Abstract
Background/Aims
Biliary atresia (BA) is a progressive disease characterized by bile duct inflammation and fibrosis. The aetiology is unknown and may be due to a virus-induced, autoimmune-mediated injury of cholangiocytes. Cholangiocytes are not only targets of injury but may also modulate hepatic inflammation. The aim of this study was to determine the immune profile of murine cholangiocytes and the ability to function as antigen-presenting cells (APCs) in culture with Rhesus rotavirus (RRV), poly I:C (viral mimic) or interferon-γ/tumour necrosis factor-α.
Methods/Results
Both the cholangiocyte cell line (long-term culture) and fresh, ex vivo cholangiocytes expressed APC surface markers major histocompatibility complex (MHC)-class I and II and CD40, while only the cultured cell line expressed costimulatory molecules B7-1 and B7-2. Despite APC expression, cultured cholangiocytes were unable to function as competent APCs in T-cell proliferation assays. Furthermore, both cultured and ex vivo cholangiocytes expressed RNA transcripts for many pro-inflammatory cytokines and chemokines.
Conclusions
Although cholangiocytes contain APC molecules, they are incompetent at antigen presentation and cannot elicit effective T-cell activation. Upregulation of MHC-class I and II found in BA mice may serve to prime the cholangiocyte as a target for immune-mediated injury. Cholangiocytes produced many pro-inflammatory cytokines and chemokines in the setting of RRV infection and T-helper type 1 cytokine milieu, suggesting a role of cholangiocytes as immune modulators promoting the ongoing inflammation that exists in RRV-induced BA.
Keywords: antigen presentation, autoimmunity, chemokines, cholangitis, cytokines
Biliary atresia (BA) is a progressive disease of infancy that leads to an inflammatory obliteration and fibrosis of the extrahepatic and intrahepatic bile ducts. The disease process is characterized by fibrotic obliteration of the extrahepatic bile duct and intrahepatic periductal inflammation and injury. The current incidence worldwide is estimated to occur between 1:5000 and 1:18 000 live births. The majority of patients will exhibit progressive inflammation and fibrosis of the intrahepatic bile ducts as well, leading to biliary cirrhosis. Approximately 70–80% of children with BA will require liver transplantation, making this disease the most common indication for paediatric liver transplantation (1–5).
The aetiology of BA is currently unknown. Bile duct infection or injury from toxins may be possible inciting factors that lead to progressive, autoimmune-mediated damage of the biliary tree (5). Rotavirus has been investigated as a possible putative virus involved in the pathogenesis of human BA and serves as the inciting event in the murine model of BA, in which newborn mice infected with Group A Rhesus rotavirus (RRV) develop a biliary disease that closely mimics human disease (6–8). Investigations of the immune response in both human and murine BA have shown that the portal tract infiltrate is composed of macrophages and T cells, with lymphocytes invading cholangiocytes (9–12). The cytokine milieu within the portal tracts includes pro-inflammatory interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α), leading to progressive bile duct injury (11–13).
Cholangiocytes are not only the targets of injury, but may also be intricately involved with modulating the ongoing portal tract inflammation. For example, in other diseases, it has been shown that cholangiocytes have aberrant expression of major histocompatibility complex (MHC)-class II, suggesting a role as nonprofessional antigen-presenting cells (APCs). In addition cholangiocytes may have the ability to express immune recognition elements leading to T-cell activation through alternative pathways and may produce a variety of cytokines that contribute to ongoing immune activation and recruitment (14–20). In this study, we sought to establish the role of cholangiocytes in the setting of rotavirus infection and T-helper type 1 (Th1) inflammation, mimicking the immunological environment of murine BA. The aims were two-fold: firstly, to identify APC markers expressed on murine cholangiocytes under various inflammatory conditions and to determine competency as antigen presenters and secondly, to characterize the cytokine and chemokine profile of cholangiocytes in the setting of viral infection and inflammation.
Materials and methods
Cell and virus culture
Cultured cholangiocytes are a SV40, large T antigen immortalized cell line on a BALB/cJ background kindly provided by Yoshiyuki Ueno (Sendai, Japan). Cells were maintained in minimum essential medium (MEM) (Gibco, Grand Island, NY, USA)/10% fetal calf serum. Cultured cholangiocytes were grown in media alone or supplemented with IFN-γ (150–500 U/ml), TNF-α (20 ng/ml) (eBiosciences, San Diego, CA, USA), poly I:C (50 μg/ml) (Sigma, St Louis, MO, USA) or RRV at various time points. The DO11.10 hybridoma T cell line was kindly provided by Brian Kotzin, University of Colorado Health Sciences Center. Virus culture: RRV strain MMU 18006 was grown in MA-104 African green monkey kidney cells (ATCC) and concentration determined by infectious plaque assays as previously described (12).
Mice
Eight-week-old BALB/cJ, DO11.10 and timed-pregnant female BALB/cJ mice were purchased from rotavirus-free colonies of Jackson Laboratory (Bar Harbor, ME, USA). BALB/cJ newborn mice were injected intraperitoneally at 12–18 h of life with 40 μl of RRV (4.0 × 104 PFU) or Hank's balanced salt solution alone and sacrificed at day 14 of life. Livers and extrahepatic bile ducts were harvested, pooled together (10–20 mice) and tissue disrupted into single cell suspension for future fluorescent-activated cell sorting (FACS) analysis or RNA extraction. All animals were housed and handled in accordance with the UCHSC Office of Laboratory Animal Medicine.
