Abstract
The effects of arsenic exposure during rapid brain growth period (RBGP) (postnatal period 4-11) on pyramidal neurons of cornu ammonis (specifically CA1 and CA3 regions) and granule cells of dentate gyrus (DG) of rat hippocampus were studied. Wistar rat pups, subdivided into the control (group I) and the experimental groups (group II, III, and IV), received distilled water and sodium arsenite (aqueous solution of 1.0, 1.5, and 2.0 mg/kg body weight, respectively) by intraperitoneal (i.p.) route. On postnatal day (PND) 12, the animals were sacrificed and brain tissue obtained. Paraffin sections (8 μm thick) stained with Cresyl Violet (CV) were observed for morphological and morphometric parameters. Arsenic induced programmed cell death (apoptosis) was studied using Terminal deoxyribonucleotidyl transferase mediated dUTP biotin Nick End Labeling (TUNEL) technique on the paraffin sections. Microscopy revealed decreased number and isolation of pyramidal neurons in superficial layers, misalignments of pyramidal cells in stratum pyramidale (SP) of CA1 and CA3 in experimental group III and IV, and presence of polymorphic cells in subgranular zone of ectal limb of dentate gyrus (suggestive of arsenic induced proliferation and migration of granule cells in the dentate gyrus). Morphometric assessments quantified and confirmed the microscopic findings. The mean nuclear area of pyramidal cells was increased and cell density was decreased in the CA1, CA3, and DG of experimental groups in comparison to the control group. Increase in the TUNEL positive cells in DG was observed in the experimental group IV, suggestive of increased apoptosis. These observations confirm vulnerability of pyramidal (CA1, CA3) and granule cells (DG) of hippocampus during RBGP.
Keywords: Apoptosis, arsenic, dentate gyrus, granule cells, hippocampus, morphometry, pyramidal cells
INTRODUCTION
The ubiquitous distribution of arsenic (a metalloid) in the soil, air and water[1] makes it an environmental contaminant of global concern. The environmental levels of arsenic and its derivatives keep on changing on account of a number of dynamic processes by natural and human activities.[2,3] Hazardous waste sites (HWS) are also a source of arsenic.[3] Arsenic levels keep on getting added to the environment through weathering of rocks, burning of fossil fuels, smelting of ores, etc.[4] Arsenic affects all organ systems of the body including central nervous system (CNS). In the nervous system seizures, encephalopathy, peripheral neuropathies, and behavioral changes have been reported.[5]
Nevertheless, the increased levels of arsenic in drinking water (permissible levels of arsenic-10 parts per billion)[6,7] at several places around the globe[8,9] contribute predominantly towards increased incidence of exposure to arsenic. Del Razo et al.,[9] assessed daily intake of arsenic along with ingested food.
Arsenic has been reported to inactivate various enzymes essential in cellular energy pathways such as replication and repair of deoxyribonucleic acid (DNA).[5,10] Arsenic exposure in rats has been reported to decrease glutathione (GSH) levels and increase lipid peroxidation, thereby resulting in oxidative stress.[5,11,12,13] Neurons are in particular vulnerable to free radical attack because of polyunsaturated fatty acids forming a major constituent of neural cell membranes and acting as substrates for free radicals.[14] Neurotoxicant effects observed following exposure to various environmental factors such as (behavior changes) lead, (low birth weight, mental retardation) methyl mercury on the development of humans.[15] Various reports, based on morphological, biochemical, and behavioral studies are suggestive of increased vulnerability of developing nervous system towards teratogens at different stage of development.[16] Developing nervous system is more susceptible to teratogens because it lacks the blood brain barrier.[17] Hippocampus (highly plastic structure) is susceptible towards various environmental stresses.[18,19]
The first 2 weeks after birth in rats signify the period of rapid brain growth and correspond to ‘brain growth spurt’ in humans extending through last trimester of pregnancy to early postnatal period.[20] During this period, the developing nervous system passes simultaneously through the phases of extensive development and maximum differentiation and these ongoing processes influence the vulnerability towards a number of insults.[21] Accordingly, the present work was planned to investigate the effects of arsenic exposure during rapid brain growth period (RBGP) on the two neuronal populations of rat hippocampus such as pyramidal cells (generated prenatally and undergoing differentiation during early postnatal period) and the granule cells (which continue to be generated throughout postnatal and adult life),[22] and to assess if the developmental stage of cells has any role in determining the differential vulnerability towards an insult.
MATERIALS AND METHODS
Animals
The present study was carried out in Wistar rat (Rattus norvergicus) pups. Pregnant female Wistar rats (Gestation day 17-19) were procured from the Experimental Animal Facility (EAF) of the All India Institute of Medical Sciences (AIIMS) after obtaining ethical clearance from Institute Ethical Committee (IEC).
Experimental diet
The animals were housed in cages and kept in temperature (25°C) and humidity (60%) controlled rooms (EAF, AIIMS) with a constant 12/12 hour light and dark cycle. The animals were fed standard rodent diet along with drinking water ad libitum.
