Abstract
84–year-old man with rheumatoid arthritis (RA) treated with methotrexate, developed progressive confusion and cerebellar symptoms, and died approximately two months later. Neuropathological examination revealed progressive multifocal leukoencephalopathy (PML) involving the cerebellum and brainstem. The affected tissues displayed intense infiltrations by CD8+ T-cells and microglia. JC virus was localized in oligodendroglia and cerebellar granule cells. This case illustrates unusual localization of inflammatory PML in a patient with RA treated with methotrexate.
Keywords: Progressive Multifocal Leukoencephalopathy, Demyelination, Cerebellar syndrome, Methotrexate, Rheumatoid Arthritis
Introduction
Progressive multifocal leukoencephalopathy (PML) is a demyelinating, usually non-inflammatory disorder of the central nervous system caused by reactivation of a latent JC virus (JCV), in the setting of immunosuppression1–4. The most frequent underlying conditions are HIV/AIDS, myelo- and lymphoproliferative disorders, autoimmune and chronic granulomatous diseases, as well as the use of immunomodulatory medications1–4. Among autoimmune disorders, the most common is systemic lupus erythematosus5–7. PML as a complication of rheumatoid arthritis (RA) treated with immunosuppressive medication is rare8–19. We present a patient with rheumatoid arthritis treated with methotrexate who developed an uncommon form of inflammatory PML limited to the infratentorial compartment.
Clinical History
An 84-year-old man with approximately one year of subtle symptoms of cognitive decline was admitted to the hospital with worsening confusion, hallucinations and progressive incoordination with frequent falls. He had been taking methotrexate (20mg/week) for rheumatoid arthritis for one year, and continued until his demise. The patient had a past history of myocardial infarction, spontaneous deep vein thrombosis and pulmonary embolus. Examination revealed an afebrile, alert, cachectic man oriented to time and person but not to place. The patient displayed moderate paratonia, mild reduction of vibration sense in big toes, drifting of the left arm up and down when eyes were closed, dysdiadochokinesis and striking bilateral dysmetria in the arms and legs, left worse than right. He had an ataxic gait with marked truncal instability and inconsistent stimulus-sensitive myoclonus.
Laboratory investigations were negative for ANNA-1, ANNA-2 and Purkinje cell antibodies, as well as for Lyme disease and HIV. Levels of serum gamma globulins were normal. Cerebrospinal fluid (CSF) glucose, WBC and protein level were within normal limits. The CSF was negative for JC and BK viruses but was positive for 14-3-3 protein, raising the suspicion of Creutzfeldt-Jacob disease (CJD). Brain magnetic resonance imaging (MRI) revealed non-enhancing white matter hyperintensities in the left cerebellar hemisphere. A repeat MRI scan twelve days later revealed “progressive vasogenic edema” suggestive of an acute progressive demyelinating disease. A CT of the chest, abdomen, and pelvis was noncontributory. Due to his advanced age and the possibility of CJD, no further aggressive diagnostic procedure or treatment was undertaken. He continued to deteriorate and died at home two months after presentation.
Methods
Standard set of neuropathology sections from all brain areas as well as samples of grossly described abnormalities were removed for microscopic examination. The sections were processed to paraffin embedding and stained with hematoxylin and eosin, and in luxol fast blue with PAS methods. Selected sections were routinely immunostained for the following tissue antigens with commercially available primary antibodies (all from DAKO, Carpenteria, CA, USA): glial fibrillary acidic protein (GFAP, polyclonal, 1:3000 dilution), ferritin (polyclonal 1:500), P53 (clone DO-7, 1:50) and neurofilament (NF, monoclonal, 1:4000, clone 2F11). Monoclonal antibodies against SV-40 T antigen (Calbiochem, 1:400) were used for initial detection of the virus. For the identification of inflammatory cells, monoclonal antibodies against CD3, CD4, CD8, CD45 and CD68 (Novocastra, Newcastle-upon-Tyne, 1:50) were also applied. The Streptovidin/biotin detection system (Invitrogen, “Histostatin Plus”) was used for visualization of the immune reactions and followed by a light hematoxylin counter stain. Immunohistochemistry was performed using LabVision autostainer. Cellular localization of the virus was performed by double immunostains combining antibodies against JCV protein Agno 48–7120, T Ag or VP1 (Santa Cruz Biotechnology, Santa Cruz, CA) with cellular markers: CNPase C5922, 11-5B (oligodendroglia and myelin, Sigma), MAP-2 (neurons), and GFAP(astrocytes), as previously reported21.