Viral infection of cholangiocytes
Cholangiocytes were grown in six-well plates and over-layed with RRV at the desired multiplicity of infection (MOI) in MEM (Gibco). Ten microgram per millilitre of endotoxin-free trypsin (CalBiochem, San Diego, CA, USA) was added to each overlay to activate the virus (30 min, 5% CO2), followed by addition of 10 μg/ml of trypsin inhibitor (Sigma) for 1 h. The viral overlay was removed and replaced with serum-free media for the desired time (3–72 h). Mock infections consisting of serum-free media alone with trypsin and trypsin inhibitor were also performed.
Electron microscopy
Rhesus rotavirus-infected cultured cholangiocytes or mock controls were prepared as above at 3 and 24 h. After incubation, cells were pelleted, placed in glutaraldehyde, sectioned and examined with a Zeiss EM-10 electron microscope (Zeiss, Thornwood, NY, USA).
Flow cytometry
Single cell suspension of the cholangiocyte cell line or liver cells ex vivo were incubated with Fc block followed by staining with fluorochrome-conjugated antibodies: MHC-class I, MHC-class II, CD40, CD80, CD86 and isotype matched controls (eBiosciences). The hybridoma to Cytokeratin 19 (anti-TROMA III) was obtained from the Developmental Studies Hybridoma Bank (NICHD and University of Iowa, Iowa City). This hybridoma was used to produce high-titre IgG-containing ascites from SCID mice (Jackson Labs) using standard protocols and purified using the Melon Gel Monoclonal IgG purification kit (Pierce, Rockford, IL, USA). The purified IgG was fluorochrome-conjugated with an Alexa Fluor 555 Protein Labeling Kit (Molecular Probes, Eugene, OR, USA). Confirmation of the cholangiocyte cell line as a pure population was confirmed by cell surface expression of cytokeratin 19. Expression of cell surface markers was determined on a FACS Caliber flow cytometer (Becton-Dickinson, Mountain View, CA, USA) using cellquest software for analysis. All flow cytometry studies were repeated three times.
Antigen-specific lymphoproliferation assays
Mitomycin C-treated bulk splenocytes from BALB/cJ mice functioned as control APCs. Cultured cholangiocytes were incubated with RRV, poly I:C or IFN-γ as described above, treated with mitomycin C and functioned as experimental APCs (2.5 × 105 cells/well). Responder ovalbumin (OVA)-specific T cells from DO11.10 mouse spleens were isolated using the T-cell isolation kit as per the manufacturer's protocol on an AutoMACS cell sorter (Miltenyi Biotec, Auburn, CA, USA) with a final purity of > 98%. 2.5 × 105 T cells were cultured with cholangiocytes or splenocyte APC controls in 96-well plates. OVA323–339 peptide antigen (GenScript, Piscataway, NJ, USA) was added to the appropriate wells (1 μg/ml), cells incubated for 72 h and pulsed with 1 μCi/well of thymidine-[methyl-3H] (Perkin Elmer; Boston, MA, USA) for 18 h. Cells were harvested with a cell harvester (Tomtek, Orange, CT, USA) and T-3H uptake was measured by liquid scintillation on a beta counter (Trilux 450 Microbeta; Wallac, Turku, Finland). All T-cell proliferation studies were performed in triplicate and repeated three times.
Enzyme-linked immunosorbent assay
Supernatants from cultured cholangiocytes, DO11.10 hybridoma lymphocyte assays in conditions described above were collected and used with commercial kits for interleukin (IL)-2, IL-6 and transforming growth factor-β (TGF-β) as per the manufacturer's instructions (eBiosciences). All enzyme-linked immunosorbent assay (ELISA) studies were performed in duplicate and repeated twice.
Isolation of cholangiocytes ex vivo
Mice inoculated with either balanced salt solution (BSS) (n = 6–10, pooled) or RRV (n = 6–10, pooled) at birth were sacrificed at day 14 of life; livers and extrahepatic bile ducts were harvested and tissue disrupted into single cell suspension. Liver cells were mixed with 40% Percoll and underlaid with 60% Percoll. The resultant gradient yielded cholangiocytes collected from the uppermost layer, distinctly separate from the immune cell-rich interface. Cholangiocytes were incubated with rat anti-mouse TROMA III followed by goat anti-rat magnetic microbeads (Miltenyi Biotec). Cholangiocytes were purified with the AutoMACS magnetic cell sorter (two passes over magnetic column). Purity of the sorted cell population was determined by post-sort FACS analysis. The cells collected were > 98% TROMA-III positive, < 2% CD11b positive and CD3 negative. Purified cell populations were the source for RNA extraction.
