receptors, melatonin receptors MT1 and MT2. Because melatonin is produced at night in both nocturnal and diurnal animals, and pinealectomy fails to consistently alter sleep [64], the necessity of melatonin signalling in the direct regulation of sleep remains controversial [65, 66]. However, several melatonin-based therapeutics for insomnia have emerged, including extended-release melatonin (Circadin) and ramelteon (TAK-375; Rozerem), and is often used as a jet-lag aid.
Recent work with knockout mice and selective MT receptor agonists predominantly implicate the MT2 receptor in mediating melatonin’s soporific effects. Mice lacking MT1 receptors have less REM sleep, while MT2 knockouts have a modest reduction only in non-REM sleep [67]. However, double knockouts are only slightly more awake than wild type animals over 24-hours [67]. Consistently, the MT2 selective agonist IIK7, induces NREM sleep in rat [68], as does the MT2 selective compound UCM765 in mouse [69*]. In addition, UCM765’s sedative effect is abolished in MT2, but not MT1, receptor knockout mice. MT1 and MT2 receptors are widely expressed in many brain areas, including the circadian pacemaking suprachiasmatic nucleus (SCN) and other hypothalamic areas, the hippocampus, cerebellum, retina, prefrontal cortex, and others [70].
Which neuronal populations are critical for melatonin’s sleep effects is not known, although infusion of the MT2-selective drug UCM765 into the sleep-active reticular thalamic nucleus (Figure 1A) increased GABAergic neuron burst firing and NREM sleep [69]. Conversely, direct action of melatonin on the circadian pacemaker in the SCN has been proposed by numerous studies to mediate the phase-shifting effects of melatonin (reviewed in [71]). Thus, distinct target circuits may underlie melatonin’s hypnotic and phase-shifting properties, although more work is needed to disentangle these processes. Viral-mediated focal replacement of functional melatonin receptors in mutant backgrounds will be an attractive experimental paradigm for dissecting these complexities. Additionally, as melatonin is strongly hyponotic in zebrafish larvae [3**, 72–73], the zebrafish may be an excellent model for disentangling melatonin’s endogenous roles in sleep.
Orexin/hypocretin receptor antagonists
Orexin/hypocretin is a wake-promoting neuropeptide produced by a small number of neurons in the hypothalamus that send projections to many wake-promoting brain areas (Figure 1A). Dysfunction of the orexin system in humans leads to a sleep disorder, narcolepsy, associated with excessive daytime sleepiness, unstable sleep/wake transitions, and cataplexy. Orexin also promotes wakefulness in zebrafish; overexpression of orexin dramatically increases waking [74], orexin neurons are maximally active during the wake phase [75], and loss of orexin neurons leads to increased sleep/wake transitions [76]. Although Drosophila do not have the orexin system, the neuropeptide PDF may fulfill a conserved role in promoting wakefulness [16**, 17**]. In mammals, orexin signalling is mediated by two G-protein coupled receptors, orexin receptor 1 and 2 (OX-1 and OX-2, also called HcrtR-1 and -2). Recently, several dual OX-1/OX-2 antagonists have been developed as possible hypnotics, including almorexant (ACT-078573, but no longer clinically pursued; [77]) and suvorexant (MK-4305; [78]). At the end of 2012, Merck announced that suvorexant was accepted for FDA review based on promising clinical data that suvorexant improved sleep in patients [79, 80].
Pharmacological and receptor mutant analyses indicate that antagonism at the OX-2 receptor is particularly critical for non-REM sleep effects of these drugs. OX-2 receptor knockout mice have stronger phenotypes than OX-1 mutants [81, 82] and renders mice insensitive to the sleep effects of almorexant [83]. Consistently, OX-2 selective antagonists induce stronger sleep effects than OX-1 antagonists in both mice [84] and rats [85], although blocking both receptors may be even more effective [82, 84]. Viral-mediated replacement of OX-2 receptors in the histaminergic TMN of OX-2 knockout mice increases wake consolidation but does not rescue sleep fragmentation, indicating these phenotypes are regulated by discrete brain areas [86*]. Consistent with the idea that these are experimentally separable, expression of orexin peptide in the zona increta (which connects to the wake-promoting LC), or in the pons of orexin knockouts selectively rescues cataplexy but not wake or sleep fragmentation [87, 88].
