Abstract
BACKGROUND
In the National Lung Screening Trial (NLST), screening with low-dose computed tomography (CT) resulted in a 20% reduction in lung-cancer mortality among participants between the ages of 55 and 74 years with a minimum of 30 pack-years of smoking and no more than 15 years since quitting. It is not known whether the benefits and potential harms of such screening vary according to lung-cancer risk.
METHODS
We assessed the variation in efficacy, the number of false positive results, and the number of lung-cancer deaths prevented among 26,604 participants in the NLST who underwent low-dose CT screening, as compared with the 26,554 participants who underwent chest radiography, according to the quintile of 5-year risk of lung-cancer death (ranging from 0.15 to 0.55% in the lowest-risk group [quintile 1] to more than 2.00% in the highest-risk group [quintile 5]).
RESULTS
The number of lung-cancer deaths per 10,000 person-years that were prevented in the CT-screening group, as compared with the radiography group, increased according to risk quintile (0.2 in quintile 1, 3.5 in quintile 2, 5.1 in quintile 3, 11.0 in quintile 4, and 12.0 in quintile 5; P = 0.01 for trend). Across risk quintiles, there were significant decreasing trends in the number of participants with false positive results per screening-prevented lung-cancer death (1648 in quintile 1, 181 in quintile 2, 147 in quintile 3, 64 in quintile 4, and 65 in quintile 5). The 60% of participants at highest risk for lung-cancer death (quintiles 3 through 5) accounted for 88% of the screening-prevented lung-cancer deaths and for 64% of participants with false positive results. The 20% of participants at lowest risk (quintile 1) accounted for only 1% of prevented lung-cancer deaths.
CONCLUSIONS
Screening with low-dose CT prevented the greatest number of deaths from lung cancer among participants who were at highest risk and prevented very few deaths among those at lowest risk. These findings provide empirical support for risk-based targeting of smokers for such screening. (Funded by the National Cancer Institute.)
Lung cancer is the most common cause of cancer-related death in the United States, accounting for 28% and 26% of all cancer deaths among men and women, respectively.1 Recent results from the National Lung Screening Trial (NLST), which showed a 20% reduction in lung-cancer mortality with low-dose computed tomography (CT) screening, as compared with chest radiography, highlighted the opportunity to reduce the burden of death from lung cancer.2 With 94 million current and former smokers in the United States,3 deciding which smokers to target for low-dose CT screening remains an important public health challenge, given the potential costs and harms of such screening.
Although it is generally agreed that screening should be limited to high-risk persons for whom the potential benefits of low-dose CT screening would outweigh its potential harms, there is uncertainty as to how a high-risk target population should be defined. A number of professional societies have endorsed the use of the NLST inclusion criteria (smokers between the ages of 55 and 74 years who have smoked a minimum of 30 pack-years and quit for no more than 15 years) as minimum4-6 or sufficient7,8 criteria for consideration of lung-cancer screening. However, several researchers have proposed that a more refined risk assessment, which would account for additional risk information not considered in the NLST entry criteria, could improve the selection process for lung-cancer screening.9-14 This position is supported by indirect calculations of variation in screening benefit that projected an increase by a factor of 10 in the number of screening-prevented lung-cancer deaths among NLST participants at highest risk for death from lung cancer, as compared with those at lowest risk.15 Despite well-recognized4-8 theoretical grounds for tailoring screening recommendations to the individual risk of lung-cancer death, empirical evidence for risk-based lung-cancer screening is lacking.
We investigated whether the benefits and harms of low-dose CT screening in the NLST differed according to participants’ prescreening risk of lung-cancer death, which was estimated with a validated prediction model. Across risk groups for lung-cancer death, we evaluated the effect of low-dose CT screening on the number of prevented lung-cancer deaths, the number of participants with false positive results, and the number of participants who would need to be screened to prevent one lung-cancer death.
METHODS
STUDY PARTICIPANTS
The NLST was a randomized trial that compared the efficacy of low-dose CT for lung-cancer screening with that of chest radiography in 53,454 smokers between the ages of 55 and 74 years with a minimum of 30 pack-years of smoking and no more than 15 years since quitting.2,16,17 Participants were randomly assigned to receive three annual lung-cancer screenings with either low-dose CT (26,722 participants) or chest radiography (26,732 participants). In the current study, we evaluated 26,604 participants in the CT group and 26,554 in the radiography group in the trial's intention-to-screen sample, excluding 118 of the original participants (0.4%) in the CT group and 178 participants (0.7%) in the radiography group who had not completed a baseline health questionnaire. As in the NLST report,2 our primary end point was the rate of death from lung cancer from August 2002 to January 15, 2009.