Results
Due to the clinical suspicion of CJD, the autopsy was limited to the brain. The fresh brain weighed 1376 grams and was cut after two weeks of fixation (CJD was excluded after preliminary examination of multiple brain samples). The cerebral hemispheres showed only mild ventricular dilatation. The cerebellum displayed minimal atrophy of the superior vermis and large geographic areas of poorly demarcated, greyish discoloration of the white matter, more in the left hemisphere.
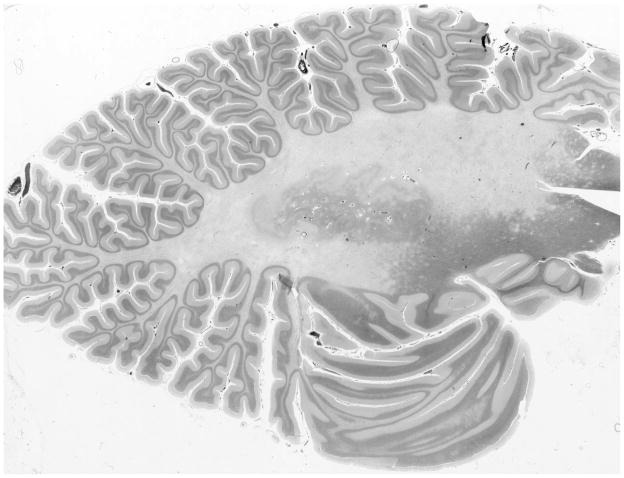
Microscopic examination revealed extensive loss of myelin involving the white matter of both cerebellar hemispheres, slightly more on the left side (Fig 1). Demyelination was accompanied by a significant dropout of axons, numerous axonal retraction balls, accumulation of ferritin-positive microglia and CD68+ foamy macrophages, and a moderate to severe degree of astrocytosis. These changes were most expressed in the centers of the lesions and gradually blended with relatively normal white matter with numerous small satellite foci of early myelin loss. The periphery of the demyelinated areas displayed many oligodendroglial cells with enlarged nuclei filled by homogeneous, intensely purple intranuclear viral inclusions that were weakly immunoreactive for P53 and strongly positive for JCV antigens. Scattered vessels at the edge of the lesions were surrounded by mild CD8+ inflammatory infiltrations, with few CD3+ and CD4+ T-cells, and no CD20+ B-cells. The population of Purkinje cells and granule cells, as well as neurons in the dentate nucleus appeared normal. Cerebellar cortex contained scattered axonal torpedoes of Purkinje cells. The overall pathological changes were consisted with chronic PML lesions.
Fig. 1.
Section of cerebellar hemisphere displaying marked demyelination. Luxol Fast Blue combined with PAS.
