Abstract
A classical paradigm of tissue engineering is to grow tissues for implantation by using human stem cells in conjunction with biomaterial scaffolds (templates for tissue formation) and bioreactors (culture systems providing environmental control). A reverse paradigm is now emerging through microphysiological platforms for preclinical testing of drugs and modeling of disease that contain large numbers of very small human tissues. We discuss the biomimetic approach as a common underlying principle and some of the specifics related to the design and utilization of platforms with heart micro-tissues for high-throughput screening in vitro.
Introduction
From its inception, tissue engineering has been largely driven by the need to repair our worn-out and missing tissues. The need is steadily growing in our aging population, with advances being made at the interface between biology, engineering and clinical sciences. After some spectacular successes [1,2], some failures, and the development of some promising new approaches [3–5], engineering of biological substitutes of human tissues is increasingly plausible but is still far from being a routine clinical practice. Some of the challenges the field is facing include the need for immediate blood perfusion of clinically sized tissues, robust sources of cells that can be predictably derived and matured to mimic adult human phenotypes, and better control of the interactions between the implanted tissue and the systemic environment of the host, as well as the reality of complex regulatory procedures that lead to long times and high costs of clinical translation.
In recent years, an entirely new field of application is emerging: the use of microphysiological platforms with human tissues designed for preclinical testing of drug toxicity and efficacy and modeling of disease (Figure 1). Instead of “classical” tissue engineering focused on producing one single large graft customized to the defect and patient being treated, here we see the same technique being employed to produce large numbers (hundreds to thousands at a time) of small yet functional tissues for high-throughput testing in vitro. Further progress with this “reverse paradigm” of tissue engineering could in fact rapidly accelerate translation of discovery to the patients in need. We discuss here, in the context of cardiac tissue engineering, the design of microtissues that need to combine biological complexity (multicellular composition; normal and disease phenotypes; tissue specific architecture and function; vascularization) and capacity for high-throughput, high-content screening platforms (small size; easy handling; on-line readouts).
Figure 1. In vitro platforms for studying physiological and pathological responses of engineered heart tissues.
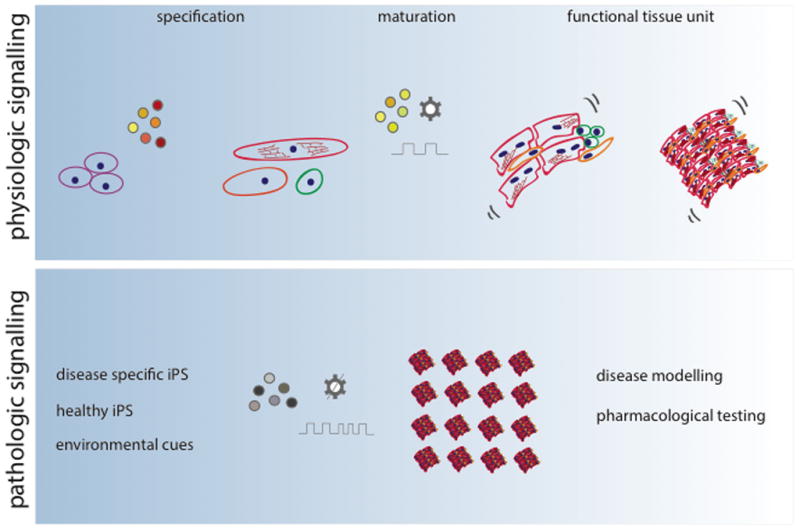
Studies of physiologic signals (top panel) include staged application of molecular regulatory factors (to induce differentiation of human iPS cells into cardiomoyocytes), the application of additional molecular factors, extracellular matrix and electromechanical factors to mature the cells and the use of the resulting tissues for drug screening. Studies of pathologic signals (bottom panel) involve the same steps as physiologic testing, except that the goal is modeling disease, by either using iPS cells from patients, or using factors (molecular, electrical, mechanical) causing specific pathological responses.