All participants provided written informed consent. All the authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
STATISTICAL ANALYSIS
We developed an absolute risk-prediction model for lung-cancer mortality in the NLST's radiography group. The absolute risk of death from lung cancer accounted for a participant's specific risk characteristics and life expectancy by incorporating Cox proportional-hazards models of death from lung cancer and competing causes of death.18 We selected predictors for lung-cancer death among a set of previously identified demographic and clinical risk factors for lung cancer (including smoking history) using Lasso regression.19 The prediction model was externally validated20 with outcome data from 15,114 NLST-eligible and 22,649 NLST-ineligible smokers between the ages of 55 and 74 years who were enrolled in the radiography group of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial.21
We stratified the participants into five quintiles for the predicted 5-year risk of death from lung cancer (with quintile 1 having the lowest risk and quintile 5 having the highest risk). Within each risk quintile, we evaluated the efficacy of low-dose CT according to the intention-to-screen principle, analyzing participants according to their risk-group assignment. Measures of efficacy were the rate ratio for lung-cancer death and the difference in the rate of death, expressed as the number of lung-cancer deaths that were prevented by low-dose CT per 10,000 person-years, as compared with radiographic screening. We used inverse-variance weighted linear regression to evaluate trends in mortality ratios and mortality differences across risk quintiles.22 For each risk quintile in the CT group, we calculated the number of participants who would need to be screened to prevent one death from lung cancer as the number of participants who were offered screening as compared with the number of lung-cancer deaths that were prevented.
To investigate the influence of coexisting pulmonary conditions on the efficacy of low-dose CT screening, we performed analyses stratified according to the total number of such pulmonary conditions, as defined in Table 1. In addition, we examined the efficacy of low-dose CT screening across risk quintiles as defined according to the risk of lung-cancer incidence, rather than the risk of lung-cancer death, using four published prediction models of lung-cancer incidence (see the Supplementary Appendix, available with the full text of this article at NEJM.org).23-26
Table 1.
Characteristics of the Study Participants.*
| Characteristic | Low-Dose CT (N = 26,604) | Radiography (N = 26,554) |
|---|---|---|
| no. (%) | ||
| Risk of lung-cancer death at 5 yr | ||
| Quintile 1: 0.15–0.55% | 5,276 (19.8) | 5,356 (20.2) |
| Quintile 2: 0.56–0.84% | 5,310 (20.0) | 5,321 (20.0) |
| Quintile 3: 0.85–1.23% | 5,396 (20.3) | 5,236 (19.7) |
| Quintile 4: 1.24–2.00% | 5,314 (20.0) | 5,317 (20.0) |
| Quintile 5: >2.00% | 5,308 (20.0) | 5,324 (20.0) |
| Age—yr† | ||
| 55–59 | 11,368 (42.7) | 11,335 (42.7) |
| 60–64 | 8,143 (30.6) | 8,145 (30.7) |
| 65–69 | 4,742 (17.8) | 4,735 (17.8) |
| 70–74 | 2,348 (8.8) | 2,333 (8.8) |
| Sex | ||
| Male | 15,701 (59.0) | 15,664 (59.0) |
| Female | 10,903 (41.0) | 10,890 (41.0) |
| Race or ethnic group‡ | ||
| Non-Hispanic white | 23,920 (89.9) | 23,902 (90.0) |
| Non-Hispanic black | 1,169 (4.4) | 1,158 (4.4) |
| Hispanic | 478 (1.8) | 456 (1.7) |
| Other or unspecified | 1,037 (3.9) | 1,038 (3.9) |
| Number of first-degree relatives with lung cancer | ||
| 0 | 22,348 (84.0) | 22,325 (84.1) |
| 1 | 3,958 (14.9) | 3,929 (14.8) |
| ≥2 | 298 (1.1) | 300 (1.1) |
| Smoking status | ||
| Former | 13,815 (51.9) | 13,758 (51.8) |
| Current | 12,789 (48.1) | 12,796 (48.2) |
| Pack-yr of smoking§ | ||
| 30–39.9 | 6,796 (25.5) | 6,866 (25.9) |
| 40–49.9 | 7,154 (26.9) | 6,945 (26.2) |
| 50–59.9 | 3,659 (13.8) | 3,699 (13.9) |
| ≥60 | 8,989 (33.8) | 9,029 (34.0) |
| Years since smoking cessation | ||
| <1 | 13,867 (52.1) | 13,858 (52.2) |
| 1–4.9 | 3,747 (14.1) | 3,703 (13.9) |
| ≥5 | 8,990 (33.8) | 8,993 (33.9) |
| Number of coexisting pulmonary conditions¶ | ||
| 0 | 17,118 (64.3) | 17,162 (64.6) |
| 1 | 6,615 (24.9) | 6,535 (24.6) |
| ≥2 | 2,871 (10.8) | 2,857 (10.8) |
Participants who were enrolled in the National Lung Screening Trial (NLST) who did not meet the NLST inclusion criteria were not included in the screening trial but were included in the study analyses in keeping with the intention-to-screen principle. There were no significant differences between the two study groups. Percentages may not total 100 because of rounding.
