Abstract
In advanced age, the resting myocardial oxygen consumption (M V̇O2) and cardiac work (CW) in the rat remain intact. However, M V̇O2, CW and cardiac efficiency achieved at high demand are decreased with age, compared to maximal values in the young. Whether this deterioration is due to decrease in myocardial ATP demand, ATP supply, or the control mechanisms that match them, remains controversial. Here we discuss evolving perspectives of age-related changes of myocardial ATP supply and demand mechanisms, and critique experimental models used to investigate aging. Specifically, we evaluate experimental data collected at the level of isolated mitochondria, tissue, or organism, and discuss how mitochondrial energetic mechanisms change in advanced age, both at basal and high energy demand levels.
Keywords: Aging, cardiac work, bioenergetics, mitochondria, respiration
Changes in ATP supply-to-demand matching with age
Mitochondria and their integrated control pathways in the heart are designed to adequately and rapidly supply ATP, to fuel the contractile work needed to achieve a cardiac output (CO, see glossary), and to match the body’s demand for blood flow (Fig. 1). Among common energetic indexes are the changes in M V̇O2 and myocardial work, which when taken together with myocardial efficiency provide an index of myocardial ATP demand. The myocardial efficiency is an important component of the efficiency of ATP supply-to-demand matching.
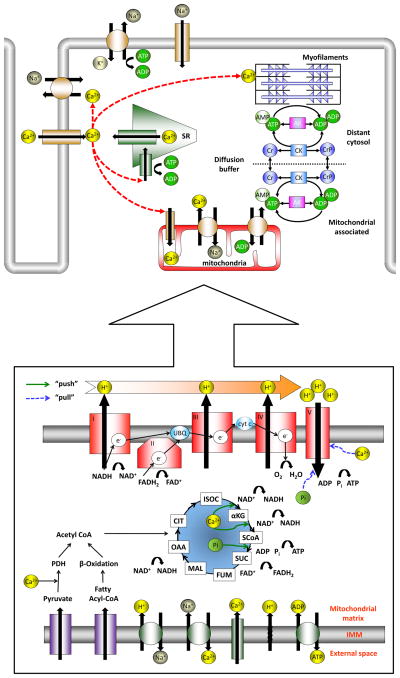
Figure 1. Excitation-contraction-bioenergetics scheme in the heart.
Majority of ATP is consumed by SERCA, Na+/K+ pump and contractile myofilaments. “Shuttlelike” diffusion of ADP and ATP in the cytosol is facilitated by CK and AK systems. In the cytosol, glucose is transformed to pyruvate, and fatty acids are converted to fatty acyl-coenzyme A. Pyruvate and fatty acyl-coenzyme A are, in turn, transported across the inner mitochondrial membrane to the mitochondrial matrix and are converted to acetyl-coenzyme A (pyruvate is oxidized by PDH, and fatty acyl-coenzyme by β-oxidation) while creating NADH and FADH2. Acetyl-coenzyme A from both substrates is oxidized to carbon dioxide and water in the Krebs cycle, resulting in additional formation of NADH and FADH2. The redox-potential energy of these reducing equivalents is, in turn, harnessed by the electron transport chain (complexes I–IV). Electrons are supplied to complex I in the form of NADH, and to complex II in the form of FADH2. These redox electrons are passed downhill (in a energy-flow sense) to complex III by coenzyme Q. Electrons from complex III are transferred to complex IV by cytochrome c. The energy released from this electron flow is used to transport protons across the inner mitochondrial membrane, out from the matrix, creating a large electrochemical gradient across that membrane. Under normal conditions, the electron transport chain flux is paralleled by the M V̇O2. The proton gradient and Δψm, form an electrochemical gradient of stored energy that is responsible to drive complex V to make ATP. ATP is transported to the cytosol across the inner mitochondrial membrane by ANT (in exchange for ADP, Pi and a proton) and the outer membrane by VDAC. The complement of ion transporters that maintain the other mitochondrial ionic gradients across the membrane, including the Ca2+ uniporter, Na+/Ca2+ exchanger and (regulated and unregulated) proton leak.
In this paper we discuss changes in the ability to make, deliver, and utilize fuel between young and old mammals, at both low and high energy demand. Reduction in maximal M V̇O2, energy production/delivery, or cardiac efficiency may cause the aged heart to run short of energy, specifically at high workload, which may contribute in part to a diminished capacity to perform CW (i.e., a diminished reserve capacity). We also examine the major age-associated alterations in the heart’s ability to contract in order to meet the body’s demand for fuel; try to clarify if ATP demand, supply and/or key regulators that control ATP supply-to-demand match are altered with aging, in both basal and high demand conditions; and discuss how alteration in kinetics may affect the cytosolic-mitochondrial energy transfer and production pathways. Finally, we critique the available experimental models used to study aging.
To allow the reader to compare the chronological age, a table with the age groups used in these experiments was added to the supplement. While animals are typically referred as “senescent” at that age when approximately 50% colony mortality occurs, different diet, environment and physical fitness may affect the mortality curves obtained in different labs and species.
Understanding age-associated alternations in cardiac energy production and utilization may help to better elucidate the cardiovascular aging process, to help develop future therapeutic interventions to improve energy supply to match the body’s demands with the goal of improving the quality of life in the elderly.
Direct and indirect regulatory control mechanisms to match energy supply with demand
ADP, Pi, Phosphocreatine levels
The natural byproducts of CW, ADP and Pi, have been suggested as candidate regulators of energy matching mechanisms, because they correlate with both ATP utilization and production. In this scenario, an increase in ATP utilization produces a sensed change in the accumulation of ADP and Pi in the cytosol, which effects ATP production both in mitochondria via ATP synthase (box 1) and in the cytosol via CK [1, 2] (box 2). However, no age-dependent changes in Pi and ADP levels have been found in tissues isolated from heart at basal state, and only marginal decreases occurred at high-energy demand conditions [1, 2]. During aging or pathological conditions, restricted diffusion of the intermediates may lead to a decrease in the availability of the substrates, and thus to changes in the intermediate concentrations (see box 1). Moreover, under these conditions any restriction in the diffusion of the substrates would also lead to a buildup of byproducts of fuel utilization and therefore reduce the ΔG available to drive the “end-use” ATPase reaction. Based on NMR studies, although the total creatine level remains constant, and the PCr level marginally decreases in aged heart tissue, compared to young at basal state, the ratio of PCr to ATP decreases in advanced age, in human [3] and rat tissue [4] (see table S1). The mechanisms of ATP utilization/production in the aged tissue are unlikely to keep pace with that of the young at high demand/workload levels (discussed below). Therefore, the more likely scenario to explain a decrease in PCr/ATP ratio (where ATP is constant or marginally reduced) is an apparent decrease in CK activity. This would be compounded by less efficient biochemical fluxes through AK, and glycolytic and guanine nucleotide high-energy phospho-transport, resulting in increased dependency on the ATP-buffering property of PCr. Note that it can be misleading to compare the CK activity alone in isolated mitochondria vs. isolated heart, because the flux also depends on the creatine level in the cytosol (vs. that supplied in the experimental model). Future experiments using the stable isotope 18O2-assisted 31P NMR, and mass spectrometry techniques, may reveal the important role of CK as a control mechanism of matching ATP supply-to-demand, in aging (reviewed in [1, 2]). Ultimately, future techniques that might reveal the existence of local nucleotide concentrations (i.e., close to the mitochondria and across the myofilaments) within the heart tissue, are needed to resolve such questions.
Text Box 1. ATP/Pi and energetics.