Experimental design
The day of delivery was considered as postnatal day 0 (PND 0). The delivered pups were observed for any gross malformation present and divided into the control (Group I) and the experimental (Group II, III, and IV) groups randomly (six animals in each group; n = 6). Sodium arsenite was administered as per protocol.[23,24] The dosage of Arsenic selected for the present study was based on World Health Organization (WHO) guidelines according to which Lethal dose 50 (LD50) of Sodium arsenite in rats is 10 mg/kg body weight and Effective dose (ED; 1/10th of LD) is 1 mg/kg.[25] ED (1 mg/kg) was administered to group II animals, whereas doses higher by 0.5 (1.5 mg/kg) and 1.0 (2.0 mg/kg) than ED were administered to group III and IV, respectively. Sodium arsenite as an aqueous solution was administered to the experimental animals by daily intraperitoneal (i.p.) injection between 10 am and 11 am, starting from PND 4 to 11 (RBGP) as per the dosage schedule. Distilled water was administered to the control animals for similar duration and through the same route. During the experimental period, the pups from both the groups were observed for the signs of physical development (ear unfolding, development of fur and spontaneous quadruped walking with the ventral surface of the body off the floor) to ascertain their general well being.
Sampling
The pups were randomly assigned into each of the four groups (Group I; control and Groups II, III, IV; experimental). Each group having six pups (n = 6) in total. Post sacrifice, Cresyl Violet (CV) staining and Terminal deoxyribonucleotidyl transferase mediated dUTP biotin Nick End Labeling (TUNEL) assay was performed on the paraffinized hippocampal sections of each group.
Terminal deoxyribonucleotidyl transferase mediated dUTP biotin Nick End Labeling assay
The sections at predefined periodicity were subjected to TUNEL staining. These sections were deparaffinized using xylene reagent. Briefly, the deparaffinized sections were incubated with proteinease K (30 μg/ml) followed by treatment with 0.3% hydrogen peroxide (to prevent endogenous peroxide activity) for 30 minutes each at room temperature. Incubation with TUNEL reaction mixture was carried out in humidified chamber at 37°C. Converter-pod was added for signal conversion and incubation was carried out in 3,3’-Diaminobenzidine (DAB) reaction mixture, serving as a chromogen. After dehydration, the sections were mounted in DPX. Sections from adult rat hippocampus were processed simultaneously with no enzyme in TUNEL reaction mixture as negative controls and sections treated with deoxyribonuclease (DNAse) prior to TUNEL reaction mixture as the positive controls. The sections stained with TUNEL technique were observed for qualitative features of TUNEL positive cells in cornu ammonis 1 (CA1), cornu ammonis 3 (CA3), and the dentate gyrus (DG) region (ectal (DGEC) and endal (DGEN) limbs).
Histology and histochemistry
At the end of the experimental period (PND 12), the animals were sacrificed under ether anesthesia by trans-cardiac perfusion with 4% para-formaldehyde in 0.1 M phosphate buffer. The brains were dissected out, weighed, and immersed in the same fixative for post fixation.
The tissue blocks containing hippocampus (−3.3 mm to −3.8 mm posterior to the bregma)[26] were further processed for paraffin embedding and 8 μ thick serial sections were cut in a coronal plane under a microtome. The sections were stained with CV using the conventional method and mounted.
Photomicrography
The CV stained coronal sections of the rat hippocampus were observed under the light microscope (Nikon Eclipse E600) and photographed using a digital camera (DS-Fi1, Nikon, Melville, NY, USA) for studying the morphological features of pyramidal neurons in the CA region (CA1 and CA3) and the granule cells of DG region (DGEC and DGEN limbs).
Morphometric assessments
For morphometric parameters, CV stained sections were viewed under the Nikon Microphot-FX microscope, mounted with Cool SNAP Procf color digital camera (Roper scientific, USA), and attached to an image analysis system driven by Image Pro-Plus software (version 4.5, Media Cybernetics, USA). The calibration was done with the Carl Zeiss stage micrometer. High-resolution high-power (40 × objective) digital photomicrographs were captured and used for determining the nuclear area and density of pyramidal cells (CA1 and CA3) and the granule cells (DGEC and DGEN limbs of DG). Polygon function of the measurement in the image proplus software was used to manually delineate the nuclear profiles. The nuclei of the pyramidal and granule cells with well-defined nuclear membrane and clearly visible nucleoli were taken into consideration for measurement [Figure 1].[27] The boxed areas [Figure 2] represent the parts of sub regions of hippocampus taken into consideration for estimation of nuclear area and cell density.
Figure 1.