Optogenetic studies of mouse orexin neurons and the noradrenergic LC demonstrate the importance of this particular connection in modulating sleep to wake transitions. Orexin neurons do fire predominately during wakefulness but also at a low frequency during sleep [89]. Optogenetic stimulation of either orexin or LC neurons during sleep shortens the latency to wakefulness, although orexin neuron activation wakes animals more slowly than LC stimulation [90, 91]. These neurons likely work in the same circuit, because stimulating orexin neurons while optogenetically blocking activity of the LC prevents the increases in sleep-to-wake transitions [92**]. Meanwhile, stimulation of orexin neurons in histamine deficient mice can still increase sleep-to-wake transitions [93], demonstrating that different sleep/wake functions can be ascribed to discrete orexin receptor-expressing neurons. More generally, optogenetically inhibiting and activating candidate sleep sub-circuits during drug exposure may be a powerful way to pinpoint neuronal drug targets without altering long-term brain activity, as in traditional knockout studies.
Prospects
Many of the experiments reviewed here point to the existence of discrete neuronal substrates as critical targets for small molecules’ sleep effects and suggest that sleep drugs do not require global modulation of brain activity to regulate sleep. In mice, viral-mediated focal knockouts and replacement strategies combined with optogenetics avoid problems of developmental compensation often seen in whole animal knockouts, allowing specific circuits to be experimentally manipulated in adults. Expanding these local knockout strategies to study additional small molecules presents both an opportunity and a challenge, as success requires choosing the correct few neurons from the whole brain to manipulate. Targeting previously described sleep-wake regulatory neurons serves as a good starting point, but small molecules (e.g. benzodiazepines, caffeine, and modafinil) are implicating novel and unexpected circuits in sleep-and wake-promoting drug activity. Furthermore, potential redundancy among multiple sleep circuits poses an obstacle for a locus-by-locus approach.
Insights from non-mammalian model systems offer some clues as to the functional and neuroanatomical properties of the neurons targeted by sleep drugs. In Drosophila, identifying the neurons responsible for drugs’ arousing or sedating actions provides a topology for how these circuits interact with other internal and external behavioural regulatory systems, such as food, light, motivational states, homeostatic processes, circadian rhythms, and memory formation. For example, linking the hypnotic effects of GABA directly onto the wake-promoting PDF cells in Drosophila [16**, 17**] highlights the intimate association between sleep and circadian rhythms and draws parallels to the mammalian circadian pacemaker in the SCN. Similarly, dopamine signalling networks, which play a fundamental in Drosophila sleep [60, 61], should also receive more attention as direct sleep/wake regulators in mammals [45].
Zebrafish larvae are also well-suited for small molecule sleep research. Compounds can be directly delivered in the water in 96-well plates and reach the brain, as these larvae lack the blood-brain barrier at this time [4]., which facilitates screens for novel sleep/wake drugs. For example, one screen of nearly 6000 small molecules identified several hundred structures that reliably alter larval fish locomotor and sleep behavior [3**]. These small molecules included many of the well-established sleep drug classes discussed in this review, such as modulators of the GABA, dopamine, histamine, noradrenaline, adenosine, and melatonin systems, as well as numerous modulators of NfkB, which is a likely integrator of cytokine, prostaglandin, and adenosine signals [30, 31]. The screen also identified the ether-a-go-go gene related potassium channel (ERG) and L-type calcium channel inhibitors as novel regulators of sleep and wake [3**]. The challenge now is to link these myriad small molecules to specific molecular and neuronal substrates. Drug-activated neurons can be mapped using immediate early gene expression [94] or by direct calcium imaging of neuronal dynamics in the whole brain [95**]. Once key drug-altered neurons are identified, they can be ablated by chemical genetics with nitroreductase [76] or by laser [96] and optogeneticallly modulated [97] to test their necessity in both drug-induced and normal sleep. Because of the considerable homology between zebrafish and mammalian brain structures, such a sleep-drug activation map in fish could have a direct correspondence to key sleep circuitry in mammals. In addition, the low cost and high-thoughput afforded by the simpler zebrafish and Drosophila models facilitate both drug discovery screens as well as the functional characterization of novel compounds [4].
In conclusion, a greater understanding of the molecular and neuronal substrates for major sleep and wake drugs not only will advance our knowledge of how sleep is regulated, it could also aid in the development of more target-selective sleep therapeutics. While this search continues using the techniques of modern mouse genetics, insights from non-mammalian systems, such as flies and fish, will also facilitate targeted approaches in mammals by pinpointing critical features likely to be shared by sleep circuits in all species.
Highlights.
