Abstract
Altered bile acid (BA) concentrations in the colon may cause diarrhea or constipation. BA malabsorption (BAM) accounts for >25% of patients with irritable bowel syndrome (IBS) with diarrhea and chronic diarrhea in Western countries. As BAM is increasingly recognized, proper diagnostic methods are desired in clinical practice to help direct the most effective treatment course for the chronic bowel dysfunction. This review appraises the methodology, advantages and disadvantages of 4 tools that directly measure BAM: 14C-glycocholate breath and stool test, 75Selenium HomotauroCholic Acid Test (SeHCAT), 7 α-hydroxy-4-cholesten-3-one (C4) and fecal BAs. 14C-glycocholate is a laborious test no longer widely utilized. 75SeHCAT is validated, but not available in the United States. Serum C4 is a simple, accurate method that is applicable to a majority of patients, but requires further clinical validation. Fecal measurements to quantify total and individual fecal BAs are technically cumbersome and not widely available. Regrettably, none of these tests are routinely available in the U.S., and a therapeutic trial with a BA binder is used as a surrogate for diagnosis of BAM. Recent data suggest there is an advantage to studying fecal excretion of the individual BAs and their role in BAM; this may constitute a significant advantage of the fecal BA method over the other tests. Fecal BA test could become a routine addition to fecal fat measurement in patients with unexplained diarrhea. In summary, availability determines the choice of test among C4, SeHCAT and fecal BA; more widespread availability of such tests would enhance clinical management of these patients.
Keywords: SeHCAT, C4, glycocholate, fecal, diarrhea
Introduction
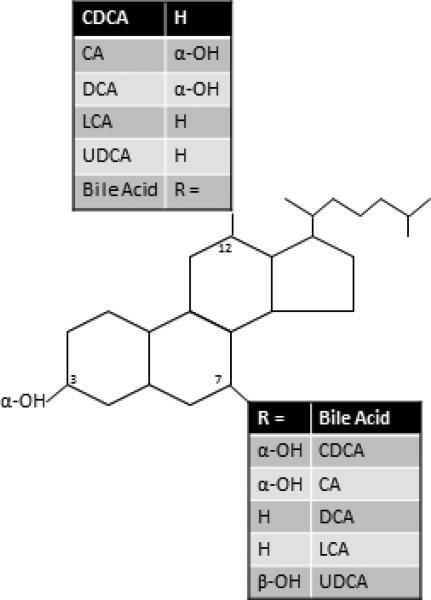
Bile acids (BAs) are detergent molecules1 excreted from the liver and are responsible for fat emulsification, aiding lipid absorption and digestion in the small intestine. There are several BA species that are differentiated structurally by their hydroxylation and conjugation status. Chenodeoxycholic acid (CDCA) and cholic acid (CA) are the primary BAs (Figure 1) which are synthesized in the liver from cholesterol.2 The majority of BAs excreted from the liver are conjugated to the amino acids, taurine or glycine, and remain ionized in the duodenum, which increases their solubility. This allows a high enough concentration of BAs to reach the critical micellar concentration, allowing for spontaneous formation of micelles whereby the polar BAs surround fat molecules and can present the hydrophobic fat molecules (which are insoluble in the aqueous phase) to the brush border membrane of the small intestine for digestion and absorption. The colonic bacteria avidly deconjugate and dehydroxylate BA; therefore, the major proportion of fecal BAs consists of the deconjugated secondary BAs, deoxycholic acid (DCA) and lithocholic acid (LCA).
Figure 1.
Bile acid (BA) chemistry: chenodeoxycholic acid (CDCA), cholic acid (CA), deoxycholic acid (DCA), lithocholic acid (LCA) and ursodeoxycholic acid (UDCA). Reproduced from Bajor A, et al. Scand J Gastroenterol 2010;45:645-664.
Sulfonation is an additional conjugation reaction that can occur in a subset of BAs; the reaction is catalyzed by sulfotransferase 2A1 (SULT2A1), preferentially transferring a sulfonate group (SO3-) to the 3α hydroxyl group on the BA molecule (Figure 1). Sulfonated BAs maintain a permanent negative charge, which influences their solubility but also decreases their affinity for the apical Na+-dependent bile salt transporter (ASBT, also called ileal BA transporter [IBAT] or SLC10A2 [solute carrier family 10, member 2]). IBAT is responsible for the active reuptake of BAs in the terminal ileum. Due to reduced affinity to IBAT and extraction by the liver, sulfonated BAs have limited enterohepatic recirculation, 3 and de-sulfonation occurs in the colon as a result of bacterial hydrolysis.4 Sulfonation has been shown to decrease the secretory effects of CDCA, CA and DCA in rats.5
A functional enterohepatic circulation (Figure 2) reabsorbs ~95% of BAs in the terminal ileum6 and transports the BAs back to the liver; interruption of the enterohepatic circulation results in cholerheic or BA diarrhea7. Bile acid malabsorption (BAM) is one of the mechanisms underlying the pathophysiology of diarrhea associated with ileal disease (Crohn's disease, surgical resection, radiation ileitis) and occurs in 32% of irritable bowel syndrome diarrhea (IBSD), 30-50% of chronic diarrhea and up to 35% of microscopic colitis8. Perfusion of BAs in the human colon results in colonic secretion of water and electrolytes9 and high amplitude, propagated contractions10. The presence of two α hydroxyl groups at the 3, 7 or 12 positions in the BA molecules (CDCA, CA, DCA) are responsible for their secretory effects.11 When the colon is exposed to an increased or decreased amount of those BAs, their presence promotes or decreases fluid and electrolyte secretion which resembles symptoms of chronic diarrhea or constipation2.
