Implications Summary Statement
Racial and SES differences in AL exist during adolescence, confirming variation in the accumulation of stress biomarkers at younger ages. These findings contribute to the understanding of how early life adverse factors “get under the skin”, and are possibly translated into increased risk for diseases later in life.
Eliminating and reducing health disparities is a primary goal of national public health priorities [1], including addressing health issues occurring during adolescence. The social conditions present and behavioral patterns developed during adolescence set the stage for lifetime health trajectories that contribute to adult risk of chronic health conditions [2]. Adolescence is a critical developmental period in the life course with numerous physiological, social, and psychological changes [2, 3]. Between the ages of 12–19 adolescents experience puberty, as well as emotional and social challenges while struggling with independence and a sense of identity [4, 5]. Additionally, nearly one in five adolescents experience significant emotional distress, and among adolescents and young adults, over 18% are obese [3]. There is also substantial racial, ethnic, and SES variability in many of key developmental transitions and in health outcomes [6]. For example, Blacks experience earlier onset puberty than Whites [6]. There exists evidence that this variation may be due, in part, to differences in socio-environmental conditions, including low parental SES, and limited social interaction during youth [7, 8]. Moreover, there is emerging research demonstrating differences in allostatic load (AL) appearing during late childhood and early adolescence [9–11]. AL is a useful framework for revealing potential biological pathways through which environmental and social stressors ‘get under the skin’ to impact health [12]. When the body perceives a stressful event, the sympathetic nervous system, and the hypothalamic pituitary aderenocortical axis activate numerous physiological systems, including the cardiovascular, metabolic, and inflammatory systems [13]. As the body responds to stressors over time, these biological systems may fail to adapt to future stressors, resulting in unbalanced regulatory biomarkers. AL focuses on the interconnection of these numerous biological systems cumulatively quantifying elevated levels of regulatory biomarkers [13]. As biomarker levels reach dysregulated concentrations, AL increases, eventually leading to negative health outcomes and possible health disparities [14, 15]. Numerous studies have examined sociodemographic profiles of AL among adult and aging populations [16–18], revealing significant associations between AL and increased morbidity and mortality with age [19, 20].
Although much of the previous research on AL has focused on adults and the elderly [15, 16], a few studies have investigated AL among younger populations. Evans and colleagues found that middle school children in rural New York exposed to greater accumulated physical risk factors (such as crowding, substandard housing, and poverty) had higher levels of AL; these effects remained consistent over time [21]. Social inequalities, such as parental education, have are also associated with metabolic and cumulative risk among a small sample of suburban Midwestern adolescents [10]. Thus, there is growing evidence that differentials in AL may emerge at young ages.
Adolescent health across the life course is shaped by social and environmental conditions [22]. Black adolescents experience greater health disparities as a result of greater levels of poverty and stressors associated with racial discrimination [22]. Geronimus and colleagues propose that racially related stressors (i.e., ‘weathering’) further contribute to earlier health problems among racial minorities, especially Blacks [18]. The present study drew from this line of research by extending the ages of investigation into adolescence. With regard to Hispanic youth, there is substantial variability in health contingent on country of origin [23]. Specifically, Mexican American adolescents have higher rates of several health conditions when compared to other Hispanic groups [24]. Because of heterogeneity across Hispanic subgroups, the current study focused exclusively on Mexican American teens.
The objectives of this study were to examine profiles of AL among adolescents age 12–19 years and to assess the racial, ethnic, and SES differentials in AL. We hypothesized that Black teens will have higher AL relative to White and Mexican American teens, and that adolescents from lower SES family backgrounds will have higher AL scores. We also investigated possible gender differences in AL. Although no significant effects of gender on AL among adolescents have been shown in previous research [10, 25], adult females generally have higher AL than males [26]. Additionally, we examined the extent to which racial and ethnic differences in AL change as teens age. We anticipated Black adolescents will have higher AL than White or Mexican American teens across adolescence.
Methods
Sample population
This study uses data from the U.S. National Health and Nutrition Examination Survey (NHANES). NHANES is administered by the National Center for Health Statistics and is a bi-annual cross-sectional survey that monitors the health status of the civilian, non-institutionalized population. NHANES used a complex, multistage, probability sampling design to provide detailed information on national health and nutritional status [27]. The NHANES design oversampled Mexican Americans, non-Hispanic Blacks, and adolescents to improve estimates for these groups. Adolescents randomly selected to participate in the study also had an adult household representative (most commonly a parent) act as a proxy to complete household socioeconomic information. NHANES collected data using an in-home questionnaire on diverse health and social factors, and a physical examination within fully equipped medical examination centers collecting measurements of blood pressure, height, weight, and blood samples, which were analyzed by offsite contract laboratories using approved and reliable procedures [27].