An increase in ATP utilization could produce a sensed change in the accumulation of ADP and Pi in the cytosol. In the mitochondria, ADP and Pi “push” the ATP synthase because they act as a substrate [56]; furthermore, ADP can activate the PDH complex, isocitrate dehydrogenase, and complex IV, to produce an “upstream” boost in ATP production (for review see [57]). The concept that ADP and Pi are the regulatory mechanisms responsible for matching ATP supply to demand, has been based primarily upon data from isolated heart mitochondria. However, in situand in vivo mitochondria can behave differently from those in isolated suspension (e.g., due to loss of normal cytoskeleton architecture and cytosolic signaling components; for review see [58]). Specifically, results from working heart models have shown that increasing ATP utilization by more than 10-fold is associated with only minimal changes in bulk PCr, ATP, ADP and Pi levels (for review see [5]). Thus, based on this data, it is difficult to reconcile how the constant concentrations of ADP, Pi or bulk PCr, per se, serve as the major control mechanism to match ATP supply to demand, in the working range of the heart. Note, however, that the time-averaged NMR method has two relevant limitations: 1) this method does not allow resolution of beat-to-beat oscillation in nucleotides. 2) The typical spatial resolution of these NMR methods probably does not allow detection of subcellular and, more importantly, sub-compartment nano-scale domain gradients of nucleotides in the cell that may exist. On the other hand, using gated 31P NMR (synchronized to different stages of the cardiac cycle), cyclic changes of high-energy phosphate concentration as a function of the phase in the cardiac cycle were resolved in the rat model (for review see [59]). Under resting conditions, the extent of cycling changes of ATP, PCr and Pi was observed to vary between ~10–15%. Because an oscillatory system can convey information encoded in its frequency and amplitude fluctuations, it is hypothetically possible that this factor may actually be able to contribute to control the supply-demand balance and that discrete changes in the nature or sensing of these signals with aging could degrade this. Note, however, that such oscillations in metabolites could not be detected in the hearts of larger mammals such as dog, sheep and human, using similar methods (for review see [59]). The heart has built-in redundancy with ample auxiliary enzyme-systems to interconvert high-energy species with fast reaction kinetics and high reaction-fluxes allowing the concentration of the intermediates to be essentially constant across a wide operating range. These intermediates of course govern reaction rates and fluxes in kinetic equations related to their concentration. Therefore, these intermediates would not serve as a direct mechanism to regulate ATP production under normal operating conditions, if their concentrations are essentially steady. Under rapidly changing dynamic conditions, however, the intermediates would no longer be constant and now their concentration would serve as a signal to match ATP supply to demand.
Text Box 2. CK and energetics.
In the cytosol, ATP can be regenerated using the energy stored in PCr via CK (for review see [60]). Cytosolic CK appears to facilitate a “shuttle-like” role in delivering ATP from mitochondria to the myofilaments. In young mice, near-total knockout of mitochondrial, cytosolic or both CK isoforms, has modest if any measurable effect in cardiac performance measurements (left ventricular pressure and systolic or diastolic function), under normal as well as the somewhat increased demand conditions of dobutamine challenge (a sympathomimetic drug used in the treatment of heart failure), compared to littermate controls (although when dP/dtmax was analyzed by 2-way ANOVA a small but significant difference was found between the genotypes) [61]. Therefore, the relative role of cytosolic CK activity across a modest range of workloads (that are far from maximal) to produce ATP in the mouse heart is probably small based on this result. In skeletal muscle, however, the CK role appears to be important because mice with near-total knockout of either mitochondrial or cytosolic CK do less voluntary wheel running. Thus, it is plausible that in the closely-related cardiac muscle, CK might play a similar role, especially at very high work load. As mentioned, dobutamine stress may actually not achieve the whole range of cardiac workload, therefore, the small or modest changes seen in near-total knockout CK mice [61] may underestimate what happens at the highest states of demand/workload. Moreover, adult non-senescent transgenic mice with creatine deficiency did neither reveal any meaningful deficit of cardiac function at baseline nor, in terms of cardiac remodeling, after myocardial infarction [62]. However, without direct experimental proof we cannot eliminate that at high workload, particularly in the aged heart, a deficit in PCr level would not limit cardiac performance or reserve capacity. Future experiments (e.g., with these mice during aging) are needed to clarify this point. Stable isotope 18O-assisted 31P NMR and mass spectrometry techniques have enabled simultaneous recording of ATP hydrolysis together with phosphotransfer fluxes (i.e., via the activities of adenylate kinase, creatine kinase, etc.) [63], at different levels of myocardial workloads. Using this technique, mice lacking cytosolic CK had lower PCr turnover, with increased glucose-6-phosphate turnover, glucose utilization and inorganic phosphate compartmentalization, compared to wild type. In mice lacking both cytosolic and mitochondrial CK, the PCr level and phospho-transfer capacity were lower, but the fluxes through adenylate kinase, glycolytic and guanine nucleotide phosphortransport increased, compared to WT. Therefore, the energy that is carried by PCr under normal physiological or pathologic conditions, and in null CK mice, and the PCr-CK pathway, can be replaced by other, less efficient phosphotransfer pathways. This can explain, in part, the modest if at all measurable change in cardiac performance measurements found in these CK deficient mice, and emphasizes the role of CK as a possible control mechanism to match ATP supply to demand at high demand.
Δψm and NADH levels
A second mechanism that might control energy-matching is the interaction between the NADH level and Δψm (reviewed in [5]). In isolated heart mitochondria, stimulation of dehydrogenases increases NADH levels, which in turn increase both electron transport chain flux and Δψm, that “push” the ATP synthase to make ATP. Direct measurements of Δψm in the isolated perfused rat heart model demonstrated a slight depolarization of Δψm with increasing workload with unchanged levels of ADP, Pi and NADH [6]. The authors suggest that this occurs by a direct effect on complex-V, which we interpreted as a “pull” mechanism. Because a hyperpolarized Δψm would be required to “push” ATP synthase during increases in heart workload, the experimental results of a slight mitochondrial depolarization suggest that Δψm is the effect, rather than the cause, and thus is not a key regulator. Because, 1) the only available measurements of Δψm changes during aging are from isolated mitochondria (Table S1), and 2) there is considerable divergence of experimental results regarding Δψm changes between the isolated mitochondria model vs. that under in vivo conditions (even under normal conditions) [5], additional confirmatory measurements under in situ or in vivo conditions are necessary to clarify that these Δψm results accurately reflect the actual changes in aged heart. Moreover, experimental evidence suggests that the NADH/NAD+ ratio remains stable in response to varying Ca2+ concentrations or substrate availability in mitochondrial suspensions, and during a period of significantly increased ATP demand as in the in vivo working heart model (reviewed in [7, 8]). Consequently, the NADH level may not be the major regulatory mechanism of energy demand. Furthermore, measurements of NADH levels in isolated mitochondria are comparable to those under in vivo conditions, and these concordances suggest that healthy isolated mitochondria might serve as a reliable model to explore NADH-related mechanistic questions, in advanced age models. In the aged heart and under basal conditions, NAD+ levels decrease while NADH levels increase, thus the NADH/NAD+ ratio increases [9]. Examination of age-dependent variation in the protein profile of heart tissue revealed down-regulation of NADH dehydrogenase expression, the enzyme that catalyzes the oxidation of NADH to NAD. This may account (in part) for the increase in NADH, discussed above [10], and a decrease in the ability to produce ATP by the mitochondria, and therefore a decrease in ATP level in aging, specifically at high demand.