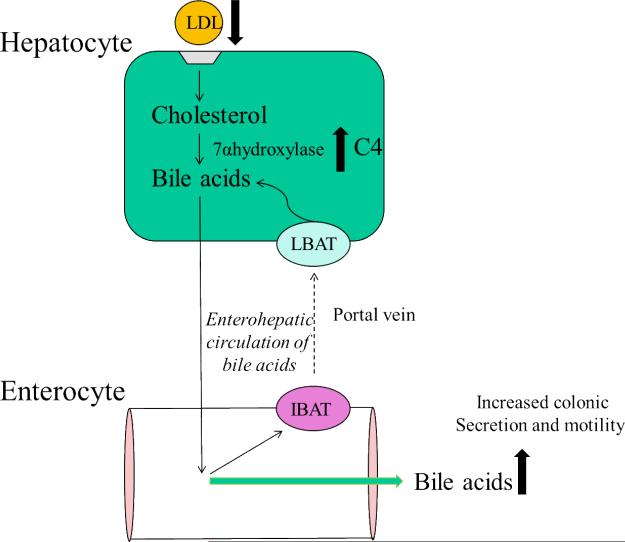
Figure 2.
Hepatic synthesis of BA from cholesterol involves entry into hepatocytes of lowdensity lipoprotein (LDL) cholesterol by binding to LDL receptors on the hepatocyte cell surface. Upregulation of hepatic BA synthesis promotes maintenance of BA pool size and leads to an increase in serum 7 α-hydroxy-4-cholesten-3-one (C4), a surrogate for the activity of cholesterol 7 α-hydroxylase, the rate-limiting enzyme in hepatic BA synthesis. Adapted from Wong BS, et al. Am J Gastroenterol 2011;106:2154-2164.
Although BAM is recognized in practice, the most popular current method of diagnosis includes a therapeutic trial of BA binders with symptom improvement; this approach is prevalent and the only resource available in countries like the United States where the noninvasive imaging based on scintigraphic BA retention is unavailable. Unfortunately in certain disease states, symptoms may only improve with high doses of a BA sequestrant or binder, and the diagnosis of BAM may be missed. Patients report poor palatability and side effects of borborygmi, flatulence and abdominal pain when using certain BA binders,7 which decrease compliance and ability to diagnose BAM. In addition, resin formulations such as cholestyramine may also bind and inactivate other etiological agents non-specifically, including Clostridium difficile toxin12,13. Therefore, a definitive diagnosis of BAM is desirable to assess disease severity and direct appropriate treatment modalities.
We briefly review the rationale, analytical methodology, and advantages/disadvantages of four methods that directly measure BAs: 14C-glycocholate breath and stool test, 75Selenium HomotauroCholic Acid Test (75SeHCAT), 7 α-hydroxy-4-cholesten-3-one (C4) and fecal BAs. Indirect measurements of BAM have been proposed, such as the serum fibroblast growth factor 19 test, 14 but this is not considered as it does not directly measure a BA entity.
14C-glycocholate Breath and Stool Test
Clinical Utility
The 14C-glycocholate breath and stool test is a method to determine bacterial dependent deconjugation within the gastrointestinal tract, due to bacterial overgrowth in the small bowel or BAM.15, 16
The 14C-glycocholate solution is orally administered and incorporates into the intraluminal pool of BAs. Bacteria can enzymatically cleave the bond between cholic acid and glycine. 14C-glycine is released, absorbed into the portal circulation, rapidly metabolized in the liver, and the end-product is exhaled as 14CO2. Since bacterial deconjugation is the rate limiting step, early breath excretion prior to the expected active absorption of the BA by the ileal BA transporter is highly suggestive of small bowel bacterial overgrowth.
Conversely, if the 14C-BA is not reabsorbed in the terminal ileum and enters the large intestine, the 14C-BA will be deconjugated by colonic bacteria, and a smaller proportion proceeds intact through the colon to be excreted in stool15. This situation may occur in cases of dysfunction of the IBAT (e.g. ileitis due to Crohn's or radiation) or ileal resection. Thus, stool collection after the 14C-glycocholate breath test can identify BAM.
Test Methodology
14C-glycocholate is ingested with a standard meal. Exhaled air is blown every hour for a minimum of 6 hours into a drying tube and is collected in a vial containing a solution that extracts the 14CO2. Stool is collected for 24 hours and is combusted to create 14CO2. A liquid beta scintillation counter quantifies the amount of 14C and provides estimate of fecal bile acid excretion.17
Advantages and Disadvantages
Unfortunately, the amount of exhaled 14CO2 during the first 2 to 4 hours after ingestion does not completely differentiate deconjugation occurring in the small or large intestine (Table 1). The small bowel transit time for liquids in a mixed meal was 181±28 (SEM) minutes;18 therefore, detection of 14CO2 in breath from colonic bacterial deconjugation (which would be increased in the presence of BAM as a result of the delivery of 14C-glycocholate to the colon) would occur about 3 hour after meal ingestion. Since the timing of breath excretion does not completely differentiate BAM from bacterial overgrowth, this test necessitates measurement of fecal intact 14C-BA.15
Table 1.