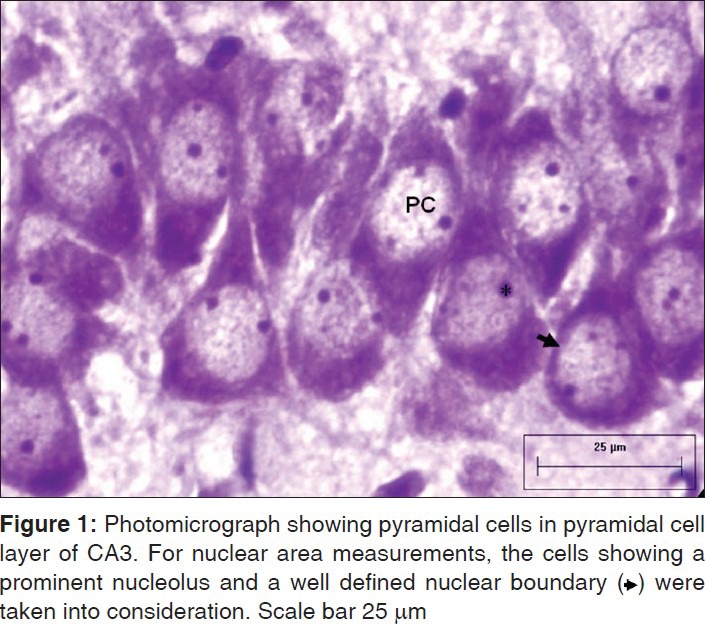
Photomicrograph showing pyramidal cells in pyramidal cell layer of CA3. For nuclear area measurements, the cells showing a prominent nucleolus and a well defined nuclear boundary ($$$) were taken into consideration. Scale bar 25 μm
Figure 2.
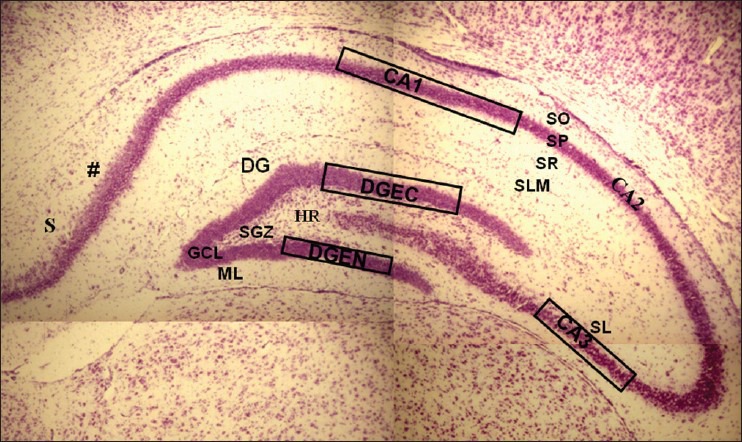
Photomicrograph of CV stained coronal section of hippocampus (−3.8 mm behind bregma) showing the boxed areas in CA1, CA3, and DG (DGEC=ectal limb, DGEN=endal limb) SO = Stratum Oriens, SP=Stratum Pyramidale, SR=Stratum Radiatum, SLM=Stratum Lacunosum-moleculare, SL=Stratum Lucidum, ML=Molecular Layer, GCL=Granule Cell Layer, SGZ=Subgranular zone, HR=Hilar Region, (#)=A transition in SP thickness marks the junction between CA1 and subiculum (S). Scale bar 240 μm
Briefly, a total of 36 coronal sections from each group (6-7 sections/animal) were scanned and in each subdivision of hippocampus (CA1, CA3, DGEC, and DGEN); approximately, 70 neurons per region per animal were analyzed.[28] In each animal, the first section was chosen randomly at the level of −3.3 mm posterior to bregma and every fifth section (40 μm caudal to the first one) was taken subsequently to avoid repeat measuring of the cells according to the systematic random sampling procedure.[29]
Counting frames with areas of 0.012 mm2 (12,150 μm2) and 0.010 mm2 (10,125 μm2) were used for CA (CA1 and CA3 regions) and DG (DGEC and DGEN regions), respectively. The total numbers of neuronal nuclear profiles falling in the counting frames of micrographs were counted. All counting procedures were carried out using the “Forbidden Line” rule.[30] In order to obtain acceptable precision of the estimates of cell density, about 100-200 neurons per animal[31] were counted. It was ensured that the total counts (Q−) were more than 100 in each region (CA1, CA3 and DGEC and DGEN) per animal by selecting 10 sections in a systematic random manner. Estimates of the cell densities of pyramidal and granule cells were expressed as number of cells per millimeter square (mm2).[32]
Statistical analysis
The data obtained for body weight, mean nuclear area of pyramidal cells (CA1 and CA3); granule cells (DGEC and DGEN) and cell densities (per mm2) was represented as Mean ± SE and analyzed by one way analysis of variance (ANOVA). Bonferroni post hoc test was used to compare the means wherever needed. P < 0.05 was considered significant. Statistical Package for Social Sciences (SPSS) 17 software was used for statistical analysis.
Reagents and stains
All reagents and stains were from Sigma Aldrich (St. Louis, MO, USA).
RESULTS
General features
During the experimental period (PND 4-11), the features of normal physical development such as ear unfolding, development of fur and locomotor activity appeared at scheduled time periods (PND 4, 6, and 11) in the control and the treated animals. There was no observed difference in the two groups’ general activities such as food intake, alertness, and movement.