Approximately 12,000 respondents are screened to participate in NHANES each bi-annual cycle, and roughly 20% (2,400) are adolescents age 12–19 years. The sample design for NHANES makes it possible to combine two or more survey cycles to increase the sample size and analytic power. This study utilized data from five NHANES cycles from 1999–2008 [27], and included adolescents age 12–19 years who completed both the in-home interview and physical examination components of the study.
Study Variables
Outcome Variable
AL was operationalized to include multiple biomarkers based on their representation of physiological systems and was guided by prior research and data availability [16, 18, 28]. Nine biomarkers were used to create a summary AL score [16, 18, 28]. Cardiovascular markers included diastolic and systolic blood pressure. Metabolic functioning markers included body mass index, waist-circumference, total cholesterol, high-density lipoprotein (HDL), and glycosylated hemoglobin. Inflammatory markers included serum albumin and C-reactive protein. Detailed measurement and laboratory assessment techniques have been published elsewhere [27].
In this study AL was operationalized as an index, using empirical cut-points based on the aggregated sample distribution [16, 18, 28] to capture cumulative physiological dysregulation at more rigorous levels than clinical cut-points. While clinically-defined “high risk” biological profiles are common among adults, only that for hypertension and body mass index among children and adolescents are known by gender, and age-height [29, 30]. For each of the 9 indicators, empirical cut-points were determined by the 75th percentile value, identified as high risk, with the exception of HDL and albumin, whose high risk cut-points were defined as below the 25th percentile. The decision to use quartile cut-points was based on previous studies, and is considered the preferred approach [18, 28]. Adolescents who exhibited high risk levels of biological markers received a score of 1 for that parameter. A composite AL index was then created by summing the number of parameters identified as high risk. For a composite score with 9 biomarkers, the range of AL scores was 0 to 9, with higher values signifying greater systemic dysregulation. High risk cut-points for individual biomarkers and AL scores were done according to the unweighted empirical sample to develop an AL index specific to the population [18, 28].
Independent Variables
Independent variables in this study included age, gender, race, ethnicity, nativity status, household income, and parental education. Age was measured in years at the time of the screening interview, and was included here in two different forms. First, as a categorical variable in increments of 2 year age groups, and second as a mean-centered continuous variable to facilitate the interpretation of AL variation across age among Blacks and Mexican Americans compared to Whites. Mean age of the sample was 15.4 years. Gender was coded as female and male. Race and ethnicity was coded into three categories, giving priority to Hispanic ethnicity: non-Hispanic White, non-Hispanic Black, and Mexican American. Nativity status was coded as U.S.-born and foreign-born. SES was measured using annual family income and educational attainment of the household representative that acted as the adolescent proxy. Annual family income was divided into five categories: <$20,000, $20,000–$44,999, $45,000–$74,999, and ≥$75,000. Educational attainment of the household representative was categorized as: less than high school, high school graduate or GED, and more than high school.
Missing values (n=17) for nativity status numbered less than one percent, and were coded into the modal category of U.S.-born. Results did not differ whether missing cases were coded to the modal category or dropped. Approximately 8.7% of the final sample was missing either education, annual family income, or both. To retain these cases multiple imputation using chained equations (ICE) was employed. This method assumes data are missing at random, and utilizes an iterative algorithm to approximate imputation of missing values [31]. A total of five datasets were imputed to estimate accurate standard errors.
Analytic Sample
A total of 10,397 adolescents age 12–19 years completed both the interview and examination components of NHANES. Respondents who self-identified as non-Hispanic White, non-Hispanic Black, and Mexican American were included in our analyses. Adolescents identified as “Other” race (n=347) and those identified as “Other Hispanic” (n=379) were excluded due to the difficulty in interpreting results of AL among a non-specific racial or ethnic group. Pregnant females (n=158) were also excluded because fluctuating and/or elevated biomarker levels are considered normal during pregnancy, are not indicative of physiological dysregulation [32]. Adolescents missing any of the 9 biological markers used to calculate AL score were also excluded (n=1,461). Of these 1,461 missing: 876 did not have their blood drawn, 285 were missing blood pressure, 119 were missing waist circumference, 10 were missing BMI, and 171 were missing individual blood markers (albumin, cholesterol, HDL, C-reactive protein or glycosylated hemoglobin). Selection analysis using logistic regression showed modest differential selection where non-Hispanic Black females were under-represented. In sum, the analytic sample included 8,052 adolescents.