The mechanisms and sites of action for sleep-altering drugs are poorly understood
Conditional mouse mutants and optogenetics are identifying critical sleep circuits
Sleep drugs may act on critical sets of neurons, instead of globally
Small molecules affect Zebrafish and Drosophila sleep in conserved ways
Acknowledgements
We thank Thomas Scammell, David Prober, and members of the Rihel lab for critical reading of the manuscript and the McKnight Endowment Fund for Neuroscience, NIH, Life Sciences Research Foundation, and the European Research Council for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Annotated Bibliography
- 1.Ioachimescu OC, El-Solh AA. Pharmacotherapy of insomnia. Expert Opinion on Pharmacotherapy. 2012;13:1243–1260. doi: 10.1517/14656566.2012.683860. [DOI] [PubMed] [Google Scholar]
- 2. Ellenbogen JM, Pace-Schott EF. Drug-induced sleep: theoretical and practical considerations. Pflügers Archiv - European Journal of Physiology. 2011;463:177–186. doi: 10.1007/s00424-011-1033-3.. This review emphasizes that drug-induced sleep may not recapitulate all the important features of normal sleep. Knowing which features of sleep are important for health—and which drugs best encapsulate those features—is of major clinical interest.
- 3. Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, Haggarty SJ, Kokel D, Rubin LL, Peterson RT, et al. Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation. Science. 2010;327:348–351. doi: 10.1126/science.1183090.. The first large scale small molecule screen for sleep regulators identified hundreds of both known and novel small molecule regulators of zebrafish sleep. Automated tracking of zebrafish larvae in 96-well plates allowed for the assessment of small molecules effects on behavior for several days. Multiple sleep/wake parameters for each compound were used to generate behavioral profiles that facilitated comparisons of the effects of hundreds of small molecules simultaneously.
- 4.Rihel J, Schier AF. Behavioral screening for neuroactive drugs in zebrafish. Developmental Neurobiology. 2012;72:373–385. doi: 10.1002/dneu.20910. [DOI] [PubMed] [Google Scholar]
- 5.Bushey D, Cirelli C. From genetics to structure to function: exploring sleep in Drosophila. Int. Rev. Neurobiol. 2011;99:213–244. doi: 10.1016/B978-0-12-387003-2.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nature Reviews Drug Discovery. 2011;10:685–697. doi: 10.1038/nrd3502.. An excellent review of GABA receptor subtype diversity and how these different subtypes contribute to various clinical and behavioural outcomes, such as addiction, anxiety, and sleep.
- 7.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat. Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 8.van Rijnsoever C, Täuber M, Choulli MK, Keist R, Rudolph U, Mohler H, Fritschy JM, Crestani F. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J. Neurosci. 2004;24:6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy J-M, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zecharia AY, Yu X, Gotz T, Ye Z, Carr DR, Wulff P, Bettler B, Vyssotski AL, Brickley SG, Franks NP, et al. GABAergic Inhibition of Histaminergic Neurons Regulates Active Waking But Not the Sleep-Wake Switch or Propofol-Induced Loss of Consciousness. Journal of Neuroscience. 2012;32:13062–13075. doi: 10.1523/JNEUROSCI.2931-12.2012.. Zecharia et al. used a Cre-lox strategy to remove all GABA-A and GABA-B signalling spefically from the histamine producing neurons. First, they used a knock-in strategy to express Cre recombinase under the control of the HDC promoter, which expresses in the histamine producing neurons. Crossing this transgene into mice that have a floxed allele of the gamma-2 subunit of GABA-A receptors or a floxed allele of the GABA-B1 subunit selectively removed GABA-A or GABA-B signalling from the histaminergic neurons. Neither the sleep-wake cycle nor the sensitivity to propofol were affected in these mice. Because this strategy removes GABA signalling throughout development, compensatory processes at a systems level might complicate the interpretation of these results.