Microscopic observations (Cresyl violet stained sections)
Cornu ammonis (CA1, CA3)
No remarkable abnormality was observed in the hippocampal sections from animals of the control group. Qualitative observations revealed preservation of laminar stratification in CA1 and CA3 region of experimental animals [Figure 3c, 3e, 3g and 3d, 3f, 3h]; were being largely comparable to that of controls [Figure 3a and b]. On the whole, the Pyramidal cell layer (PCL) thickness was apparently decreased with certain amount of misalignment of pyramidal cells in the experimental groups as against the controls. The normal neurons were identified by their rounded and pale nuclei, whereas degenerating neurons had smaller cell bodies and pyknotic nuclei. There was evidence of vacuolation of neuropil surrounding the degenerating neurons. Few pyramidal cells could be spotted ectopically [Figure 3d, f, h.]
Figure 3.
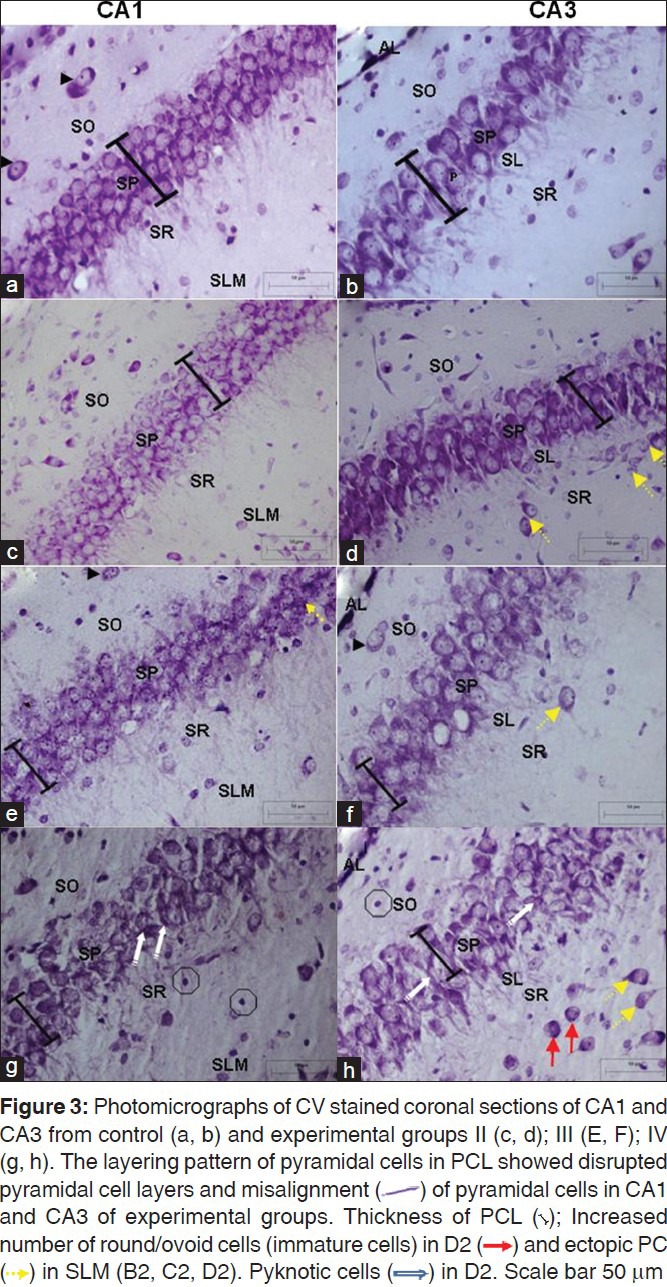
Dentate gyrus
The stratification was preserved in DG across the control and the experimental animals [Figure 4]; In DGEN and DGEC, the granule cells in the outer part of granule cell layer (GCL) were larger and lightly stained as against the smaller and darker cells in the deeper layers, suggestive of presence of neurons being generated in deeper layers. On the whole, the thickness of GCL was decreased in DGEC and DGEN of experimental animals as against those of controls [Figure 4a and b]. Also, disruption of granule cell layer was evident in DG of experimental animals with increased number of migrating cells in sub granular zone (SGZ) along lower border of GCL [Figure 4c and g.]
Figure 4.
