Abstract
Mice with a mutation in the Clock gene (ClockΔ19) have been identified as a model of mania, however, the mechanisms that underlie this phenotype, and the changes in the brain that are necessary for lithium’s effectiveness on these mice remain unclear. Here we find that Cholecystokinin(Cck) is a direct transcriptional target of CLOCK and levels of Cck are reduced in the ventral tegmental area (VTA) of ClockΔ19 mice. Selective knock-down of Cck expression via RNA interference (RNAi) in the VTA of wild type mice produces a manic-like phenotype. Moreover, chronic treatment with lithium restores Cck expression to near wild type and this increase is necessary for the therapeutic actions of lithium. The decrease in Cck expression in the ClockΔ19 mice appears to be due to a lack of interaction with the histone methyltransferase, MLL1, resulting in decreased histone H3K4me3 and gene transcription, an effect reversed by lithium. Human postmortem tissue from bipolar subjects reveals a similar increase in Cck expression in the VTA with mood stabilizer treatment. These studies identify a key role for Cck in the development and treatment of mania, and describe some of the molecular mechanisms by which lithium may act as an effective anti-manic agent.
Keywords: Bipolar disorder, dopamine, lithium, chromatin structure, gene expression
Introduction
Bipolar disorder (BPD) is a chronic psychiatric disease that afflicts approximates 1–3% of the United States population.1 The underlying cause of BPD is unknown, though there is a growing body of evidence linking disruptions in circadian rhythms with the disease.2 Within the suprachiasmatic nucleus (SCN) and other regions, circadian rhythms are controlled by the molecular clockwork, which is comprised of a series of autoregulatory transcriptional-translational feedback loops. The transcription factors CLOCK and BMAL1 heterodimerize and activate transcription of target genes, including the Period (Per) and Cryptochrome (Cry) genes which act to inhibit the activity of the CLOCK/BMAL1 complex.3 Recent human genetic studies have linked elements of the molecular clockwork to BPD. Polymorphisms in CLOCK and other circadian genes are associated with various aspects of bipolar disorder.4–12 In addition, rhythm disruptions and sleep disturbances are common in BPD and often precipitate manic or depressive episodes.13, 14
Mice bearing a dominant negative mutation (Clock ∆19) in the Clock gene15 have a behavioral profile which is very similar to human mania.16, 17 These mice exhibit hyperactivity, decreased anxiety-related and depression-related behavior, and increased preference for rewarding stimuli.16, 17 Furthermore, the majority of these behavioral abnormalities can be reversed with chronic lithium treatment.17 Previous studies from our group have identified an important role for the VTA in the development of this manic-like phenotype. When CLOCK levels are decreased specifically in the VTA of wild type (WT) animals, behaviors similar to Clock∆19mice, including hyperactivity and decreased anxiety are induced.18 Conversely, when a functional CLOCK protein is expressed only in the VTA of Clock∆19 mice, locomotor activity and anxiety-related behavior are restored to wild type levels.17 Interestingly, the Clock∆19 mice also have increased firing and bursting of VTA dopamine neurons which is reversed with chronic lithium treatment.19
Microarray analysis of VTA tissue from Clock∆19 mice and WT littermates revealed altered transcription of many genes involved in dopaminergic transmission.16 One of the genes identified as significantly down regulated was the neuropeptide transmitter, cholecystokinin (CCK).16 The sulphatedcarboxy terminal octapeptide, CCK-8S, is the most commonly expressed form in the brain, with larger forms expressed in the gut. The primary action of CCK in the brain is thought to be mediated through the CCKB receptor, which has been shown in cultured striatal neurons to increase intracellular calcium levels.20 Within the VTA and the substantia nigra, CCK is highly co-localized with dopaminergic neurons that project to the nucleus accumbens (NAc), with 40–80% of the cells co-expressing dopamine and CCK.21, 22 At VTA dopaminergic terminals, CCK is co-released with dopamine, specifically upon burst firing.23 CCK acts as a negative modulator of dopaminergic transmission in vivo, as infusions of CCK-8S into the NAc inhibit K+-stimulated dopamine release and reduce extracellular dopamine concentrations.24 Behavioral studies using CCK agonists and antagonists find that increased CCK results in increased anxiety and depression-like behavior while CCK receptor blockade is anxiolytic and antidepressant.25 Thus, it is possible that the decreased CCK levels in the VTA of the Clock∆19 mice are responsible for their overall manic-like phenotype. Here we wanted to determine the role of CCK in the development of manic-like symptoms in the Clock∆19 mutant mice, as well as the reversal of these phenotypes with lithium treatment. Moreover, we wanted to know the mechanism by which CLOCK regulates CCK expression.
Materials and Methods
Animals
Clock ∆19 mutant mice were created by N-ethyl-N-nitrosurea mutagenesis and produce a dominant-negative CLOCK protein defective in transcriptional activation activity as described.26 For all experiments using Clock∆19 mutants, 8 to 16 week old adult male mutant (Clock∆19; Mut) and wild-type (WT) littermate controls on a mixed BALBc; C57BL/6 background were used. Mice were group housed in sets of 2–4 per cage on a 12:12 h light/dark cycle (lights on 6:00 a.m., lights off at 6:00 p.m) with food and water provided ad libitum. All mouse experiments were performed in compliance with National Institute of Health guidelines and approved by the Institutional Animal Care and Use Committees of UT Southwestern Medical Center. All behavioral and molecular assays were performed between ZT 7–11.
Lithium Administration
Lithium treated mice received 600 mg/l of LiCl in drinking water for 10 days prior to behavioral testing, and throughout the course of the testing. This administration results in a stable serum concentration of lithium in the low therapeutic range for human patients (0.41±0.06 mmol/l), with little to no adverse health consequences.17
Chromatin Immunoprecipitation (ChIP)
ChIP assays were performed according to methods described previously.27, 28 Additional details are in the Supplemental Material.
Behavioral Assays
The locomotor response to novelty, Elevated Plus Maze, Dark/Light test and Forced Swim test all utilized standard protocols and were performed as described previously.18 Additional details are in the Supplemental Material.
Quantitative PCR
cDNA or purified genomic DNA was mixed with buffers, primers, SYBR green, and hot start Taq polymerase in a master mix prepared by a manufacturer (Applied Biosystems, Foster City, CA). Using a Real-Time PCR machine (7500 Real Time PCR machine, Applied Biosystems) PCR reactions were run followed by a dissociation reaction to determine specificity of the amplified product. The amount of gene expression was quantified using the ∆∆Ct method as previously described.29
Reporter and Expression Plasmids
The wild-type Cck luciferase reporter was described previously.27 Additional details are in the Supplemental Material.