Mitochondrial Ca2+ level
Cytosolic-mitochondrial Ca2+ cross-talk could potentially play a role in matching ATP supply and demand (box 3). It is well documented that in the aged rodent heart, the peak of the cytosolic Ca2+ transient remains unchanged, but the action potential and Ca2+ transient durations are significantly prolonged ([11]; reviewed in [12]). Moreover, SR load may decrease as consequence of an increase in Ca2+ extrusion by the sarcolemmal Na+-Ca2+ exchanger, which further prolongs the action potential, and through the sarcolemmal Ca2+-pump (reviewed in [12]). The up-regulation of myosin isoform V3 that has a lower ATPase activity compared to V1 [13], and a decrease in SERCA pump activity [14], may slow the intracellular Ca2+ kinetics. Slower Ca2+ kinetics will slow the force production and relaxation which decreases the rate of ATP utilization by the myofilaments, and also Ca2+ sequestration by the SR (which may reduce the Ca2+ gradient between the cytosol and SR), resulting in further decrease in the activity of SERCA and hence its ATP utilization. Although slowing the cytosolic Ca2+ transient in advanced age, compared to young [11] may decrease the heart’s ATP utilization, slowing cytosolic Ca2+ transients and mitochondrial Ca2+ uptake may reduce rapid Ca2+ uptake when the demand rapidly increases, resulting in reduction in the rate of ATP production vs. the young. Therefore, even if the maximal demand level declines, at high workload decreases in ATP supply below a certain threshold can still induce an ATP supply-and-demand mismatch and cause a decrease in ATP content.
Text Box 3. Ca2+ and energetics.
Because the major consumers of ATP in the heart are the contractile myofilaments (responsible for ~2/3 of ATP hydrolyzed [64]), and most of the cytosolic Ca2+ is bound to the myofilaments [29], Ca2+ might be well positioned to regulate energy matching. Depending on the species, mitochondrial Ca2+ levels can also respond to relatively rapid changes in cytosolic Ca2+ levels (i.e., over a train of beats) [21]. Ca2+ enters mitochondria through the mitochondrial Ca2+-uniporter ([65, 66]) and is extruded by the mitochondrial Na+-Ca2+ exchanger ([67]) (reviewed in [68]). Because changes in cytosolic Ca2+ concentrations reflect changes in ATP demand, and Ca2+ accumulates inside mitochondria, the mitochondrial Ca2+ level is an indicator of ATP demand (reviewed in [69]). Ca2+ can affect ATP supply by stimulating PDH activity and other dehydrogenase mechanisms, such as isocitrate and α-ketoglutarate (reviewed in [69]). Whether Ca2+ directly activates ANT remains controversial [70]. It has also been reported that an increase in mitochondrial Ca2+ level can regulate mitochondrial matrix volume [71] which in turn can elevate respiration in isolated mitochondria suspensions. Recently, however, it was shown that in mechanically unloaded intact cardiomyocytes during light pacing contraction-related work, there is no detectable change in mitochondrial volume accompanying any small pacing-related increase in mitochondrial Ca2+ [72].
The relative role of mitochondrial Ca2+ in controlling ATP supply-to-demand matching may be largely independent of changing the workload by Frank-Starling-related ventricular filling mechanisms [57]. However, a decrease in mitochondrial Ca2+ (by inhibition of the mitochondrial Ca2+ uniporter with Ru360) decreased the NADH level at high pacing rate (4 Hz) while maintaining constant NADH level at low pacing rate (1 Hz) [73]. Moreover, mathematical modeling shows that both Ca2+ and ADP can potentially serve as controlling mechanisms that match ATP supply/demand [74]. In isolated trabeculae an increase in pacing frequency produced an undershoot in the level of NADH, followed by a recovery phase until a steady-state NADH level was reached [75]. Upon a decrease in pacing frequency an overshoot in the NADH level occurred followed by a recovery phase until a steady-state NADH level was reached. Thus, the apparent predictive behavior of this mathematical model in the forgoing experiments suggests that ADP-stimulated respiration is a reasonable mechanism to explain the rapid overshoot and undershoot, while the mitochondrial Ca2+ accumulation accounts for the slower recovery of NADH.
In the isolated rat mitochondria model, the rates of Ca2+ uptake (likely due to changes in Vmax of the Ca2+-uniporter and not in its Km) and release, are reduced in advanced age [15] [16]. However, the total Ca2+ content of the mitochondria does not change with age in the basal state [16], and even increases after treatment with norepinephrine, compared to the young rat [17]. It is possible that a parallel loss of activity of both Ca2+ uptake and release may explain the lack of age-linked change in Ca2+ content, in resting conditions. Note, however, that there is a lack of experimental data on the mitochondrial Ca2+ content under in situ/in vivo conditions in advanced age, where the cytosolic Ca2+ naturally oscillates, versus the constant external Ca2+ exposure in isolated mitochondria models [18]. Specifically, because there is an important cross-talk between the SR and the mitochondria (reviewed in [19, 20]), a decrease in beat-to-beat SR Ca2+ release can decrease the mitochondrial Ca2+ levels. Whether mitochondrial Ca2+ uptake varies substantially on a beat-to-beat manner or over a longer time frame (reviewed in [21]), slower SR Ca2+ kinetics could slow Ca2+ accumulation into mitochondria, reduce the steady-state mitochondrial Ca2+ level and delay and diminish the activation of Ca2+-regulated ATP production mechanisms. In summary, although Ca2+ activates mitochondrial dehydrogenases and other enzymes and primes mitochondria for the anticipated increase in energy demand (see box 3), and cytosolic Ca2+ kinetics become substantially altered in advanced age potentially causing its priming mechanism to become deficient, more experimental data under in situ/in vivo conditions are needed to clarify if changes in mitochondrial Ca2+ dynamics can explain alternations in the regulatory mechanisms responsible for matching energy supply-to-demand in advanced age, specifically at high demand.
Phosphorylation and acetylation of mitochondrial proteins
Post-translational modifications, including phosphorylation and acetylation are important modifications that affect protein function. The relative role of protein phosphorylation within the cardiac mitochondrial matrix, and in particular the respiratory complexes, has become more appreciated over the last several years. Changes in phosphorylation might modulate the activity of respiratory complexes and the rate of ATP production (for review see [22]). Currently, several molecules including ANT, VDAC, cytochrome C, complex I and complex V have been found to be phosphorylated [22]. Also, changes in the level of protein phosphorylation in the cytosol may affect mitochondrial ATP production via the traffic of phosphorylated signal proteins and enzymes such as PKA, GSK-3β, AMPK, etc., to the mitochondria. At high demand, defects in β-adrenergic receptor signaling, in advanced age, reduce cAMP/PKA production in the rat heart [23] which could in principle affect mitochondrial protein and myofilament phosphorylation related to PKA signaling. To date, many of the respective regulatory sites have been found in proteins residing in the mitochondrial intermembrane space (reviewed in [22]).
Another important mechanism of post-translational modification is acetylation or deacetylation of proteins. A well-studied family of deacetylases includes the NAD-dependent enzymes sirtuins (Sirt1 and Sirt3 are two examples) [24]. Sirtuin activation is directly linked to the energetic and redox status, and thus the NAD/NADH ratio, and SIRT3 is the major mitochondrial enzyme in this class (reviewed in [24]). Potential changes in both mitochondrial protein phosphorylation and acetylation may adversely affect mitochondrial bioenergetics in advanced age [25]. SIRT3 has been linked to the activation of fatty-acid β-oxidation, oxidative-phosphorylation, deacetylation and activation of enzymes in complex I, II and V [24]. Therefore, an increase in SIRT3 or its activity can potentially lead to an increase in mitochondrial ATP production. Although the effect of aging on signaling of the cardiac NAD-dependent deacetylase SIRT3, and the related member SIRT1, has not been determined [24], at least in skeletal muscle, SIRT3 and SIRT1 signaling is decreased during aging [25]. Recently (reviewed in [26]), the importance of the regulation of peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1α/β) activity by SIRT1-mediated deacetylation in skeletal muscle was established. PGC-1α/β is a crucial transcriptional co-activator that plays central role in the regulation of mitochondrial biogenesis and turnover. This modification of PGC-1α can increase mitochondrial biogenesis and protects against metabolic disorders associated with a high-fat diet.