- 11.Heldt SA, Ressler KJ. Amygdala-specific reduction of alpha1-GABAA receptors disrupts the anticonvulsant, locomotor, and sedative, but not anxiolytic, effects of benzodiazepines in mice. J. Neurosci. 2010;30:7139–7151. doi: 10.1523/JNEUROSCI.0693-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Alam MN, Rai S, Bashir T, McGinty D, Szymusiak R. Central nervous system sites of the sleep promoting effects of eszopiclone in rats. Neuroscience. 2011;181:67–78. doi: 10.1016/j.neuroscience.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 13. Moore JT, Chen J, Han B, Meng QC, Veasey SC, Beck SG, Kelz MB. Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr. Biol. 2012;22:2008–2016. doi: 10.1016/j.cub.2012.08.042.. Using c-fos mapping, Moore et al. discovered that the sleep-promoting GABA-ergic VLPO neurons are activated during isoflurane-induced anaesthesia. Remarkably, this was not an indirect recruitment of these sleep-active neurons, but rather a direct activation, as demonstrated by electrophysiology. Ablation of the sleep-active VLPO neurons caused animals to be resistant to isoflurane, demonstrating that these neurons are critical for the hypnotic effects of this drug. Thus, not only do volatile anaesthetics engage sleep centers, they do so by direct action to increase GABAergic tone in sleep circuits.
- 14.Kottler B, Bao H, Zalucki O, Imlach W, Troup M, Van Alphen B, Paulk A, Zhang B, Van Swinderen B. A Sleep/Wake Circuit Controls Isoflurane Sensitivity in Drosophila. Current Biology. 2013 doi: 10.1016/j.cub.2013.02.021. http://dx.doi.org/10.1016/j.cub.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nature Neuroscience. 2008;11:354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJL, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF Cells Are a GABA-Responsive Wake-Promoting Component of the Drosophila Sleep Circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042.. Parisky et al., 2008 and Chung et al., 2009 showed that circadian clock cells that express the neuropeptide PDF are regulated by GABA to control sleep and wake in Drosophila. RNAi knockdown of the Drosophila GABA-A receptor gene, Resistant to dieldrin (Rdl) specifically in PDF neurons reduced total sleep. The small and large ventral lateral neurons (LNvs), circadian clock cells that express PDF form a wake-promoting circuit, as mutants lacking PDF the receptor, or the PDF neurons sleep more. Parisky et al further showed that specifically manipulating the excitiability of these neurons alters sleep. Together, these data suggest a model similar to the mammalian “flip-flop” switch, with the wake-promoting LNvs inhibited by (as yet unidentified) sleep-promoting GABAergic inputs.
- 17. Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABAA Receptor RDL Acts in Peptidergic PDF Neurons to Promote Sleep in Drosophila. Current Biology. 2009;19:386–390. doi: 10.1016/j.cub.2009.01.040.. Parisky et al., 2008 and Chung et al., 2009 showed that circadian clock cells that express the neuropeptide PDF are regulated by GABA to control sleep and wake in Drosophila. RNAi knockdown of the Drosophila GABA-A receptor gene, Resistant to dieldrin (Rdl) specifically in PDF neurons reduced total sleep. The small and large ventral lateral neurons (LNvs), circadian clock cells that express PDF form a wake-promoting circuit, as mutants lacking PDF the receptor, or the PDF neurons sleep more. Parisky et al further showed that specifically manipulating the excitiability of these neurons alters sleep. Together, these data suggest a model similar to the mammalian “flip-flop” switch, with the wake-promoting LNvs inhibited by (as yet unidentified) sleep-promoting GABAergic inputs.
- 18.Sullivan S. Update on emerging drugs for insomnia. Expert Opin Emerg Drugs. 2012;17:295–298. doi: 10.1517/14728214.2012.693158. [DOI] [PubMed] [Google Scholar]
- 19. Krystal AD, Richelson E, Roth T. Review of the histamine system and the clinical effects of H(1) antagonists: Basis for a new model for understanding the effects of insomnia medications. Sleep Med Rev. 2013 doi: 10.1016/j.smrv.2012.08.001. *A review that grapples with the complex features of various H1-antagonists’ effects on sleep.