Reverse transcriptase-polymerase chain reaction
RNA from cultured cholangiocytes or cholangiocytes isolated ex vivo was prepared using the RNeasy kit (Qiagen, Valencia, CA, USA). RNA was treated with DNAse (Sigma), 1.5 μg RNA per sample was transcribed to cDNA using the Advantage RT for polymerase chain reaction (PCR) kit (Clontech, Mountain View, CA, USA), each PCR reaction utilized 3 μl of cDNA and PCR was performed using IL-1β, IL-6, TNF-α, IFN-γ, TGF-β, monokine induced by γ-interferon (MIG), macrophage inflammatory protein-1α (MIP-1α), γ-interferon inducible protein-10 (IP-10), regulated upon activation, normal T-cell expressed and secreted (RANTES), glyceraldehyde-3-phosphate dehydrogenase primer pairs (Maxim Biotech, Rockville, MD, USA), RRV viral structural protein 4 (VP-4) (GenBank accession number AY033150) and nonstructural protein 1 (NSP-1) (Gen-Bank accession number AY117048). All PCR studies were repeated three times.
Results
Cholangiocytes are targets of Rhesus rotavirus infection
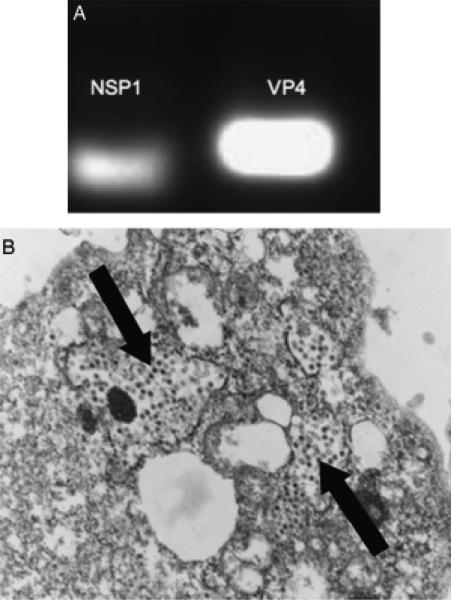
Rhesus rotavirus infection of mice leads to biliary obstruction that mimics BA; however it was not known if the RRV was actually infecting the cholangiocyte. Therefore, investigation of RRV infection and replication within cultured cholangiocytes was performed by detection of viral RNA transcripts through PCR and viral particles by electron microscopy. Cholangiocytes cultured with RRV at an MOI of 50 for 3–24 h consistently expressed mRNA transcripts for VP4 and NSP1 (Fig. 1A). At time courses > 30 h at the same MOI, the majority of the cholangiocytes lysed and died in culture. In addition to PCR evidence of cholangiocyte viral infection, ultra-structural examination of RRV-infected cholangiocyte cell pellets at 24 h revealed intracytoplasmic rotavirus particles (Fig. 1B). Confirmation of cholangiocyte viral infection was essential in the experimental design as the goal was to mimic in vitro the viral and cytokine milieu present in murine BA that would allow cholangiocytes to function as APCs or immune modulating cells.
Fig. 1.
Rhesus rotavirus (RRV) infection of cholangiocytes. (A) Cultured cholangiocytes infected with RRV (multiplicity of infection of 50, 3 h) contained mRNA transcripts for nonstructural protein 1 (NSP1) and viral structural protein 4 (VP4). (B) Electron microscopy of RRV-infected cholangiocytes revealed intracytoplasmic RRV particles (black arrows).
Cultured cholangiocytes express all antigen-presenting cell surface markers in vitro
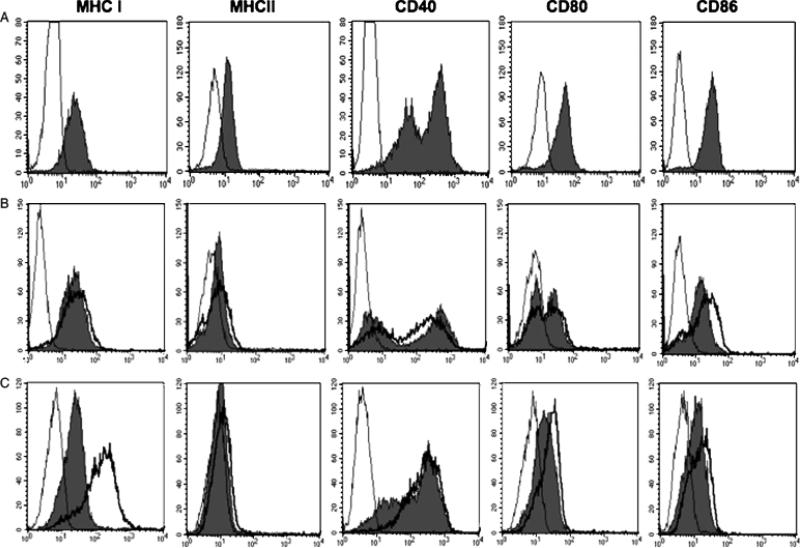
In the setting of virus infection and periductal inflammation, it was hypothesized that cholangiocytes may express the necessary cell surface markers of APCs. Murine cholangiocytes cultured alone were analysed by flow cytometry and showed cell surface expression of MHC-class I, MHC-class II, CD40, CD80 (B7-1) and CD86 (B7-2) (Fig. 2A). Comparison of RRV-infected cholangiocytes to uninfected controls revealed a mild increase in CD86 expression with a two-fold increase in mean channel fluorescence (MCF) (Fig. 2B). Poly I:C (viral mimic of single-stranded RNA viruses) did not alter APC marker expression (data not shown). Cholangiocytes cultured with IFN-γ (100 U/ml) and TNF-α (20 ng/ml) for 48 h led to a marked increase in MHC-class I expression with a six-fold increase in MCF (Fig. 2C). Therefore, in the setting of rotavirus infection and pro-inflammatory cytokines (murine BA), MHC-class I and CD86 costimulation are upregulated on cultured cholangiocytes.