At randomization, three participants in the CT group and six in the radiography group did not meet the criterion for the screening study of being between the ages of 55 and 74 years.
Race or ethnic group was self-reported.
At randomization, 6 participants in the CT group and 15 in the radiography group did not meet the criterion for the screening study of having a smoking history of at least 30 pack-years.
Coexisting pulmonary conditions included asbestosis, bronchiectasis, chronic bronchitis, chronic obstructive pulmonary disease, emphysema, fibrosis, pneumonia, sarcoidosis, silicosis, and tuberculosis. The frequencies of each condition are provided in Table S1 in the Supplementary Appendix.
In the CT group, we summarized screening outcomes across risk quintiles for lung-cancer death. Outcome measures included the characteristics of lung-cancer cases (the number of diagnoses and the proportion of early-stage cancers [stages IA and IB combined]), measures of the benefit of low-dose CT screening (the number of prevented lung-cancer deaths and the number of participants who would need to be screened to prevent one lung-cancer death), a measure of potential harm of low-dose CT screening (the number of participants with false positive results), and a harm-to-benefit measure (the ratio of the number of participants with false positive results to the number of prevented lung-cancer deaths).
The classification of positive results and false positive results on low-dose CT screening used a linked-year method that considered screening-linked cases of lung cancer as those identified within 1 year after a diagnostic follow-up initiated within 1 year after the screening (for details, see the Supplementary Appendix). Screening characteristics were individual-based and accounted for all three screening rounds. We defined a positive result as the detection of lung cancer on screening, a false positive result as the detection of lung cancer in a participant who was subsequently found not to have the disease, and a negative result as no detection of lung cancer during all screening rounds. Trends across risk quintiles were assessed with the use of the Cochran–Armitage test for proportions and weighted linear regression22 for continuous outcomes. All analyses were performed with the use of R software.27
RESULTS
STUDY PARTICIPANTS
Participants in the two study groups were well balanced with respect to selected demographic and clinical variables (Table 1, and Table S1 in the Supplementary Appendix). Over a median of 5.5 years of follow-up, lung-cancer deaths were reported in 354 participants in the CT group and in 442 in the radiography group. The rate of death from lung cancer was 24.6 per 10,000 person-years in the CT group, as compared with 30.9 per 10,000 person-years in the radiography group, with a relative reduction of 20.4% in the CT group (rate ratio, 0.80; 95% confidence interval [CI], 0.69 to 0.92; P = 0.001) and 6.3 fewer lung-cancer deaths per 10,000 person-years (95% CI, 2.4 to 10.1; P = 0.001).
PREDICTION MODEL FOR LUNG-CANCER DEATH
Selected risk factors for the hazard-ratio model for lung-cancer death were age, body-mass index, family history of lung cancer, pack-years of smoking, years since smoking cessation, and emphysema diagnosis (Table 2). For the hazard-ratio model for competing causes of death, sex and race were selected in addition to these risk factors, with the exception of a family history of lung cancer. The prediction model was well calibrated and had good discrimination for NLST-eligible and NLST-ineligible smokers in the radiography group of the PLCO trial (ratio of expected-to-observed lung-cancer deaths, 0.97 [95% CI, 0.87 to 1.07]; C-statistic, 0.80 [95% CI, 0.77 to 0.82]) (Tables S2 and S3 in the Supplementary Appendix).
Table 2.
Cause-Specific Hazard Models Used in the Risk-Prediction Model for Lung-Cancer Death in the Radiography Group of the NLST.*
| Factor | Coding | Death from Lung Cancer | Death from Another Cause |
|---|---|---|---|
| hazard ratio (95% CI) | |||
| Age | Continuous | 1.08 (1.06–1.10) | 1.09 (1.08–1.10) |
| Female sex | Binary | NA† | 0.50 (0.44–0.58) |
| Race | Categorical | NA† | |
| Non-Hispanic white | 1.00 (reference) | ||
| Non-Hispanic black | 2.22 (1.78–2.76) | ||
| Hispanic | 1.34 (0.89–2.03) | ||
| Other | 1.21 (0.91–1.60) | ||
| Body-mass index‡ | |||
| Linear term | Continuous | 0.75 (0.66–0.86) | 0.89 (0.82–0.97) |
| Quadratic term | Continuous | 1.05 (0.99–1.11) | 1.06 (1.04–1.09) |
| Pack-years of smoking | Continuous | 1.02 (1.01–1.02) | 1.01 (1.01–1.01) |
| Years since smoking cessation | Trend§ | 0.62 (0.55–0.70) | 0.76 (0.70–0.81) |
| Presence of emphysema | Binary | 1.56 (1.20–2.04) | 1.52 (1.28–1.80) |
| First-degree relative with lung cancer | Trend¶ | 1.27 (1.00–1.62) | NA∥ |
NA denotes not applicable.