Advantages and Disadvantages of BAM Diagnostic Methods
| BAM Diagnostic Methods | Advantages | Disadvantages |
|---|---|---|
| 14C glycocholate | May identify small bowel bacterial overgrowth | Radiation exposure, β emission, long t1/2 |
| Varying normal values | ||
| Positive breath excretion at 2-4h does not differentiate BAM from small bowel bacterial overgrowth | ||
| Laborious test method (stool collection) | ||
| 75SeHCAT | γ emission, short t1/2, with decreased radiation to extra-abdominal organs | Not available in U.S. |
| Well-defined normal values; level of isotope retention predicts response to bile acid sequestrant | Radiation exposure | |
| Simple test method: 2 patient visits | ||
| Serum C4 | No radiation | Fasting sample, diurnal variation |
| Normal values reported in adults | Requires further validation | |
| Not dependent on age, gender or cholesterol | False-positive in liver disease, treatment with statins and altered circadian rhythm | |
| Simple blood test: 1 patient visit | ||
| Fecal BA | No radiation | Variable daily fecal BA excretion, requires at least 48h sample |
| Measures total and individual BAs | Cumbersome method (stool collection) |
One of the primary disadvantages of the test is the concern over radiation exposure and disposal. Studies have shown that there is a minor concern about radiation exposure to patients,15 but the extensive half-life of 5,730 years makes disposal of 14C taxing to the environment.20 In addition, the complexity and laborious nature of 14C-glycocholic breath and stool test reduced its application for diagnosing small bowel bacterial overgrowth or BAM. As other tests were developed to diagnose BAM, increasing the ease and improved accuracy and reliability19, the 14C-glycocholic method was discontinued in many centers.
Interpretation
Interpretive reference range values were not available for normal subjects with different ages and genders, thus making interpretation of results complex and individualized to each study center.
75Selenium HomotauroCholic Acid Test(75SeHCAT)
Clinical Utility
75SeHCAT utilizes a synthetic 75selenium homotaurocholic BA that is resistant to bacterial degradation21 and passive diffusion.22 Thus, 75selenium homotaurocholic BA can be either actively absorbed in the terminal ileum to enter the enterohepatic circulation or excreted into stool, unaltered by its passage through the colon.
Unlike 14C-glycocholate, 75selenium decays through gamma emission and thus can be measured with an external counter, a gamma camera, which does not require a collimator, therefore reducing the radiation dose necessary to estimate BA retention.
Test Methodology
The patient ingests a capsule of 75selenium homotaurocholic acid (gamma radiolabeled BA). The 75selenium is assumed to be appropriately distributed in the gut after approximately 1 hour, at which time a baseline scan is obtained and represents 100% retention. The number of subsequent scans has decreased over the years following introduction of the 75SeHCAT test from daily for a period of 7 days to a single follow-up scan on Day 7. The amount of radioactivity from 75selenium on subsequent scans is divided by the baseline scan on Day 1, indicating the percentage of 75selenium homotaurocholate remaining in the body and, indirectly, how much was lost in the stool. Sciarretta et al. have shown that a single subsequent scan on Day 7 has a sensitivity of 89% and specificity of 100% using whole body retention value.23 BA may undergo 5 enterohepatic circulations per day with ~5% loss in the stool with each circulation. In healthy subjects, 83% of 75selenium homotaurocholic BA is passed into the colon by Day 7. Thus, even a minor decrease in BA reabsorption will result in a substantial loss of BA.24
The test can be conducted using a single-headed camera, requiring the patient to remain prone and immobile for the duration of the test, and then return to that same position for follow-up imaging. Alternatively, use of a dual-headed camera allows the patient to be in a supine position while the anterior and posterior cameras capture the images. There are three ways to measure 75selenium: whole body, abdominal, or stool measurements. Currently, abdominal imaging from the chest to the knees is preferred compared to the whole body, due to the lack of customized shielded rooms. However, it is imperative that the patient and camera be in the same position for each image acquisition for abdominal imaging. Stool measurements require 24-hour stool collections for a period of 5 days. Following stool collection, a gamma camera is positioned ~20cm away from the stool container, and measurements are taken in duplicate to quantitate fecal excretion of the radiolabeled BA. Benefits of the stool measurement include patient convenience and visualization of the stool, which assists in characterizing stool type and form, potentially allowing for additional testing for electrolytes and individual BA measurements if indicated.24 The most common practice is either abdominal and/or whole body scans.
Advantages and Disadvantages
The avoidance of multiple stool collections makes the test less laborious and improves patient compliance. In addition, this practicality allowed for development of normative data in healthy volunteers, as well as a thorough appraisal of the diagnosis and severity of the BAM.