Analyses
Descriptive statistics employed included the range, mean, quartiles, and empirical cut-points of each of the 9 AL biomarkers. Mean differences in AL for each sociodemographic characteristic were assessed using bivariate regression and an adjusted Wald F-test. As the measure of AL was a non-negative integer count-based summation with a non-normal distribution, negative binomial regression models were applied in multivariate regression analyses to examine main effects of sociodemographics and interactive effects between mean-centered age and race and ethnicity on AL in adolescence.
All regression estimates were weighted using the NHANES individual-level sampling weights, which adjust for complex sample design, selection, and non-response [27]. Predicted values of AL for race and ethnicity interacted with age were estimated and graphically presented. Predicted values were estimated using a regression model that included: race; ethnicity; mean-centered age; interaction terms for race, ethnicity, and mean-centered age; nativity status; household representative education level; and annual household income. All analyses were conducted using Stata 12 [33].
Results
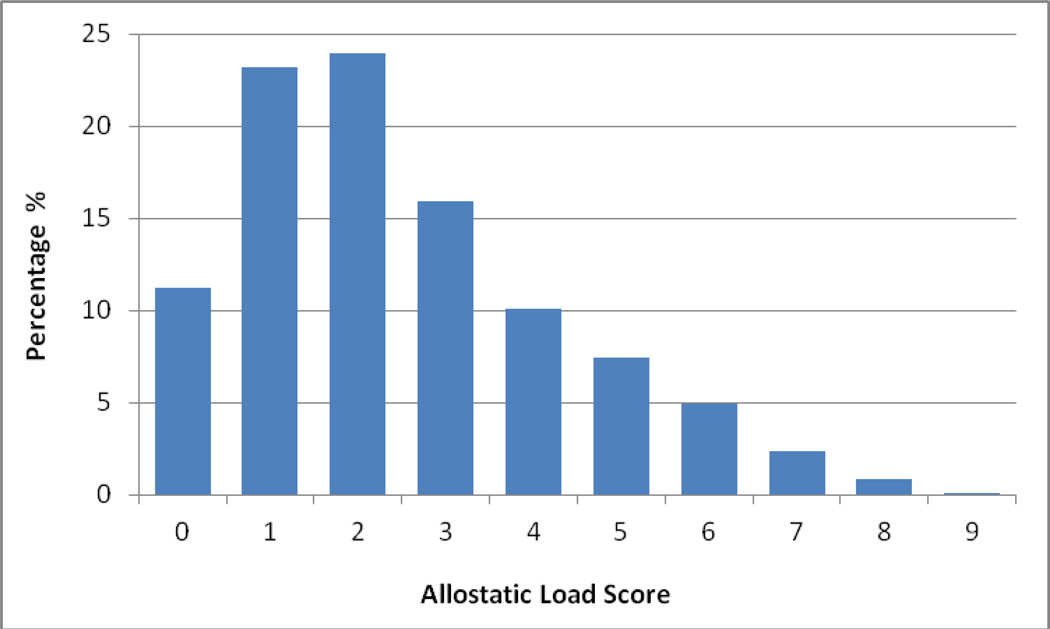
Table 1 presents descriptive statistics, including range, mean, standard deviations, and quartiles of each of the 9 biomarkers used to compile AL score among adolescents age 12–19 years. Individual biomarker distributions are shown by physiological system. Mean AL score among the adolescent sample was 2.50 (SD = 1.84), with a range from 0 to 9. Figure 1 shows the percentage sample distribution of AL score. The majority of adolescents had an AL score of four or less, although some adolescents could have a score as high as 9.
Table 1.
Descriptive Statistics of Individual Biomarkers and Summary Allostatic Load Score among Adolescents Ages 12–19, NHANES, 1999–2008a
| Standard Deviation |
Quartiles | |||||
|---|---|---|---|---|---|---|
| Biomarker | Range | Mean | 25% | 50% | 75% | |
| Cardiovascular Markers | ||||||
| Diastolic Blood Pressure (mmHg) | 10–104 | 61.10 | 11.42 | 54.00 | 62.00 | 69.00 |
| Systolic Blood Pressure (mmHg) | 78–158 | 109.88 | 10.41 | 103.00 | 110.00 | 116.00 |
| Metabolic Markers | ||||||
| Total Cholesterol (mg/dL) | 68–548 | 161.29 | 30.73 | 140.00 | 158.00 | 179.00 |
| High-density Lipoprotein (mg/dL) | 15–122 | 51.27 | 12.30 | 42.00 | 50.00 | 58.00 |
| Glycosylated Hemoglobin (%) | 2.5–15.1 | 5.17 | 0.46 | 5.00 | 5.10 | 5.30 |
| Body Mass Index (kg/m2) | 13.14–60.85 | 23.86 | 5.82 | 19.83 | 22.39 | 26.56 |
| Waist Circumference (cm) | 47.1–165.6 | 81.50 | 14.64 | 71.20 | 77.90 | 88.80 |
| Inflammatory Markers | ||||||
| C-reactive Protein (mg/dL) | 0.01–11.2 | 0.19 | 0.50 | 0.02 | 0.05 | 0.16 |
| Albumin (g/dL) | 1.2–5.7 | 4.46 | 0.32 | 4.30 | 4.50 | 4.70 |
| Allostatic Load Scoreb | 0–9 | 2.50 | 1.84 | 1.00 | 2.00 | 4.00 |
n=8,052
AL score is determined by counting the number of biomarkers where the respondent has a value ≥75% on the corresponding biomarker, also identified as high risk. Albumin and HDL have a high risk of ≤25%.