- 20.Huang Z-L, Mochizuki T, Qu W-M, Hong Z-Y, Watanabe T, Urade Y, Hayaishi O. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103:4687–4692. doi: 10.1073/pnas.0600451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unno K, Ozaki T, Mohammad S, Tsuno S, Ikeda-Sagara M, Honda K, Ikeda M. First and second generation H1 histamine receptor antagonists produce different sleep-inducing profiles in rats. European Journal of Pharmacology. 2012;683:179–185. doi: 10.1016/j.ejphar.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Stahl SM. Selective histamine H1 antagonism: novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr. 2008;13:1027–1038. doi: 10.1017/s1092852900017089. [DOI] [PubMed] [Google Scholar]
- 23.Ramesh V, Thakkar MM, Strecker RE, Basheer R, McCarley RW. Wakefulness-inducing effects of histamine in the basal forebrain of freely moving rats. Behav. Brain Res. 2004;152:271–278. doi: 10.1016/j.bbr.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Lin JS, Hou Y, Sakai K, Jouvet M. Histaminergic descending inputs to the mesopontine tegmentum and their role in the control of cortical activation and wakefulness in the cat. J. Neurosci. 1996;16:1523–1537. doi: 10.1523/JNEUROSCI.16-04-01523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundvik M, Kudo H, Toivonen P, Rozov S, Chen Y-C, Panula P. The histaminergic system regulates wakefulness and orexin/hypocretin neuron development via histamine receptor H1 in zebrafish. FASEB J. 2011;25:4338–4347. doi: 10.1096/fj.11-188268. [DOI] [PubMed] [Google Scholar]
- 26.Sundvik M, Panula P. Organization of the histaminergic system in adult zebrafish (Danio rerio) brain: neuron number, location, and cotransmitters. J. Comp. Neurol. 2012;520:3827–3845. doi: 10.1002/cne.23126. [DOI] [PubMed] [Google Scholar]
- 27.Griebel G, Decobert M, Jacquet A, Beeské S. Awakening properties of newly discovered highly selective H3 receptor antagonists in rats. Behav. Brain Res. 2012;232:416–420. doi: 10.1016/j.bbr.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 28.Gondard E, Anaclet C, Akaoka H, Guo R-X, Zhang M, Buda C, Franco P, Kotani H, Lin J-S. Enhanced Histaminergic Neurotransmission and Sleep-Wake Alterations, a Study in Histamine H3-Receptor Knock-Out Mice. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2012.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J-S, Sergeeva OA, Haas HL. Histamine H3 receptors and sleep-wake regulation. J. Pharmacol. Exp. Ther. 2011;336:17–23. doi: 10.1124/jpet.110.170134. [DOI] [PubMed] [Google Scholar]
- 30.Krueger JM. The role of cytokines in sleep regulation. Curr. Pharm. Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urade Y, Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med Rev. 2011;15:411–418. doi: 10.1016/j.smrv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Bjorness TE, Greene RW. Adenosine and Sleep. Curr Neuropharmacol. 2009;7:238–245. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halassa MM, Florian C, Fellin T, Munoz JR, Lee S-Y, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 35.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of Sleep and Wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawryluk JM, Ferrari LL, Keating SA, Arrigoni E. Adenosine inhibits glutamatergic input to basal forebrain cholinergic neurons. J. Neurophysiol. 2012;107:2769–2781. doi: 10.1152/jn.00528.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J. Neurosci. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rai S, Kumar S, Alam MA, Szymusiak R, McGinty D, Alam MN. A1 receptor mediated adenosinergic regulation of perifornical-lateral hypothalamic area neurons in freely behaving rats. Neuroscience. 2010;167:40–48. doi: 10.1016/j.neuroscience.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oishi Y, Huang Z-L, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallopin T, Luppi P-H, Cauli B, Urade Y, Rossier J, Hayaishi O, Lambolez B, Fort P. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience. 2005;134:1377–1390. doi: 10.1016/j.neuroscience.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 41.Methippara MM, Kumar S, Alam MN, Szymusiak R, McGinty D. Effects on sleep of microdialysis of adenosine A1 and A2a receptor analogs into the lateral preoptic area of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1715–R1723. doi: 10.1152/ajpregu.00247.2005. [DOI] [PubMed] [Google Scholar]
- 42.Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 43.Huang Z-L, Qu W-M, Eguchi N, Chen J-F, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 44. Lazarus M, Shen H-Y, Cherasse Y, Qu W-M, Huang Z-L, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, et al. Arousal Effect of Caffeine Depends on Adenosine A2A Receptors in the Shell of the Nucleus Accumbens. Journal of Neuroscience. 2011;31:10067–10075. doi: 10.1523/JNEUROSCI.6730-10.2011.. It had been previously shown that adenosine A2A receptor knockout mice were insensitive to caffeine’s arousal effects. In this paper, Lazarus et al. showed that A2A receptors in the shell of the nucleus accumbens are mediators of caffeine’s effects in both mice and rats. First, they used the Cre-Lox system to selectively knockout adenosine A2A receptors in the basal ganglia of mice and showed that this abolished caffeine-induced arousal. Next, they injected AAV-Cre to the nucleus accumbens of mice carrying a floxed allele of the A2A receptor; this, too, blocked caffeine-induced arousal. Finally, they delivered short hairpin interfering RNAs (shRNA) targeting the A2A receptor into the nucleus accumbens of rats and showed that A2A knockdown in the shell, but not the core, blocked caffeine-induced arousal. Detailed anatomical and behavioral implications of this discovery are elaborated upon in [45].