This category was not a selected risk factor for lung-cancer death.
The body-mass index is the weight in kilograms divided by the square of the height in meters. In the hazard models, the body-mass index was centered at 25, and hazard ratios are for an increase of 5.
This category was scored as 0 for less than 1 year, 1 for 1 to 5 years, and 2 for more than 5 years.
This category was scored as 0 for no relatives, 1 for one relative, and 2 for two or more relatives.
This category was not a selected risk factor for death from another cause.
The quintiles for the 5-year risk of lung-cancer death were as follows: 0.15 to 0.55% in quintile 1, 0.56 to 0.84% in quintile 2, 0.85 to 1.23% in quintile 3, 1.24 to 2.00% in quintile 4, and more than 2.00% in quintile 5 (Table 2). Within each risk quintile, participants in the CT and radiography groups were balanced with respect to the risk factors in the prediction model (data not shown).
EFFICACY OF CT SCREENING ACCORDING TO RISK
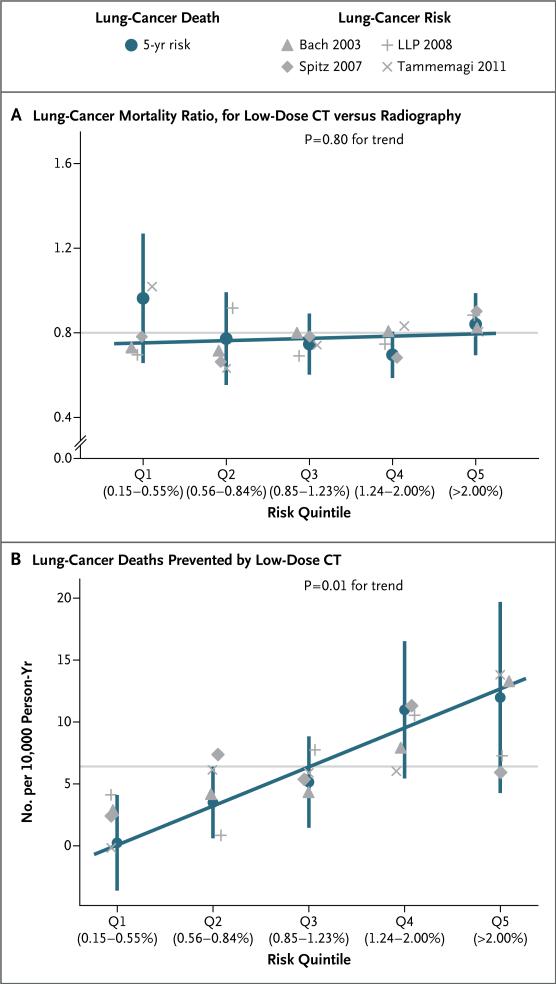
Lung-cancer mortality ratios in the low-dose CT group, as compared with the radiography group, did not differ significantly across risk quintiles (0.97 in quintile 1, 0.78 in quintile 2, 0.75 in quintile 3, 0.70 in quintile 4, and 0.84 in quintile 5; P = 0.80 for trend) (Fig. 1A). However, since there was an increase in the risk of lung-cancer death across risk quintiles, the overall 20% reduction in the rate of lung-cancer death in the CT group meant that the number of lung-cancer deaths per 10,000 person-years that were prevented by low-dose CT screening increased significantly across risk quintiles (0.2 in quintile 1, 3.5 in quintile 2, 5.1 in quintile 3, 11.0 in quintile 4, and 12.0 in quintile 5; P = 0.01 for trend) (Fig. 1B). Similar trends in lung-cancer mortality ratios and mortality differences were observed whether risk quintiles were based on the predicted risk of lung cancer or on the risk of lung-cancer death (P = 0.90 for heterogeneity for both efficacy comparisons).
Figure 1. Efficacy of Low-Dose CT Screening, According to Risk Quintile.