The clinical need for human tissue models
There is a growing interest in the in vitro applications of engineered tissues, because these applications are seemingly easier and more likely to be successful, at least in the near term. Tissues used for in vitro testing are small enough (typically just several hundred micrometers in size) so that the diffusional constraints of oxygen supply can be avoided, and the regulatory requirements are minimal. The need is clear. Animal models to study drug efficacy and toxicity often fail to predict human physiology, and this lack of predictive power leads to high cost, slow pace, and substantial risk in pre-clinical testing of prospective new drugs and treatment modalities. Currently used testing systems with human cells and tissues lack much of the structural and signaling features of native tissues, the temporal and spatial sequences of molecular and physical regulatory factors, and the dynamic forces and systemic factors provided by blood circulation. As a result, the predictive power of drug testing using simple cell and tissue culture remains limited. Also, these systems are now finding utility in modeling disease. Differences in cellular responses recorded in in vitro studies, animal models, and human clinical studies, decrease effectiveness of developing new therapeutic strategies. This is particularly true for the heart, an organ of immense complexity, the function of which involves physical forces and differs between various species, and with age and state of health. Developing faithful models of human heart physiology for drug testing and study of disease is a major need, that is now being addressed by designing creative screening platforms with human tissues engineered from human iPS cells.
While it is unreasonable to expect 3D tissue models to exactly match the native tissue [6], by recapitulating certain physiological functions in vitro tissue models can be used to investigate the efficacy, safety and mode of action of therapeutic agents. Instead of attempting to mimic the entire complexity of the organ, a reasonable goal would be to replicate the tissue-specific architecture (as a basis for function in most tissues) and a subset of most relevant functions in a way predictable of human physiology. A fundamental question we are now facing is what is the simplest functional tissue unit that allows high-throughput, high-contents in vitro studies. Such minimally functional units of different tissue types (e.g., heart, vasculature, liver, lung, kidney) will need to be combined into multi-organ platforms providing an environment predicting human physiology with sufficient fidelity (for example to capture cardiotoxic effects of a drug after it is metabolized by liver) [7].
Learning from the development
The heart is the first functional organ in human body – it starts to beat only three weeks into gestation, and much of its development occurs in the presence of physical regulatory factors. It is logical to expect that biologically sound in vitro models of heart tissue will take into account the complex hierarchical organization of the molecular, electrical, and mechanical signals, a challenge which complicated by the presence of different cell types and many interplaying factors and mediators such as metabolites, protein networks, and extracellular matrix. For example, the intracellular calcium compartmentalization and calcium concentration waveforms trigger signal-transduction pathways with outcomes covering several hierarchical levels. This small ion plays fundamental roles in processes such as excitation–contraction coupling, activation of fetal gene expression programs and cardiac remodeling, and transmission of biomechanical stress, while disruptions and imbalances in calcium handling can trigger dysfunction and abnormalities [8.9].
Cardiac precursor cells are specified via spatially and temporally defined sequences of multiple signaling events, conformational and mechanical rearrangements that commit pluripotent cells to the mesodermal and endodermal lineage. Wnts, Nodal and BMPs play highly conserved roles in cell specification and definitive commitment into cardiac lineages, by acting in a topologically defined fashion and with time-dependent activation and inhibition of genes [10–14]. Some of the key regulators – including Notch, Activin/Nodal, Wnt, BMP, and FGF pathways present themselves in the form of concentration gradients over both short and long distances, and pattern the cell behavior in concentration-dependent manner [7]. The complexity of developmental processes was nicely described as “signalling ballet in space and time” [16].
Years of progress in developmental biology and signaling network discovery are bringing us closer to a good level of control in deriving cardiac progenitors from pluripotent cell sources, but we are just scratching the surface of the understanding of regulatory networks involved in gene activation and control of commitment programs. This knowledge is being used to design differentiation protocols for obtaining cardiac cell populations from human pluripotent stem cells (hESCs and iPSc) [17]. Keller group was the first one to implement in vitro the regulation of Activin/Nodal and BMP pathways in human stem cells to generate cardiac mesoderm, and stage-specific induction/inhibition towards cardiac specification [14–18]. The wide use of hESCs and iPSc and the optimization of protocols used to obtain cardiac progenitors from those sources, allowed leaps forward in studies of cardiac development [13], the onset of disease, and the development of new therapeutic approaches [19–21].