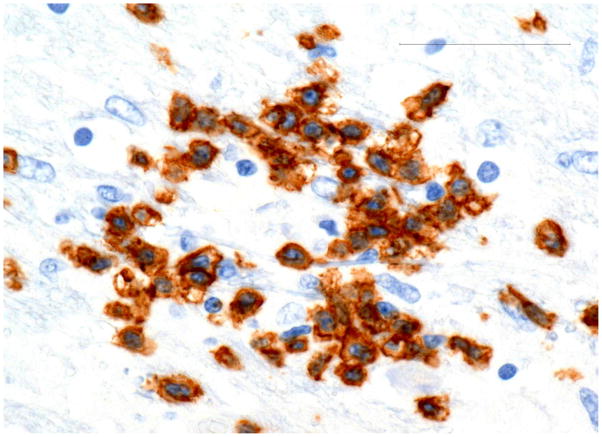
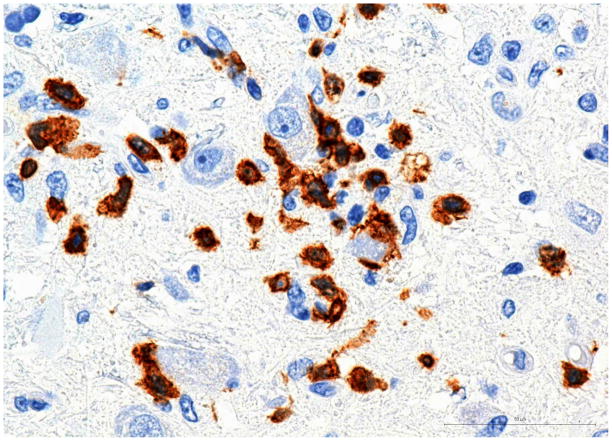
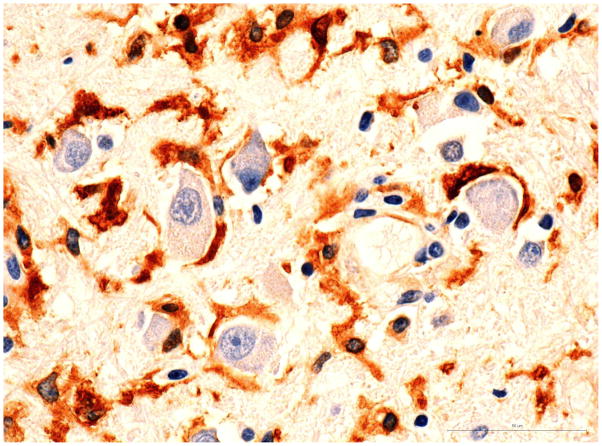
The brainstem showed multiple small patches of demyelination with centrifugal distribution of oligodendroglial intranuclear inclusions (Fig 2A and B) and numerous foci of perivascular infiltrations by CD8+ T-cells, and less abundant CD3+ and CD4+ T-cells (Fig 3A and 3B). CD20+ B-cells were entirely absent. The perivascular myelin was not affected. Clusters of normal-appearing neurons outside of areas of demyelination were surrounded by CD8+ T-cells and microglia (Fig 4A and 4B). In addition, the parenchyma of the pons was sprinkled with small collections or individual CD8+ cells without relation to the vessels or neurons. Very careful screening of sections of the brainstem revealed no direct contact of CD8+ T-cells with the oligodendroglial cells containing intranuclear inclusions. CD68+ macrophages and ferritin-positive microglia were massively increased in foci of demyelination and, to a lesser extend, diffusely throughout the entire brainstem. Scattered, well-formed microglial nodules were present as well. Double immunostains of the sections removed from the pons and cerebellum demonstrated viral infections limited to the oligodendroglial cells and few cerebellar granule cells. Very thorough screening of multiple slides revealed only two microscopic foci of early demyelination present in the midbrain and in the deep white matter of the frontal lobe. The meninges showed mild lymphocytic infiltrates slightly more prominent at the base of the brain.