Fig. 2.
Fluorescent-activated cell sorting (FACS) analysis of antigen-presenting cell (APC) surface markers on the cultured cholangiocyte cell line. (A) The cultured cholangiocyte cell line was incubated with fluorescein isothiocyanate-labelled antibodies to major histocompatibility complex (MHC) I, MHC II, CD40, CD80 or CD86 followed by FACS analysis that revealed cell surface expression of all APC markers (solid grey) compared with isotype controls (thin black line). (B) Cultured cholangiocytes infected with RRV (multiplicity of infection of 50, 24 h) expressed higher levels of CD86 (heavy black line) compared with uninfected controls (solid grey) and isotype controls (thin black line). (C) Cultured cholangiocytes treated with interferon-γ and tumour necrosis factor-α showed marked increase in MHC I expression (heavy black line) compared with uninfected controls (solid grey) and isotype controls (thin black line).
Cholangiocytes isolated ex vivo from murine biliary atresia constitutively express major histocompatibility complex -class I and CD40, with aberrant expression of major histocompatibility complex class II
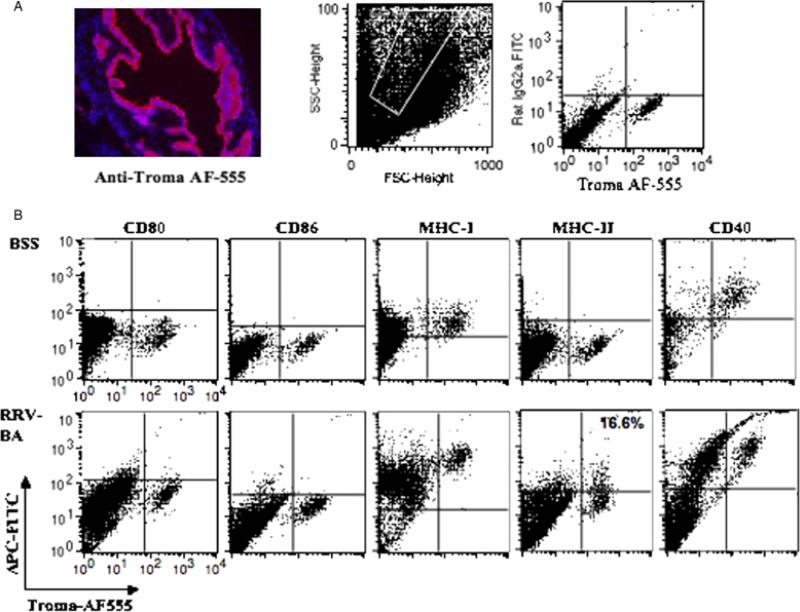
Livers and extrahepatic bile ducts from 14-day-old BSS control or RRV-induced BA mice were pooled and cholangiocytes identified by flow cytometry based on surface expression of TROMA III, a cytokeratin 19 marker (Fig. 3A). Costaining of TROMA III positive cholangiocytes with APC surface markers revealed that cholangiocytes from normal BSS mice expressed only MHC class I and CD40 (Fig. 3B). Interestingly, ex vivo cholangiocytes from RRV-induced BA mice had increased expression of MHC class I and CD40 (1/2 — 1 log-fold increase) and aberrant expression of MHC class II in approximately 16–20% of the population (range with repeat FACS analysis). In contrast to cultured cholangiocytes, freshly isolated cholangiocytes ex vivo from either BSS or BA mice did not express the costimulatory molecules CD80 or CD86.
Fig. 3.
Antigen-presenting cell (APC) surface markers on cholangiocytes isolated ex vivo from livers of balanced salt solution (BSS) control and Rhesus rotavirus (RRV)-induced biliary atresia (BA) mice. (A) Cholangiocytes were identified based on cell surface expression of TROMA III (anticytokeratin antibody). The specificity of this antibody was confirmed by immunohistochemistry staining of mouse liver and bile duct tissue. On the left is an extrahepatic bile duct incubated with anti-TROMA III-AF555 (red) and counterstained with 4,6-diamidino-2-phenylindole nuclear stain (blue) (magnification: × 200). On the right, fluorescent-activated cell sorting analysis of liver cells revealed that approximately 5–7% of all liver cells were cholangiocytes (TROMA III positive). (B) Ex vivo cholangiocytes from BSS and BA mice were double stained with TROMA III-AF555 and various APC markers-fluorescein isothiocyanate (FITC) and analysed by flow cytometry. Normal cholangiocytes express major histocompatibility complex (MHC) class I and CD40 while cholangiocytes from BA mice showed upregulation of these APC molecules as well as aberrant expression of MHC class II in approximately 16% of the population. Isotype controls were negative (data not shown).