Figure 1.
Percentage Distribution of Allostatic Load score Among Adolescents Age 12–19 Yearsa
a n=8,052; National Health and Nutrition Examination Survey 1999–2008
Table 2 presents the unweighted frequencies and weighted distributions of sociodemographic characteristics of U.S. adolescents 12–19 and mean AL scores for each sociodemographic variable. Mean AL scores increased significantly with each two-year age increment. Adolescents age 12–13 years had an AL mean of 2.08, while older adolescents age 18–19 had a mean AL score of 2.84. There was no significant gender difference in mean AL. Non-Hispanic Blacks had the highest mean AL score (2.81), and Mexican Americans had slightly higher AL scores (2.41) than non-Hispanic Whites (2.28) (p≤.001). U.S.-born adolescents had significantly higher mean AL scores (2.40) than foreign-born adolescents (2.11). Mean AL scores significantly increased with lower household representative educational status and lower annual household income.
Table 2.
Unweighted Frequencies, Weighted Percentage and Mean Distribution of Sociodemographic Characteristics and Mean AL among Adolescents, NHANES, 1999–2008a
| N | % Distribution | Mean AL Score | |
|---|---|---|---|
| Age (Years) | |||
| 12–13 | 2,085 | 24.64 | 2.08*** |
| 14–15 | 2,017 | 26.05 | 2.19 |
| 16–17 | 2,079 | 26.50 | 2.45 |
| 18–19 | 1,871 | 22.81 | 2.84 |
| Gender | |||
| Male | 4,178 | 52.31 | 2.39 |
| Female | 3,874 | 47.69 | 2.37 |
| Race/ethnicity | |||
| Non-Hispanic White | 2,334 | 70.59 | 2.28*** |
| Non-Hispanic Black | 2,735 | 16.36 | 2.81 |
| Mexican American | 2,983 | 13.05 | 2.41 |
| Nativity status | |||
| U.S.-born | 6,985 | 93.31 | 2.40*** |
| Foreign-born | 1,067 | 6.69 | 2.11 |
| Household Representative Education | |||
| Less than high school | 2,952 | 20.64 | 2.69*** |
| High school/GED | 1,973 | 26.24 | 2.48 |
| More than high school | 3,127 | 53.12 | 2.20 |
| Family income | |||
| <$20,000 | 2,682 | 23.43 | 2.67*** |
| $20,000–44,999 | 2,440 | 24.77 | 2.54 |
| $45,000–74,999 | 1,451 | 21.25 | 2.36 |
| ≥$75,000 | 1,479 | 30.55 | 2.03 |
p≤ .001 on Adjusted Wald F-test
n=8,052
Table 3 presents results from the negative binomial regression model of AL score, specifically including mean-centered age by race and ethnicity interactions (F = 3.22, p = 0.045; results not shown). AL significantly increased with each year of age among Whites. Compared to Whites age 15.4 years, Blacks had significantly higher AL; there was no difference between Whites and Mexican American teens. However, relative to Whites, the amount of increase in AL by year of age was significantly lower for Blacks (p < 0.05). The interaction term was not significant for Mexican Americans. There were no significant gender differences. Compared to U.S.-born teens, those who were foreign-born had significantly lower AL. Compared to adolescents living in households in which the adult representative had the highest education, those living with lower educated adults had significantly higher AL. Similarly, compared to teens living in the highest income household, those in lower incomes had significantly higher AL. This was significant for each category of education and income.
Table 3.