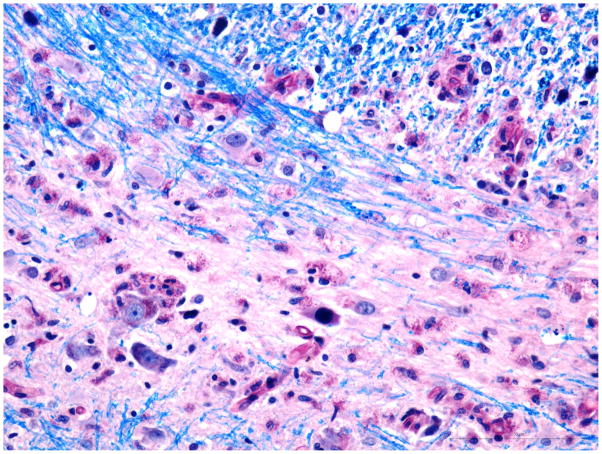
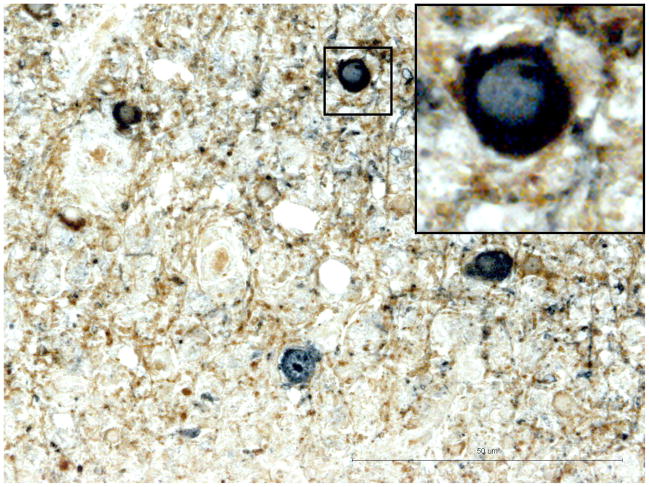
Fig. 2. Pons.
A) Intranuclear inclusion in oligodendroglial cells in a small focus of demyelination. Luxol Fast Blue combined with PAS. Additional inclusion at the edge of demyelination in upper part of the photograph. Bar 50 μm.
B) Double immunostaining for CNPase (Brown) and JCV Agno protein (blue). JCV antigen co-localizes with reaction for CNPase indicates infection of oligodendrocytes. Bar 50μ. Window – details of JCV infected oligodendroglial cell.
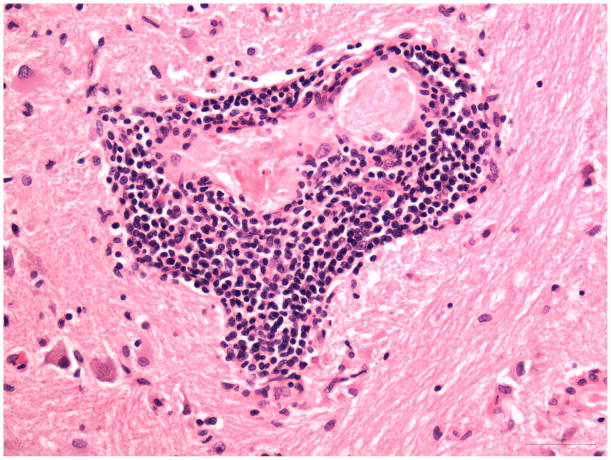
Fig. 3. Pons.
A) Perivascular mononuclear inflammatory infiltrate (H&E).
B) Capillary surrounded by CD8+ T-cells. Bar 50μm
Fig. 4. Pons.
A) Group of normal appearing neurons surrounded by CD8+ T-cells. Please note absence of intranuclear inclusions in any cell type in this microscopic field.
B) Morphologically normal neurons surrounded by ferritin positive microglia.
Discussion
The present case is remarkable for the association of PML with RA, intense inflammation in the progressing lesions in the brainstem, and selective involvement of subtentorial compartments. There have only been a few case reports of PML in patients with RA. Amend et al22 did not find a single case of RA with PML in studies of 138,469 patients with autoimmune disease. However, in a review of 57 HIV-negative PML patients from the Mayo Clinic, Aksamit reported approximately 5% with RA, without details about the topography of lesions, pathology, or specific treatment23. Until 2008, only seven patients with PML associated with RA were described, all with the typical clinical and pathological presentation8–14. Subsequently, eight additional PML cases were found in the group of RA patients treated with humanized monoclonal antibodies, including five patients taking methotrexate15–19. All the RA patients developed typical cerebral lesions and only two (treated with Rituximab), displayed inflammatory changes with the presence of T- and B-cells15,18.