Cultured cholangiocytes do not function as competent antigen-presenting cells in T-cell stimulation assays
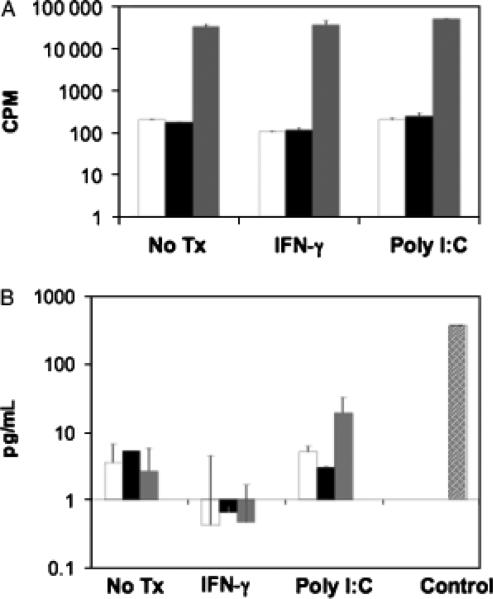
Cultured cholangiocytes expressed all of the necessary surface markers required for effective antigen presentation. Therefore, analysis of APC function was performed with cholangiocytes as the stimulator (APC), OVA peptide-specific T cells from DO11.10 BALB/cJ mice as the responders and OVA323–339 peptide as the stimulating antigen. This system tested the ability of cholangiocytes to present OVA antigen in the context of MHC-class II to OVA-specific T cells. Despite the presence of APC markers on cholangiocytes, neither resting cholangiocytes, nor cholangiocytes cultured with RRV, poly I:C or IFN-γ stimulated the T cells to proliferate in the presence of OVA antigen (Fig. 4A). A complementary assessment of cholangiocyte APC function was performed utilizing a DO11.10 T-cell hybridoma line that produces IL-2 upon stimulation with OVA peptide presented in the context of MHC-class II. In parallel with the proliferation assay, the cholangiocytes (resting or cultured with RRV, poly I:C, IFN-γ) failed to stimulate IL-2 production from the hybridomas in the presence of OVA antigen (Fig. 4B).
Fig. 4.
Functional antigen presentation of cultured cholangiocytes as measured by lymphoproliferation assay and interleukin (IL)-2 production. (A) Cholangiocytes functioned as experimental antigen-presenting cells (APCs) and were pretreated with media alone, poly I:C or interferon-γ (IFN-γ) before mitomycin C treatment. 2.5 × 105 cholangiocytes were cultured with 2.5 × 105 DO11.10 T cells in the absence (white bars) or presence (black bars) of 1 μg/ml ovalbumin (OVA)323–339 antigen. As a positive control, DO11.10 T cells were cultured with 2.5 × 105 mitomycin C-treated bulk splenocytes with OVA antigen (grey bar). Cultures were pulsed with 1 μCi/well of thymidine-[methyl-3H] at 72 h and harvested 18 h thereafter. Values represent c.p.m. ± SEM of triplicate cultures. Pretreated cholangiocytes were unable to induce OVA antigen-specific T-cell proliferation compared with control APCs. (B) Cholangiocytes were pretreated with media alone, poly I:C or IFN-γ before mitomycin C treatment. 2.5 × 105 cholangiocytes were cultured alone (white bar), or cocultured with 2.5 × 105 OVA-specific DO11.10 hybridoma cells and without (black bars) or with OVA antigen (grey bars). As a positive control, DO11.10 hybridoma cells were cultured with 2.5 × 105 mitomycin C-treated bulk splenocytes with OVA antigen (hatched bar). Culture supernatants were collected at 24 h and analysed by enzyme-linked immunosorbent assay for IL-2 protein. Values represent the mean pg/ml ± SEM. Cholangiocytes were unable to induce IL-2 secretion from DO11.10 hybridomas in the presence of OVA antigen compared with control APCs.
Cultured and ex vivo isolated cholangiocytes produce pro-inflammatory cytokines and chemokines
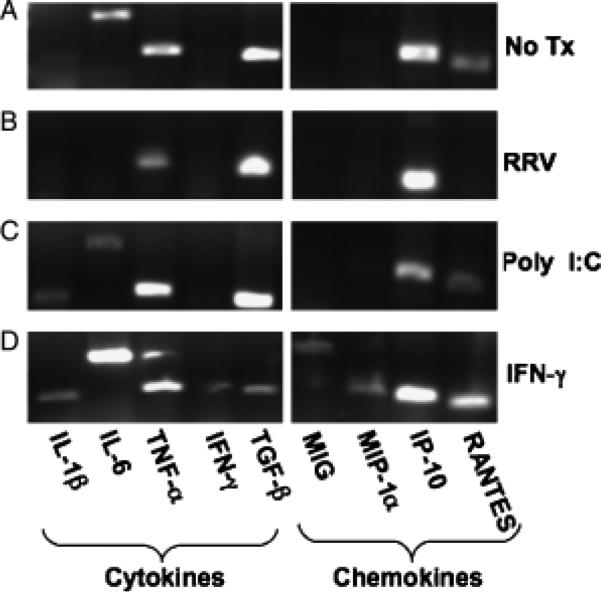
A second role that cholangiocytes may play in liver disease is through modulation of the immune response during inflammation. Investigation of the generation of pro-inflammatory cytokines and chemokines was performed by PCR analysis of mRNA transcripts from cultured and ex vivo cholangiocytes and ELISA analysis of specific cytokine proteins produced from cultured cholangiocytes. At baseline, resting cultured cholangiocytes produced RNA for cytokines IL-1, IL-6, TNF-α, TGF-β and chemokines IP-10 and RANTES. RRV-infected cholangiocytes resulted in downregulation of IL-6 and RANTES and no change was found in poly I:C-treated cholangiocytes. IFN-γ-treated cholangiocytes generated RNA transcripts for all cytokines and chemokines, including MIG and MIP-1α (Fig. 5).