Changes of ATP utilization in advanced age
In advanced age and assuming that Ox-Phos remains fully coupled, there is an apparent decrease in ATP demand by the myofilaments at high workloads, compared to the young. In the isolated working heart, CW and efficiency decline with age [27]. In humans and under high-demand conditions in vivo, CO and stroke volume at maximal exercise and maximal work load capacity are reduced in the aged subjects, compared to the young [28]. Additionally, in isolated rat papillary muscle the maximal power output, maximal shortening velocity and maximal force are decreased (reviewed in [13]). Taken together, the maximal CW at high peripheral-tissue ATP-demand decreases in advanced age.
Proteomics approaches have identified a change in myosin isoforms in aging rodents [10] (see above; reviewed in [13]). Because most of the cytosolic Ca2+ is bound to the myofilaments [29], cytosolic Ca2+ dynamics are directly related to force generation and relaxation kinetics. Changes in gene expression to lower myosin ATPase activity may explain, in part, changes in the kinetics of contraction and also of the Ca2+ transient.
Another point of interest is that of arterial stiffening, which during aging is associated with increased aortic impedance that increases the pulse wave velocity and results in increased left ventricular loading. The increase in pulse-wave velocity causes the diastolic-reflected pulse pressure wave to arrive back at the central aorta earlier (i.e., prior to closure of the aortic valve, instead of during diastole and supporting coronary flow), leading to an increase in systolic pressure, left ventricular wall stress, and a maladaptive increase in M V̇O2 per unit of CW performed [13, 30]. Collectively, these data suggest that increased systolic pressure during aging, together with increases in cardiac interstitial matrix, might contribute to a decrease in the efficiency of CW. Note, that the reduction in efficiency may be partially related to change in substrate metabolism (fat vs. carbohydrate). Therefore, the heart needs to utilize more ATP to perform the same amount of CW as the young, and this places heavier demands on the mitochondria to produce and supply adequate ATP levels. These factors diminish the cardiac reserve capacity that would be necessary to perform augmented work during aging.
ATP supply in advanced age
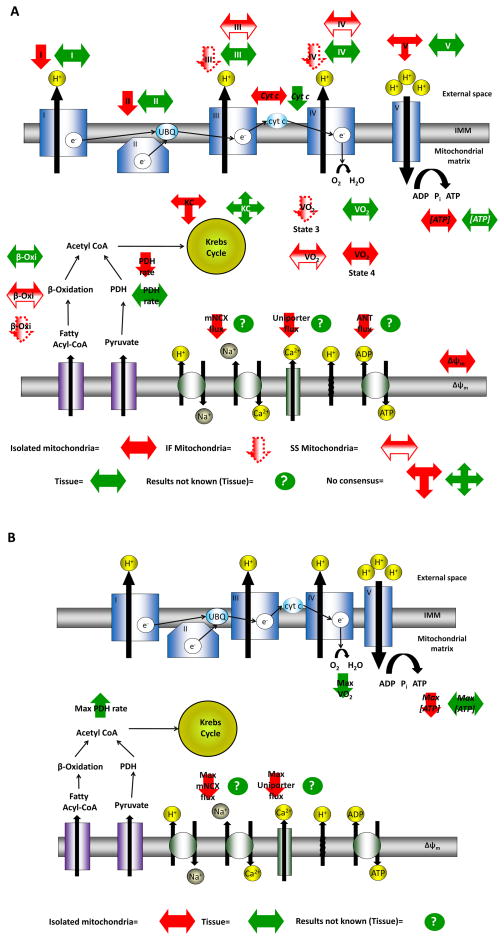
A decline in mitochondrial function may affect the production of cellular energy, which in turn can interfere with ATP-dependent cellular physiology (see above). While there is a wide array of data suggesting that mitochondrial energetic mechanisms decline in advanced age (see online supplement Table for extensive listing), the relative importance of these changes are still controversial. To better understand the source of this controversy, changes reported in both tissue and in isolated mitochondria, under rest and high demand conditions, were compared (Fig. 2; see online supplement Table for extensive listing). Indeed, the results in isolated mitochondria are often remarkably different than those from tissue (discussed above).
Figure 2. Changes in mitochondrial energetic mechanisms.
While there is a wide array of evidence that mitochondrial energetic mechanisms decline in advanced age, the relative importance of these changes are still controversial. To better understand the source of this controversy we compared the changes in mitochondrial energetic mechanisms reported in both tissue (green) and isolated mitochondria (red) (see online supplement Table for extensive annotated listing and literature citations). The summary is separated into panels (A) basal conditions, and (B) high demand conditions. Note that the results in isolated mitochondria are often remarkably different than those from tissue. Moreover, in some cases the data are controversial even at the same level (i.e., tissue or isolated mitochondria) therefore a double arrow was used to reflect these existing discrepancies. Sometimes data are available only for isolated mitochondria and therefore a question mark was used for tissue data. Finally, two mitochondrial sub-populations (i.e., SSM and IFM) have been described in the myofilament compartments of cardiomyocytes and can be studied in isolated population. The age-dependent mitochondrial energy production is different for the two populations and therefore different symbols were used.
Under in situ conditions in aged rat hearts, the ATP level remains constant at rest, but slightly decrease at high demand, compared to that in young [31, 32]. Because ATP consumption likely increases to perform the same amount of CW, due to the age-dependent efficiency decrease at high demand, any concurrent deterioration in the ability to make or deliver ATP would further compromise cardiac function.
As described above, changes in M V̇O2 might be considered a reliable index of ATP production. In isolated rat mitochondrial suspension, most of the experimental data support that the state 4 respiratory rate (when ADP is fully consumed), does not significantly decline in advanced age, but the state 3 respiratory rate (after ADP addition), has been variably reported to either decrease or remain constant [33–35]. However, there is no significant change in the respiratory control ratio (M V̇O2 -state 3/state 4). In the isolated aged-heart model there is no significant decrease in M V̇O2 in resting conditions, but the maximal M V̇O2 is reduced at high demand (workload), compared to that in the young [31]. This is consistent with steady-state ATP data [31, 32].
Experiments in isolated rat mitochondria have shown that the import of fuel (i.e., oxidizable substrates), the ability to oxidize fatty acids to produce ATP, and the rate of ATP export from the mitochondria, deteriorates in advanced age [33, 36]. In mitochondria, the pyruvate uptake and oxidation are lower compared to the young [37], but remain intact in tissue biopsy samples [17]). ANT activity was found to decrease in advanced age in isolated mitochondria (Table S1). Hexokinase II is bound to, or very near, the outer membrane protein VDAC (for review see [38]). In the presence of glucose and ATP, it can make glucose-6-phosphate (via glycolytic metabolism) and increase anaerobic ATP production. However, no significant change in hexokinase II abundance was found in advanced age rat compared to young [39]. More tissue-based model-data are needed to understand the change in ATP production and export in advanced age.