Classical PML lesions in immunocompromised patients show minimal or no inflammation1–3. However, intense inflammation develops in PML cases with immune reconstitution inflammatory syndrome (IRIS), following initiation of HAART in the setting of HIV/AIDS, as well as in HIV-negative patients treated with monoclonal antibodies24–26. Clinically, focal inflammation has been reported in about 15% of PML cases using gadolinium-enhanced MRI2,27. Although PML is often defined as a non-inflammatory demyelinating disease, some studies suggest that the frequency of inflammation in non-AIDS patients is probably underestimated28, and it appears to be more common in the individuals with minimal immunosuppression or without immunodeficiency. Several reports indicate that inflammatory PML is associated with better prognosis14,28–31.
In the inflammatory form of PML, virus specific CD8+ T-cells concentrate in largest number at the borders of progressing demyelination, known to harbor the greatest load of the virus30. Furthermore, CD8+ T-cells can be localized in direct contact with the inclusion bearing oligodendroglia30. Although the inflammatory cells were concentrated at the progressive edge of the glial infection, direct contact of T-cells and oligodendroglia could not be demonstrated in this patient. This phenomenon could be explained by immune response mounted against the viral antigen released from disintegrated oligodendroglial cells, rather than against intact virus- bearing oligodendroglia. Double immunostains of cerebellum and brainstem for JCV-specific antigens and cell markers (MAP-2, oligodendroglial CNPase or GFAP), revealed viral presence only in the oligodendroglia. With the exception of a few granule cells, there was no sign of invasion of other neurons or astrocytes. Massive neuronal infection has been demonstrated in several entities associated with JCV, such as granule cell neuronopathy21,32,33 and fulminant encephalopathy with productive infection of cortical pyramidal neurons34. The striking CD8 and microglial perineuronal infiltrates in the pons, may suggest greater sensitivity of the hosts immunological system to recognize early JCV neuronal invasion, than the ability of the immunohistochemical methods to detect the virus at the light microscopic level. PML tends to involve subcortical white matter, mostly in the frontal and parieto-occipital areas1–3. Predominantly infratentorial localization of PML in non-AIDS patients is approximately ten times less common than the cerebral form35. Since 1958, when PML was first described36, close to 30 case reports of infratentorial PML have been listed in Medline, however, none in RA patients.
In view of the increasing array of new and powerful immunomodulators in the treatment of autoimmune diseases, this case highlights the importance of considering PML in the differential diagnosis for acute or subacute onset of cerebellar or brainstem symptoms in patients with RA on immunosuppressant therapy. Although the frequency of PML with methotrexate use is very low, given the almost uniformly fatal consequences of this infection, patients should be warned of the risk of this complication.
Acknowledgments
Supported in part by NIH grants R56 NS 041198, R01 NS 047029, R01074995 and K24 NS 060950 to IJK.
We would like to thank Ms. Bruna Capretta for her help in preparation of the manuscript and Cyprian Estrada for his assistance with photographic documentation.