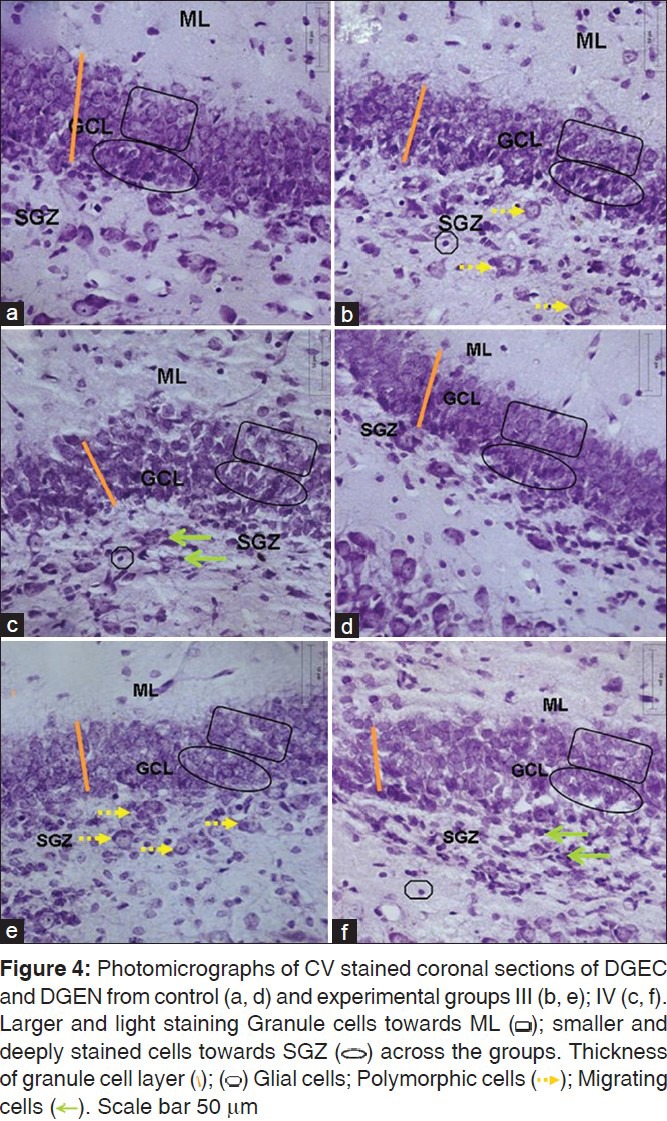
Morphometric observations
The estimates of mean nuclear area of the pyramidal cells in the CA1 and CA3 regions of control Group (I) as well as the experimental groups (II, III, and IV) are presented in Figure 5. On the whole, an increase in mean nuclear area of pyramidal cells was evident in CA1 and CA3 regions of experimental groups (II, III, and IV) as against the control group. However, across the treatment groups, a statistically significant increase in mean nuclear area was noted in group III and IV for CA1 and group IV for CA3 in comparison to control group (I).
Figure 5.
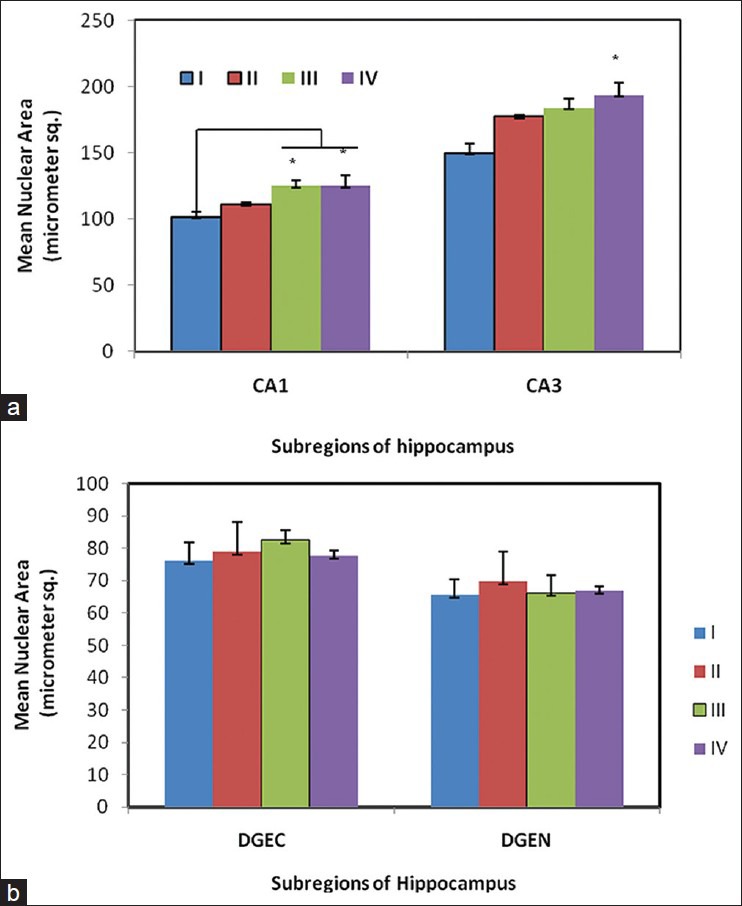
Mean nuclear area (μm2) of neurons in hippocampus of pyramidal cells (CA1 and CA3) (a) and granule cells (DGEC and DGEN) (b) in hippocampus of Wistar rat pups exposed to sodium arsenite (PND 4-11); n = 6/group. Mean ± SE, * P< 0.05 Vs Group I (One way ANOVA). Statistically significant increase was observed in CA1 (group III and IV) and CA3 (group III)
The mean nuclear area of the granule cells in DGEC and DGEN of experimental groups (II, III, and IV) and control group (I) is presented in Figure 5. The estimates are indicative of an apparent increase in the mean nuclear area of the granule cells in DGEC and DGEN of experimental groups though the difference is not statistically significant as against the control values.