Fig. 5.
Cultured cholangiocytes produce pro-inflammatory cytokines and chemokines. RNA was extracted from the cholangiocyte cell line alone (A) or cholangiocytes pretreated with Rhesus rotavirus (RRV) (B), poly I:C (C) or interferon-γ (IFN-γ) (D). RT-PCR was performed on cDNA using interleukin (IL)-1β, IL-6, tumour necrosis factor-α (TNF-α), IFN-γ, transforming growth factor-β (TGF-β), monokine induced by γ-interferon (MIG), macrophage inflammatory protein-1α (MIP-1α), γ-interferon inducible protein-10 (IP-10) and regulated upon activation, normal T-cell expressed and secreted (RANTES) primer pairs.
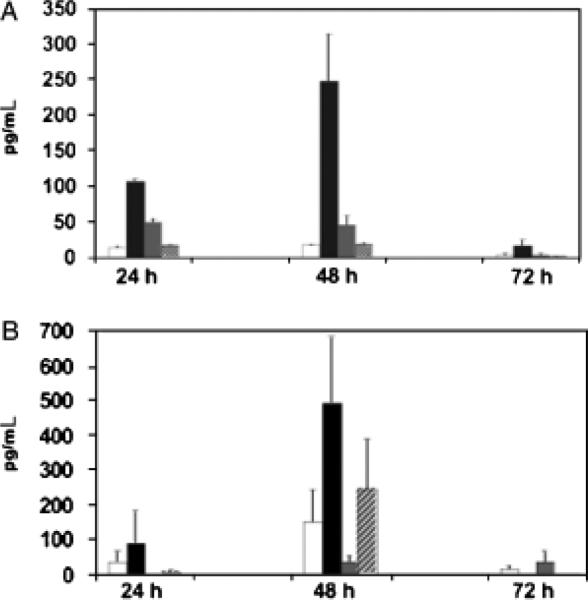
RNA transcripts for IL-6 and TGF-β were present in the majority of cultured cholangiocytes under the different experimental conditions. It is known that IL-6 and TGF-β act synergistically to promote the differentiation of a naïve T cell into a Th17 cell with subsequent production of IL-17. This IL-17 pathway of inflammation has been associated with eliciting autoimmune disease (21). Therefore, we investigated if the cholangiocyte was capable of making large quantities of IL-6 and TGF-β proteins as well. Cholangiocytes treated with poly I:C generated modest amounts of IL-6 proteins and cholangiocytes treated with IFN-γ produced abundant amounts of IL-6 and TGF-β proteins compared with unstimulated controls at both 24 and 48 h in culture (Fig. 6).
Fig. 6.
Cultured cholangiocytes produce interleukin (IL)-6 and transforming growth factor-β (TGF-β) proteins. Cholangiocytes from the cell line were cultured alone (white bar) or with interferon-γ (IFN-γ) (black bar), poly I:C (grey bar) or Rhesus rotavirus (hatched bar) and culture supernatants were collected at 24, 48 and 72 h to assess for IL-6 (A) and TGF-β (B) proteins by enzyme-linked immunosorbent assay. Values represent the mean pg/ml ± SEM. Cholangiocytes treated with poly I:C generated modest amounts of IL-6 and those treated with IFN-γ produced abundant amounts of IL-6 and TGF-β compared with unstimulated controls at 24 and 48 h in culture.
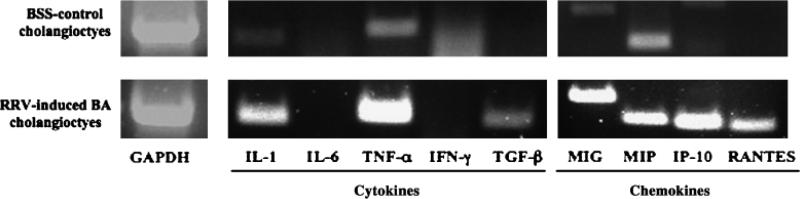
Furthermore, in order to characterize the immune modulator capabilities of freshly isolated (ex vivo) cholangiocytes, RNA was extracted from cholangiocytes isolated in high purity (> 98%) by magnetic cell sorting from 2-week-old BSS control and RRV-induced BA livers. Similar to untreated cultured cholangiocytes, ex vivo BSS cholangiocytes contained mRNA transcripts for cytokines IL-1 and TNF-α (Fig. 7). However, unlike the cultured cholangiocytes, the ex vivo BSS cholangiocytes did not express IL-6 or TGF-β. In addition, chemokines MIG and MIP-1β were present in ex vivo BSS cholangiocytes, while IP-10 and RANTES were present in untreated cultured cholangiocytes. In RRV-induced murine BA the cytokine milieu within the liver consists of Th1 cytokines (12). Therefore, the cholangiocytes are not only exposed to RRV, but also to IFN-γ and TNF-α in the diseased animal. Cholangiocytes isolated from 2-week-old BA mice expressed mRNA transcripts for IL-1, TNF-α and TGF-β and all chemokines, a similar profile to that of the IFN-γ-treated cultured cholangiocyte cell line.