Weighted Negative Binomial Regression Results for AL by Sociodemographic Characteristics, NHANES, 1999–2008a
| Sociodemographic Characteristics (reference group) |
Estimated Count Ratiosb (95% CI) |
|---|---|
| Age Centered (15.4 Years) | 1.06 (1.05, 1.08)*** |
| Race/Ethnicity (Non-Hispanic White) | |
| Non-Hispanic Black | 1.17 (1.11, 1.23)*** |
| Mexican American | 1.03 (0.96, 1.10) |
| Age Centered by Race and ethnicity (15.4 Years × Non-Hispanic White) | |
| Age Centered × Non-Hispanic Black | 0.98 (0.96, 1.00)* |
| Age Centered × Mexican American | 0.99 (0.97, 1.01) |
| Gender (Male) | |
| Female | 0.98 (0.94, 1.04) |
| Nativity Status (U.S.-born) | |
| Foreign-born | 0.81 (0.75, .88)*** |
| Household Representative Education (More than high school) | |
| Less than high school | 1.13 (1.05, 1.22)** |
| High school grad/GED | 1.07 (1.00, 1.14)* |
| Family Income (≥$75,000) | |
| <$20,000 | 1.18 (1.11, 1.26)*** |
| $20,000–$44,999 | 1.18 (1.10, 1.27)*** |
| $45,000–$74,999 | 1.12 (1.04, 1.20)** |
| Intercept | 2.00 (1.89, 2.12)*** |
p≤ .05
p≤ .01
p ≤ .001
n=8,052; all analyses weighted.
Estimated Count Ratios are interpreted as: Holding all other variables in the model constant, each 1-unit change in the predictor variable is expected to change the estimated AL score by a factor of the respective count ratio relative to the reference category.
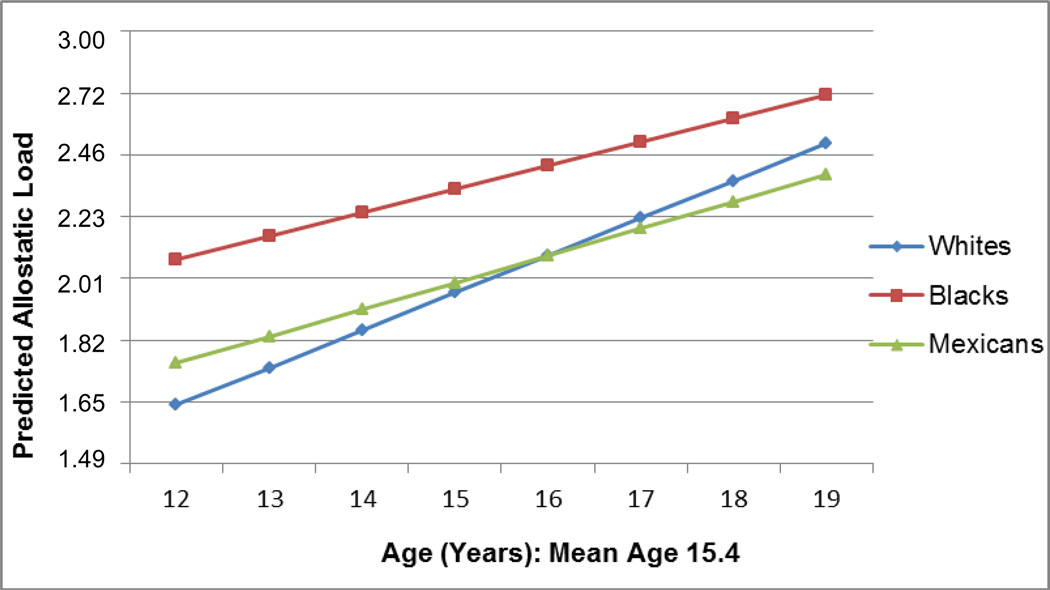
Figure 2 graphically demonstrates the findings presented in Table 3, with an emphasis on characterizing the predicted AL values by age for each racial and ethnic group. For all groups, AL increased with age and Blacks had higher predicted AL at each age than Whites or Hispanics. However, at older ages, predicted AL values start to converge, reducing the racial and ethnic differences. Although not significant, Mexican American teens’ predicted AL score, higher than Whites at younger ages, becomes lower than Whites during later adolescence.
Figure 2.
Predicted Allostatic Load by Mean-Centered Age and Race/Ethnicitya
a n=8,052; National Health and Nutrition Examination Survey 1999–2008; all analyses weighted.
Note: Predicted values of AL are based on coefficients within the negative binomial regression model of the interaction between age and race/ethnicity. The interaction is interpreted as predicted AL score by race/ethnicity conditional on age, with covariates set equal to the mean or the reference category.
Discussion
This study highlights a national profile of AL among a representative sample of U.S. adolescents. As predicted, higher AL scores, indicative of greater cumulative physiological dysregulation, occur at older ages of adolescence, among Black teens, U.S.-born adolescents, and those of lower SES. Black adolescents also had higher AL scores across all ages of adolescence than Whites. However, Whites had a higher rate of AL score increase compared to Blacks and Mexican Americans. Taken together, these findings demonstrate that differences in AL occur early in the life course and that the relative advantage of Whites and Mexican Americans over Blacks declines over adolescence.