The estimates of cell density (pyramidal and granule cells) in the CA1 and CA3 and DGEC and DGEN of the experimental groups (II, III, and IV) and control group (I) are presented in Figure 6. A negative trend in the packing density of pyramidal and a granule cell was noted in the experimental groups as against the control group. However, significant difference was noted in packing density of pyramidal cells in CA1 region (group I vs. group III and IV * P < 0.05), whereas in CA3, the difference in packing density of pyramidal cells was not significant across the groups. The cell density of granule cells in the DG was not statistically altered as well among the groups [Figure 6].
Figure 6.
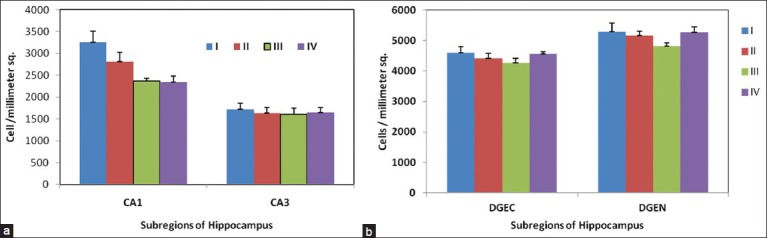
Cell density (number of cells/mm2) of pyramidal cells (CA1 and CA3) (a) and granule cells (DGEC and DGEN) (b) in hippocampus of Wistar rat pups exposed to sodium arsenite (PND 4-11); n= 6/group. Mean ± SE, *P< 0.05 Vs Group I (One way ANOVA). Statistically significant increase was observed in CA1 (group IV)
Terminal deoxynucleotidyl transferase mediated dUTP biotin Nick End Labeling assay for deoxyribonucleic acid damage
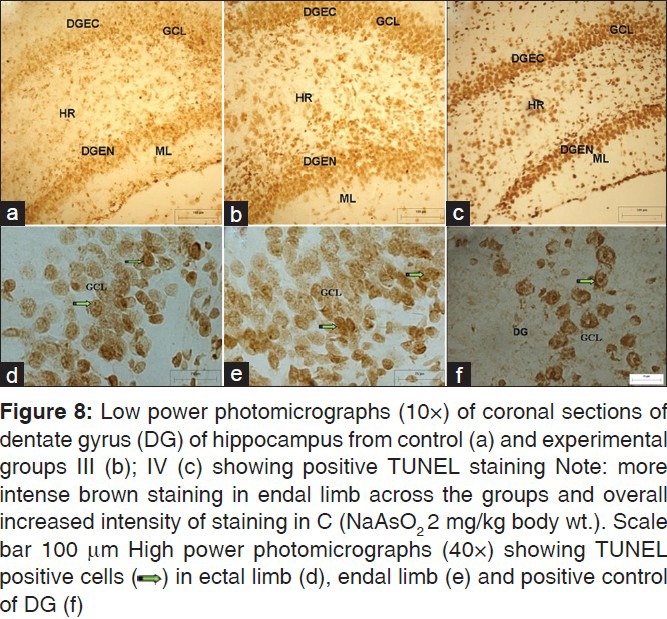
TUNEL positive cells depicting apoptotic nuclei containing condensed chromatin were spotted throughout the extent of CA in the control as well as the experimental animals, though qualitative observations suggested increased number of positive cells in the hippocampi of experimental animals. Also, TUNEL positive neurons were more abundant in the CA1 region of experimental animals in comparison to CA3 [Figure 7], apparently due to overall increased density of pyramidal cells in CA1. In DG, TUNEL positive cells were spotted in DGEC, DGEN, and hilar regions in the control as well as the experimental animals [Figure 8]. On the whole, the staining was more intense in the DGEC and DGEN limbs than the hilar region across the groups and in particular in DGEC and DGEN of group IV.
Figure 7.
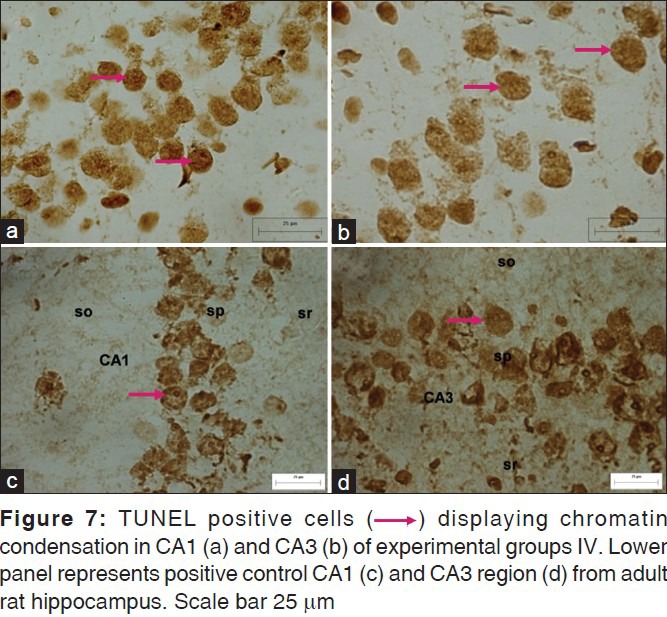
Figure 8.