Changes in the activity/level of mitochondrial proteins can also affect ATP production. In advanced age, there is a decrease in Krebs-cycle enzyme activities: e.g., NAD+-isocitrate dehydrogenase and 3-hydroxyacyl-CoA dehydrogenase activities (see Fig. 2). It is debatable whether the activities of any or all mitochondrial complexes decrease with age. In human tissue, some reports indicate no change in complex I–IV activities [40, 41], while others suggested that there may be a decrease in complex IV content ([42] vs. [40]). Most of the data from both isolated mitochondria and tissue models agree that complex V activity does not change in advanced age [43, 44]. However, proteomics data revealed that the expression of three mitochondrial enzymes was up-regulated in aged mouse heart tissue, namely 3-oxoacid-CoA transferase 1; α-subunit of ATP synthase; and cytosolic CK2. A reduced expression was found for NADH-dehydrogenase; flavoprotein 1; subunit-8 of ATP synthase; and cytochrome c [10]. Recent proteomics data in mice also show down-regulation of complex 1 (Ndufa5, Ndufb2, Ndufb4 and Np 15), complex III (Uqcrb), complex IV (Cox7a2 and Cox7b) and complex V (Atp5a1 and Atp5f1) [45, 46] in advanced age. Recent gene array data show down regulation of mitochondrial electron transport chain genes in both mice and human in advanced age [31, 47]. These data suggest that a changed pattern of mitochondrial gene expression and proteins may contribute to the decrease in the ability to produce ATP at high demand with aging. On the other hand, increase in CK2 or in some mitochondrial enzymes could be a compensatory mechanism to increase ATP delivery or production, respectively, at high demand. Note, however that in aged rat down-regulation of complex II, III and IV proteins and genes were not correlated with changes in their activity [47].
As mentioned above PGC-1α/β has been identified as a crucial transcriptional co-activator that regulates genes involved in energy metabolism. Increased expression of PGC-1α has been shown to protect against age-related sarcopenia [48], to improve respiration and gluconeogenesis under conditions of telomere dysfunction [49] and in increased longevity in drosophila [50]. Therefore, PGC-1 may function as an integrator of essential pathways involved in age-related decline in mitochondrial energy production and may be an important determinant of longevity [51, 52].
The concentration of mitochondrial respiratory complexes, the availability of substrates for ATP production (ADP and Pi) and the level of their control regulators have been shown to be proportional to the maximal metabolic rate and therefore the ability of the heart to have “excess” mitochondrial energy production [53]. Therefore, even if the concentrations of mitochondrial respiratory complexes remain intact (see above), a reduction in availability of substrates (i.e., in time and space) and/or an alteration in Ca2+ kinetics, may alter the ability of the heart to utilize this potential “excess” mitochondrial energy production capacity, specifically at high demand. Therefore, these regulators could potentially contribute to the loss of “excess” mitochondrial energy production during aging that potentially exists in young, especially at high-demand. More experimental data are needed to resolve the underling mechanisms.
Two mitochondrial populations have been described in the myofilament compartments of cardiomyocytes and can be studied in isolated population: SSM that lie beneath the plasma membrane, and IFM that are ensconced among the myofibrils (reviewed in [54]). Although the ratio of SSM to IFM remains constant in aged rats compared to young, there appear to be a decrease in mitochondrial energy production in IFM. Moreover, IFM display a decrease in Ca2+ content in advanced age when glutamate or malate are used as a fuel (compared to succinate), whereas SSM Ca2+ content is unaffected with age [55]. Unfortunately, isolation techniques can vary between laboratories, yielding arbitrary alternations in the SSM to IFM ratio, which may lead to confounding interpretations based on comparison of these different data sets [54]. Additionally, different levels of fibrosis may interfere with the isolation procedures, and different degrees of mitochondrial purity may undermine conclusions, based on measurements performed without proper normalization procedures. Moreover, aging myocytes are more sensitive to stress damage compared to young (e.g., the threshold to open the mitochondrial permeability transition pore is lower in aged cardiomyocytes vs. young adult [12]), and therefore during mitochondrial isolation the aging mitochondria might be expected to have a generally higher tendency to be damaged versus the young, which in turn, may alter their biophysical and energetic properties, or even alter their true (native) abundance after isolation procedures.
Concluding remarks
In this review we discuss relevant energetic mechanisms that may change during aging, from energy (ATP) production, to delivery and ATP utilization, and analyze whether and how the mechanical and chemical efficiencies change.
Related to energy production in advanced age, (assuming that Ox-Phos is tightly coupled (Table S1), and if the same mitochondrial flux of substrates is maintain to make ATP), the inability to augment the maximal M V̇O2 to the same degree as the young would indicate that ATP production and delivery are limited at high demand. This is consistent with the interpretation that there is an age-related decline in mitochondrial energy production, contributing, in part, to a decline in maximal contractility. During aging there is also a change in control mechanisms that match ATP supply and demand. The changes in mitochondrial energetic mechanisms also challenge what is the most suitable experimental model to explore aging, given the discrepancies discussed here (Fig. 2). Unfortunately, published data addressing these issues is incomplete, and comparison of the various experimental models frequently produces conflicting results. The experimental data obtained from isolated mitochondria, cells, and tissue optimally should be validated in the whole-organ models to provide a measure of confidence that the simple models are suitable for aging research. After this validation is achieved, the simple model results could be more reliably translated to the organ and organism levels.
Supplementary Material
Text Box 4. Human clinical application.
In humans, CO and stroke volume at maximal exercise and maximal work load capacity are reduced in aged subjects, compared to the young [28]. In human tissue, some reports indicated no change in complex I–IV activities [40, 41], while others suggested that there may be a decrease in complex IV content ([42] vs. [40], respectively). Moreover, in parallel to the reduction in maximal cardiac work, maximal peripheral (body) oxygen consumption is also reduced (Table S1). This reduction in peripheral oxygen consumption, however, does not necessary imply that M V̇O2 ecreased because even if the efficiency of cardiac ATP utilization, production and delivery remain intact, the maximal capacity of the body to perform work may also be reduced by aging mechanisms and therefore less CO would be required. In the case that the heart’s efficiency of ATP utilization decreases, more ATP would be needed per unit work, and thus the M V̇O2 would increase accordingly. If the heart’s ATP production efficiency is reduced M V̇O2 increases, but CO may still decrease because of insufficient amounts of ATP. Finally, if ATP delivery in the heart is reduced M V̇O2 can increase or remain constant, but the CO may decrease because of insufficient amounts of ATP. Future experiments that include ATP production rate, oxygen consumption and work during aging are needed to better define the physiology.
In aging humans, as a fraction of total energy expenditure, myocardial fatty acid oxidation and utilization decline with no change in myocardial glucose utilization [76], but with increased myocardial triglyceride content [77]. Therefore, the overall contribution of fatty acids (which need more oxygen per ATP produced compared to glucose) relatively to glucose as a fuel for ATP production decreases in advanced age. Note, that the age-related shift in fuel preference (i.e., fatty acid to carbohydrates) would affect cardiac efficiency by virtue of the differences in ATP/O ratio.
In aged human heart tissue, compared to young at the basal state, the PCr/ATP ratio decreases [3] (see discussion in the main text). It was clinically shown that a decrease in PCr/ATP ratio in patients with dilated cardiomyopathy is a predictor of increased mortality [78]. A recent study has found that sedentary men undergoing long-lasting sustained aerobic oxidative training have higher PCr/ATP ratio vs. untrained control [79]. Therefore, a link between the beneficial effects of exercise and changes in ATP supply-to-demand mechanisms may exist in aging human.