The increase in AL across adolescence from ages 12–19 years is consistent with prior research on increasing AL among adult and aging populations [16–18]. The mean AL of the sample, 2.50, while low on a scale of 0 to 9, is relatively high compared to prior studies of AL among adult populations. Chyu and Upchurch found a mean AL score of 2.71 among midlife women [16]. This increase in AL with age, and high mean value, suggest that accumulation of stress biomarkers occurs at earlier ages, prior to adulthood. Increased levels of AL above 3 throughout adulthood and aging may then be associated with increased risk for cardiovascular disease [34]. The finding also supports the notion that AL is cumulative [17]. As predicted, regression results show that Black teens had higher AL scores compared to Whites, a finding uniform with previous AL research among adults and the aging [16, 18] suggesting that Blacks may experience greater stress due to living in a stigmatizing race-conscious society. However, the present findings are somewhat contrary to prior work on AL among a convenience sample of Midwestern adolescents, which found that AL score did not differ significantly by race, ethnicity or age [10]. The differences may be partially explained by the differences in sampling strategies across the studies or differences in the ways in which AL was assessed. The research presented here characterizes a nationally representative sample of adolescents, while the Midwestern study is less generalizable to the larger U.S. population.
White and Mexican American adolescents do not statistically differ in their AL levels. However, as found in other studies, nativity status is significantly associated with AL [16, 28]. Foreign-born adolescents have healthier biological risk profiles than U.S.-born. These results support previous research assessing AL among adults [28], adult women [16], and a representative sample of adults living in Texas [35]. Additional research shows differences by nativity status not only for AL, but also for metabolic markers including BMI. Foreign-born children ages 10–17 years have lower BMI, and subsequent lower risk for obesity than U.S.-born kids [36]. Possible explanations for healthier AL profiles among foreign-born adolescents are protective cultural practices and health behaviors, or selective migration of healthier children or infants migrating with healthy parents from the country of origin [37]. In prior work analyzing NHANES to investigate explanations for nativity status differences in AL among adults, Crimmins and colleagues found stronger support for selective immigration hypotheses [28]. These findings were also confirmed in a regional sample of Mexican Americans [35], showing that acculturation did not account for the differences in AL score between U.S.-born Mexican American adults and foreign born Mexican Americans. While this may also be true for Mexican American teens, it is left to further research to specifically test these competing hypotheses.
As expected, SES was significantly associated with AL score. Adolescents who lived in a family with lower SES had higher AL scores than their higher SES counterparts; similar to previous findings among adolescents and adults [10, 19]. Such findings support Link and Phelan’s theory of social conditions and fundamental causes of health differences [7]. Positive linkages between low SES and negative “downstream” physiological outcomes suggest that individuals with fewer socioeconomic resources are at risk for poor health due to limited access to health care, greater exposure to environmental and psychosocial stressors, and less support [38]. The increased stress of lower SES, coupled with the developmental period of adolescence, potentially contributes to higher levels of AL and ultimately, poorer health in adulthood [19].
Adolescent boys and girls are no different from one another with respect to AL. Previous work in adult samples find a female disadvantage in general [18], although some studies find that men have higher AL than women [39]. In the other adolescent studies, no significant gender effects on AL were found [10, 25]. Possible explanations why gender differences in AL appear in adulthood but not adolescence include undifferentiated biological stress responses by gender during adolescent development [40], or complementary and limited early life social experiences for girls and boys, that may become divergent and prevalent with age, contributing to gender differences in physiological dysregulation [16]. These mixed results among adolescents and adults point to the need for continuing work to better understand gender differences (or lack thereof) in AL.
Age-by-race and ethnicity interaction terms within our regression model reveal significant racial and ethnic differences in AL scores across adolescence from 12–19 years. As expected, for all racial and ethnic groups, AL score increased with age. Black adolescents had higher AL scores relative to Whites across all ages of adolescence, consistent with the weathering hypothesis [18]. However, White and Mexican American adolescents appear to lose their advantage over time, with the disparity in AL score between Blacks and Whites declining after age 15. Much of this reduction in the racial and ethnic differentials at older ages is due to the increasing percentages of White and Mexican American teens who have more detrimental metabolic marker profiles as they age, especially increases in waist circumference and lower (worse) levels of HDL (data not shown).
This study is not without limitations. First, the data used in this study are cross-sectional, limiting causal inferences or linkages over time. Second, data limitations precluded the inclusion of neuroendocrine biomarkers, which have been identified as key parameters of dysregulation [9, 10, 15]. The third limitation of this study is the use of the AL index. Although this is the traditional and most often used method of AL score creation, it allots each biomarker an equal weight in the AL score; and it is unlikely that each biomarker contributes equally to AL. Last, this study created an AL score based on an aggregated sample of adolescents in NHANES, and does not take into account that select biomarkers, such as body mass index, may increase temporally. It is noted that results of this study are specific to the adolescent population, and are not generalizable to other populations such as adults or the aging.