DISCUSSION
During the experimental period, the features of normal physical development appeared at scheduled time periods, thereby suggesting apparently uneventful postnatal development. We did not observe any dose related increase or decrease in the locomotor activity of experimental animals It could be further assumed that the short exposure time, as in the present study, might not be sufficient to produce grossly noticeable changes.
Pyramidal cell layer thickness was observed to be somewhat reduced with disruption of cells in experimental animals and this reduced thickness seemed to be dose related. Somewhat similar observations have been reported in neonatal rats exposed to formaldehyde,[33] where investigators noticed significant decrease in volume of pyramidal cell layer in exposed animals (at PND 30 and PND 90). Darkly stained non pyramidal neurons, presumably inter-neurons, were most abundantly dispersed in Stratum Radiatum (SR) and Stratum Lacunosum-moleculare (SLM) of tissue sections obtained from experimental group IV. The increased expression of glial cells in the experimental group is substantiated by the earlier reports,[34,35] according to which glial cells increase in aged brain and neurodegenerative conditions. Few pyramidal cells were spotted in the superficial layers (SR and SLM) in tissue sections of experimental animals; irrespective of doses of arsenic administrated, being maximally evident in group IV. These could be indicative of delayed migration and delayed settlement of these cells in the PCL, which otherwise is completed either by late embryonic or early postnatal period. However, delayed settlement could be due to altered neuronal neuroglial interaction subsequent to arsenic exposure. The healthy glial guided neuronal migration during development is considered as an important step in determining the final destination of neurons and astrotactin, a neuronal cell surface antigen is supposed to function as neuron glia ligand.[36] It could be presumed that arsenic exposure could decrease the viability of astroglial cells, thereby hampering the glial guided neuronal migration. Falluel-Morel et al.,[37] observed the effects of methylmercury (MeHg) on the hippocampus during developmental period and concluded that loss of cells in the dentate gyrus of hippocampus especially in the hilar region and GCL and also observed lesser sensitivity of glial cells (molecular layer). Effects of hypothyroidism on cell proliferation and neuroblasts in the hippocampal dentate gyrus showed significantly alleviated reduction of cell proliferation and neuronal progenitors in the DG in a rat model of type 2 diabetes.[38] Delayed migration of purkinje cells in rat cerebellum following arsenic exposure during RBGP was observed by.[23] Upregulation of glial cells has also been associated with pathogenesis of senile neurodegenerative conditions like Alzheimer's disease. Hence, we presume that in the present study, upregulation of glial cells could be suggestive of neurodegenerative effects induced by the sodium arsenite.
The present study revealed an increase in nuclear area of the pyramidal cells (CA1, CA3) and granule cells (DGEC, DGEN) in experimental animals receiving different doses of sodium arsenite. Increase in soma size of pyramidal cells (CA1 region) and granule cells (DG) have previously been reported by[28] following chronic stress. Increase in neuronal nuclear size (reflecting increase in cell size) could be linked to increase in organelle size (hypertrophy). Proper functioning of sodium-potassium adenosine triphosphatase (Na-K ATPase) pump is one of the important requisite for the maintenance of intracellular fluid/substance balance. Cellular adenosine triphosphate (ATP) level decrease as arsenite inhibit pyruvate dehydrogenase (PDH)[11] and arsenate competes with phosphate in the process of binding to adenosine.[39] This decrease in cellular ATP jeopardizes, the role of Na-K ATPase pump, the functioning of which is determined by available ATP levels. Thus, impaired functioning of Na-K ATPase pump could precipitate Na-K imbalance across the cell,[10] thereby resulting in increase in cell size. Also, interaction of arsenite with thiol groups;[40,41] could block the sulfhydryl groups with context to dihydrolipoate (part of pyruvate dehydrogenase complex), thereby preventing its oxidation to lipoate. Since, lipoate helps in the formation of acetyl CoA from pyruvate and alpha ketoglutarate, its nonavailability interferes with Krebs cycle, thereby interrupting oxidative phosphorylation and causing depletion of cellular ATP and decreased activity of Na-K ATPase pump. Cellular swelling has also been suggested as an early feature of impending cell death. Piao et al.,[42] while working on mice (9 weeks age) exposed to arsenic (1 or 2 ppm arsenic via drinking water for forty days) concluded that it could induce oxidative stress leads to DNA damage in the brain tissues in vivo. Interestingly, it was observed that the rats pretreated with GSH precursors prior to the exposure to arsenic, perform better with regard to maintaining GSH levels and reducing lipid peroxidation.[43] Arsenic induced increase in lipid peroxidation accompanied by reduced antioxidant activity of enzyme superoxide dismutase and glutathione peroxidase could also inhibit Na-K ATPase pump in brain cells. Miyake et al.,[44] based on their work dealing with porcine cerebral cortex suggested that lipid peroxidation products of neural cell membrane could alter the physical conformation of Na-K ATPase molecule. Also, chronic arsenic exposure has been observed to induce decrease in plasma membrane Na-K ATPase activity in mice hepatocytes. In the present study, a significant increase was observed in the nuclear size of pyramidal cells of the experimental animals. The nuclear size of the granule cells was not significantly altered in the experimental animals in comparison to control value. It could be hypothesized that the cells in the process of differentiation show increased vulnerability to arsenic induced toxicity as compared to cells that are in the process of being generated, it is supported by previous studies that cerebellar purkinje cells are more vulnerable during their maturation period following alcohol exposure rather than generation period in rat pups.[45]