Luciferase Assays
Cell culture and performance of the luciferase assay were carried out as described previously.27 Additional details are in the Supplemental Material.
Construction of AAV- Cck-shRNA and Virus Purification
A small hairpin RNA (shRNA) directed against Cck was designed using previously published criteria.18 For the CckshRNA, a 15 base pair sequence in the coding region of the Cck gene (5’-CTTGAGCGGTTCGG-3’) was identified as a target region. A previously published scrambled RNA sequence (5’-CGGAATTTAGTTACGGGGATCCAC-3’) that has no known sequence similarities was used as a negative control. An antisense sequence of selected region and a miR23 loop of 10 nucleotides (CTTCCTGTCA) were added to the 5’ end of these sequences. The annealed oligonucleotides were cloned into an adeno-associated virus (AAV) plasmid expressing enhanced green fluorescent protein (Stratagene, La Jolla, CA). Viral production was carried out using a helper-free triple transfection method. Additional details are in the Supplemental Material.
Laser Capture Microdissection
Laser capture microdissection (LCM) to assess levels of in vivo Cck knockdown were performed as in previous studies.18 Additional details are in the Supplemental Material.
Stereotaxic Surgery
Surgery was performed as described previously.18 Additional details are in the Supplemental Material.
Immunohistochemistry and validations of injections and infections
Validation of injections was performed as published previously.18 Additional details are in the Supplemental Material.
Subject selection and tissue acquisition
Human brain tissue from depression and control cases was obtained from the Dallas Brain Collection.30 The tissue was collected only after acquiring consent from the next of kin along with permission to review medical records and to conduct a telephone interview with a primary caregiver. All clinical information on each case was evaluated by at least three research psychiatrists and diagnoses made using DSM IV criteria. Blood screens for drugs of abuse, alcohol and prescription medications were conducted on each case. Cases were excluded when there was a known history of neurological disorders or of an axis I psychiatric condition other than major depression. The method of collection and storage of human brain tissue is approved by the Institutional Review Board of the University of Texas Southwestern Medical Center.
Human tissue preparation
In each case, cerebral hemispheres were cut coronally into 1–1.5 cm blocks and the midbrain was cut into 1–1.5 cm blocks in a plane perpendicular to its long axis as previously described31. Additional details are in the Supplemental Material.
Statistical analysis
For the comparison of two groups, two-tailed unpaired Student’s t tests were used,and one-way ANOVAs followed by Bonferroni post tests for multiple comparisons were performed for the comparison of three or more groups. When more than one factor was examined simultaneously, two-way ANOVAs were performed followed by Bonferroni post hoc tests. * p < 0.05, ** p < 0.01, *** p < 0.001.In all figures error bars show S.E.M.
Results
CCK is a direct CLOCK target gene in the VTA
To determine if the previously observed decrease in Cckm RNA levels was a direct or indirect result of the Clock∆19 mutation, we measured CLOCK binding at the Cck promoter. The Cck promoter contains an E-box element (CANNTG) that is part of a 110 bp proximal promoter region that is over 80% conserved from mouse to human.32 In addition to the E-box, there is also a GC-rich region, CRE/TRE site, and putative TATA box (Figure 1a). To assess potential CLOCK binding at the Cck promoter, chromatin immunoprecipitation (ChIP) assays were carried out on VTA-containing tissue from WT andClock∆19 littermates. CLOCK was enriched at the Cck promoter above background in both genotypes with no significant difference between the two (Figure 1b). To further elucidate the role of CLOCK in the transcriptional regulation of the Cck gene, luciferase assays were performed in PC12 cells using Cck-luc reporter plasmids containing approximately 300 bp of the Cck proximal promoter region with either an intact or a mutated E-box site. When this reporter plasmid was co-transfected into cells along with CLOCK and BMAL1 expression constructs, a significant increase in Cck-luc reporter activity was observed when the E-box was intact (Figure 1c). When the E-box was mutated, the ability of transfected CLOCK/BMAL1 to induce Cck-luc reporter activity was abolished (Figure 1c). These results demonstrate that CLOCK acts as a direct, positive regulator of the Cck gene in an E-box dependent manner. Furthermore, the decrease in Cck gene expression observed in the Clock∆19 mice is not due to a loss of CLOCK binding at the promoter, but rather a loss of function of the CLOCK protein as a transcriptional regulator.
Figure 1. Cck is a CLOCK target gene and is regulated by the E-box element.