Given significant health disparities that exist across the life course from adolescence to adulthood, this study provides an initial examination of associations between major sociodemographic factors and AL among a nationally representative sample of adolescents, focusing on racial and ethnic differences in age patterns of AL. Much research has yet to be done on AL, and even more so among younger populations. Future studies should incorporate a comprehensive set of biological parameters with longitudinal designs that afford investigation of how AL accumulates over time during adolescence. The concept of AL offers great promise toward expanding our understanding of how social and environmental factors are embodied within biological regulatory systems during adolescence, and translated into disease outcomes and health disparities across the life course. Additional studies examining AL among adolescents and youth are warranted.
Acknowledgements
This study was funded by a California Center for Population Research (CCPR) predoctoral traineeship to Dr. Bethany K. Wexler Rainisch from the NIH National Institutes on Aging (1T32AG033533). The study was also funded by a grant to Dr. Upchurch from the NIH National Center of Complementary and Alternative Medicine (K01AT002156). Special thanks to Dr. Rainisch’s peers for their guidance and editorial assistance. Dr. Rainisch affirms that all contributors to this manuscript have been acknowledged.
Abbreviations
- AL
Allostatic Load
- NHANES
National Health and Nutrition Examination Survey
- SES
Socioeconomic Status
- HDL
High-Density Lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. [cited 2012 January 20];Healthy People 2020 Objectives. 2011 Available from: http://www.healthypeople.gov/2020/topicsobjectives2020/default.aspx.
- 2.Halfon N, Hochstein M. Life course health development: an integrated framework for developing health, policy, and research. Milbank. 2002;80(3):433–479. doi: 10.1111/1468-0009.00019. iii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulye TP, Park MJ, Nelson CD, et al. Trends in adolescent and young adult health in the United States. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2009;45(1):8–24. doi: 10.1016/j.jadohealth.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 4.DiVall SA, Radovick S. Pubertal development and menarche. Ann Ny Acad Sci. 2008;1135:19–28. doi: 10.1196/annals.1429.026. [DOI] [PubMed] [Google Scholar]
- 5.McNeely C, Blanchard J. The teen years explained: A guide to healthy adolescent development. [cited 2012 April 12];2009 Available from: http://www.jhsph.edu/adolescenthealth. [Google Scholar]
- 6.Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys: estimates from the national health and nutrition examination survey III, 1988–1994. Archives of pediatrics & adolescent medicine. 2001;155(9):1022–1028. doi: 10.1001/archpedi.155.9.1022. [DOI] [PubMed] [Google Scholar]
- 7.Phelan JC, Link BG, Tehranifar P. Social Conditions as Fundamental Causes of Health Inequalities. J Health Soc Behav. 2010;51(1 suppl):S28–S40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- 8.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–398. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- 9.Evans GW, Kim P. Childhood poverty and health-Cumulative risk exposure and stress dysregulation. Psycho Sci. 2007 Nov;18(11):953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 10.Goodman E, McEwen BS, Huang B, et al. Social Inequalities in Biomarkers of Cardiovascular Risk in Adolescence. Psychosom Med. 2005;67(1):9–15. doi: 10.1097/01.psy.0000149254.36133.1a. [DOI] [PubMed] [Google Scholar]
- 11.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Green TL, Darity WA., Jr Under the Skin: Using Theories From Biology and the Social Sciences to Explore the Mechanisms Behind the Black-White Health Gap. Am J Public Health. 2010 doi: 10.2105/AJPH.2009.171140. Commentaries:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 14.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 15.Seeman T, Gruenewald T, Karlamangla A, et al. Modeling multisystem biological risk in young adults: The Coronary Artery Risk Development in Young Adults Study. Am J Hum Bio. 2010;22(4):463–472. doi: 10.1002/ajhb.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chyu L, Upchurch DM. Racial and Ethnic Patterns of Allostatic Load Among Adult Women in the United States: Findings from the National Health and Nutrition Examination Survey 1999–2004. J Womens Health. 2011;20(4):575–583. doi: 10.1089/jwh.2010.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crimmins E, Johnston M, Hayward M, et al. Age differences in allostatic load: an index of physiological dysregulation. Exp Gerontol. 2003;38(7):731–734. doi: 10.1016/s0531-5565(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 18.Geronimus AT, Hicken M, Keene D, et al. "Weathering" and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States. Am J Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeman TE, Epel E, Gruenewald T, et al. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann Ny Acad Sci. 2010;1186(1):223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- 20.Taylor SE, Way BM, Seeman TE. Early adversity and adult health outcomes. Dev Psychopathol. 2011;23(3):939–954. doi: 10.1017/S0954579411000411. [DOI] [PubMed] [Google Scholar]