Physical forces being generated and acting at the cells and their extracellular matrix play major roles in regulating development and the function of healthy and diseased heart. Among extracellular forces, fluid shear and hydrostatic pressure originated by fluid flows are of great importance in both tissue pattern formation and in the transport of soluble morphogens to target cells or regions [22,23]. Heart development emerges from a dynamic interplay between cascades of signals and transduction systems that can be triggered by various physical stimuli. For example, surface tensions have a proven fundamental role for the proper looping of the developing heart [24]. In another context, mechanical stress (due to increased hemodynamic load) is one of the causes of pathological hypertrophy, associated with the mechanical overload that can induce release of growth-promoting factors and further stimulate pathological cellular responses [25]. At the same time, normal hypertrophy, the developmental increase in size of cardiomyocytes, is a major mechanism of heart growth after birth.
Mechanotransduction, the process by which cells sense and respond to mechanical signals, is a complex set of events involving the extracellular matrix, integrins, cytoskeleton and downstream signaling molecules [26]. Major known mediators of mechanotransduction are integrins that are coupling with the cytoskeleton via sarcolemmal proteins, as well as through direct action of the cytoskeleton on the cell nucleus [25]. It is now well established that the structure and molecular regulation are inextricably linked at all times, and all size scales. In the heart, macroscopic stimuli dictate the sensing and responding of individual cells with intercellular, microscopic signals providing a feedback to behavioral changes. Alterations in the delicate processes of mechanotransduction have been linked to the onset of disease, and have lead to the identification of physical signals as putative mediators of potential therapeutic targets [26,27].
From engineering perspective, heart development could be viewed as a three-step process consisting of a (i) proliferative and migratory phase, (ii) structural phase, and (iii) mechanical loading phase. Beginning from the blastocyst and ending with a functioning organ (heart), the embryo’s complexity increases quite substantially. The embryo forms via a series of coordinated migratory patterns and rearrangements of undifferentiated cells establishing physical boundaries. This first phase of heart development, dominated by molecular regulation of undifferentiated cells within homogeneous and static matrix, leads to the formation of the three germ layers: endoderm, ectoderm, and mesoderm. In the second phase, the differentiation of the cells progresses towards establishing the orientation of the embryo (e.g., anterior vs. posterior), and the structural specification with the axes and boundaries. This structural phase results in the formation of primitive streak and is associated with gradients of multiple regulatory factors. The third phase brings into the picture mechanical loading, with electrical signals spreading over the heart and depolarizing the cells to induce their macroscopic contractions. Looping of the initial heart tube with further structural differentiation, orchestrated by electrical and mechanical forces, leads to a functioning heart that pumps the blood. A lesson we learn from heart development is that physical factors – the structural template and physical forces - need to be incorporated into our tissue engineering models, and in a timely fashion.
Bioengineering tissue platforms: providing the right context for studying disease
Drug toxicity often goes undetected, both in simple cultures of human cells and in animal models, in many case until late into clinical trials. A new generation of preclinical models - in form of integrated platforms with functional human tissues designed for high-throughput work would be transformative to drug screening and predictive modeling of disease. Recent advances in tissue engineering and microtechnologies are making the advent of human tissue platforms increasingly plausible.
Tissue engineering has made major strides from a largely empirical discipline to effective incorporation of biological principles into the design of in vitro culture environments. Two premises provide the conceptual underpinning to many effective approaches to tissue engineering:
(i) Both in vitro and in vivo, the cells respond to and interact with the entire context of their environment - surrounding cells, extracellular matrix, molecular factors, and physical signals;
(ii) If we are to elicit biologically meaningful cell responses in vitro, the complexity and dynamics of the native cell environment need to be reconstructed, at least in part, in experimental systems used in vitro.
The selection of factors, their combinations, spatial distribution and timing of application comes from what has been learned about heart development. The tools come from bioengineering, through implementation of human stem cells (and their differentiated progeny), in conjunction with biomaterial scaffolds (templates for cell differentiation and functional assembly) and bioreactors (culture systems providing environmental control, the application of molecular and physical signals, and measurements of cell/tissue readouts). Together, the biological principles and engineering designs form what we call the “biomimetic approach” to tissue engineering. Advanced culture systems that combine high biological fidelity with tight control over the cellular environment have begun to take center stage in efforts to observe stem cell responses that predict their behavior in vivo [28–30].