Panel A shows the lung-cancer mortality ratio for the low-dose CT group versus the radiography group among risk quintiles 1 through 5 (Q1 through Q5, each 20% of the screening group) for the prescreening 5-year risk of lung-cancer death. Panel B shows the number of lung-cancer deaths prevented by low-dose CT (i.e., the between-group difference) among the risk quintiles. The exact boundaries for the risk of 5-year lung-cancer death for each quintile, as percentages, are shown on the x axis. Bars denote 95% confidence intervals. The horizontal gray lines indicate the overall efficacy of low-dose CT screening. The gray symbols indicate the efficacy outcomes in risk quintiles as defined by four published prediction models of lung-cancer incidence: Bach et al.,23 Spitz et al.,24 Cassidy et al. (for the Liverpool Lung Project [LLP]),25 and Tammemagi et al.26
COEXISTING PULMONARY CONDITIONS
At baseline, 64.5% of participants in the NLST had no coexisting pulmonary conditions, 24.7% had one pulmonary condition, and 10.8% had two or more conditions (Table 1). Overall, low-dose CT screening was efficacious among participants with no pulmonary conditions (6.2 CT-prevented lung-cancer deaths per 10,000 person-years [95% CI, 1.7 to 10.7]) or with one pulmonary condition (9.6 CT-prevented lung-cancer deaths per 10,000 person-years [95% CI, 1.5 to 17.7]). Such screening was not efficacious among participants with multiple coexisting conditions (CT-prevented lung-cancer deaths per 10,000 person-years, –0.5; 95% CI, –15.4 to 14.3). However, the differences in the efficacy of low-dose CT screening according to the number of coexisting pulmonary con ditions were not significant (P = 0.50 for heterogeneity) (Table S4 in the Supplementary Appendix). The presence of multiple pulmonary conditions increased significantly across risk quintiles (11.1% in quintile 1 vs. 35.7% in quintile 5, P<0.001 for trend). We could not detect significant differences in trends of CT-prevented lung-cancer deaths according to the status of coexisting illnesses (P = 0.70 for heterogeneity) (Table S4 in the Supplementary Appendix).
CT-PREVENTED LUNG-CANCER DEATHS
The number of CT-prevented lung-cancer deaths increased in tandem with the risk of lung-cancer death (1 in quintile 1 vs. 33 in quintile 5, P = 0.01 for trend) (Table 3). Consequently, the number of participants who would need to be screened to prevent one lung-cancer death decreased significantly with an increasing risk of lung-cancer death (5276 in quintile 1, 531 in quintile 2, 415 in quintile 3, 171 in quintile 4, and 161 in quintile 5; P<0.001 for trend).
Table 3.
Outcomes of Three Rounds of Annual Low-Dose CT Screening, According to Risk Quintile.*
| Quintile of 5-Year Risk of Lung-Cancer Death | Participants | Lung-Cancer Cases | Lung-Cancer Deaths | Positive Screening Results | Number of False Positives per Prevented Lung-Cancer Death† | Number Needed to Screen†‡ | |||
|---|---|---|---|---|---|---|---|---|---|
| Total No. | Stage I† | Total No. | Prevented† | Total No. | False Positive†§ | ||||
| no. (%) | no. (%) | no. (%) | no. (%) | ||||||
| All quintiles | 26,604 (100) | 1083 | 530 (48.9) | 354 | 88 (24.9) | 10,151 | 9484 (93.4) | 108 | 302 |
| Quintile 1: 0.15–0.55% | 5,276 (19.8) | 71 | 40 (56.3) | 20 | 1 (5.0) | 1,699 | 1648 (97.0) | 1648 | 5276 |
| Quintile 2: 0.56–0.84% | 5,310 (20.0) | 105 | 59 (56.2) | 35 | 10 (28.6) | 1,879 | 1806 (96.1) | 181 | 531 |
| Quintile 3: 0.85–1.23% | 5,396 (20.3) | 182 | 84 (46.2) | 45 | 13 (28.9) | 2,024 | 1911 (94.4) | 147 | 415 |
| Quintile 4: 1.24–2.00% | 5,314 (20.0) | 263 | 132 (50.2) | 73 | 31 (42.5) | 2,123 | 1973 (92.9) | 64 | 171 |
| Quintile 5: >2.00% | 5,308 (20.0) | 462 | 215 (46.5) | 181 | 33 (18.2) | 2,426 | 2146 (88.5) | 65 | 161 |
The tabulated outcomes include all three rounds of low-dose CT screening and all events that occurred through January 15, 2009.
P<0.05 by means of a linear test of trend in continuous outcomes.
The number needed to screen is the number of participants in the CT group divided by the number of lung-cancer deaths prevented by low-dose CT screening.
The number of participants with positive screening results includes all those with any positive result during the trial. P<0.05 by means of the Cochran–Armitage test of trend in proportions.
The increase in the number of CT-prevented lung-cancer deaths across risk quintiles was correlated with the number of stage I tumors. Approximately 50% of diagnosed lung cancers in the CT group were stage I tumors, and this proportion was constant across risk quintiles (Table 3). Nevertheless, owing to an increase in the number of diagnosed lung cancers in higher-risk quintiles, the number of stage I lung cancers increased significantly with an increasing risk of lung-cancer death (40 in quintile 1 vs. 215 in quintile 5, P<0.001 for trend).