- 21.Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39(5):924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- 22.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Ann Rev Pub Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 23.Lara M, Gamboa C, Kahramanian MI, et al. Acculturation and Latino Health in the United States: A Review of the Literature and its Sociopolitical Context. Ann Rev Public Health. 2005;26(1):367–397. doi: 10.1146/annurev.publhealth.26.021304.144615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delva J, Wallace JM, O’Malley PM, et al. The Epidemiology of Alcohol, Marijuana, and Cocaine Use Among Mexican American, Puerto Rican, Cuban American, and Other Latin American Eighth-Grade Students in the United States: 1991–2002. Am J Public Health. 2005;95(4):696–702. doi: 10.2105/AJPH.2003.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans GW, Kim P, Ting AH, et al. Cumulative Risk, Maternal Responsiveness, and Allostatic Load Among Young Adolescents. Dev Psychol. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 26.Karlamangla AS, Singer BH, Seeman TE. Reduction in Allostatic Load in Older Adults Is Associated With Lower All-Cause Mortality Risk: MacArthur Studies of Successful Aging. Psychosom Med. 2006;68(3):500–507. doi: 10.1097/01.psy.0000221270.93985.82. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Content. [cited 2011 February 8];1999–2008a Available from: http://www.cdc.gov/nchs/data/nhanes/survey_content_99_08.pdf.
- 28.Crimmins E, Kim JK, Alley DE, et al. Hispanic Paradox in Biological Risk Profiles. Am J Public Health. 2007;97(7):1305–1310. doi: 10.2105/AJPH.2006.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falkner B, Daniels SR. Summary of the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Hypertension. 2004;44(4):387–388. doi: 10.1161/01.HYP.0000143545.54637.af. [DOI] [PubMed] [Google Scholar]
- 30.Dinsdale H, Ridler C, Ellis LJ. A simple guide to classifying body mass index in children. Oxford: National Obesity Observatory; 2011. [Google Scholar]
- 31.Heeringa SG, West BT, Berglund PA. Applied Survey Data Analysis. Boca Raton, FL: Chapman & Hall/CRC; 2010. [Google Scholar]
- 32.Petraglia F, Florio P, Nappi C, et al. Peptide Signaling in Human Placenta and Membranes: Autocrine, Paracrine, and Endocrine Mechanisms. Endocr Rev. 1996;17(2):156–186. doi: 10.1210/edrv-17-2-156. [DOI] [PubMed] [Google Scholar]
- 33.StataCorp. Stata Statistical Software: Release 12.0. College Station, TX: Stata; 2011. [Google Scholar]
- 34.Seeman TE, Singer BH, Rowe JW, et al. Price of Adaptation--Allostatic Load and Its Health Consequences: MacArthur Studies of Successful Aging. Arch Intern Med. 1997;157(19):2259–2268. [PubMed] [Google Scholar]
- 35.Peek MK, Cutchin MP, Salinas JJ, et al. Allostatic Load Among Non-Hispanic Whites, Non-Hispanic Blacks, and People of Mexican Origin: Effects of Ethnicity, Nativity, and Acculturation. Am J Public Health. 2010;100(5):940–946. doi: 10.2105/AJPH.2007.129312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh GK, Kogan MD, Yu SM. Disparities in Obesity and Overweight Prevalence Among US Immigrant Children and Adolescents by Generational Status. J Commun Health. 2009;34(4):271–281. doi: 10.1007/s10900-009-9148-6. [DOI] [PubMed] [Google Scholar]
- 37.Crimmins E, Soldo B, Ki Kim J, et al. Using anthropometric indicators for Mexicans in the United States and Mexico to understand the selection of migrants and the hispanic paradox. Biodem and Soc Behav. 2005;52(3–4):164–177. doi: 10.1080/19485565.2005.9989107. [DOI] [PubMed] [Google Scholar]
- 38.Thoits PA. Stress and Health. J Health Soc Behav. 2010;51(1 suppl):S41–S53. doi: 10.1177/0022146510383499. [DOI] [PubMed] [Google Scholar]
- 39.Goldman N, Weinstein M, Cornman J, et al. Sex differentials in biological risk factors for chronic disease: Estimates from population-based surveys. J Womens Health. 2004;13(4):393–403. doi: 10.1089/154099904323087088. [DOI] [PubMed] [Google Scholar]
- 40.Santrock JW. Adolescence. 13th Edition. New York, NY: McGraw-Hill; 2010. [Google Scholar]