Abbreviations used
- CJD
Creutzfeld-Jacob Disease
- JCV
JC Virus
- GFAP
Glial Fibrillary Acidic Protein
- MAP-2
Microtubule Associated Protein
- IRIS
Immune Reconstitution Inflammatory Syndrome
- HAART
Highly Active Antiretroviral Therapy
References
- 1.Koralnik IJ. Progressive multifocal leukoencephalopathy revisited: has the disease outgrown its name? Ann Neurol. 2006;60:162–173. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- 2.Weber T. Progressive multifocal leukoencephalopathy. Neurol Clin. 2008;26:833–54. doi: 10.1016/j.ncl.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–37. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng PM, Turnbull BR, Cook SF, Davidson JE, Kurth T, Seeder JD. Characteristics and antecedents of progressive multifocal leukoencephalopathy in an insured population. Neurology. 2006;67(5):884–6. doi: 10.1212/01.wnl.0000233918.21986.9c. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese LH, Molloy ES, Huang D, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases. Evolving clinical and pathologic patterns of disease. Arthritis & Rheumatism. 2007;56:2116–2128. doi: 10.1002/art.22657. [DOI] [PubMed] [Google Scholar]
- 6.Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy. A national estimate of frequency in systemic lupus erythematosus and other rheumatic diseases. Arthritis & Rheumatism. 2009;60:3761–3765. doi: 10.1002/art.24966. [DOI] [PubMed] [Google Scholar]
- 7.Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimm Rev. 2008;8:144–146. doi: 10.1016/j.autrev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Sponzilli EE, Smith JK, Malamud N, McCulloch JR. Progressive multifocal leukoencephalopathy: a complication of immunosuppressive treatment. Neurology. 1975;25:664–668. doi: 10.1212/wnl.25.7.664. [DOI] [PubMed] [Google Scholar]
- 9.Riskind PN, Richardson PE. Case 20-1995 - A 66-year-old man with a history of rheumatoid arthritis treated with adrenocorticosteroids, with the development of aphasia and right-sided weakness. N Engl J Med. 1995;332:1773–80. doi: 10.1056/NEJM199506293322609. [DOI] [PubMed] [Google Scholar]
- 10.Rankin E, Scaravilli F. Progressive multifocal leukoencephalopathy in a patient with rheumatoid arthritis and polymyositis. J Rheumatol. 1995;22:777–9. [PubMed] [Google Scholar]
- 11.Yamamoto M, Takahashi H, Wakasugi H, et al. Leukoencephalopathy during administration of etanercept for refractory rheumatoid arthritis. Mod Rheumatol. 2007;17:72–74. doi: 10.1007/s10165-006-0530-2. [DOI] [PubMed] [Google Scholar]
- 12.Nived O, Bengtsson AA, Jönsen A, Sturfelt G. Progressive multifocal leukoencephalopathy – the importance of early diagnosis illustrated in four cases. Lupus. 2008;17:1036–1041. doi: 10.1177/0961203308089445. [DOI] [PubMed] [Google Scholar]
- 13.Rahmlow M, Shuster EA, Dominik J, et al. Leflunomide-associated progressive multifocal leukoencephalopathy. Arch Neurol. 2008;65:1538–1539. doi: 10.1001/archneur.65.11.1538. [DOI] [PubMed] [Google Scholar]
- 14.Marzocchetti A, Wuthrich C, Tan CS, et al. Rearrangement of the JC virus regulatory region sequence in the bone marrow of a patient with rheumatoid arthritis and progressive multifocal leukoencephalopathy. J NeuroVirology. 2008;14:455–458. doi: 10.1080/13550280802356837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischmann RM. Progressive multifocal leukoencephalopathy following rituximab treatment in a patient with rheumatoid arthritis. Arthritis & Rheumatism. 2009;60:3225–3228. doi: 10.1002/art.24906. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Okomoto Y, Inoue H, Usui T. Leukoecephalopathy with Cognotove Impairment following Tocilizumab for Threatment of Rheumatoid Arthritis (RA) Inter Med. 2009;48:1307–1309. doi: 10.2169/internalmedicine.48.1926. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis & Rheumatism. 2010;62:3191–3195. doi: 10.1002/art.27687. [DOI] [PubMed] [Google Scholar]