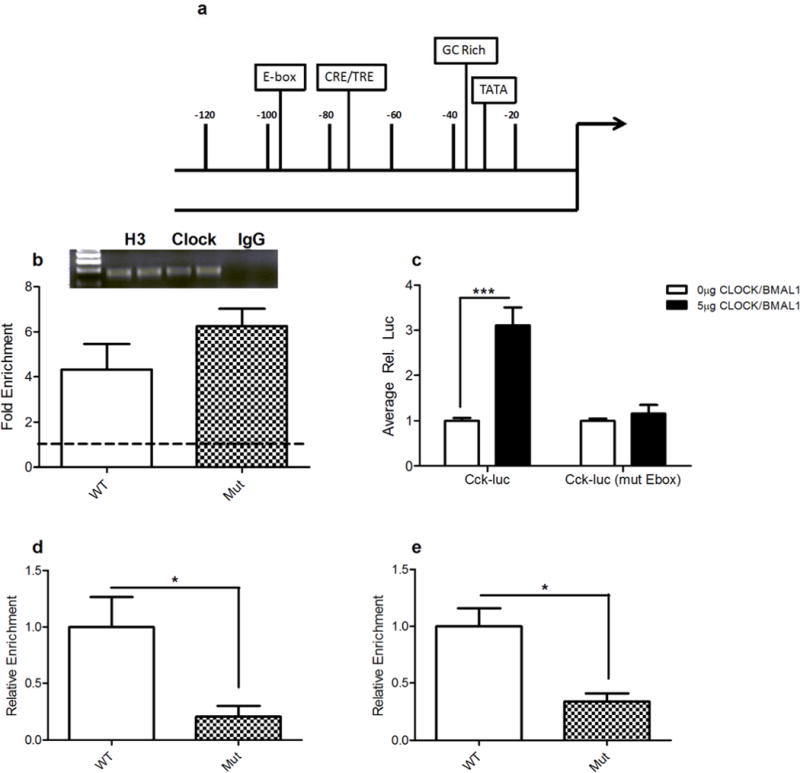
(a) Diagram of the Cck promoter. The region containing the proximal promoter, including the E-Box, was amplified by quantitative qPCR after ChIP assays were performed. Additional important transcription factor binding sites and regulatory regions are highlighted. (b) Fold enrichment at proximal promoter region following ChIP with a CLOCK specific antibody comparing Clock∆19 mutants and wild-type (WT) littermate controls. One sample t-tests revealed that CLOCK is significantly enriched at the Cck promoter regions (~4–6 fold) above background in both WT (t4=2.920, p = 0.0432) and Clock∆19 (Mut) (t3=6.754. p = 0.0066) mice. Inset are representative agarose gels of q-PCR products from ChIP assays showing AcH3 positive control IPs, IgG negative control IPs, and CLOCK IPs; (n=4–6 per genotype). (c) Relative luciferase activity of PC12 cells transfected with a Cck-luc construct (318 bp) containing either an intact or mutated E-box element. Co-transfection of 5μg of CLOCK and BMAL1 expression constructs resulted in a significant increase in Cck-luc activity (t14=5.314, p =0.0001) when the E-box element was intact. Induction of Cck-luc activity was not detected when the E-box element was mutated; (n=5–8 per group). d) Relative enrichment of MLL1 at the Cck promoter in Clock∆19 mice and WT littermates was assessed by performing ChIP assays with an MLL1-specific antibody. A significant decrease in MLL1 at the Cck promoter was observed in Clock∆19 mice (t8=2.827, p = 0.0223); (n= 5 per group). e) Relative enrichment of H3K4me3 at the Cck promoter in Clock∆19 mice and WT littermates was assessed by performing ChIP assays with an H3K4me3-specific antibody. A significant decrease in MLL1 at the Cck promoter was observed in Clock∆19 mice (t8=2.377, p = 0.0415); (n= 5 per group). In all panels error bars show S.E.M.
Cck expression in the ClockΔ19 mice is reduced due to a lack of MLL1 binding at the promoter
Previous in vitrostudies have found that CLOCK activates transcription via interactions with histone methyltransferase mixed lineage leukemia 1(MLL1), which then causes tri-methylation of histone H3 at lysine 4 (H3K4me3), and allows for transcriptional activation of CLOCK target genes.33 To determine if this is the case in vivo at the Cck promoter, ChIP assays were carried out using an antibody against MLL1. We found a significant decrease in both MLL1 and H3K4me3 levels at the Cck promoter in the midbrain of the Clock∆19 mice compared to WT animals (Figure 1d, e). Thus, Cck levels are likely reduced in the ClockΔ19 mice due to the inability of MLL1 to bind CLOCKΔ19 protein, leading to a decrease in H3K4me3.
Knock-down of CCK in the VTA of WT animals results in a manic-like phenotype
To determine if a loss of Cck expression in the VTA is sufficient to induce manic-like behaviors, an AAV-shRNA directed specifically against Cck was generated (AAV-Cck-shRNA). An AAV-scrambled sequence (AAV-Scr) that does not match any known gene was used as a control.18 Stereotaxic injection of AAV-Cck-shRNA into the VTA of C57BL6J mice resulted in a significant (~6-fold) decrease in Cck mRNA levels after 3 weeks incubation compared to AAV-Scr controls (Supplemental Figure 1). Following the confirmation of knock-down, a separate cohort of mice injected with AAV-Cck-shRNA or AAV-Scr were then subjected to a battery of behavioral tests. When exposed to a novel environment, mice injected with AAV-Cck-shRNA in the VTA were significantly hyperactive compared to those injected with AAV-Scr over the course of two hours (Figure 2a). Mice were then subjected to two anxiety-related behavioral tests: the elevated plus maze and light/dark box. AAV-Cck-shRNA injected mice spent significantly more time in the open arms of the elevated plus maze and in the light side of the light/dark box, suggesting that they have decreased anxiety (Figure 2b, c). We then determined the effects of Cck knock-down on depression-related behavior by subjecting animals to the Porsolt forced swim test. Decreased immobility time and an increase in latency to first bout of immobility was observed in mice injected with AAV-Cck-shRNA in the VTA compared to AAV-Scr control mice (Figure 2d, 2e). Together these results indicate that a knock-down of Cck in the VTA decreases anxiety and depression-related behavior.
Figure 2. Knockdown ofCckin the VTA of wild type animals results in a manic-like phenotype.
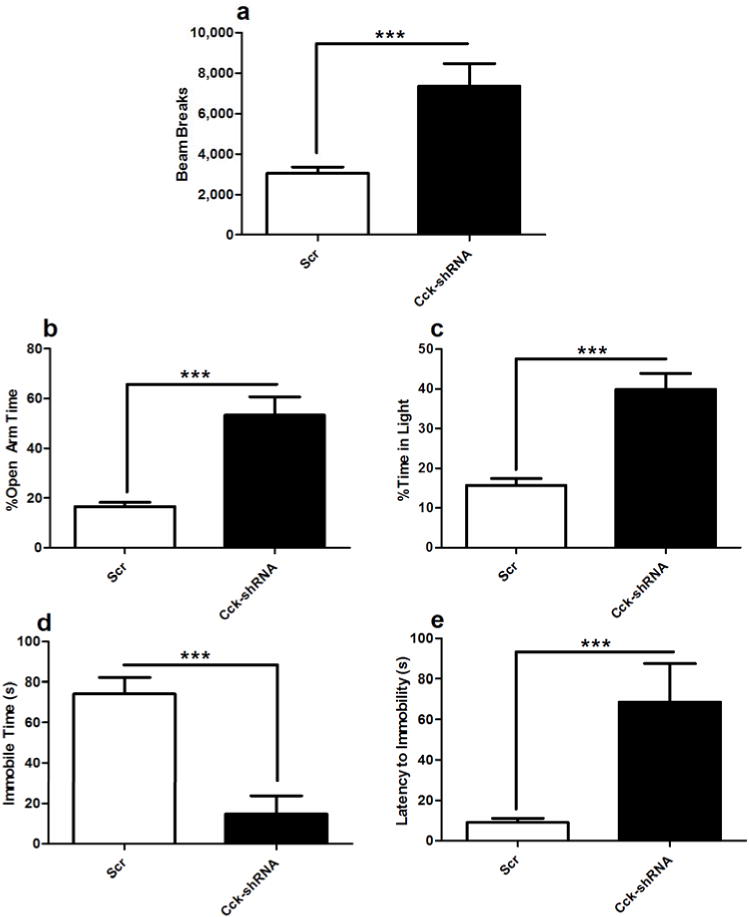
(a) Locomotor activity of AAV-Cck-shRNA and AAV-Scr injected C57BL/6J animals was assessed for two hours, 3 weeks after stereotaxic injection. AAV-Cck-shRNA injected animals are hyperactive when compared to AAV-Scr controls (t18= 3.756, p < 0.01). (b–c) Anxiety-related behavior was assessed in AAV-Cck-shRNA and AAV-Scr injected animals using (b) the elevated plus maze (EPM)and (c) dark/light box. AAV-Cck-shRNA injected animals are significantly less anxious than AAV-Scr controls as seen by (b) an increase in open arm time on the EPM (t16=4.90, p < 0.001) and (c) time spent in the light side of the dark/light box (t20=5.528, p < 0.0001). (d-e) Depression-related behavior was assessed in AAV-Cck-shRNA and AAV-Scr injected mice using the forced swim test. AAV-Cck-shRNA injected animals display less depression-related behavior than AAV-Scr controls as evidenced by (d) a decrease in total immobile time (t32=4.935, p < 0.0001) and (e) and increased latency to first bout of immobility (t14=3.126, p < 0.01); (n=15–20 per group). In all panels error bars show S.E.M.