Microtechnologies enable support and tight control of highly sophisticated experiments at biologically relevant scales, with real-time insights into cellular responses [31]. They have been developed to precisely manipulate the cellular microenvironment and study cellular responses in real time and in a quantitative fashion [32,33]. Small scale brings several fundamental advantages over conventional tissue culture devices. First, small scale allows the use of large numbers of micro-tissues in high-throughput screening studies, with detailed studies of large numbers of factors, their ranges and combinations, while minimizing the amounts of cells and materials. Second, miniaturization is a major step towards accurate control in tissue culture, because of small transport distances that enable accurate control of cell environment [34]. Third, at small scale, it is possible to support cell viability and function by simple diffusional transport of nutrients, and most critically [35,36], while still manipulating microfluidic flows to provide medium exchange and integration with analytics. To take advantage of such simple and effective microfluidic designs, the critical size of the tissue needs to be significantly smaller than the diffusional penetration depth of oxygen. For cardiac microtissues, the geometries under consideration are cardiac organoids that are a few millimeters long and only ~100 μm in diameter. These systems allow modeling and optimization of flow and transport [31] and can be fine-tuned to meet the specific requirements of cell and tissue culture.
One emerging application of bioengineering platforms is to provide time sequences of space-resolved gradients of multiple molecular factors in three-dimensional (3D) cell culture settings, along with a versatile, high-throughput operation and imaging compatibility. One such system with 120 culture wells (for example, 15 concentration levels with 8 replicates per group) on a platform that has a size of a microscope slide enabled detailed study of early cardiac differentiation of human embryonic and induced pluripotent stem cells exposed to concentration gradients of Wnt3a, Activin A, BMP4 and their inhibitors [37]. Another platform combined inducible gene expression with a microfluidic technology to pattern gene expression embryonic stem cells and investigate cell decisions between self-renewal and differentiation [38]. Boundaries between Nanog expressing cells (pluripotency zone) and Nanog suppressed cells (early differentiation zone) were established within the same cell population, with a gradient of Nanog across the individual cell colonies, to serve as a mimic of the developmental process. Such studies of cellular commitment at the boundaries between gene expression domains, a phenomenon critical for early development, are important for derivation of differentiated cell populations and their application in studies of development and disease.
Maturation of human cardiomyocytes remains a challenge. Engineered cardiac tissues using neonatal rat heart cells have reached the level of advanced morphologic differentiation and maturation typical of their age-matched in vivo counterparts [39]. As of now, however, embryonic- and iPS-derived human cardiomyocytes cannot be differentiated beyond the immature, neonatal-like stage. One approach currently being investigated is focusing on the role of non-myocytes in the heart, as the multicellular makeup of myocardium – population ratios, anatomic location, and cell-level organization - is both an indicator of the proper heart function and contributing player in failing myocardium. In addition, the architecture and cell organization at the very smallest scales determine cardiac function. The micropatterning, microfabrication and 3D printing of cells (alone and in hydrogels) now allow building tissues bottom-up. Applying these techniques to cardiac tissue engineering is still in its infancy, but the tissue architecture could in fact be built through serial patterning, or the use of micro-scaled building blocks, assembled in repetitive arrays [40,41].
Another approach to maturation of cardiomyocytes is through modulation of the composition and mechanical properties of the extracellular matrix, in form of polymerizable hydrogels that are biocompatible, easy to use, and have tunable properties. Composite hydrogels, made by blending decellularized ECM from porcine hearts and collagen I at varying ratios, were capable of enhancing the differentiation and maturation of hESC-CM [42]. A PEGylated fibrinogen (PF) hydrogel supported growth and maturation of functional 3D hESC-CM constructs, with improved biomolecular organization and contractile behaviors, and propagation of action potentials through well-formed gap junctions [43]. In addition, these constructs demonstrated measurable responses to chronotropic agents, thus proving useful for pharmacological testing and (patho)physiological studies [43]. Tuning the hydrogel compliance (by varying hydrogel cross-linking density without altering cell-adhesion ligand density) could be used to modulate spontaneous cell contractility. Data suggest that an adaptation period might be needed for cardiomyocytes to adjust to a stiffer microenvironment, mature and exert contractile forces on the surrounding matrix [44].