FALSE POSITIVE RESULTS ON SCREENING
Among participants in the CT group with a positive result, the proportion of false positive results was high, as has been reported previously.28 This proportion decreased with an increasing risk of lung-cancer death (97% in quintile 1 vs. 88% in quintile 5, P<0.001 for trend). However, since there were more positive results in the higher-risk quintiles, the total number of participants with false positive results increased across risk quintiles (1648 in quintile 1 vs. 2146 in quintile 5, P<0.001 for trend) (Table 3, and Table S5 in the Supplementary Appendix). The ratio of the number of participants with false positive results to the number of CT-prevented lung-cancer deaths (a measure of the ratio of harm to benefit) decreased significantly across risk quintiles (1648 in quintile 1 vs. 65 in quintile 5, P<0.001 for trend).
RISKS VERSUS BENEFITS OF CT SCREENING
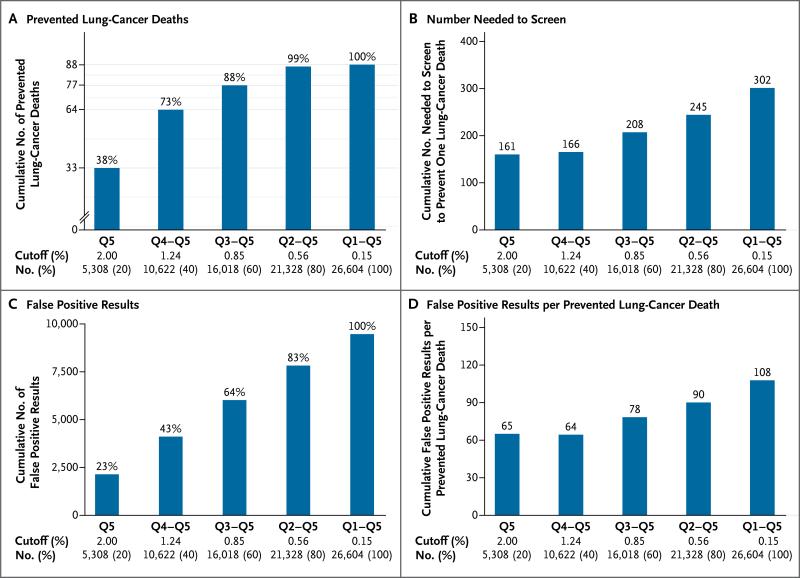
Figure 2 shows the performance of low-dose CT screening in cumulative risk groups, beginning with the highest-risk quintile (quintile 5) to the left and expanding the risk group from left to right by cumulatively adding the next-highest risk quintile. These cumulative results show two key observations. First, participants at highest risk for lung-cancer death accounted for a disproportionate share of the benefits of low-dose CT screening (Fig. 2A). For example, 77 of 88 CT-prevented lung-cancer deaths (88%) occurred among the 60% of participants with a 5-year risk of lung-cancer death of 0.85% or more (i.e., in quintiles 3 through 5), whereas only 1% of prevented lung-cancer deaths occurred among the 20% of participants at lowest risk (i.e., in quintile 1). Also, the number of participants who would need to be screened to prevent one lung-cancer death decreased from 302 for the overall CT group to 208 among the 60% of participants at highest risk (Fig. 2B). Second, the 60% of participants at highest risk accounted for 64% of total false positive results in the CT group (Fig. 2C). As a result, the number of participants with false positive results per CT-prevented lung-cancer death decreased from 108 overall to 78 in the three highest-risk quintiles (Fig. 2D).
Figure 2. Cumulative Screening Outcomes in the Low-Dose CT Group.
Each panel shows cumulative screening outcomes in the low-dose CT group, from highest risk in quintile 5 (Q5, on the left) to overall risk in quintiles 1 through 5 (Q1–Q5, on the right). The risk cutoff defining each group and the represented number and proportion of patients are indicated below the x axis. Panel A shows the cumulative number of lung-cancer deaths that were prevented in the low-dose CT group, as compared with the radiography group, with the corresponding percentage over each bar. Panel B shows the number of participants who would need to undergo three annual screenings with low-dose CT to prevent one lung-cancer death. Panel C shows the cumulative number of participants with false positive results on screening, with the corresponding percentage over each bar. Panel D shows the cumulative number of participants with false positive results on screening per CT-prevented lung-cancer death.
DISCUSSION
Our study provides empirical evidence for the potential utility of targeting smokers at highest risk for lung cancer for low-dose CT screening. The number of CT-prevented lung-cancer deaths strongly increased with an increase in the prescreening risk of death from lung cancer. Consequently, the number of participants who would need to be screened to prevent one lung-cancer death decreased from 5276 among the 20% of participants at lowest risk to 161 among the 20% of those at highest risk. Also, the number of participants with false positive results on screening per CT-prevented lung-cancer death decreased from 1648 among the 20% of participants at lowest risk to 65 among the 20% of those at highest risk.