Several recent studies report that ROS production increases in advanced age [80, 81]. ANT was found to be the only mitochondrial protein to exhibit an age-associated increase of oxidative modification in flight muscles of the housefly [82]. However, lacking of ANT genes is accompanied by increase in ROS production due to upregulation of oxidative phosphorylation genes that compensate for reduction in ANT [83]. Although the contribution of overexpression of SOD2 (antioxidant defense mechanism that scavenges ROS) to life-span is controversial, it decreased age-related decline in mitochondrial ATP production [84].
The progression of HF is associated with diminished energy metabolism and Ca2+ transient amplitude [73], a decrease in ATP synthesis capacity and a decrease in overall ATP levels and oxygen consumption [85] in both basal and high-demand conditions, but the deterioration of these functions occurs only at high-demand in healthy aging. Additionally, in chronic HF ROS levels increase, myocardial anti-oxidant reserve decreases, the PCr/ATP ratio decreases and NADH/NAD+ ratio increases (review in [86]) similar to aged heart over the entire range of heart work. It may be that the failing heart, depending on the degree of impairment, may look like the aging heart not only when the failing heart is working at high-demand, but in severe cases also at low demand.
Outstanding questions.
Future experiments facilitated by mathematical modeling are needed to answer the following questions:
What specifically deteriorates during aging: the ability of the heart to contract (the “engine”, which encompasses mechanical efficiency), the ability to produce and deliver ATP (the fuel) and/or the biochemical efficiency to utilize ATP to perform work?
What are the relative roles of control mechanisms that match ATP supply to demand during aging?
Are there important regulatory pools of local nucleotide or metabolite concentrations or gradients in subcellular domains (i.e., close to the mitochondria and across the myofilaments) in the working range of the heart, and do they change with aging?
Is there a role of Δψm as a control mechanism of energy matching during aging?
How does mitochondrial Ca2+ content/gradient under in situ/in vivo conditions change during aging (see [87]) ?
In primates, caloric restriction improves healthspan, and potentially lifespan [88] (reviewed in [89]). Regular aerobic exercise can improve healthspan [90]. Is there an important mechanistic link between the beneficial effects of caloric restriction and exercise, and changes in ATP supply-to-demand mechanisms?
Overexpression of CK improves ATP supply-to-demand balance in postischemic myocardium [91]. Would treatment aimed at maintaining and/or boosting the high-energy-intermediate phosphoryl-exchange capacity (e.g., by AK, CK, etc.) be beneficial in the aging heart to maintain ATP supply-to-demand matching (especially at high demand)?
VDAC function is controlled by the cytoskeleton properties and phosphorylation level [92, 93]. Could alteration in cytoskeleton structure/function or phosphorylation level (see above) in advanced age affect VDAC and in turn, HK function resulting in diminished energy production capacity?
Deletion of mitochondrial intermembrane enzyme AK 2 is embryonically lethal [94]. Could alteration in AK 1 and 2 activities affect ATP supply-to-demand matching in advanced age?
ROS production may increase in advanced age. Would a decrease in cardiac ROS production or an increase in ROS scavenger mechanisms improve healthspan and/or lifespan in humans?
How to compare aging across species? It would be valuable if aging data would not only be given in chronologic age, but also as a fraction of that species maximal lifespan to better compare species-independent aging variables.
Highlights.
Age-related changes in cardiac energetics are discussed.
It is controversial if ATP production, delivery or utilization changes during aging.
Results from isolated mitochondria frequently differ from those obtained in tissue.
Unresolved and controversial questions requiring further studies are highlighted.
Acknowledgments
We thank Dr. Edward G. Lakatta for useful discussions.
Sources of Funding
The work was supported entirely by the Intramural Research Program of the National Institute on Aging, NIH.
Glossary
- Adenine nucleotide translocase (ANT)
also called the ADP/ATP carrier, is an antiporter that facilitates the exchange of ADP for ATP, across the inner mitochondrial membrane
- Adenylate cyclase (AK)
phosphotransfer enzyme playing an important role in cellular energy homeostasis that catalyzes the inter-conversion of 2 ADP ⇌ ATP+AMP
- Creatine kinase (CK)
an enzyme that consumes ATP to catalyze the conversion of creatine to phosphocreatine (PCr) and ADP. This “shuttle function” is reversible, such that ATP can be regenerated from PCr and ADP, as needed
- Cardiac output (CO)
the effective volume of blood pumped out by the ventricles in a given period of time
- Cardiac work (CW)
measurement of myocardial work, which when taken together with myocardial efficiency, provides an index of myocardial ATP demand
- Efficiency
the ratio between CW and the amount of fuel used to do that work
- Frank–Starling law of the heart
defines the relationship between the strength of the heart’s contraction to volume. The greater the heart volume (muscle length) the greater is the heart’s contraction (muscle force)
- Heart failure (HF)
a condition in which the heart is incapable to pump sufficient blood to meet the requirements of the rest of the body
- Hexokinase (HK)
important enzyme initiating the energy metabolism of glucose utilization by catalyzing the phosphorylation of glucose to yield glucose-6-phospate
- Interfibrillar mitochondria (IFM)
mitochondria sub-population located between the contractile the myofibrils inside cardiomyocytes
- Myocardial oxygen consumption rate (M V̇O2)
an index of mitochondrial respiration, and in a highly coupled oxidative-phosphorylation system, the ATP production rate
- Nuclear magnetic resonance (NMR)
a spectroscopy technique to explore chemical and biochemical properties of organic molecules by using the magnetic properties of certain atomic nuclei
- Oxidative phosphorylation (Ox-Phos)
The process in which respiratory enzymes in the mitochondria harness energy from NADH and FADH2, consuming O2, to generate a gradient of protons across the mitochondrial inner membrane that drives ATP synthase to make ATP from ADP+Pi
- Phosphocreatine (PCr)
A high-energy storage molecule that can donate a Pi group to ADP to form ATP rapidly under the enzyme catalysis of CK
- Pyruvate dehydrogenase (PDH)
an enzyme that transports the end product of glycolysis (pyruvate) to produce fuel (acetyl-CoA) that is used by citric acid cycle to make ATP in the mitochondria
- Reactive oxygen species (ROS)
chemically reactive oxygen-containing molecules (including oxygen ions and peroxide) that can form as natural byproduct of the Ox-Phos metabolism. Increase in ROS production may results in a significant damage to cellular constituents
- Sarcoplasmic reticulum (SR)
a Ca2+ store and release organelle inside excitable cells
- Sarco-endoplasmic reticulum Ca2+ ATPase (SERCA)
Ca2+ ATPase pump that transfers Ca2+ from the cytosol to the SR
- Subsarcolemmal mitochondria (SSM)
mitochondria sub-population that exists in close proximity to the cell membrane
- Transmembrane potential difference (Δψm)
the electrical potential difference across the mitochondrial inner membrane inside cardiomyocytes
- Voltage dependent anion channel (VDAC)
Large internal channels (about 2–3 nm), located in the mitochondrial outer membrane that allows ions, metabolites, and certain small molecules to move between the cytoplasm and mitochondria
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Beek JH. Adenine nucleotide-creatine-phosphate module in myocardial metabolic system explains fast phase of dynamic regulation of oxidative phosphorylation. Am J Physiol Cell Physiol. 2007;293:C815–829. doi: 10.1152/ajpcell.00355.2006. [DOI] [PubMed] [Google Scholar]