There are many additional attractive properties about the 75SeHCAT retention test. First, there is little influence of isotope distribution due to body build or position of the patient, as well as decreased radiation dose administered to extra-abdominal organs (Table 1). In the presence of BAM, there is increased fecal excretion of the intact radioactive BA, further decreasing the radioactivity exposure to the body, in contrast to 14C which could be absorbed from the colon by diffusion. Second, 75SeHCAT has a half-life of 118 days, thus, remaining in the body for less time as well as simpler disposal in the environment. Third, when directly compared to the 14C-glycocholate test, the 75SeHCAT test resulted in less false negatives and less borderline results.21 The major disadvantage is that, unfortunately, this test is approved and used in most European and many other countries, but not in the United States.19 Secondly, it is conceivable that false positive SeHCAT results may occur in patients with accelerated transit. There is no published evidence that experimentally-induced diarrhea affects SeHCAT results. However, there are conflicting reports of the non-specific effects of diarrhea or accelerated transit on BAM: there is abnormal SeHCAT in post-vagotomy diarrhea,25 but there was no association between small bowel transit and BAM, analyzed by SeHCAT.26
Interpretation
Retention at 7 days of ≥15% BA is consistent with a normal result. Mild BAM is considered 10-15%, moderate 5-10% and severe <5% retention. Wedlake et al. analyzed 18 studies and found response to cholestyramine, a BA binder, in 96% of patients with <5% retention, 80% with <10%, and 70% with <15%, indicating that the more severe the BAM the greater the response to BA binders. Response was assessed differently in the 15 studies that provided the information and is detailed elsewhere.7 The studies did not evaluate the response to cholestyramine in non-BAM patients.7
Serum 7 α-hydroxy-4-cholesten-3-one (C4)
Clinical Utility
C4 was initially developed to measure BA synthesis and the associated removal of circulating LDL cholesterol in clinical trials investigating modalities to reduce cardiovascular risk.27 BA synthesis occurs via neutral and acidic pathways. In humans, 90% of BA synthesis occurs through the neutral pathway, which is regulated by the rate-limiting enzyme, cholesterol 7α hydroxylase (CYP7A1). C4 is a downstream product of CYP7A1 (Figure 2). The correlation of C4 with BA synthesis has been validated in multiple studies, even in the presence of rapid CYP7A1 activity.28
Unlike the 14C and SeHCAT tests, which require multiple visits and testing with special equipment, serum C4 is a simple blood test, requiring only a standardized specimen collection time. Galman et al. reported diurnal variability in C4, with peak concentrations lasting for ~1.5 to 3 hours postprandially at 1:00 and 9:00 p.m. These C4 peaks reflect a true diurnal pattern, as they were observed in patients who fasted, and they were not related to postprandial excretion of bile. C4 levels in the gallbladder were minimal, and the diurnal peak was also seen in cholecystectomized patients.29 Similarly, Ma et al. demonstrated alteration of the circadian rhythm or a severed connection between the central and peripheral ‘clock’ changes in the diurnal rhythm for BA synthesis in mice.30 Thus, it is hypothesized that C4 is not released directly into the bile, but reaches the bloodstream directly. Although peaks are found during regular meal hours, C4 release is not dependent on meal ingestion, but reflects the circadian rhythm.
Test methodology
Quantitation of C4 requires a single blood draw in the morning following an overnight fast. C4 is isolated using liquid chromatography-tandem mass spectrometry (LCMS/MS). Although there are many methods and reagents that are utilized, the most recent method has an average of 99% recovery and is described elsewhere.8 Briefly, lipids and proteins are precipitated out with HPLC grade water, acetonitrile and saturated ammonium sulfate, and the specimen is vortexed and centrifuged. The supernatant is dried, reconstituted with 100% methanol, and injected onto a LC-MS/MS system (AB Sciex API 5000 MS/MS) coupled with an electrospray ionization (ESI) interface on a Cohesive HPLC System (Thermo Fisher Scientific, Franklin, MA) with a Phenomenex MAX-RP column (150 × 2.0mm, 4μm; Phenomenex).8
Advantages and Disadvantages
The clinical performance of the C4 assay demonstrated a sensitivity of 90%, specificity of 79%, negative predictive value of 98%, and positive predictive value of 74% when compared to the SeHCAT test, identifying BAM when the half-life remaining in the body was ≤1.2 days. The high NPV makes the assay attractive as a screening test to rule out BAM. C4 was unrelated to age, gender or serum cholesterol when analyzed against potential covariates (Table 1).19 Unlike other diagnostic tests, measurement of C4 allows for a dynamic understanding of the CYP7A1 enzyme.31 The availability of a test like C4 would be particularly advantageous in the pediatric population because of the lack of radiation exposure, and it is relatively simple to obtain normal values in children.
Quantitation of C4 requires specialized equipment and personnel, a disadvantage to the assay. There may be false-positive or false-negative C4 results in patients with liver disease (cholestatic disease with hypertriglyceridemia, AST or ALT >2x upper limit of normal), in patients taking medications which alter BA production (statins),8 or in individuals with an altered circadian rhythm. The utility of the test is still relatively limited, and it is unclear whether age, emotional conditions, or environmental factors such as shift work or jet lag may alter the circadian rhythm and thus the synthesis of BAs. The 9:00 a.m. fasting blood draw is a routine practice in most labs.
Patients who have slow colonic transit constipation (STC) have C4 levels which are often difficult to interpret. While recent studies demonstrated lower C431 and lower fecal BAs in a subset of patients with constipation phenotypes,31,32 STC was associated with an elevated C4 in the morning (9:00 a.m.) and decreased peak values at noon33. This change in pattern was hypothesized to depict the body's attempt to increase production, but no conclusive evidence has been reported.33
Interpretation
C4 is reported as a serum concentration (ng/mL). Two studies have reported the median (5th, 95th %) as 14.3ng/mL (6, 60.7)8 and 16.9ng/mL (5, 50.5)31 in 111 and 23 healthy volunteers respectively.8, 31 In our laboratory, an elevated serum C4 concentration is >60.7ng/mL. Unfortunately, there are no data on the response to BA binders in patients with an elevated C4.
Fecal BA
Clinical Utility
Apart from the fecal measurement of 14C-glycocholate, the tests discussed above indirectly assist in the diagnosis of BAM by measuring BA synthesis or retention. In contrast, it is now possible to quantify fecal total and individual BA. Increased total fecal BAs is reported in patients with chronic functional diarrhea;31 moreover, recent data show that functional diarrhea or diarrhea-predominant irritable bowel syndrome (IBS-D) are associated with higher fecal levels of secretory BAs (CA,CDCA, DCA), whereas functional constipation and constipation-predominant irritable bowel syndrome (IBS-C) are associated with higher fecal LCA levels.32 Measurement of fecal BAs is technically challenging and requires skilled technicians to perform the test. Although in time fecal BAs may become the gold standard assay, it is currently a test which is only available at a few clinical laboratories.
Test methodology
Stool is collected during the last 48 hours of the high fat intake diet. There are two predominant approaches to measure fecal BAs: enzymatic and chromatographic.