In our study, the overall relative reduction of 20% in the rate of lung-cancer death among participants in the CT group, as compared with the radiography group, did not differ significantly across risk quintiles. Nevertheless, owing to an increase in the risk of lung-cancer death across risk quintiles, the constant 20% reduction in death rate translated into a significant increase in the total number of CT-prevented lung-cancer deaths across risk quintiles. A similar phenomenon was observed for the number of stage I lung cancers, as well as the number of false positive results across risk quintiles. These observations underscore the importance of absolute measures (e.g., risk differences and counts) over relative measures (e.g., ratios) for evaluating the public health benefits of screening interventions.
Although there is currently a consensus among published screening guidelines on recommending low-dose CT screening for patients who meet the NLST entry criteria,29 some experts have speculated that further refinement of selection criteria may be appropriate.4,15 Our results confirm that tailoring of low-dose CT screening to a patient's predicted risk of lung-cancer death could narrow the NLST-eligible population without a loss in the potential public health benefits of screening or a disproportionate increase in the potential harms. For example, we found that restricting screening to the 60% of participants at highest risk for death from lung cancer within 5 years (>0.85%), as compared with the entire CT group, captured 88% of CT-preventable lung-cancer deaths, reduced the number of participants who would need to be screened to prevent one lung-cancer death from 302 to 161, and reduced the number of false positive results per CT-prevented lung-cancer death from 108 to 65. In contrast, the 20% of participants at lowest risk for lung-cancer death accounted for almost none of the CT-prevented lung-cancer deaths. These observations argue for the use of individualized risk assessment of lung-cancer death instead of the NLST entry criteria to increase the efficiency of low-dose CT screening.
Furthermore, a risk-based strategy for low-dose CT screening could provide a rational, empirical framework for the inclusion of NLST-ineligible smokers at high risk for lung-cancer death. However, such a strategy would depend on the generalizability of the benefits and harms of screening that were observed in NLST participants, as compared with NLST-ineligible persons at similar risk, for whom there are no empirical data.30,31
Risk-based low-dose CT screening could be based on a patient's risk of either lung-cancer incidence or lung-cancer death. We focused on the risk of lung-cancer death because the primary benefit of low-dose CT screening is the prevention of lung-cancer death. Yet, given the high case fatality rate for lung cancer, prediction models for lung-cancer incidence and death are likely to have similar discriminatory ability. Indeed, we found similar trends in the number of CT-prevented lung-cancer deaths across risk quintiles that were defined according to the risk of lung-cancer death and the risk of lung-cancer incidence. Thus, although there is evidence to support the use of risk assessment for screening selection, further study of the comparative performance of available tools for assessing lung-cancer risk is needed to determine which tool to recommend for risk-based screening strategies.
Our findings need to be interpreted within the context of low-dose CT screening in the NLST. This limits extrapolation of our results to alternative screening and follow-up schedules. Furthermore, beyond false positive results, we did not consider other potential harms of low-dose CT screening, such as the psychological burden of false positive results, complications with invasive follow-up procedures, and radiation-induced cancers.32 In addition, our assessment of the efficacy of low-dose CT screening in patients with coexisting pulmonary conditions had limited power. Thus, additional study of the benefits and risks of low-dose CT screening in the presence of coexisting pulmonary conditions is needed.
Our results have public health implications. In 2011, there were 8.9 million NLST-eligible and 20.3 million NLST-ineligible smokers between the ages of 55 to 74 years and 94 million current and former smokers of all ages in the United States.33 Since the publication of the NLST findings, a key question has been which of these smokers should be targeted for low-dose CT screening. Our observation that both the potential benefits and harms of such screening strongly depend on a patient's risk of lung-cancer death underscores the potential utility of risk-based low-dose CT screening. Our estimates of the expected benefits and potential harms of such screening across risk groups provide the empirical framework for evaluating the cost-effectiveness of low-dose CT screening, investigating optimal risk cutoffs for screening, and communicating the potential benefits and harms of such screening tailored to each patient's individual risk.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), National Institutes of Health, Department of Health and Human Services. The PLCO trial was supported by the NCI. The American College of Radiology Imaging Network component of the NLST was supported by grants (U01-CA-80098 and U01-CA-79778) under a cooperative agreement with the Cancer Imaging Program, Division of Cancer Treatment and Diagnosis, NCI. The Lung Screening Study sites of the NLST were funded through contracts with the Early Detection Research Group and Biometry Research Group, Division of Cancer Prevention, NCI (N01-CN-25514, to University of Colorado—Denver; N01-CN-25522, to Georgetown University; N01-CN-25515, to Pacific Health Research and Education Institute; N01-CN-25512, to Henry Ford Health System; N01-CN-25513, to University of Minnesota; N01-CN-25516, to Washington University in St. Louis; N01-CN-25511, to University of Pittsburgh; N01-CN-25524, to University of Utah; N01-CN-25518, to Marshfield Clinic Research Foundation; N01-CN-75022, to University of Alabama at Birmingham; N01-CN-25476, to Westat; N02-CN-63300, to Information Management Services).