Lithium selectively restores Cck expression levels in Clock∆19 mice
Since we were able to determine that a decrease in Cck is sufficient to induce manic-like behaviors, and lithium treatment rescues Clock∆19 mutant behavior17, we investigated whether Cck levels were altered by lithium treatment. mRNA was isolated from the VTA of Clock∆19 and littermate control mice receiving either chronic lithium (600 mg/L in drinking water) or normal drinking water, and qPCR was performed to assess Cck mRNA levels. Consistent with our previous microarray results16, Cck expression was decreased in Clock∆19 mice relative to WT littermates (Figure 3a). When Clock∆19 mice were administered lithium, Cck mRNA levels were restored to near WT levels (Figure 3a). Interestingly, lithium treatment had no detectable effect on WT Cck mRNA levels (Figure 3a) suggesting a specific effect in mice with a manic-like phenotype.
Figure 3. Effect of the Clock∆19 mutation and lithium treatment on Cck expression and H3k4me3 and MLL1 binding at the Cck promoter.
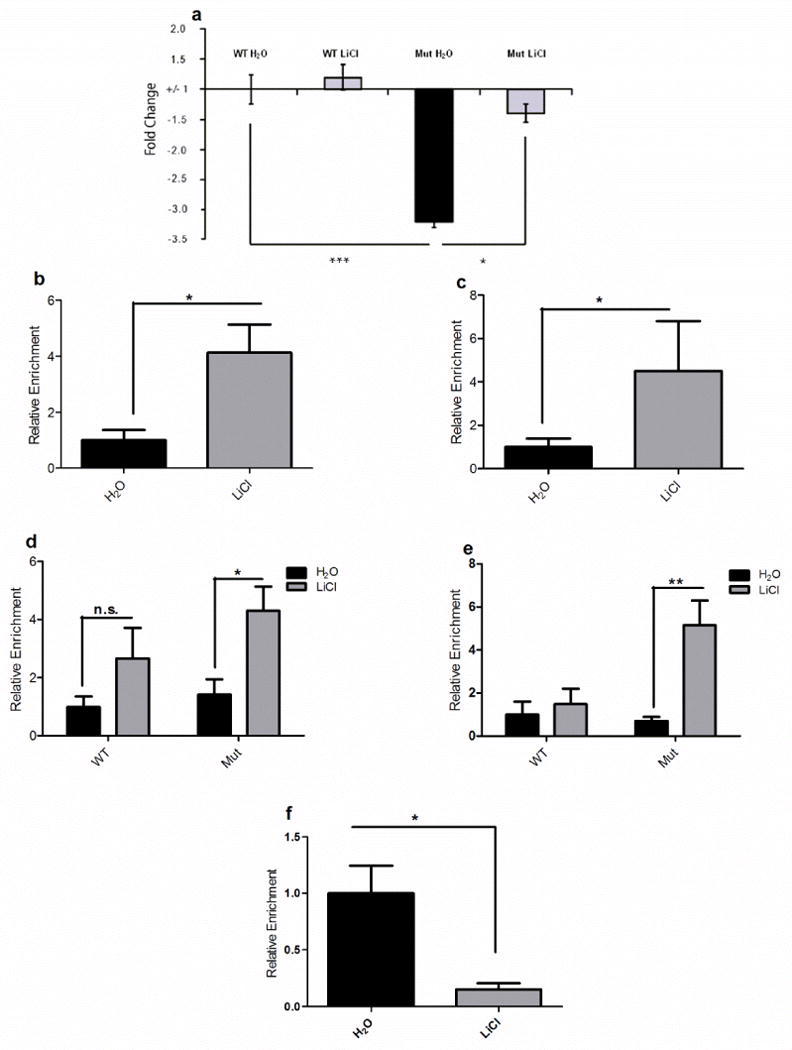
(a) Relative mRNA levels of Cck in Clock∆19 mice and WT littermates receiving 10 days of water or lithium treatment (600 mg/L). Levels were normalized to an internal control, Gapdh. Analysis by two-way ANOVA revealed a significant decrease in Cck mRNA levels in untreated Clock∆19 mice compared to WT animals (main effect of genotype F1,20=16.99; p < 0.001). Bonferroni post hoc tests revealed that lithium treatment caused a significant increase in Cck expression in Clock∆19 mice relative to water alone, restoring it to near WT levels (t=2.600, p < 0.05). Lithium treatment had no detectable effect on WT Cck expression. In all panels error bars show S.E.M. (b) Relative levels of histone H3K4me3 at the Cck promoter in Clock∆19 mice following lithium treatment were assessed by performing ChIP assays with a H3K4me3 specific antibody. Lithium treatment caused a significant increase in levels like H3K4me3 at the Cck promoter (t9 = 2.690, p = 0.0248); (n=5–6 per group).(c) Relative enrichment of MLL1 at the Cck promoter in Clock∆19 mice following lithium treatment was assessed by performing ChIP assays with an MLL1-specific antibody. There was no significant change in MLL1 levels in lithium-treated Clock∆19 mice (t8=1.865, p = 0.0992); (n=5–6 per group). (d) Relative levels of acetylated histone H3 (AcH3) and acetylated histone H4 (AcH4) at the Cck promoter in Clock∆19 (Mut) mice and WT littermates following lithium (LiCl) treatment were assessed by performing ChIP assays with a AcH3 and AcH4 specific antibodies. Analysis by two-way ANOVA revealed a main effect of lithium treatment on AcH3 (d,F1,20=9.4, p=0.0061) and AcH4(e,F1,17=11.32, p=0.0037) levels. Bonferroni post-tests revealed a significant increase in levels of AcH3 (t = 2.744, p < 0.05) and AcH4 (t=4.198, p < 0.01) at the Cck promoter in Clock∆19 mice following lithium treatment, while lithium had no detectable effect on WT animals; (n= 5–6 per group). (f) Relative enrichment of CLOCK at the Cck promoter in Clock∆19 mice following lithium treatment was assessed by performing ChIP assays with a CLOCK-specific antibody. A significant decrease in CLOCK binding at the Cck promoter was observed in lithium-treated Clock∆19 mice (t9=3.137,p < 0.05); (n=5–6 per group). In all panels error bars show S.E.M.