Enzymatic Assays
The assays utilize a NAD+ dependent steroid dehydrogenase enzyme to oxidize deconjugated BAs and produce NADH. Since the sensitivity of detecting NADH is low, another reaction is coupled that utilizes the reducing potential of NADH to create a product that is detected with greater sensitivity. With a variety of BA conjugations (sulfonation, glucuronidation) and hydroxyl groups, the quantified total BAs are underestimated because this method requires proper stereotactic alignment of enzyme and substrate. When hydrolysis time and BA concentration were increased, there was a significant correlation between enzymatic assay and GC-MS when measuring 3α hydroxyl BAs.34
Chromatographic Assays
The stool sample is weighed and frozen at -25°C until analysis. The chemical measurement of fecal BA involves separate steps of extraction, deconjugation, derivatization and quantification. The specifics of each chromatography process are described in detail elsewhere35,36 and are briefly described below. The stool specimen is homogenized with water, deconjugated, and separated from proteins, lipids and salts. Unfortunately, these steps may be complicated by the formation of molecules that may interfere with subsequent steps and result in loss of BAs.
Quantitation can be accomplished using 3 methods: gas chromatography-mass spectrometry (GC-MS), liquid chromatography-tandem mass spectrometry (LC-MS/MS), or high-performance liquid chromatography-mass spectrometry (HPLC-MS).
GC-MS requires additional steps to deconjugate all the fecal BAs and derivatize the BAs to allow for improved chromatographic separation.35,36 Derivatization provides the best analysis of complex mixtures of BAs. GC-MS also measures sulfonated BAs. Hoffman et al. studied pediatric functional constipation and found that, in a small subset of children, 3-sulfonate (sulfate) CDCA was the predominant fecal BA. This was accomplished by use of electrospray ionization-single ion monitoring-mass spectrometry, and individual BAs were measured by GCMS.37
Compared to LC-MS/MS, GC-MS provides more structural information and allows proper quantification of individual BAs. Because GC and LC focus on two different aspects of the analysis, it is plausible that they can be performed sequentially.
HPLC-MS requires limited sample preparation (protein extraction), but focuses more on the number of observable features and less on the structure or character of the BAs. HPLC-MS allows simultaneous analysis of free and conjugated BAs.35
Advantages and Disadvantages
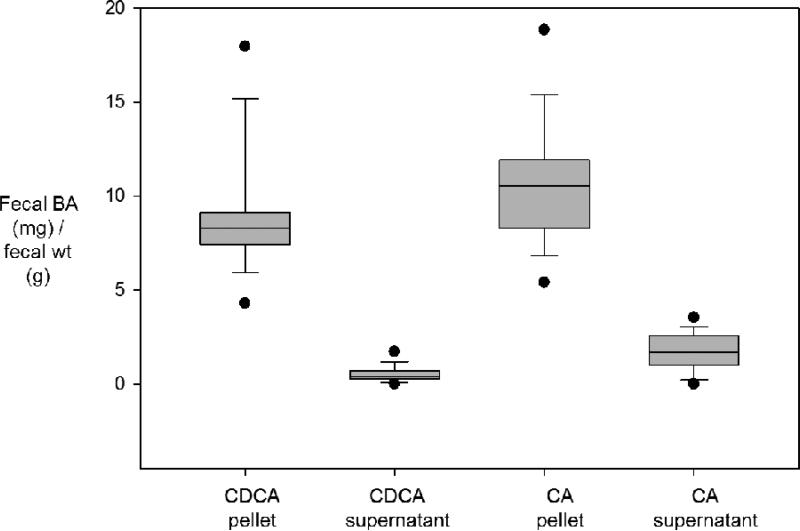
An unresolved aspect of fecal BA measurement includes variation in daily BA excretion (Table 1).35 As mentioned earlier, serum C4 studies suggest that there are diurnal variations in BA synthesis.29 Mitchell et al. measured fecal BAs in individual bowel movements of 3 patients (total 20 bowel movements) with ileal resections, and noted substantial variations between samples from the same individual (Figure 3). Patient G reported in their study had 11 stool samples that were homogenized and centrifuged to separate the supernatant and pellet. These were separately analyzed for chenodeoxycholic and cholic acid.38 There was a wide range of BA concentrations in the pellet for each BA (Figure 3). Due to the variability, a single stool sample to quantify fecal BA is insufficient to assess BA excretion or achieve an accurate diagnosis of BAM. Although there is no documented evidence in the literature, multiple sources recommend a stool collection of about 3-5 days to accurately diagnose BAM from fecal BA measurements.35, 39 Currently, at our institution, we collect a 48-hour stool sample, allowing for measurement of both fecal fat and BAs. This increases the practicality of the test and appears to be a reasonable approach for patients with suspected BAM who have diarrhea, since scintigraphic transit measurements show that the majority of isotope is excreted in stool at 48 hours in patients with diarrhea.40 However, more studies must be conducted to evaluate how many days of stool collection are required in order to produce precise, accurate, and clinically representative fecal BA measurements, particularly in patients with constipation.
Figure 3.
Variation in BA excretion per gram fecal weight in each bowel movement (BM) in a single patient. Data show median, interquartile range, 5th and 95th percentiles, and each individual value. Reproduced from Mitchell WD, et al. Gut 1973;14:348-353.
Another methodological question pertains to the measurement of BAs in stool supernatant, compared to the solid phase. From Mitchell et al. (Figure 3), the fecal BAs present in the supernatant are minimal and always less than the minimum values in the pellet or solid phase of the stool.38 These observations argue for homogenization of the solid and liquid phase of the stool in water during the extraction step of the assay and for collection of stool over 48 hours rather than a random sample for fecal BA measurement.