We thank the participants in the NLST and PLCO for making this research possible.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.The National Lung Screening Trial Research Team Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cigarette smoking among adults and trends in smoking cessation — United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1227–32. [PubMed] [Google Scholar]
- 4.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–29. doi: 10.1001/jama.2012.5521. [Erratum, JAMA 2012; 308:1324.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wender R, Fontham ET, Barrera E, Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107–17. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couraud S, Cortot AB, Greillier L, et al. From randomized trials to the clinic: is it time to implement individual lung-cancer screening in clinical practice? A multidisciplinary statement from French experts on behalf of the French Intergroup (IFCT) and the Groupe d'Oncologie de Langue Francaise (GOLF). Ann Oncol. 2013;24:586–97. doi: 10.1093/annonc/mds476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw. 2012;10:240–65. doi: 10.6004/jnccn.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–8. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 9.Raji OY, Duffy SW, Agbaje OF, et al. Predictive accuracy of the Liverpool Lung Project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med. 2012;157:242–50. doi: 10.7326/0003-4819-157-4-201208210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–36. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19:154–6. doi: 10.1258/jms.2012.012010. [DOI] [PubMed] [Google Scholar]
- 12.Maisonneuve P, Bagnardi V, Bellomi M, et al. Lung cancer risk prediction to select smokers for screening CT: a model based on the Italian COSMOS Trial. Cancer Prev Res (Phila) 2011;4:1778–89. doi: 10.1158/1940-6207.CAPR-11-0026. [DOI] [PubMed] [Google Scholar]
- 13.Young RP, Hopkins RJ, Midthun DE. Computed tomographic screening for lung cancer. JAMA. 2012;308:1320. doi: 10.1001/2012.jama.11892. [DOI] [PubMed] [Google Scholar]
- 14.Jett JR, Midthun DE. Screening for lung cancer: for patients at increased risk for lung cancer, it works. Ann Intern Med. 2011;155:540–2. doi: 10.7326/0003-4819-155-8-201110180-00367. [DOI] [PubMed] [Google Scholar]
- 15.Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Ann Intern Med. 2012;157:571–3. doi: 10.7326/0003-4819-157-8-201210160-00524. [DOI] [PubMed] [Google Scholar]
- 16.National Lung Screening Trial Research Team The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–53. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aberle DR, Adams AM, Berg CD, et al. Baseline characteristics of participants in the randomized National Lung Screening Trial. J Natl Cancer Inst. 2010;102:1771–9. doi: 10.1093/jnci/djq434. [Erratum, J Natl Cancer Inst 2011;103: 1560.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benichou J, Gail MH. Estimates of absolute cause-specific risk in cohort studies. Biometrics. 1990;46:813–26. [PubMed] [Google Scholar]
- 19.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385–95. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JM, Ankerst DP, Andridge RR. Validation of biomarker-based risk prediction models. Clin Cancer Res. 2008;14:5977–83. doi: 10.1158/1078-0432.CCR-07-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oken MM, Marcus PM, Hu P, et al. Baseline chest radiograph for lung cancer detection in the randomized Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Cancer Inst. 2005;97:1832–9. doi: 10.1093/jnci/dji430. [DOI] [PubMed] [Google Scholar]
- 22.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 23.Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95:470–8. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 24.Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99:715–26. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- 25.Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 2008;98:270–6. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst. 2011;103:1058–68. doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Development Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2012. [Google Scholar]
- 28.Silvestri GA. Screening for lung cancer: it works, but does it really work? Ann Intern Med. 2011;155:537–9. doi: 10.7326/0003-4819-155-8-201110180-00364. [DOI] [PubMed] [Google Scholar]
- 29.Boiselle PM. Computed tomography screening for lung cancer. JAMA. 2013;309:1163–70. doi: 10.1001/jama.2012.216988. [DOI] [PubMed] [Google Scholar]
- 30.Heuvers ME, Stricker BH, Aerts JG. Generalizing lung-cancer screening results. N Engl J Med. 2012;366:192–3. doi: 10.1056/NEJMc1111935. [DOI] [PubMed] [Google Scholar]
- 31.Sox HC. Better evidence about screening for lung cancer. N Engl J Med. 2011;365:455–7. doi: 10.1056/NEJMe1103776. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor GT, Hatabu H. Lung cancer screening, radiation, risks, benefits, and uncertainty. JAMA. 2012;307:2434–5. doi: 10.1001/jama.2012.6096. [DOI] [PubMed] [Google Scholar]
- 33.Barnes PM, Ward BW, Freeman G, et al. Early release of selected estimates based on data from the January–March 2011 National Health Interview Survey. National Center for Health Statistics; Sep, 2011. ( http://www.cdc.gov/nchs/nhis.htm) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.