The methods for assembling cardiac micro-tissues necessarily involve the application of electrical and mechanical forces, individually or in combination. Recent successful studies engineered cardiac tissue constructs starting from both hESC- and iPSc-derived cardiomyocytes (hESC-CM and iPS-CM, respectively) encapsulated in collagen-based matrix and subjected to uniaxial static and cyclic stress conditioning [45]. The presence of mechanical forces induced formation of tissue constructs with highly aligned cells and matrix, increased spontaneous beating rates, higher DNA synthesis and hypertrophy. All those parameters are determinants of bioengineered cardiac tissues resembling in vivo cardiac muscle. Analogous approaches were followed to generate 3D miniaturized, force-generating human heart muscle from hESC-CM [46,47]. A combined microfabrication-hydrogel molding technique was used to generate 3D hESC-CM-based constructs cultured under passive tension conditions [48]. Cells oriented along the direction of the force lines developing longer sarcomeres, exhibiting higher conduction velocities (CVs), and generally higher maturation (reported by the enhanced expression of genes involved in cardiac contractile function).
Multiple reports demonstrated how electrical stimulation can improve the quality of engineered cardiac tissues by enhancing cell-cell interactions (via gap junctions), improving tissue structure, alignment and organization, and enhancing both the amplitude and synchronicity contractions [49,50]. In addition, electrical stimulation pushed hESCs differentiation towards a mesodermal fate through mechanisms associated with the intracellular generation of reactive oxygen species (ROS) [51]. In a micropatterned hESC-CM system, electrical stimulation was used to induce a more mature cell phenotype and control both the frequency and synchrony of contraction, enabling the use of the platform for screening studies of pathological conditions and responses to drugs [52].
Pathologic physical stresses were recently used to induce a disease phenotype [53]. Indeed, through simple physical manipulation, a model of increased after-load could be created, and validated at the morphologic, phenotypic and genotypic level [53]. Using physical stimuli, we can recreate these ‘end-point’ conditions, bypassing the decades-long process that led to that state in the human. We don’t yet know if that difference matters in the realm of testing drugs and novel therapeutics, and if the tissue-engineering models can account for the effects of epigenetic modifications that occur throughout prenatal development and adult life and affect cardiovascular disease [54]. The protocols for preclinical toxicology studies using platforms with such pre-stressed cardiac tissues generated from human stem cells are being developed. One such model that used fibrin-based human engineered heart tissues in a 24-well format showed that proarrhythmic compounds E-4031, quinidine, procainamide, cisapride, and sertindole exerted reversible, concentration-dependent decreases in the velocity and regularity of beating, thus establishing a model for heart research [46].
Bioengineering platforms for preclinical drug screening are being designed to meet several requirements. Human iPS cells are used to derive both the cardiomyocytes and nonmyocytes for microtissues, which allows us to reach into a large genotype pool and use both the healthy cells and cells with genetic mutations when testing drugs and modeling disease. The interface with molecular and functional imaging is critical, as it provides real-time insights into cellular processes at multiple hierarchical levels - from molecular all the way to organ levels. The platform also needs to interface with the on-line analytics for measuring functional responses (such as the frequency and amplitude of contractions, maximum capture rate and regularity of contractions, force generation, calcium flux), and allow sampling for any end-point assays.
One of the key needs for achieving utility of microtissues in high-throughput studies is to define the simplest, minimally functional tissue unit providing responses representative of human physiology. The main criteria for such a minimally functional cardiac tissue unit relate to contractile behavior and signal propagation in microtissues, for several reasons. Expression of cardiac-specific functional properties means that the necessary molecular and structural features are also established, these properties change in response to drugs and environmental factors much sooner than cell viability, and they are easy to measure in real time. Reasonable benchmarks for minimally functional microtissues would include: (a) size (millimeters in length, 100 μm in diameter), and (b) electromechanical readouts (excitation threshold and maximum capture rate within 50% of native tissue values; conduction velocity within 75% of the native tissue value; force generation within 25% of the native tissue values; resting potential close to that of the adult human ventricular cardiomyocytes, −84mV). Recent studies indicate that these properties are within reach [55]. Based on the known structure-function relationships for engineered cardiac tissues, one would expect that these functional benchmarks would be complemented by the expression of mature cardiac genes and proteins, cell alignment, ultrastructural organization, functional junctions and channels. A probable design of drug screening studies would be based on high-throughput evaluation of functional properties measured on line, followed by the second tier of confirmatory end-point assays of additional cell and tissue properties for the conditions of interest.