Interpretation
Fecal BAs are reported as a concentration in stool. Wong et al. showed a median (5th, 95th%) of 363μmol/24 hours (83, 2008) in 20 healthy volunteers, suggesting an elevated fecal BA measurement is >2.0mmol/24 hours.31
Cross-Validation of Methods to Detect Bile Acid Malabsorption
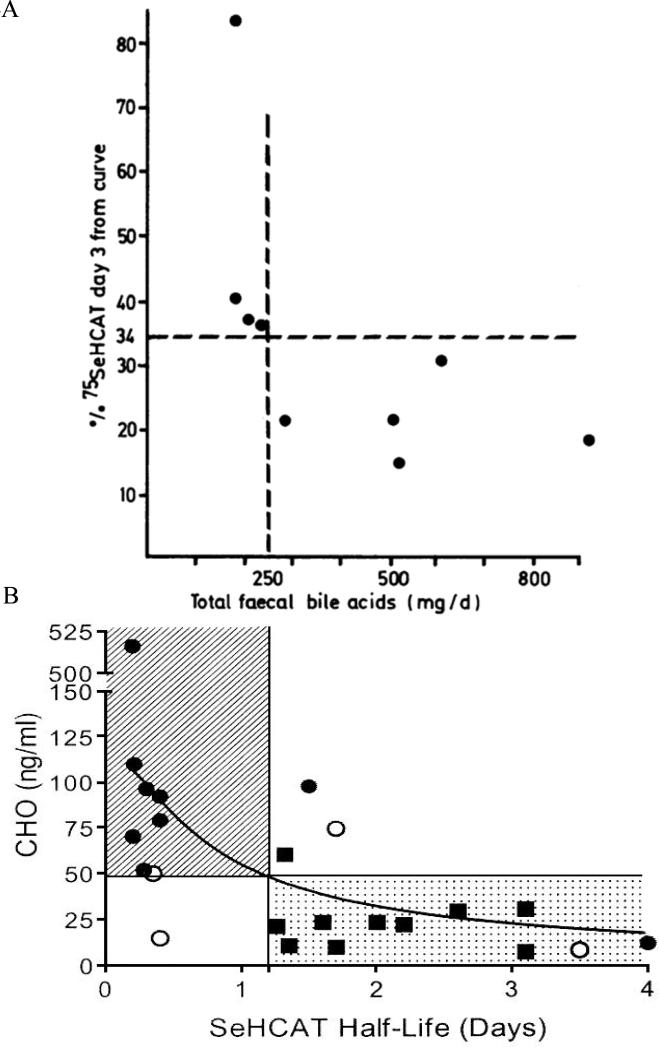
A number of studies published in the literature describe cross-validation between the various methods described; the comparative data are shown in Figures 4 and 5. Figure 4A compares 75SeHCAT retention at 3 days with total fecal BAs. The decrease in 75SeHCAT retention at Day 3 is significantly associated with an increase in fecal BAs. Following these initial validation studies, in which the cut-off at 3 days was 34% retention, subsequent studies focused on the 75SeHCAT retention at 7 days and described different degrees of BAM based on the proportion retained, with 5% or lower being associated with severe BAM. Figure 4B demonstrates the inverse relationship between serum C4 (a reflection of BA synthesis) and 75SeHCAT half-life in days.19 A shorter half-life reflects lower retention of the radioisotope in the body.
Figure 4A and B.
(A) 75SeHCAT values and total fecal bile acids of nine patients. Vertical dotted line marks the lower normal limit for total fecal bile acids (250mg/day). Reproduced from Sciaretta G, et al. Gut 1987;28:970-975. (B) Relationship between half-life of 75SeHCAT and HCO (7 α-hydroxy-4-cholesten-3-one) serum concentrations in patients with diarrhea of unknown origin. The response to treatment is indicated by different symbols (  = response to treatment, ■ = no response to treatment, ● = response not evaluated). The hatched area denotes pathologic values for both tests; the dotted area denotes normal values for both tests. Reproduced from Sauter GH, et al. Dig Dis Sci 1999;44:14-19.
= response to treatment, ■ = no response to treatment, ● = response not evaluated). The hatched area denotes pathologic values for both tests; the dotted area denotes normal values for both tests. Reproduced from Sauter GH, et al. Dig Dis Sci 1999;44:14-19.
Figure 5A, B and C.
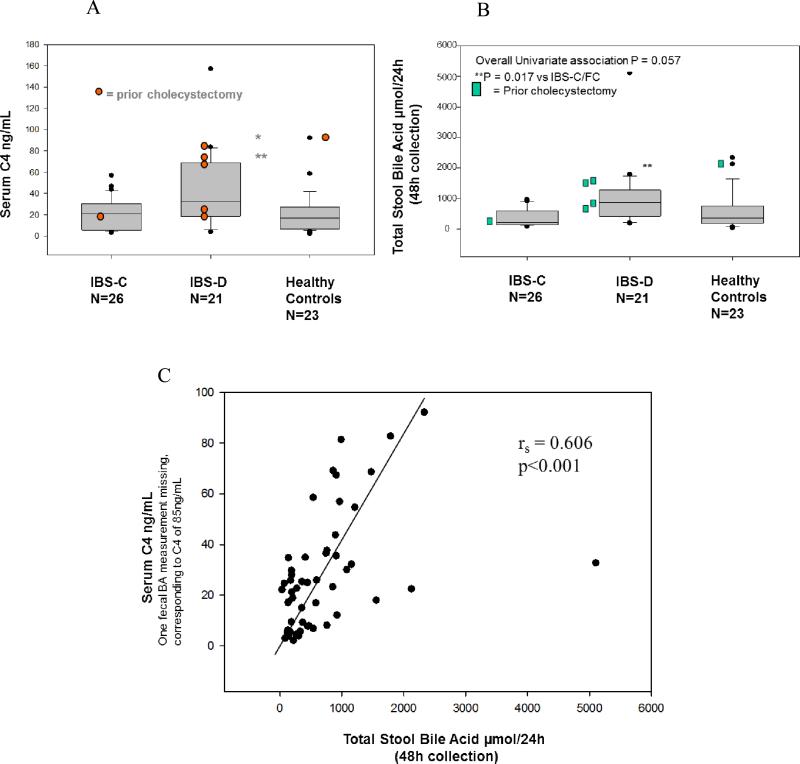
(A and B) Quantification of serum C4 and total stool bile acids in IBS-C, IBS-D and healthy controls. Data show median and interquartile ranges, 5th and 95th percentiles. Note the higher serum C4 and fecal total bile acids excreted over 48 hours in patients with IBS-D. (C) Relationship between fasting serum C4 and total 48 hour stool BA excretion. Adapted from Wong BS, et al. Clin Gastroenterol Hepatol 2012;10:1009-1015.