Fig. 7.
Cytokine and chemokine profile of purified cholangiocytes isolated ex vivo from murine balanced salt solution (BSS) control and Rhesus rotavirus (RRV)-induced biliary atresia (BA) mice. Cholangiocytes were isolated by magnetic cell sorting (purity > 98%) from pooled liver samples of either BSS control or BA mice. RNA was extracted and RT-PCR was performed on cDNA using interleukin (IL)-1β, IL-6, tumour necrosis factor-α (TNF-α), interferon-γ (IFN-γ), transforming growth factor-β (TGF-β), monokine induced by γ-interferon (MIG), macrophage inflammatory protein-1α (MIP-1α), γ-interferon inducible protein-10 (IP-10) and regulated upon activation, normal T-cell expressed and secreted (RANTES) primer pairs.
Discussion
Currently, the pathogenesis of BA remains unknown; however there is a growing body of evidence to suggest that an initial viral insult leads to a progressive auto-immune-mediated destruction of the biliary tree (22, 23). The cholangiocyte may be a critical component of the ongoing inflammation by regulation of the immune response through cytokine and chemokine production, lymphocyte recruitment and adhesion, and possibly through antigen presentation and activation of T cells. This study provided evidence for the cholangiocyte as an immune modulator with generation of a variety of cytokines and chemokines. Furthermore, cultured cholangiocytes expressed all the necessary surface markers for antigen presentation but failed to function as competent antigen presenters in the context of MHC-class II. Cultured cholangiocytes had greater expression of MHC-class I and it is possible that these cells could present antigen to CD8+ T cells in the context of MHC-class I. However, the OVA antigen-specific system analysed only MHC-class II presentation, a limitation of this study. Previous studies have suggested that epithelial cells, including cholangiocytes, may present antigen in the context of MHC-class II leading to either T-cell activation or anergy dependent upon the presence or absence of costimulation via traditional or alternative activation pathways (14–20, 24, 25). For example, Kamihira et al. (26) showed that biliary epithelial cells did not induce proliferation of autoreactive T-cell clones. Leon et al. (18) were unable to induce T-cell proliferation using cultured human biliary epithelial cells as APCs without the addition of anti-CD28 antibodies that served to augment costimulation. The presence of T-cell anergy could explain why the cultured cholangiocytes in this study expressed APC markers but did not stimulate the T cell to proliferate or produce IL-2.
Lazaridis et al. (27) proposed a ‘reactive cholangiocyte’ model in which activated cholangiocytes lose their differentiated phenotype, acquire expression of adhesion molecules and are capable of producing pro-inflammatory cytokines and chemotactic mediators. In this study, proinflammatory cytokines and chemokines were identified from both the cultured cholangiocyte cell line under various conditions as well as from 2-week-old RRV-induced mouse livers. Cholangiocyte cell line coculture with IFN-γ was associated with the widest spectrum of expression of cytokines and chemokines. IFN-γ is present in the livers of infants with BA and is a key mediator of the inflammatory injury to bile ducts in the murine model (11, 13). Based on these studies, one could postulate that the IFN-γ stimulates the cholangiocyte to produce additional cytokines and chemokines, augmenting the inflammatory response. IFN-γ stimulation of cultured cholangiocytes also led to significant increases in IL-6 and TGF-β protein expression. In cholangiocytes isolated ex vivo from BA mice, TGF-β was upregulated; however IL-6 was not. The lack of IL-6 in the ex vivo cholangiocytes compared with cultured cholangiocytes may be due to timing of collection of ex vivo cholangiocytes. It is possible that this cytokine was upregulated earlier in the disease process (i.e. in the first week of life), and future experiments will analyse cytokine and chemokine expression at various time points. IL-6 and TGF-β can synergistically promote the naïve CD4+ T cell to differentiate into a Th17 cell. The Th17 effector cell has been associated with eliciting autoimmunity (21). In murine BA, we have recently shown that autoreactive T cells specific to bile duct epithelia contribute to the biliary injury. Future studies will determine if the IL-6/TGF-β pathway of Th17 cellular differentiation plays a role in the bile duct targeted autoimmunity of murine BA.
Acknowledgements
The authors wish to acknowledge Dr Gary Mierau for performing the electron microscopy studies.
Grants: This research was supported by a research grant no. NIH T32 DK067009-02.