Conclusion
Bioengineering platforms with human cardiac tissues are emerging as a new modality for screening the toxicity and efficacy of drugs, in settings mimicking the native milieu of development or disease. One current challenge is to understand what functionality we really need and how much is enough in terms of replicating the complexity of the native tissue function. Methods are needed for measuring direct and indirect effects of drugs, in real time and over long cultivation times. To this end, there is a clear need for multi-organ platforms that can give us an environment predictable of human physiology (for example to measure cardiotoxic effects of a drug after it is metabolized by liver).
*Highlights (for review).
Functional human tissues can be grown using cells, scaffolds and bioreactors
In vitro and in vivo, cells interact with their entire environment
Designs of biomimetic systems are guided by biological principles
Microphysiological platforms allow preclinical testing of drugs
We discuss the design of engineered microtissues for drug screening.
Acknowledgments
We gratefully acknowledge NIH funding (grant UH2 EB 17103 to GVN) supporting some of the work described in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 2.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann WH, Didie M, Doker S, Melnychenko I, Naito H, Rogge C, Tiburcy M, Eschenhagen T. Heart muscle engineering: an update on cardiac muscle replacement therapy. Cardiovasc Res. 2006;71(3):419–429. doi: 10.1016/j.cardiores.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100(2):263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 5.Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 3(68):68ra69. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 6*.Gilbert PM, Blau HM. Engineering a stem cell house into a home. Stem Cell Res Ther. 2(1):3. doi: 10.1186/scrt44. The authors describe the potential of creative bioengineering approaches to expanding our basic understanding of stem cell regulation imposed by the niche. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012;12(12):2156–64. doi: 10.1039/c2lc40089h. Review. An excellent review of microscale technologies applied to the “organ on a chip” models for drug screening. The need for incorporating intercations of multiple organs is emphasized. [DOI] [PubMed] [Google Scholar]
- 8.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 9.Olson EN. A decade of discoveries in cardiac biology. Nature Medicine. 2004;10(5):467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 10.LaFlamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 11.Mercola M, Ruiz-Lozano P, Schneider MD. Cardiac muscle regeneration: lessons from development. Genes and Development. 2011;25:299–309. doi: 10.1101/gad.2018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mummery C, Ward-van-Oostwaard D, Doevendans P, Spijker R, Brink SVD, Hassink R, VanDerHeyden M, Opthof T, Pera M, DeLaRiviere AB, Passier R, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2007;107(21):2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 13**.Murry CE, Keller GM. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. This is a critical review of the important insights derived from embryology has offered into key pathways regulating embryonic stem cell differentiation. Key results include the development of in vitro protocols for efficient induction of endoderm, mesoderm, ectoderm and their downstream derivatives. [DOI] [PubMed] [Google Scholar]
- 14*.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, et al. Human cardiovascular progenitor cells develop from a KDR+embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. This is the basic protocol for derivation of cardiac lineages from human stem cells, by staged application of Activin/Nodal and BMP. [DOI] [PubMed] [Google Scholar]
- 15.Perrimon N, Pitsouli C, Shilo BZ. Signaling Mechanisms Controlling Cell Fate and Embryonic Patterning. Cold Spring Harbor Perspectives in Biology. 2012;4:a005975. doi: 10.1101/cshperspect.a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nature Reviews Molecular Cell Biology. 2010;11:414–426. doi: 10.1038/nrm2901. Excellent review of the complexity and dynamics of molecular sinaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong SS, Bernstein HS. Cardiac regeneration using human embryonic stem cells: producing cells for future therapy. Regenerative Medicine. 2010;5(5):763–775. doi: 10.2217/rme.10.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta H, Ellis J, Keller G. Stage-Specific Optimization of Activin/Nodal and BMP Signaling Promotes Cardiac Differentiation of Mouse and Human Pluripotent Stem Cell Lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Unternaehrer JJ, Daley GQ. Induced pluripotent stem cells for modelling human diseases. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:2274–2285. doi: 10.1098/rstb.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braam SR, Tertoolen L, VanDeStolpe A, Meyer T, Passier R, Mummery CL. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Research. 2010;4(2):107–116. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 21*.Davis RP, VanDenBerg CW, Casini S, Braam SR, Mummery CL. Pluripotent stem cell models of cardiac disease and their implication for drug discovery and development. Trends in Molecular Medicine. 2011;17(9):475–484. doi: 10.1016/j.molmed.2011.05.001. The authors describe genetic diseases that affect the heart and discuss how the generation of human cardiomyocytes can aid in drug discovery. In particular, we highlight the limitations of other commonly used model systems in predicting the consequences of drug exposure on the human heart. [DOI] [PubMed] [Google Scholar]
- 22.Freund JB, Goetz JG, Hill KL, Vermot J. Fluid flows and forces in development: functions, features and biophysical principles. Development. 2012;139:1229–1245. doi: 10.1242/dev.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voronov DA, Taber LA. Cardiac Looping in Experimental Conditions: Effects of Extraembryonic Forces. Developmental Dynamics. 2002;224:413–421. doi: 10.1002/dvdy.10121. [DOI] [PubMed] [Google Scholar]
- 25.Ruwhof C, VanDerLaarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovascular Research. 2000;47:23–37. doi: 10.1016/s0008-6363(00)00076-6. [DOI] [PubMed] [Google Scholar]
- 26.Ingber DE. Mechanobiology and diseases of mechanotransduction. Annals of Medicine. 2003;35:1–14. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler-Jones CPD. Cell signalling in the cardiovascular system: an overview. Heart. 2005;91:1366–1374. doi: 10.1136/hrt.2005.072280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15(2):205–219. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell Stem Cell. 8(3):252–261. doi: 10.1016/j.stem.2011.02.014. The authors propose that experimentation at the interfaces of biology, engineering, and medical sciences is critical for unlocking the full potential of stem cells, and describe the design and utilization of in vitro platforms for studying tissue development, disease, and regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–441. doi: 10.1038/nature08602. A classical paper on novel biomaterials designed to provide for spatial and temporal control of microenvironmental cues regulating cell function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cimetta E, Figallo E, Cannizzaro C, Elvassore N, Vunjak-Novakovic G. Micro-bioreactor arrays for controlling cellular environments: design principles for human embryonic stem cell applications. Methods. 2009;47(2):81–89. doi: 10.1016/j.ymeth.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. Excellent overview of microscale technologies in tissue engineering applications, with examples of the use of microscale technologies for controlling the cellular microenvironment in vitro and for performing high-throughput assays. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung PJ, Lee PJ, Sabounchi P, Aghdam N, Lin R, Lee LP. A novel high aspect ratio microfluidic design to provide a stable and uniform microenvironment for cell growth in a high throughput mammalian cell culture array. Lab Chip. 2005;5(1):44–48. doi: 10.1039/b410743h. [DOI] [PubMed] [Google Scholar]
- 34.Squires TM, Quake SR. Microfluidics: Fluid physics at the nanoliter scale. Rev Mod Phys. 2005;77(3):977–1026. [Google Scholar]
- 35.Di Carlo D, Wu LY, Lee LP. Dynamic single cell culture array. Lab Chip. 2006;6(11):1445–1449. doi: 10.1039/b605937f. [DOI] [PubMed] [Google Scholar]
- 36.Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci U S A. 2007;104(14):5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cimetta E, Sirabella D, Yeager K, Davidson K, Simon J, Moon RT, Vunjak-Novakovic G. Microfluidic bioreactor for dynamic regulation of early mesodermal commitment in human pluripotent stem cells. Lab Chip. 13(3):355–364. doi: 10.1039/c2lc40836h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Sevilla A, Wan LQ, Lemischka IR, Vunjak-Novakovic G. Patterning pluripotency in embryonic stem cells. Stem Cells. 2003 doi: 10.1002/stem.1468. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiburcy M, Didie M, Boy O, Christalla P, Doker S, Naito H, Karikkineth BC, El-Armouche A, Grimm M, Nose M, Eschenhagen T, et al. Terminal differentiation, advanced organotypic maturation, and modeling of hypertrophic growth in engineered heart tissue. Circ Res. 109(10):1105–1114. doi: 10.1161/CIRCRESAHA.111.251843. [DOI] [PubMed] [Google Scholar]