- 18.Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156–1164. doi: 10.1001/archneurol.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drugs Events and Reports project. Blood. 2009;113:4834–40. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang X, Wuthrich C, Gordon J, et al. JC virus encephalopathy is associated with a novel agnoprotein-deletion JCV variant. PLoS One. 2012;7(4):e35793. doi: 10.1371/journal.pone.0035793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wüthrich C, Cheng YM, Joseph JT, et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2009;66(1):15–25. doi: 10.1097/NEN.0b013e3181912570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amend KI, Turnbull B, Foskett N, et al. Incidence of progressive leukoencephalopathy in patients without HIV. Neurol. 2010;75:1326–1332. doi: 10.1212/WNL.0b013e3181f73600. [DOI] [PubMed] [Google Scholar]
- 23.Aksamit AJ. Review of progressive multifocal leukoencephalopathy and natalizumab. The Neurologist. 2006;12:293–298. doi: 10.1097/01.nrl.0000250948.04681.96. [DOI] [PubMed] [Google Scholar]
- 24.Nuttall JJC, Wilmshurst JM, Ndondo AP, et al. Progressive multifocal leukoencephalopathy after initiation of highly active antiretroviral therapy in a child with advanced human immunodeficiency virus infection: a case of immune reconstitution inflammatory syndrome. Pediatr Inf Dis J. 2004;23:683–5. doi: 10.1097/01.inf.0000130954.41818.07. [DOI] [PubMed] [Google Scholar]
- 25.Travis J, Varma A, duPlessis D, Turnbull I, Vilar FJ. Immune reconstitution associated with progressive multifocal leukoencephalopathy in human immunodeficiency virus: a case discussion and review of the literature. Neurologist. 2008;14:321–6. doi: 10.1097/NRL.0b013e31816e2f13. [DOI] [PubMed] [Google Scholar]
- 26.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 27.Woo HH, Rezai AR, Knopp EA, Weiner HL, Miller DC, Kelly PJ. Contrast-enhancing progressive multifocal leukoencephalopathy: radiological and pathological correlations: case report. Neurosurgery. 199(39):1031–4. doi: 10.1097/00006123-199611000-00029. [DOI] [PubMed] [Google Scholar]
- 28.Huang D, Cossoy M, Li M, et al. Inflammatory progressive multifocal leukoencephalopathy in human immunodeficiency virus-negative patients. Ann Neurol. 2007;62:34–39. doi: 10.1002/ana.21085. [DOI] [PubMed] [Google Scholar]
- 29.Tan IL, Koralnik IJ, Rumbaugh JA, Burger PC, King-Rennie A, McArthur JC, et al. Progressive multifocal leukoencephalopathy in a patient without immunodeficiency. Neurology. 2011;77:297–299. doi: 10.1212/WNL.0b013e318225ab3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wüthrich C, Kesari S, Kim W, et al. Characterization of lymphocytic infiltrates in progressive multifocal leukoencephalopathy: Co-localization of CD8+ T cells with JCV-infected glial cells. J Neurovirol. 2006;12:116–28. doi: 10.1080/13550280600716604. [DOI] [PubMed] [Google Scholar]
- 31.Du Pasquier RA, Koralnik IJ. Inflammatory reaction in progressive multifocal leukoencephalopathy: Harmful or beneficial. J Neurovirol. 2003;9(suppl 1):25–31. doi: 10.1080/13550280390195315. [DOI] [PubMed] [Google Scholar]
- 32.Du Pasquier RA, Corey S, Margolin DH, et al. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003;61(6):734–5. doi: 10.1212/01.wnl.0000081306.86961.33. [DOI] [PubMed] [Google Scholar]
- 33.Koralnik IJ, Wüthrich C, Dang X, et al. JC virus granule cell neuronopathy: A novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol. 2005;57:576–80. doi: 10.1002/ana.20431. [DOI] [PubMed] [Google Scholar]
- 34.Wüthrich C, Dang X, Westmoreland S, et al. Fulminant JC Virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol. 2009;65:742–8. doi: 10.1002/ana.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gagne F, Bouchard JP, Bernier JP. Progressive multifocal leucoencephalopathy. Observation with predominant pontocerebellar lesions and association with congenital immune deficiency. Acta Neuropathol. 1977 May 16;38:167–9. doi: 10.1007/BF00688566. [DOI] [PubMed] [Google Scholar]
- 36.Astrom KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy. A hitherto unrecognized complication of chronic lymphatic leukemia and Hodgkin’s disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]