Figures 5A and B show the results of serum C4 and fecal total BAs in patients with symptoms of chronic diarrhea or constipation, compared to healthy controls. Note that both measurements identify subgroups of patients with abnormal fecal excretion, especially in patients with IBS-D. There is also a cross-validation comparing the fecal 48 hour total BA excretion and serum C4, as illustrated in Figure 5C, plotted from the results in the same study.31
Conclusions
Currently, 75SeHCAT retention, serum C4, and fecal BA measurements are the three viable tests available to diagnose BAM. Unfortunately, 75SeHCAT is not available in several countries, including the United States. Serum C4 is a simple and accurate method for patients who do not have liver disease or take statins and maintain a normal circadian rhythm; however, it has been studied in relatively small numbers of patients, and further validation, including response to therapy in patients selected for BA binders based on serum C4 test, is desirable. Fecal BA measurement by enzymatic assay provides an estimate of total fecal BAs, but its accuracy is considered suboptimal. The chromatographic assays can quantify total and individual fecal BAs, but the method is cumbersome, not widely available, and requires further validation of the optimal collection time of stool samples for patients with constipation. Recent data suggest that there is an advantage to studying the individual BAs and their role in BAM or constipation. Regrettably, for the U.S. physician, the only current option is to continue with trials of bile acid binders in patients suspected with BAM. Hopefully, increased availability of serum C4 and fecal BAs through samples mailed to centralized clinical laboratories will enhance the opportunity to optimize treatment directed at disorders of BA homeostasis that are responsible for either chronic diarrhea or constipation.
Acknowledgments
We thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding: This work is supported by grant RO1-DK92179 (to Dr. Camilleri) from National Institutes of Health.
Abbreviations
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- LCA
lithocholic acid
- BA
bile acid
- BAM
bile acid malabsorption
- 75SeHCAT
75Selenium HomotauroCholic Acid Test
- C4 or HCO
7 α-hydroxy-4-cholesten-3-one
- ASBT
Na+-dependent bile salt transporter
- IBAT
ileal BA transporter
- CYP7A1
cholesterol 7α hydroxylase
Footnotes
Disclosures: The authors have no conflicts of interest.
Authors’ contributions: P. Vijayvargiya: writing and revising manuscript; Dr. M. Camilleri: writing and revising manuscript; Dr. Shin: writing and revising manuscript; Dr. A. Saenger: revising manuscript
References
- 1.Hofmann AF, Small DM. Detergent properties of bile salts: correlation with physiological function. Annu Rev Med. 1967;18:333–376. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- 2.Rao AS, Wong B, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alnouti Y. Bile acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108:224–246. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- 4.Robben J, Caenepeel P, Van Eldere J, et al. Effects of intestinal microbial bile salt sulfatase activity on bile salt kinetics in gnotobiotic rats. Gastroenterology. 1988;94:494–502. doi: 10.1016/0016-5085(88)90443-x. [DOI] [PubMed] [Google Scholar]
- 5.Breuer NF, Rampton DS, Tammar A, et al. Effect of colonic perfusion with sulfated and nonsulfated bile acids on mucosal structure and function in the rat. Gastroenterology. 1983;84:969–977. [PubMed] [Google Scholar]
- 6.Hofmann AF. The syndrome of ileal disease and the broken enterohepatic circulation: cholerhetic enteropathy. Gastroenterology. 1967;52:752–757. [PubMed] [Google Scholar]
- 7.Wedlake L, A'Hern R, Russell D, et al. Systematic Review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7α-hydroxy-4-cholesten-3-one (or 7αC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734–e43. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mekjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50:1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bampton PA, Dinning PG, Kennedy ML, et al. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 11.Chadwick VS, Gaginella TS, Carlson GL, et al. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94:661–674. [PubMed] [Google Scholar]
- 12.Surowiec D, Kuyumjian AG, Wynd MA, et al. Past, present, and future therapies for Clostridium difficile-associated disease. Ann Pharmacother. 2006;40:2155–2163. doi: 10.1345/aph.1H332. [DOI] [PubMed] [Google Scholar]
- 13.Weiss K. Toxin-binding treatment for Clostridium difficile: a review including reports of studies with tolevamer. Int J Antimicrob Agents. 2009;33:4–7. doi: 10.1016/j.ijantimicag.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Walters JR, Tasleem AM, Omer OS, et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Fromm H, Hoffman A. Breath test for altered bile-acid metabolism. Lancet. 1971;2:621–625. doi: 10.1016/s0140-6736(71)80068-5. [DOI] [PubMed] [Google Scholar]