Regulation of chromatin structure at the Cck promoter by lithium treatment
We wanted to begin to understand the molecular mechanisms by which lithium treatment regulates the Cck gene. ChIP assays were carried out using an antibody against H3K4me3 in Clock∆19 mice receiving either water or lithium. Lithium treatment in Clock∆19 mice led to an increase of H3K4me3 at the Cck promoter (Figure 3b) and a trend toward increased levels of MLL1 at the Cck promoter in Clock∆19 mice, though this did not reach significance (Figure 3c). This suggests that other histone methyltranferases are likely involved. Lithium treatment had no significant effect on H3K4me3 or MLL1 levels at the Cck promoter in WT animals (Supplemental figure 2). We then wanted to determine whether lithium could regulate histone acetylation at the Cck promoter. ChIP assays were carried out using antibodies against AcH3 and AcH4 in Clock∆19 mice and WT controls receiving either water or lithium (Figure 3d, e). Lithium treatment caused a selective increase in levels of both AcH3 and AcH4 at the Cck promoter in Clock∆19 mice, while levels of AcH3 and AcH4 are not significantly affected in WT mice (Figure 3d, e). To determine how lithium treatment results in recruitment of chromatin remodeling enzymes to the Cck promoter, we assessed whether there were any changes in mutant CLOCK protein binding to the Cck promoter. A significant reduction in CLOCKΔ19 protein at the Cck promoter was observed in Clock∆19 mice receiving lithium treatment, suggesting that lithium causes another DNA-binding protein to compete with CLOCKΔ19 and this results in recruitment of chromatin remodeling enzymes that rescue expression (Figure 3f).
Cck levels are altered in human subjects with bipolar disorder following treatment
Because a restoration of Cck levels in the VTA seems to correlate with the therapeutic actions of lithium in the Clock∆19 mice, we wanted to determine if Cck levels were also regulated by drugs that treat BPD in human postmortem tissue. RNA was isolated from the VTA of healthy control and BPD subjects, which were categorized as either on or off medication at the time of death. Information on individual human postmortem samples is detailed in Tables S2, S3, and S4. qPCR was performed to assess Cck mRNA levels. While there was no detectable difference in Cck levels in the VTA between control subjects and BPD patients not receiving medication (Figure 4), interestingly, BPD patients receiving pharmacological treatment had a significant increase in Cck levels in the VTA (Figure 4).
Figure 4. Regulation of Cck levels in the VTA of BPD patients by mood stabilizers.
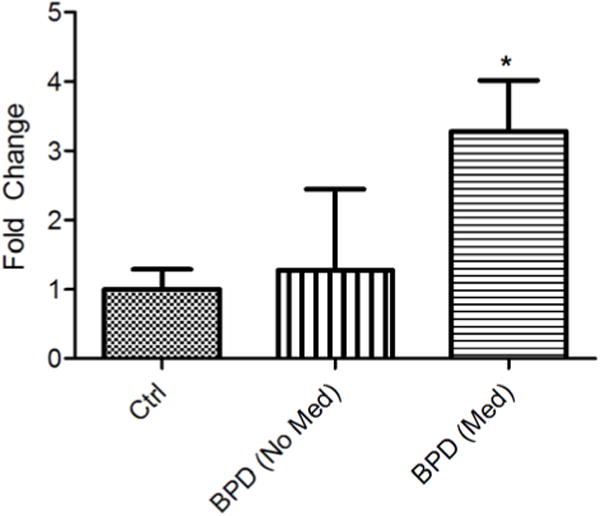
Relative mRNA levels of Cck in the VTA of BPD patients,either receiving (Med) or not receiving medication (No Med), and normal controls. Levels were normalized to an internal control,Gapdh. Analysis by one-way ANOVA a significant difference in means (F=4.5254, p = 0.0266). Bonferroni post hoc tests revealed that BPD patients receiving medication had significantly higher levels of Cck mRNA in the VTA than control patients (t = 2.903, p < 0.05). Cck levels in BPD patients not receiving medication did not differ significantly from either groups; (n = 9 for control, n= 3 for BPD (No Med), n = 8 for BPD (Med). n all panels error bars show S.E.M.
Increased Cck levels in the VTA are required for the therapeutic effects of lithium in Clock∆19 mice
We injected AAV-Cck-shRNA or AAV-Scr into the VTA of Clock∆19 mice. The mice then received chronic lithium treatment or normal drinking water to determine if lithium could still rescue Clock∆19 anxiety and depression related behavior when it was unable to restore proper Cck levels. Clock∆19 mice injected with AAV-Cck-shRNA were hyperactive compared to AAV-Scr controls (Figure 5a). Consistent with previous findings17, this lithium treatment paradigm had no detectable effect on locomotor activity in animals injected with either virus (Figure 5a). The mice were then subjected to the elevated plus maze and dark/light box to measure anxiety-related behavior. Importantly, lithium had no effect on the Clock∆19 mice injected with the AAV-Cck-shRNA in the VTA while it was able to normalize the anxiety-related behavior in the mice injected with the AAV-Scr as measured by the percent open arm time of the EPM (Figure 5b) and the percent time in the light in the dark/light box (Figure 5c). Mice were then subjected to the forced swim test to measure depression-related behavior. Similar to what was observed in the anxiety-related behavioral tests, lithium was successful in reversing the time spent immobile and the latency to immobility in the Clock∆19 mice injected with AAV-Scr but it had no effect on the mice injected with AAV-Cck-shRNA in the VTA (Figure 5d, e). These results demonstrate that an increase in Cck levels in the VTA is necessary for lithium to have therapeutic effects.