- 45.Lazarus M, Huang Z-L, Lu J, Urade Y, Chen J-F. How do the basal ganglia regulate sleep–wake behavior? Trends in Neurosciences. 2012;35:723–732. doi: 10.1016/j.tins.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J. Neurosci. 2009;29:11029–11037. doi: 10.1523/JNEUROSCI.1653-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J. Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu W-M, Huang Z-L, Xu X-H, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J. Neurosci. 2008;28:8462–8469. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young JW, Kooistra K, Geyer MA. Dopamine receptor mediation of the exploratory/hyperactivity effects of modafinil. Neuropsychopharmacology. 2011;36:1385–1396. doi: 10.1038/npp.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J. Pharmacol. Exp. Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang G-J, Jayne M, Hooker JM, Wong C, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology (Berl.) 2010;210:439–448. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitt KC, Reith MEA. The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. PLoS ONE. 2011;6:e25790. doi: 10.1371/journal.pone.0025790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu MN, Koh K, Yue Z, Joiner WJ, Sehgal A. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila. Sleep. 2008;31:465–472. doi: 10.1093/sleep/31.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lebestky T, Chang J-SC, Dankert H, Zelnik L, Kim Y-C, Han K-A, Wolf FW, Perona P, Anderson DJ. Two Different Forms of Arousal in Drosophila Are Oppositely Regulated by the Dopamine D1 Receptor Ortholog DopR via Distinct Neural Circuits. Neuron. 2009;64:522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stavropoulos N, Young MW. insomniac and Cullin-3 regulate sleep and wakefulness in Drosophila. Neuron. 2011;72:964–976. doi: 10.1016/j.neuron.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfeiffenberger C, Allada R. Cul3 and the BTB adaptor insomniac are key regulators of sleep homeostasis and a dopamine arousal pathway in Drosophila. PLoS Genet. 2012;8:e1003003. doi: 10.1371/journal.pgen.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shang Y, Haynes P, Pírez N, Harrington KI, Guo F, Pollack J, Hong P, Griffith LC, Rosbash M. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nature Neuroscience. 2011;14:889–895. doi: 10.1038/nn.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ueno T, Tomita J, Tanimoto H, Endo K, Ito K, Kume S, Kume K. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nature Neuroscience. 2012;15:1516–1523. doi: 10.1038/nn.3238.. Ueno et al. and Liu et al. identified a critical set of dopamine neurons that regulate sleep in Drosophila. Ueno et al. used the Gal4-UAS system to rescue dopamine signalling in specific sets of neurons in dopamine signalling mutant backgrounds. Expression of the dopamine D1 receptor in the dorsal fan-shaped body (dFB) rescued the behaviour. Next, using the GRASP system, in which the two halves of split-GFP are expressed in synaptic partners and will reconstitute GFP expression across the synapse, Ueno et al. identified the dopamine neuron population anatomically connected to the dFB. Finally, they used the heat-sensitive Trp channel to activate this dopamine cell population and found it reduced sleep, even when mosaic analysis was used to activate only a single neuron.Liu and colleagues used a series of progressively more specific Gal4-drivers to express the TrpA1 channel in subsets of dopamine neurons, and found that heat activating the same dFB body-projecting dopamine neurons increased wakefulness. Using an atrial natriuretic factor fused to GFP (ANF-GFP) to monitor vesicle release suggested that these neurons are active during wakefulness. Together, these two papers identify a key wake-promoting dopamine circuit in Drosophila and show that modulation of even a single neuron is sufficient to alter sleep behaviour in this species.
- 61. Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN. Two Dopaminergic Neurons Signal to the Dorsal Fan-Shaped Body to Promote Wakefulness in Drosophila. Current Biology. 2012;22:2114–2123. doi: 10.1016/j.cub.2012.09.008.. Ueno et al. and Liu et al. identified a critical set of dopamine neurons that regulate sleep in Drosophila. Ueno et al. used the Gal4-UAS system to rescue dopamine signalling in specific sets of neurons in dopamine signalling mutant backgrounds. Expression of the dopamine D1 receptor in the dorsal fan-shaped body (dFB) rescued the behaviour. Next, using the GRASP system, in which the two halves of split-GFP are expressed in synaptic partners and will reconstitute GFP expression across the synapse, Ueno et al. identified the dopamine neuron population anatomically connected to the dFB. Finally, they used the heat-sensitive Trp channel to activate this dopamine cell population and found it reduced sleep, even when mosaic analysis was used to activate only a single neuron.Liu and colleagues used a series of progressively more specific Gal4-drivers to express the TrpA1 channel in subsets of dopamine neurons, and found that heat activating the same dFB body-projecting dopamine neurons increased wakefulness. Using an atrial natriuretic factor fused to GFP (ANF-GFP) to monitor vesicle release suggested that these neurons are active during wakefulness. Together, these two papers identify a key wake-promoting dopamine circuit in Drosophila and show that modulation of even a single neuron is sufficient to alter sleep behaviour in this species.