- 2.Saks VA, et al. Functional coupling as a basic mechanism of feedback regulation of cardiac energy metabolism. Mol Cell Biochem. 2004;256–257:185–199. doi: 10.1023/b:mcbi.0000009868.92189.fb. [DOI] [PubMed] [Google Scholar]
- 3.Hollingsworth KG, et al. Left ventricular torsion, energetics, and diastolic function in normal human aging. Am J Physiol Heart Circ Physiol. 2012;302:H885–892. doi: 10.1152/ajpheart.00985.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bak MI, et al. Interaction of hypoxia and aging in the heart: analysis of high energy phosphate content. J Mol Cell Cardiol. 1998;30:661–672. doi: 10.1006/jmcc.1997.0633. [DOI] [PubMed] [Google Scholar]
- 5.Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan B, et al. Effects of cardiac work on electrical potential gradient across mitochondrial membrane in perfused rat hearts. Am J Physiol. 1993;265:H453–460. doi: 10.1152/ajpheart.1993.265.2.H453. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, et al. Regulation of myocardial substrate metabolism during increased energy expenditure: insights from computational studies. Am J Physiol Heart Circ Physiol. 2006;291:H1036–1046. doi: 10.1152/ajpheart.01382.2005. [DOI] [PubMed] [Google Scholar]
- 8.Heineman FW, Balaban RS. Effects of afterload and heart rate on NAD(P)H redox state in the isolated rabbit heart. Am J Physiol. 1993;264:H433–440. doi: 10.1152/ajpheart.1993.264.2.H433. [DOI] [PubMed] [Google Scholar]
- 9.Braidy N, et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarti B, et al. Proteomic profiling of aging in the mouse heart: Altered expression of mitochondrial proteins. Arch Biochem Biophys. 2008;474:22–31. doi: 10.1016/j.abb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Froehlich JP, et al. Studies of sarcoplasmic reticulum function and contraction duration in young adult and aged rat myocardium. J Mol Cell Cardiol. 1978;10:427–438. doi: 10.1016/0022-2828(78)90364-4. [DOI] [PubMed] [Google Scholar]
- 12.Lakatta EG, Sollott SJ. The “heartbreak” of older age. Mol Interv. 2002;2:431–446. doi: 10.1124/mi.2.7.431. [DOI] [PubMed] [Google Scholar]
- 13.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 14.Babusikova E, et al. Age-associated changes in Ca(2+)-ATPase and oxidative damage in sarcoplasmic reticulum of rat heart. Physiol Res. 2012;61:543–560. doi: 10.33549/physiolres.932320. [DOI] [PubMed] [Google Scholar]
- 15.Jahangir A, et al. Increased calcium vulnerability of senescent cardiac mitochondria: protective role for a mitochondrial potassium channel opener. Mech Ageing Dev. 2001;122:1073–1086. doi: 10.1016/s0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 16.Hansford RG, Castro F. Effect of senescence on Ca2+-ion transport by heart mitochondria. Mech Ageing Dev. 1982;19:5–13. doi: 10.1016/0047-6374(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 17.Pepe S, et al. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 18.Juhaszova M, et al. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorn GW, 2nd, Maack C. SR and mitochondria: Calcium cross-talk between kissing cousins. J Mol Cell Cardiol. 2012;55:42–49. doi: 10.1016/j.yjmcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, et al. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Rourke B, Blatter LA. Mitochondrial Ca2+ uptake: tortoise or hare? J Mol Cell Cardiol. 2009;46:767–774. doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Covian R, Balaban RS. Cardiac Mitochondrial Matrix and Respiratory Complex Protein Phosphorylation. Am J Physiol Heart Circ Physiol. 2012;303:H940–H966. doi: 10.1152/ajpheart.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao RP, et al. Age-associated reductions in cardiac beta1- and beta2-adrenergic responses without changes in inhibitory G proteins or receptor kinases. J Clin Invest. 1998;101:1273–1282. doi: 10.1172/JCI1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sack MN. The role of SIRT3 in mitochondrial homeostasis and cardiac adaptation to hypertrophy and aging. J Mol Cell Cardiol. 2012;52:520–525. doi: 10.1016/j.yjmcc.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanza IR, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurd BJ. Deacetylation of PGC-1alpha by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab. 2011;36:589–597. doi: 10.1139/h11-070. [DOI] [PubMed] [Google Scholar]
- 27.Starnes JW, Rumsey WL. Cardiac energetics and performance of exercised and food-restricted rats during aging. Am J Physiol. 1988;254:H599–608. doi: 10.1152/ajpheart.1988.254.3.H599. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa T, et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 29.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 30.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74:2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Erreish GM, et al. Fatty acid oxidation by isolated perfused working hearts of aged rats. Am J Physiol. 1977;232:E258–262. doi: 10.1152/ajpendo.1977.232.3.E258. [DOI] [PubMed] [Google Scholar]
- 32.Finelli C, et al. Effect of age on phosphorylated compounds and mechanical activity of isolated rat heart: a 31P-NMR study. Cardiovasc Res. 1993;27:1978–1982. doi: 10.1093/cvr/27.11.1978. [DOI] [PubMed] [Google Scholar]
- 33.Atlante A, et al. The rate of ATP export in the extramitochondrial phase via the adenine nucleotide translocator changes in aging in mitochondria isolated from heart left ventricle of either normotensive or spontaneously hypertensive rats. Mech Ageing Dev. 2011;132:488–495. doi: 10.1016/j.mad.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Gold PH, et al. Effect of age on oxidative phosphorylation in the rat. J Gerontol. 1968;23:509–512. doi: 10.1093/geronj/23.4.509. [DOI] [PubMed] [Google Scholar]
- 35.Guarnieri C, et al. Age-related changes in cardiac mitochondrial energetics under the influence of calcium in rat. Cardioscience. 1993;4:117–120. [PubMed] [Google Scholar]
- 36.Kim JH, et al. Age-related changes in respiration coupled to phosphorylation. II. Cardiac mitochondria. Mech Ageing Dev. 1988;46:279–290. doi: 10.1016/0047-6374(88)90130-3. [DOI] [PubMed] [Google Scholar]
- 37.Paradies G, Ruggiero FM. Age-related changes in the activity of the pyruvate carrier and in the lipid composition in rat-heart mitochondria. Biochim Biophys Acta. 1990;1016:207–212. doi: 10.1016/0005-2728(90)90060-h. [DOI] [PubMed] [Google Scholar]
- 38.Zorov DB, et al. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83:213–225. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Tata V, et al. Changes of glycogen metabolism in phosphorylcreatine-depleted muscles taken from rats fed with beta-guanidine propionate. Arch Int Physiol Biochim. 1989;97:123–132. doi: 10.3109/13813458909075056. [DOI] [PubMed] [Google Scholar]
- 40.Miro O, et al. Aging is associated with increased lipid peroxidation in human hearts, but not with mitochondrial respiratory chain enzyme defects. Cardiovasc Res. 2000;47:624–631. doi: 10.1016/s0008-6363(00)00122-x. [DOI] [PubMed] [Google Scholar]
- 41.Marin-Garcia J, et al. Human mitochondrial function during cardiac growth and development. Mol Cell Biochem. 1998;179:21–26. doi: 10.1023/a:1006839831141. [DOI] [PubMed] [Google Scholar]
- 42.Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart--an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol. 1989;134:1167–1173. [PMC free article] [PubMed] [Google Scholar]
- 43.Barogi S, et al. Lack of major changes in ATPase activity in mitochondria from liver, heart, and skeletal muscle of rats upon ageing. Mech Ageing Dev. 1995;84:139–150. doi: 10.1016/0047-6374(95)01640-6. [DOI] [PubMed] [Google Scholar]