Figure 5. Increased Cck in the VTA is required for lithium’s therapeutic actions in the Clock∆19 mice.
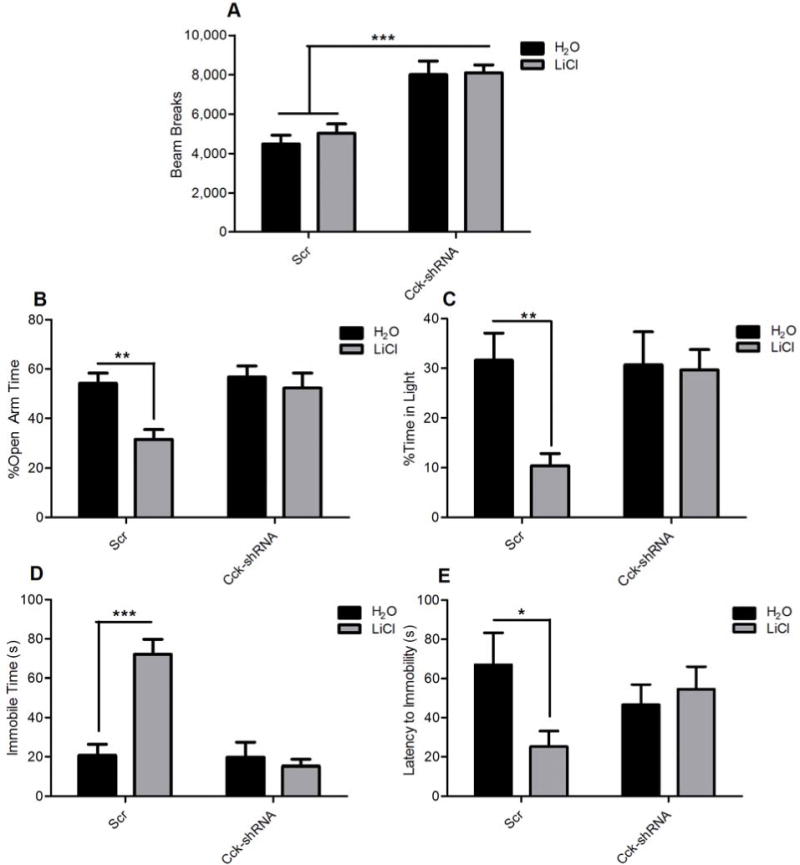
(a) Locomotor activity was measured in AAV-Cck-shRNA or AAV-Scr injected Clock∆19 mice for two hours following 10 days of lithium administration. Analysis by two-way ANOVA revealed a main effect of viral injection on locomotor activity (F1,53=36.34, p < 0.0001). Bonferroni post hoc tests revealed that there was no effect of any treatment on locomotor response to novelty. (b–c) Anxiety-related behavior was assessed in AAV-Cck-shRNA and AAV-Scr injected Clock∆19 mice following lithium treatment using the EPM (b) and dark/light box (c). Analysis by two-way ANOVA followed by Bonferonni post hoc tests revealed that lithium treatment caused a significant increase in anxiety-related behavior in AAV-Scr injected animals as seen by (b) a decrease in time spent in the open arms of the elevated plus maze (t=3.051, p < 0.01 and (c) a decrease in time spent in the light side of the dark/light box (t=3.343, p < 0.01). Lithium treatment had no detectable effect on AAV-Cck-shRNA injected animals. (d–e) Depression-related behavior following lithium treatment was assessed in AAV-Cck-shRNA and AAV-Scr injected Clock∆19 animals using the forced swim test. Analysis by two-way ANOVA followed by Bonferonni post-tests revealed that lithium treatment causes a significant increase in depression-related behavior in AAV-Scr injected animals as seen by (d) an increase in total immobile time (t=5.986, p < 0.0001), and (e) a decrease in latency to first bout of immobility (t=2.513, p < 0.05). Lithium treatment had no detectable effect on AAV-Cck-shRNA injected animals; (n=12–20 per group). In all panels error bars show S.E.M.
Discussion
Our results identify the peptide neurotransmitter Cck as a novel and direct transcriptional target of the CLOCK protein in the VTA. CLOCK acts as a positive regulator of the Cck gene via its interactions at the E-box element of the Cck promoter. It is likely that CLOCK also contributes to the strong circadian rhythm in Cck expression.34, 35 As expected by the sequence of the CLOCK∆19 protein, this protein can still bind to the Cck promoter but cannot activate transcription. We find that levels of H3K4me3, a histone modification associated with transcriptional activation, are decreased at the Cck promoter when compared to WT mice. This is likely due to the inability of the mutant form of CLOCK to associate with MLL1, which was also found to be decreased at the Cck promoter in Clock∆19 mice relative to WT littermates. Though this lack of association was thought to underlie the dominant negative function of CLOCK∆19 based on findings in cell culture at other gene promoters, we provide the first evidence to suggest that this is indeed the case in the brain, and that this interaction is important for regulation at the Cck promoter.
Furthermore, we have determined that a knock-down of Cck in the VTA produces a manic-like phenotype similar to the Clock∆19 mice. AAV-mediated knock-down of Cck specifically in the VTA results in hyperactivity, decreased anxiety-related behavior, and a decrease in depression-related behavior. These results correspond well to previous studies with systemic administration of CCKB receptor antagonists, which are anxiolytic and antidepressant.36 37 Since these changes in behavior are very similar to those seen in the Clock∆19 mice, the “manic-like” phenotype of these mice may ultimately be due to a decrease in Cck levels in the VTA. Interestingly, the behavioral effects of Cck knock-down differ somewhat from those observed in mice that have AAV-mediated knock-down of CLOCK specifically in the VTA, which results in hyperactivity and decreased anxiety, but also results in an increase in depression related behavior when compared to control animals.18 Given the nature of viral mediated gene transfer and the fact that this was a gene knock-down experiment rather than an overexpression of the CLOCK∆19 protein, there are a number of possibilities that might explain this discrepancy and it will be interesting to explore these in future studies.