- 40.Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci U S A. 2008;105(28):9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eng G, Lee BW, Parsa H, Chin CD, Schneider J, Linkov G, Sia SK, Vunjak-Novakovic G. Assembly of complex cell microenvironments using geometrically docked hydrogel shapes. Proc Natl Acad Sci U S A. 110(12):4551–4556. doi: 10.1073/pnas.1300569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan Y, Liu Z, O’Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid Gel Composed of Native Heart Matrix and Collagen Induces Cardiac Differentiation of Human Embryonic Stem Cells without Supplemental Growth Factors. Journal of cardiovascular translational research. 2011;4:605–615. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapira-Schweitzer K, Habib M, Gepstein L, Seliktar D. A photopolymerizable hydrogel for 3-D culture of human embryonic stem cell-derived cardiomyocytes and rat neonatal cardiac cells. Journal of Molecular and Cellular Cardiology. 2009;46:213–224. doi: 10.1016/j.yjmcc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Chung C, Anderson E, Pera RR, Pruitt BL, Heilshorn SC. Hydrogel crosslinking density regulates temporal contractility of human embryonic stem cell-derived cardiomyocytes in 3D cultures. Soft Matter. 2012;8:10141–10148. doi: 10.1039/C2SM26082D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KH, Pabon L, Reinecke H, Murry CE. Growth of Engineered Human Myocardium With Mechanical Loading and Vascular Coculture. Circulation Research. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Schaaf S, ShibamiyA A, Mewe M, Eder A, Stohr A, Hirt MN, Rau T, Zimmermann WH, Conradi L, Eschenhagen T, Hansen A. Human Engineered Heart Tissue as a Versatile Tool in Basic Research and Preclinical Toxicology. PLoS ONE. 2011;6(10):e26397. doi: 10.1371/journal.pone.0026397. A simple and robust protocol for generating human engineered heart tissues from embryonic stem cells in a 24-well format. The authors demonstrated physiologically relevant responses responses to calcium, isoprenaline, and five different proarrhythmic compounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 34(23):5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D, Shadrin IY, Lam J, Xian H, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34:5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, Radisic M, Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. Nature Protocols. 2009;4(2):155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences. 2004;101(52):18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serena E, Figallo E, Tandon N, Cannizzaro C, Gerecht S, Elvassore N, Vunjak-Novakovic G. Electrical stimulation of human embryonic stem cells: Cardiac differentiation and the generation of reactive oxygen species. Experimental Cell Research. 2009;315(20):3611–3619. doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serena E, Cimetta E, Zatti S, Zaglia T, Zagallo M, Keller G, Elvassore N. Micro-arrayed human embryonic stem cells-derived cardiomyocytes for in vitro functional assay. PLoS One. 2012;7(11):e48483. doi: 10.1371/journal.pone.0048483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Hirt MN, Sorensen NA, Bartholdt LM, Boeddinghaus J, Schaaf S, Eder A, Vollert I, Stohr A, Schulze T, Witten A, Stoll M, et al. Increased afterload induces pathological cardiac hypertrophy: a new in vitro model. Basic Res Cardiol. 107(6):307. doi: 10.1007/s00395-012-0307-z. An experimental model is described for studying the impact of afterload enhancement on work-performing heart muscles in vitro. Increased afterload resulted in reduced contractile force and impaired diastolic relaxation, whereas sustained afterload alone was sufficient to induce pathological cardiac remodeling with reduced contractile function and increased glucose consumption. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lorenzen JM, Martino F, Thum T. Epigenetic modifications in cardiovascular disease. Basic Res Cardiol. 107(2):245. doi: 10.1007/s00395-012-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Massé S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013 Jun 23; doi: 10.1038/nmeth.2524. [Epub ahead of print]. The authors describe a platform that combines three-dimensional cell cultivation with electrical stimulation to engineer mature hPSC-derived cardiac microtissues that are well aligned and contain frequent striations. When subjected to physical and molecular stimuli, these microtissues exhibited physiological responses similar to those of mature cardiac tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]