- 44.Capozza G, et al. Age related changes of the mitochondrial energy metabolism in rat liver and heart. Arch Gerontol Geriatr. 1994;19(Suppl 1):31–38. doi: 10.1016/s0167-4943(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 45.Brink TC, et al. Age-related transcriptional changes in gene expression in different organs of mice support the metabolic stability theory of aging. Biogerontology. 2009;10:549–564. doi: 10.1007/s10522-008-9197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancaster TL, et al. Quantitative Proteomic Analysis Reveals Novel Mitochondrial Targets of Estrogen Deficiency in the Aged Female Rat Heart. Physiol Genomics. 2012 doi: 10.1152/physiolgenomics.00184.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preston CC, et al. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev. 2008;129:304–312. doi: 10.1016/j.mad.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wenz T, et al. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Sahin E, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rera M, et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson R, Prolla T. PGC-1alpha in aging and anti-aging interventions. Biochim Biophys Acta. 2009;1790:1059–1066. doi: 10.1016/j.bbagen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Lluch G, et al. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phillips D, et al. Regulation of oxidative phosphorylation complex activity: effects of tissue-specific metabolic stress within an allometric series and acute changes in workload. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1034–1048. doi: 10.1152/ajpregu.00596.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoppel CL, et al. Dynamic organization of mitochondria in human heart and in myocardial disease. Int J Biochem Cell Biol. 2009;41:1949–1956. doi: 10.1016/j.biocel.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hofer T, et al. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev. 2009;130:297–307. doi: 10.1016/j.mad.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 57.Saks V, et al. Intracellular Energetic Units regulate metabolism in cardiac cells. J Mol Cell Cardiol. 2012;52:419–436. doi: 10.1016/j.yjmcc.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 58.Guzun R, et al. Regulation of respiration in muscle cells in vivo by VDAC through interaction with the cytoskeleton and MtCK within Mitochondrial Interactosome. Biochim Biophys Acta. 2012;1818:1545–1554. doi: 10.1016/j.bbamem.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 59.Spindler M, et al. Temporal fluctuations of myocardia high-energy phosphate metabolite with the cardiac cycle. Basic Res Cardiol. 2001;96:553–556. doi: 10.1007/s003950170006. [DOI] [PubMed] [Google Scholar]
- 60.Strumia E, et al. Creatine phosphate: pharmacological and clinical perspectives. Adv Ther. 2012;29:99–123. doi: 10.1007/s12325-011-0091-4. [DOI] [PubMed] [Google Scholar]
- 61.Lygate CA, et al. Cardiac phenotype of mitochondrial creatine kinase knockout mice is modified on a pure C57BL/6 genetic background. J Mol Cell Cardiol. 2009;46:93–99. doi: 10.1016/j.yjmcc.2008.09.710. [DOI] [PubMed] [Google Scholar]
- 62.Lygate CA, et al. Living without creatine: unchanged exercise capacity and response to chronic myocardial infarction in creatine-deficient mice. Circ Res. 2013;112:945–955. doi: 10.1161/CIRCRESAHA.112.300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nemutlu E, et al. Dynamic phosphometabolomic profiling of human tissues and transgenic models by 18O-assisted (3)(1)P NMR and mass spectrometry. Physiol Genomics. 2012;44:386–402. doi: 10.1152/physiolgenomics.00152.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Opie LH, editor. The heart: physiology and metabolism. Raven Press; 1991. [Google Scholar]
- 65.De Stefani D, et al. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2012;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palty R, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pizzo P, et al. Mitochondrial Ca(2)(+) homeostasis: mechanism, role, and tissue specificities. Pflugers Arch. 2012;464:3–17. doi: 10.1007/s00424-012-1122-y. [DOI] [PubMed] [Google Scholar]
- 69.Balaban RS. The role of Ca(2+) signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banienë R, Mildapienë V. Stimulation of ATP synthase by Ca2+ in heart mitochondria. Biologija. 2005;1:20–23. doi: 10.1049/ip-syb:20060009. [DOI] [PubMed] [Google Scholar]
- 71.Halestrap AP. The regulation of the oxidation of fatty acids and other substrates in rat heart mitochondria by changes in the matrix volume induced by osmotic strength, valinomycin and Ca2+ Biochem J. 1987;244:159–164. doi: 10.1042/bj2440159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yaniv Y, et al. Beat-to-beat Ca(2+)-dependent regulation of sinoatrial nodal pacemaker cell rate and rhythm. J Mol Cell Cardiol. 2011;51:902–905. doi: 10.1016/j.yjmcc.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008;103:279–288. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cortassa S, et al. A computational model integrating electrophysiology, contraction, and mitochondrial bioenergetics in the ventricular myocyte. Biophys J. 2006;91:1564–1589. doi: 10.1529/biophysj.105.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandes R, Bers DM. Simultaneous measurements of mitochondrial NADH and Ca(2+) during increased work in intact rat heart trabeculae. Biophys J. 2002;83:587–604. doi: 10.1016/S0006-3495(02)75194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kates AM, et al. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293–299. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 77.van der Meer RW, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J. 2008;29:1516–1522. doi: 10.1093/eurheartj/ehn207. [DOI] [PubMed] [Google Scholar]
- 78.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 79.Perseghin G, et al. Left ventricular function and energy metabolism in middle-aged men undergoing long-lasting sustained aerobic oxidative training. Heart. 2009;95:630–635. doi: 10.1136/hrt.2008.154716. [DOI] [PubMed] [Google Scholar]
- 80.Jacob MH, et al. Age-related effects of DHEA on peripheral markers of oxidative stress. Cell Biochem Funct. 2010;28:52–57. doi: 10.1002/cbf.1619. [DOI] [PubMed] [Google Scholar]
- 81.Jacob MH, et al. Redox imbalance influence in the myocardial Akt activation in aged rats treated with DHEA. Exp Gerontol. 2010;45:957–963. doi: 10.1016/j.exger.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 82.Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci U S A. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esposito LA, et al. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 1999;96:4820–4825. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jang YC, et al. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci. 2009;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen L, Knowlton AA. Mitochondria and heart failure: new insights into an energetic problem. Minerva Cardioangiol. 2010;58:213–229. [PMC free article] [PubMed] [Google Scholar]
- 86.Jeong EM, et al. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012;52:454–463. doi: 10.1016/j.yjmcc.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu X, et al. Measuring Local Gradients of Intra-Mitochondrial [Ca] in Cardiac Myocytes During SR Ca Release. Circ Res. 2012;112:424–431. doi: 10.1161/CIRCRESAHA.111.300501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pallavi R, et al. Insights into the beneficial effect of caloric/dietary restriction for a healthy and prolonged life. Front Physiol. 2012;3:318. doi: 10.3389/fphys.2012.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guiney H, Machado L. Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychon Bull Rev. 2012;20:73–86. doi: 10.3758/s13423-012-0345-4. [DOI] [PubMed] [Google Scholar]
- 91.Akki A, et al. Creatine kinase overexpression improves ATP kinetics and contractile function in postischemic myocardium. Am J Physiol Heart Circ Physiol. 2012;303:H844–852. doi: 10.1152/ajpheart.00268.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuznetsov AV, et al. Cytoskeleton and regulation of mitochondrial function: the role of beta-tubulin II. Front Physiol. 2013;4:82. doi: 10.3389/fphys.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grant JE, et al. Quantification of protein expression changes in the aging left ventricle of Rattus norvegicus. J Proteome Res. 2009;8:4252–4263. doi: 10.1021/pr900297f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang S, et al. Metabolomic profiling of adenylate kinase AK1−/− and AK2+/− transgnic mice: Effect of physical stress. Circulation. 2010;122:A20435. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.