- 16.Scarpello JHB, Sladen GE. Appraisal of the 14C-glycocholate acid test with special reference to the measurement of faecal 14C excretion. Gut. 1977;18:742–748. doi: 10.1136/gut.18.9.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Tilburg AJP, de Rooij FWM, van den Berg WO, et al. The selenium-75-homocholic acid taurine test reevaluated: combined measurement of fecal selenium-75 activity and 3α-hydroxy bile acids in 211 patients. J Nucl Med. 1991;32:1219–1224. [PubMed] [Google Scholar]
- 18.Camilleri M, Brown ML, Malagelada J-R. Impaired transit of chyme in chronic intestinal pseudo obstruction: correction by cisapride. Gastroenterology. 1986;91:619–626. doi: 10.1016/0016-5085(86)90631-1. [DOI] [PubMed] [Google Scholar]
- 19.Sauter GH, Munzing W, Von Ritter C, et al. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7[alpha]-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44:14–19. doi: 10.1023/a:1026681512303. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson J, Walker K, Thomson AB. Limitations in the use of 14C-glycocholate breath and stool bile acid determinations in patients with chronic diarrhea. J Clin Gastroenterol. 1986;8:258–262. doi: 10.1097/00004836-198606000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Thaysen EH, Orholm M, Arnfred T, et al. Assessment of ileal function by abdominal counting of the retention of a gamma emitting bile acid analogue. Gut. 1982;23:862–865. doi: 10.1136/gut.23.10.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams AJ, Merrick MV, Eastwood MA. Idiopathic bile acid malabsorption--a review of clinical presentation, diagnosis, and response to treatment. Gut. 1991;32:1004–1006. doi: 10.1136/gut.32.9.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sciarretta G, Vicini G, Fagioli G, Verri A, Ginevra A, Malaguti P. Use of 23-selena-25-homocholyltaurine to detect bile acid malabsorption in patients with ileal dysfunction or diarrhea. Gastroenterology. 1986;91:1–9. doi: 10.1016/0016-5085(86)90431-2. [DOI] [PubMed] [Google Scholar]
- 24.Notghi A, O'Brien J, Chen SL, et al. Measuring SeHCAT retention: a technical note. Nucl Med Commun. 2011;32:960–966. doi: 10.1097/MNM.0b013e32834a36af. [DOI] [PubMed] [Google Scholar]
- 25.al-Hadrani A, Lavelle-Jones M, Kennedy N, Neill G, Sutton D, Cuschieri A. Bile acid malabsorption in patients with post-vagotomy diarrhoea. Ann Chir Gynaecol. 1992;81:351–353. [PubMed] [Google Scholar]
- 26.Sciarretta G, Fagioli G, Furno A, Vicini G, Cecchetti L, Grigolo B, Verri A, Malaguti P. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut. 1987;28:970–975. doi: 10.1136/gut.28.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axelson M, Aly A, Sjövall J. Levels of 7 alpha-hydroxy-4-cholesten-3-one in plasma reflect rates of bile acid synthesis in man. Fed Eur Biochem Sci. 1988;239:324–328. doi: 10.1016/0014-5793(88)80944-x. [DOI] [PubMed] [Google Scholar]
- 28.Galman C, Arvidsson I, Angelin B, et al. Monitoring hepatic cholesterol 7α hydroxylase activity by assay of the stable bile acid intermediate 7α-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44:859–865. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Galman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology. 2005;129:1445–1453. doi: 10.1053/j.gastro.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Ma K, Xiao R, Tseng H, et al. Circadian dysregulation disrupts bile acid homeostasis. PLoS One. 2009;4:e6843–e6852. doi: 10.1371/journal.pone.0006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10:1009–1015, e3. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin A, Vijayvargiya P, Busciglio I, et al. Quantitative assessment of fecal primary and secondary bile acids in health and irritable bowel syndrome (IBS) with diarrhea or constipation. Gastroenterology. 2013 abstract in press. [Google Scholar]
- 33.Abrahamsson H, Ostlund-Lindqvist A, Nilsson R, et al. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol. 2008;43:1483–1488. doi: 10.1080/00365520802321212. [DOI] [PubMed] [Google Scholar]
- 34.Porter JL, Fordtran JS, Santa Ana CA, et al. Accurate enzymatic measurement of fecal bile acids in patients with malabsorption. J Lab Clin Med. 2003;141:411–418. doi: 10.1016/S0022-2143(03)00040-4. [DOI] [PubMed] [Google Scholar]
- 35.Griffiths WJ, Sjövall J. Bile acids: analysis in biological fluids and tissues. J Lipid Res. 2010;51:23–41. doi: 10.1194/jlr.R001941-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Story JA, Furumoto EJ. Bile acid analysis: methods and problems. Eur J Cancer Prev. 1991;1(Suppl. 2):29–33. [PubMed] [Google Scholar]
- 37.Hofmann AF, Loening-Baucke V, Lavine JE, et al. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J Pediatr Gastroenterol Nutr. 2008;47:598–606. doi: 10.1097/MPG.0b013e31816920a6. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell WD, Findlay JM, Prescott RJ, et al. Bile acids in the diarrhoea of ileal resection. Gut. 1973;14:348–353. doi: 10.1136/gut.14.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Setchell KD, Ives JA, Cashmore GC, et al. On the homogeneity of stools with respect to bile acid composition and normal day-to-day variations: a detailed qualitative and quantitative study using capillary column gas chromatography-mass spectrometry. Clin Chim Acta. 1987;162:257–275. doi: 10.1016/0009-8981(87)90045-3. [DOI] [PubMed] [Google Scholar]
- 40.Manabe N, Wong BS, Camilleri M, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:293–e82. doi: 10.1111/j.1365-2982.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]