- 62.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sigurgeirsson B, Thorsteinsson H, Arnardóttir H, Jóhannesdóttir IT, Karlsson KA. Effects of modafinil on sleep-wake cycles in larval zebrafish. Zebrafish. 2011;8:133–140. doi: 10.1089/zeb.2011.0708. [DOI] [PubMed] [Google Scholar]
- 64.Fisher SP, Sugden D. Endogenous melatonin is not obligatory for the regulation of the rat sleep-wake cycle. Sleep. 2010;33:833–840. doi: 10.1093/sleep/33.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhdanova IV. Melatonin as a hypnotic: pro. Sleep Med Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Van den Heuvel CJ, Ferguson SA, Macchi MM, Dawson D. Melatonin as a hypnotic: con. Sleep Med Rev. 2005;9:71–80. doi: 10.1016/j.smrv.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 67.Comai S, Ochoa-Sanchez R, Gobbi G. Sleep-wake characterization of double MT(1)/MT(2) receptor knockout mice and comparison with MT(1) and MT(2) receptor knockout mice. Behav. Brain Res. 2013;243C:231–238. doi: 10.1016/j.bbr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Fisher SP, Sugden D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci. Lett. 2009;457:93–96. doi: 10.1016/j.neulet.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spadoni G, Rivara S, Bedini A, Angeloni D, Fraschini F, et al. Promotion of Non-Rapid Eye Movement Sleep and Activation of Reticular Thalamic Neurons by a Novel MT2 Melatonin Receptor Ligand. Journal of Neuroscience. 2011;31:18439–18452. doi: 10.1523/JNEUROSCI.2676-11.2011.. Ochoa-Sanchez et al. showed that UCM765, a selective melatonin MT2 receptor agonist, increases sleep in rats and mice. This effect appears specific for the MT2 receptor, as sleep promotion is abolished in MT2 receptor, but not MT1 receptor, mouse mutants. Microinfusion of UCM765 into the MT2-expressing reticular thalamus increased firing of GABAergic neurons and increased sleep.
- 70.Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJM, Zisapel N, Cardinali DP. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Progress in Neurobiology. 2008;85:335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Pevet P, Challet E. Melatonin: Both master clock output and internal time-giver in the circadian clocks network. Journal of Physiology-Paris. 2011;105:170–182. doi: 10.1016/j.jphysparis.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- 73.Appelbaum L, Wang GX, Maro GS, Mori R, Tovin A, Marin W, Yokogawa T, Kawakami K, Smith SJ, Gothilf Y, et al. Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc. Natl. Acad. Sci. U.S.A. 2009;106:21942–21947. doi: 10.1073/pnas.906637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prober DA, Rihel J, Onah AA, Sung R-J, Schier AF. Hypocretin/Orexin Overexpression Induces An Insomnia-Like Phenotype in Zebrafish. Journal of Neuroscience. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naumann EA, Kampff AR, Prober DA, Schier AF, Engert F. Monitoring neural activity with bioluminescence during natural behavior. Nat. Neurosci. 2010;13:513–520. doi: 10.1038/nn.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elbaz I, Yelin-Bekerman L, Nicenboim J, Vatine G, Appelbaum L. Genetic ablation of hypocretin neurons alters behavioral state transitions in zebrafish. J. Neurosci. 2012;32:12961–12972. doi: 10.1523/JNEUROSCI.1284-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, Van Gerven J, De Haas SL, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat. Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 78.Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, Roecker AJ, Mercer SP, Bednar RA, Lemaire W, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J. Med. Chem. 2010;53:5320–5332. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- 79.Sun H, Kennedy WP, Wilbraham D, Lewis N, Calder N, Li X, Ma J, Yee KL, Ermlich S, Mangin E, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36:259–267. doi: 10.5665/sleep.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herring WJ, Snyder E, Budd K, Hutzelmann J, Snavely D, Liu K, Lines C, Roth T, Michelson D. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79:2265–2274. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 81.Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 82.Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and-2 in the regulation of non-REM and REM sleep. J. Neurosci. 2011;31:6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mang GM, Dürst T, Bürki H, Imobersteg S, Abramowski D, Schuepbach E, Hoyer D, Fendt M, Gee CE. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep. 2012;35:1625–1635. doi: 10.5665/sleep.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morairty SR, Revel FG, Malherbe P, Moreau J-L, Valladao D, Wettstein JG, Kilduff TS, Borroni E. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS ONE. 2012;7:e39131. doi: 10.1371/journal.pone.0039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gozzi A, Turrini G, Piccoli L, Massagrande M, Amantini D, Antolini M, Martinelli P, Cesari N, Montanari D, Tessari M, et al. Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PLoS ONE. 2011;6:e16406. doi: 10.1371/journal.pone.0016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mochizuki T, Arrigoni E, Marcus JN, Clark EL, Yamamoto M, Honer M, Borroni E, Lowell BB, Elmquist JK, Scammell TE. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4471–4476. doi: 10.1073/pnas.1012456108.. Mochizuki et al. generated an OX-2 receptor knock-in mouse in which the gene is disrupted by a small flox-cassette, which, when provided Cre-recombinase, allows for restoration of normal OX-2 receptor function. Prior to exposure to Cre, these mice have narcolepsy, with unstable sleep-wake states and increased daytime sleepiness. By injecting an adeno-associated viral vector to express Cre(AAV-Cre) into different brain areas, they tested whether selective rescue of OX-2 receptor function could restore wild type behavior. AAV-Cre injection into the TMN and surrounding regions rescued wake, but not sleep, fragmentation in these mice, demonstrating that OX-2 receptor function in the TMN is sufficient to rescue some, but not all, aspects of the narcolepsy phenotype.
- 87.Liu M, Blanco-Centurion C, Konadhode R, Begum S, Pelluru D, Gerashchenko D, Sakurai T, Yanagisawa M, Van den Pol AN, Shiromani PJ. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J. Neurosci. 2011;31:6028–6040. doi: 10.1523/JNEUROSCI.6069-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blanco-Centurion C, Liu M, Konadhode R, Pelluru D, Shiromani PJ. Effects of orexin gene transfer in the dorsolateral pons in orexin knockout mice. Sleep. 2013;36:31–40. doi: 10.5665/sleep.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J. Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, De Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, De Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, De Lecea L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. PNAS. 2012;109:E2635–E2644. doi: 10.1073/pnas.1202526109.. Carter et al 2012 builds on the series of optogenetic studies to investigate the role of the orexin neuron onto the locus coeruleus (LC) neuronal circuit in regulating sleep-wake transitions. By expressing channelrhodopsin in orexin neurons and halorhodopsin in LC neurons, the authors activated orexin neurons with blue light while inhibiting LC neurons with yellow light. Activation of orexin neurons during sleep increased sleep-to-wake transitions. This phenotype required a functional LC, as blocking LC activation at the same time eliminated the effect. This paper, and the ones that preceeded it, show how optogenetics can be used to understand the role of specific circuits in sleep-wake control.
- 93.Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, De Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J. Neurosci. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ellis LD, Seibert J, Soanes KH. Distinct models of induced hyperactivity in zebrafish larvae. Brain Res. 2012;1449:46–59. doi: 10.1016/j.brainres.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 95. Ahrens MB, Li JM, Orger MB, Robson DN, Schier AF, Engert F, Portugues R. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature. 2012;485:471–477. doi: 10.1038/nature11057.. This paper demonstrates the feasibility of mapping whole-brain neuronal activity during complex zebrafish behaviors. Using calcium imaging to watch neuronal activity of a zebrafish larva ficitively navigating in response to moving stripes, Ahrens et al. identify a number of neuronal populations selectively activated during different stages of the locomotor task.
- 96.Orger MB, Kampff AR, Severi KE, Bollmann JH, Engert F. Control of visually guided behavior by distinct populations of spinal projection neurons. Nat. Neurosci. 2008;11:327–333. doi: 10.1038/nn2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Del Bene F, Wyart C. Optogenetics: a new enlightenment age for zebrafish neurobiology. Dev Neurobiol. 2012;72:404–414. doi: 10.1002/dneu.20914. [DOI] [PubMed] [Google Scholar]