References
- 1.Chardot C, Carton M, Spire-Bendelac N, et al. Epidemiology of biliary atresia in France: a national study 1986–96. J Hepatol. 1999;31:1006–13. doi: 10.1016/s0168-8278(99)80312-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen SM, Chang MH, the Taiwan Infant Stool Color Card Study Group et al. Screening for biliary atresia by infant stool color card in Taiwan. Pediatrics. 2006;117:1147–54. doi: 10.1542/peds.2005-1267. [DOI] [PubMed] [Google Scholar]
- 3.Karrer FM, Price MR, Bensard DD, et al. Long-term results with the Kasai operation for biliary atresia. Arch Surg. 1996;131:493–6. doi: 10.1001/archsurg.1996.01430170039006. [DOI] [PubMed] [Google Scholar]
- 4.Karrer FM, Bensard DD. Neonatal cholestasis. Semin Pediatr Surg. 2000;9:166–9. doi: 10.1053/spsu.2000.18847. [DOI] [PubMed] [Google Scholar]
- 5.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57:87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]
- 6.Riepenhoff-Talty M, Gouvea V, Evans MJ, et al. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis. 1996;174:8–15. doi: 10.1093/infdis/174.1.8. [DOI] [PubMed] [Google Scholar]
- 7.Petersen C, Biermanns D, Kuske M, et al. New aspects in a murine model fro extrahepatic biliary atresia. J Pediatr Surg. 1997;32:1190–5. doi: 10.1016/s0022-3468(97)90680-1. [DOI] [PubMed] [Google Scholar]
- 8.Riepenhoff-Talty M, Schaekel K, Clark HF, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33:394–9. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Davenport M, Gonde C, Redkar R, et al. Immunohisto-chemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg. 2001;36:1017–25. doi: 10.1053/jpsu.2001.24730. [DOI] [PubMed] [Google Scholar]
- 10.Kotb MA, El Henawy A, Talaat S, et al. Immune-mediated liver injury: prognostic value of CD4+, CD8+, and CD68+ in infants with extrahepatic biliary atresia. J Pediatr Surg. 2005;40:1252–7. doi: 10.1016/j.jpedsurg.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Mack CL, Tucker RM, Sokol RJ, et al. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res. 2004;56:79–87. doi: 10.1203/01.PDR.0000130480.51066.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115:200–9. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivakumar P, Campbell K, Sabla G, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-γ in experimental biliary atresia. J Clin Invest. 2004;114:322–9. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruickshank S, Southgate J, Selby P, Trejdosiewicz L. Expression and cytokine regulation of immune recognition elements by normal human biliary epithelial and established cell lines in vitro. J Hepatol. 1998;29:550–8. doi: 10.1016/s0168-8278(98)80149-9. [DOI] [PubMed] [Google Scholar]
- 15.Fava G, Glaser S, Francis H, Alpini G. The immunophysiology of biliary epithelium. Semin Liver Dis. 2005;25:251–64. doi: 10.1055/s-2005-916318. [DOI] [PubMed] [Google Scholar]
- 16.Himeno H, Saibara T, Onishi S, Yamamoto Y, Enzan H. Administration of interleukin-2 induces major histocompatibility complex class II expression on the biliary epithelial cells, possibly through endogenous interferon-γ production. Hepatology. 1992;16:409–17. doi: 10.1002/hep.1840160220. [DOI] [PubMed] [Google Scholar]
- 17.Hreha G, Jefferson DM, Chang-Hong Y, et al. Immortalized intrahepatic mouse biliary epithelial cells: immunologic characterization and immunogenicity. Hepatology. 1999;30:358–71. doi: 10.1002/hep.510300216. [DOI] [PubMed] [Google Scholar]
- 18.Leon M, Bassendine M, Wilson J, et al. Immunogenicity of biliary epithelium: investigation of antigen presentation to CD4+ T cells. Hepatology. 1996;24:561–7. doi: 10.1002/hep.510240317. [DOI] [PubMed] [Google Scholar]
- 19.Paradis K, Le O, Russo P, et al. Characterization and response to interleukin 1 and tumor necrosis factor of immortalized murine biliary epithelial cells. Gastroenterology. 1995;109:1308–15. doi: 10.1016/0016-5085(95)90593-6. [DOI] [PubMed] [Google Scholar]
- 20.Reynoso-Paz S, Coppel R, Mackay I, et al. The immunobiology of bile and biliary epithelium. Hepatology. 1999;30:351–7. doi: 10.1002/hep.510300218. [DOI] [PubMed] [Google Scholar]
- 21.Hirota K, Hashimoto M, Yoshitomi H, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–7. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber RA, Kleinman RE. Genetics, immunology and biliary atresia: an opening or a diversion? J Pediatr Gastroenterol Nutr. 1993;16:111–3. doi: 10.1097/00005176-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57:87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]
- 24.Van den Oord JJ, Sciot R, Desmet VJ. Expression of MHC products by normal and abnormal bile duct epithelium. J Hepatol. 1986;3:310–7. doi: 10.1016/s0168-8278(86)80483-4. [DOI] [PubMed] [Google Scholar]
- 25.Wu CT, Davis P, Luketic V, Gershwin ME. A review of the physiological and immunological functions of biliary epithelial cells: targets for primary biliary cirrhosis, primary sclerosing cholangitis and drug-induced ductopenias. Clin Dev Immunol. 2004;11:205–13. doi: 10.1080/17402520400004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamihira T, Shimoda S, Nakamura M, et al. Biliary epithelial cell regulate autoreactive T cell: implications for biliary specific diseases. Hepatology. 2005;41:151–9. doi: 10.1002/hep.20494. [DOI] [PubMed] [Google Scholar]
- 27.Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–77. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]