Chronic lithium administration results in a restoration of Cck mRNA to near WT levels in the VTA of the Clock∆19 mice with no detectable effect on WT Cck levels. This finding makes Cck a particularly promising target as lithium treatment selectively rescues the Clock∆19 mouse behavior while having little effect on WT animals.17 This is similar to what is observed in the human population, as lithium is effective in the treatment of bipolar mania, but has little or variable effect on healthy controls.38, 39 Therefore, the selective regulation of Cck by lithium may be of particular therapeutic relevance.
Indeed, we find an increase in Cck levelsin human BPD patients receiving pharmacological treatments. Unlike Clock∆19 mice which have a large decrease in Cck in the VTA there was no detectable difference in Cck expression between normal controls and BPD patients not receiving medications. However, there were very few patients in this group with a great deal of variability in Cck levels. It is also possible that a decrease inCck may be only evident in a manic state, which is more similar to the phenotype of the Clock∆19 mice, and the mood state of the BPD patients at the time of tissue collection was not known.
Lithium treatment leads to a restoration of H3K4me3 levels at the Cck promoter in Clock ∆19 mice without affecting WT animals. This rescue of H3K4me3 is not accompanied by a significant increase in MLL1 at the Cck promoter, indicating that factors other than MLL1 likely contribute to lithium’s effects on H3K4me3 in the Clock∆19 mice. In addition to an increase in H3K4me3, an increase in AcH3 and AcH4 was observed at the Cck promoter in the Clock∆19 mice following lithium treatment. It has recently become clear that H3K4me3 and histone acetylation are linked, and there is a large amount of crosstalk between proteins that deposit these modifications on histone tails.40–43 Interestingly there is no decrease in levels of AcH3 or AcH4 at the Cck promoter in untreated Clock∆19 mice, despite the decrease in H3K4me3. This is probably due to the fact that CLOCK∆19, though unable to recruit MLL1, still retains its histone acetyltransferase (HAT) activity.33, 44,45 Interestingly, this increase in histone acetylation and methylation specifically occurs in the context of a disease state (i.e. a manic-like phenotype) and does not occur in wild type animals. This might explain why previous animal studies have not identified lithium as a drug that alters chromatin structure. The increase in AcH3 and AcH4 following lithium treatment is likely due to the recruitment of HATs rather than an inhibition of HDAC proteins which is an activity that may underlie the efficacy of valproic acid as a mood stabilizing drug.46 Lithium treatment leads to a decrease in CLOCKΔ19 levels at the Cck promoter in the Clock∆19 mice, suggesting that another protein or transcriptional-activation complex is competing with CLOCK∆19 for Cck promoter occupancy. Future studies will determine what this factor is and how lithium acts to bring this factor to the Cck promoter.
The increase in Cck produced by lithium is necessary for its therapeutic effects in the Clock∆19 mice, as Clock∆19 mice injected with AAV-Cck-shRNA into the VTA no longer respond to lithium treatment. Interestingly, Cck knock-down in wild type mice results in a hyperactive locomotor response to novelty, and the Clock∆19 mice are hyperactive, however, lithium treatment has no effect on locomotor activity in the Clock∆19 mice either with or without injection of the AAV-Cck-shRNA. These results clearly separate hyper-locomotor activity from measures of anxiety and depression-related behavior, giving us further confidence in the interpretation of these measures. Moreover, it suggests a separate mechanism by which these processes are regulated.
In conclusion, we have identified a novel CLOCK target gene, Cck, which likely contributes to the phenotype of the ClockΔ19 mice since a decrease in Cck expression in the VTA results in a manic-like phenotype. The decrease in Cck transcription in the Clock∆19 mice is likely due to the inability of CLOCK∆19 to interact with the histone methyltransferase, MLL1, which results in a decrease of H3K4me3 at the Cck promoter. In addition, we have determined that an increase in Cck mRNA levels in the VTA is necessary for lithium’s therapeutic actions in the Clock∆19 mice. Lithium regulates the Cck gene via an increase in H3K4me3, AcH3, and AcH4 at the Cck promoter in the Clock ∆19mice. The precise mechanism by which these histone modificationsare increased at the Cck promoter following lithium treatment will be the subject of future studies. The hope is that these studies will help identify more selective therapeutic targets for the development of novel mood stabilizing medications which may be more effective with fewer side effects than current treatments.
Supplementary Material
Acknowledgments
We are grateful to Dr. Joe Takahashi for the ClockΔ19 mice. We thank Elizabeth Gordon and Ariel Ketcherside for assistance with mouse husbandry and genotyping, Dr. Shari Birnbaum and Ami Petterson for assistance with behavioral testing, and Dr. Shibani Mukherjee for assistance with AAV construction and design. We also thank Dr. Syann Lee and Dr. Joel Elmquist for assistance with laser capture equipment. We also thank Dr. R. Jude Samulski and the UNC Gene Therapy Vector Core for assistance with AAV preparation. This study was funded by The McKnight Endowment Fund for Neuroscience, The Brain & Behavior Research Foundation (NARSAD), and the NIMH (MH082876).
Footnotes
The authors declare no conflict of interest.
References
- 1.Kupfer DJ, Angst J, Berk M, Dickerson F, Frangou S, Frank E, et al. Advances in bipolar disorder: selected sessions from the 2011 International Conference on Bipolar Disorder. Ann N Y Acad Sci. 2011;1242(1):1–25. doi: 10.1111/j.1749-6632.2011.06336.x. [DOI] [PubMed] [Google Scholar]
- 2.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114(2):222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–277. doi: 10.1093/hmg/ddl207. Spec No 2. [DOI] [PubMed] [Google Scholar]
- 4.Lamont EW, Coutu DL, Cermakian N, Boivin DB. Circadian rhythms and clock genes in psychotic disorders. Isr J Psychiatry Relat Sci. 2010;47(1):27–35. [PubMed] [Google Scholar]
- 5.Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, et al. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(5):631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- 6.Serretti A, Benedetti F, Mandelli L, Lorenzi C, Pirovano A, Colombo C, et al. Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2003;121B(1):35–38. doi: 10.1002/ajmg.b.20053. [DOI] [PubMed] [Google Scholar]
- 7.Soria V, Martinez-Amoros E, Escaramis G, Valero J, Perez-Egea R, Garcia C, et al. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology. 2010;35(6):1279–1289. doi: 10.1038/npp.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ, et al. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5(2):150–157. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 9.Mansour HA, Talkowski ME, Wood J, Chowdari KV, McClain L, Prasad K, et al. Association study of 21 circadian genes with bipolar I disorder, schizoaffective disorder, and schizophrenia. Bipolar Disord. 2009;11(7):701–710. doi: 10.1111/j.1399-5618.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(3):234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjoholm LK, Backlund L, Cheteh EH, Ek IR, Frisen L, Schalling M, et al. CRY2 is associated with rapid cycling in bipolar disorder patients. PLoS One. 2010;5(9):e12632. doi: 10.1371/journal.pone.0012632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci. 2007;9(3):333–342. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey AG. Sleep and circadian functioning: critical mechanisms in the mood disorders? Annu Rev Clin Psychol. 2011;7:297–319. doi: 10.1146/annurev-clinpsy-032210-104550. [DOI] [PubMed] [Google Scholar]
- 15.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264(5159):719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102(26):9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104(15):6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the Ventral Tegmental Area Through RNA Interference Results in a Mixed State of Mania and Depression-Like Behavior. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(7):1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyoshi R, Kito S, Nomoto T. Cholecystokinin increases intracellular Ca2+ concentration in cultured striatal neurons. Neuropeptides. 1991;18(3):115–119. doi: 10.1016/0143-4179(91)90102-o. [DOI] [PubMed] [Google Scholar]
- 21.Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature. 1980;285(5765):476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- 22.Lanca AJ, De Cabo C, Arifuzzaman AI, Vaccarino FJ. Cholecystokinergic innervation of nucleus accumbens subregions. Peptides. 1998;19(5):859–868. doi: 10.1016/s0196-9781(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 23.Ghijsen WE, Leenders AG, Wiegant VM. Regulation of cholecystokinin release from central nerve terminals. Peptides. 2001;22(8):1213–1221. doi: 10.1016/s0196-9781(01)00444-2. [DOI] [PubMed] [Google Scholar]
- 24.Voigt MM, Wang RY. In vivo release of dopamine in the nucleus accumbens of the rat: modulation by cholecystokinin. Brain Res. 1984;296(1):189–193. doi: 10.1016/0006-8993(84)90531-6. [DOI] [PubMed] [Google Scholar]
- 25.Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31(4):736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 26.King DP, Vitaterna MH, Chang AM, Dove WF, Pinto LH, Turek FW, et al. The Mouse Clock Mutation Behaves as an Antimorph and Maps Within the W(19H) Deletion, Distal of Kit. Genetics. 1997;146(3):1049–1060. doi: 10.1093/genetics/146.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enwright JF, 3rd, Wald M, Paddock M, Hoffman E, Arey R, Edwards S, et al. DeltaFosB indirectly regulates Cck promoter activity. Brain Res. 2010;1329:10–20. doi: 10.1016/j.brainres.2010.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24(24):5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9(4):519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 30.Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123(1):1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghose S, Crook JM, Bartus CL, Sherman TG, Herman MM, Hyde TM, et al. Metabotropic glutamate receptor 2 and 3 gene expression in the human prefrontal cortex and mesencephalon in schizophrenia. Int J Neurosci. 2008;118(11):1609–1627. doi: 10.1080/00207450802330702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen TV. Cholecystokinin gene transcription: promoter elements, transcription factors and signaling pathways. Peptides. 2001;22(8):1201–1211. doi: 10.1016/s0196-9781(01)00443-0. [DOI] [PubMed] [Google Scholar]
- 33.Katada S, Sassone-Corsi P. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol. 2010;17(12):1414–1421. doi: 10.1038/nsmb.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber M, Lauterburg T, Tobler I, Burgunder JM. Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neuroscience letters. 2004;358(1):17–20. doi: 10.1016/j.neulet.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 35.Schade R, Vick K, Ott T, Sohr R, Pfister C, Bellach J, et al. Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats--influence on dopaminergic stimulation. Chronobiol Int. 1995;12(2):87–99. doi: 10.3109/07420529509064504. [DOI] [PubMed] [Google Scholar]
- 36.Hernando F, Fuentes JA, Roques BP, Ruiz-Gayo M. The CCKB receptor antagonist, L-365,260, elicits antidepressant-type effects in the forced-swim test in mice. Eur J Pharmacol. 1994;261(3):257–263. doi: 10.1016/0014-2999(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 37.Hughes J, Boden P, Costall B, Domeney A, Kelly E, Horwell DC, et al. Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc Natl Acad Sci U S A. 1990;87(17):6728–6732. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach: Part I: Clinical efficacy in bipolar disorder. CNS Drugs. 2009;23(3):225–240. doi: 10.2165/00023210-200923030-00004. [DOI] [PubMed] [Google Scholar]
- 39.Malhi GS, Adams D, Berk M. Is lithium in a class of its own? A brief profile of its clinical use. Aust N Z J Psychiatry. 2009;43(12):1096–1104. doi: 10.3109/00048670903279937. [DOI] [PubMed] [Google Scholar]
- 40.Crump NT, Hazzalin CA, Bowers EM, Alani RM, Cole PA, Mahadevan LC. Dynamic acetylation of all lysine-4 trimethylated histone H3 is evolutionarily conserved and mediated by p300/CBP. Proc Natl Acad Sci U S A. 2011;108(19):7814–7819. doi: 10.1073/pnas.1100099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guillemette B, Drogaris P, Lin HH, Armstrong H, Hiragami-Hamada K, Imhof A, et al. H3 lysine 4 is acetylated at active gene promoters and is regulated by H3 lysine 4 methylation. PLoS Genet. 2011;7(3):e1001354. doi: 10.1371/journal.pgen.1001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6(11):838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 43.Nightingale KP, Gendreizig S, White DA, Bradbury C, Hollfelder F, Turner BM. Cross-talk between histone modifications in response to histone deacetylase inhibitors: MLL4 links histone H3 acetylation and histone H3K4 methylation. J Biol Chem. 2007;282(7):4408–4416. doi: 10.1074/jbc.M606773200. [DOI] [PubMed] [Google Scholar]
- 44.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125(3):497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 45.Zhao WN, Malinin N, Yang FC, Staknis D, Gekakis N, Maier B, et al. CIPC is a mammalian circadian clock protein without invertebrate homologues. Nat Cell Biol. 2007;9(3):268–275. doi: 10.1038/ncb1539. [DOI] [PubMed] [Google Scholar]
- 46.Moretti M, Valvassori SS, Varela RB, Ferreira CL, Rochi N, Benedet J, et al. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav Pharmacol. 2011;22(8):766–772. doi: 10.1097/FBP.0b013e32834d0f1b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