The quantitative analysis revealed a significant difference in packing density of these cells in the CA1 region of experimental groups as against the control. More vulnerability of CA1 region to insults, because high intrinsic superoxide and endogenous reactive oxygen species (ROS) production in CA1 than CA3 region.[46,47] So, those under the effect of arsenic which produce excess oxidative stress that leads to depletion of antioxidant in CA1 region, cell death ensue. Similar observations were reported following exposure of alcohol on the hippocampus.[48,49] Mitochondrial permeability transition pore of CA1 region are more sensitive to calcium homeostasis and leads to active production of ROS,[50] so that vulnerability of CA1 for cerebral ischemia and neurodegenerative disease is more in comparison to CA3 region.
However, encountering statistically non-significant change in the number of granule cells (DGEC, DGEN) of experimental animals as in the present study does not rule out deleterious effects of arsenic in the said region. On the other hand, DG represents the region of active neurogenesis (granule cells) in hippocampus which could compensate for statistically non-significant decrease in packing density in DG is high[51,52,53] replenishes the cell loss.[18] Falluel-Morel et al.,[37] reported that exposure to MeHg during the perinatal period induced apoptotic cell death in hippocampus, leading to structural and functional deficits at a later stage. These investigators noted a marked decrease (29% and 22%) in the number of cells in the GCL and hilus respectively following exposure to MeHg. A significant reduction in the neuronal number in CA1, CA3, and area dentatae (rat hippocampus) subsequent to subcutaneous and dermal exposure to chlorpyrifos has been reported earlier.[54] According to these investigators, neuronal cell death could be the underlying basis for deficit in neuronal numbers in these regions.
The observation of cell loss (decreased packing density of cells) was substantiated by the results of TUNEL assay carried out for detecting programmed death at cellular level in various regions of hippocampus (CA1, CA3, DGEC, and DGEN). An apparent enhancement of TUNEL positive cells was seen in dentate gyrus of the experimental animals as well. More prominent TUNEL positive cells in DG indicated natural apoptosis as well in DG which occurs in postnatal period.[55] The enhanced tendency to apoptosis of dentate granule cells has also been suggested by Harry et al.[56] According to these investigators, trimetlyltin (TMT) exposure could cause more pronounced neurodegenration and necrosis in DG than in CA1 and CA3 regions, assuming that natural ongoing developmental apoptosis could be the enhancing factor. Vahidina et al.,[39] reported that metabolite of arsenic inhibits PDH by production of ROS, which leads ATP depletion in the cell[41] that is responsible for the release of apoptosis inducing factor. These factors responsible for DNA fragmentation, finally leads to cell death. Sodium arsenite causes apoptosis by activation of caspase pathway,[57] similar pathway is also involved in MeHg induced programmed cell death.[37] Chattopadhyay et al.,[58] studied the effect of arsenic on human foetal brain explants exposed to arsenic in culture; they noticed the disturbance in lipid peroxidation, generation of nitric oxide (NO), ROS, and apoptosis.
Exposure to arsenic during early life postnatal period result in the laminar pattern being maintained with some amount of mal alignment, increase in nuclear area and decrease in cell density of the pyramidal cells (CA) and granule cells (DG) in experimental animals. More enhanced TUNEL reaction in the CA and DG of experimental groups; enhanced reaction being more pronounced in DG in comparison to CA.
Low doses of arsenic (as used in the present study) administered in early postnatal period might not bring about grossly evident changes in features reflecting physical development. However, such low doses might be sufficient to bring about changes at cellular level as reflected by increased nuclear size (decrease in Na-K ATPase activity), decrease in cell density (increased cell loss), and enhanced TUNEL reaction (indicative of increased apoptosis).
The exact mechanism underlying differential vulnerability of various regions of hippocampus to various insults continues to remain a controversial subject till date. Whether it is the dosage of a particular substance, the duration of its exposure or the developmental stage of the subject (animal) during which exposure is made that determines maximally the intensity of vulnerability, remains yet to be unfolded. Most importantly, early insults may have repercussions in later life, appearing only with cognitive system maturation. Indeed, this is the scenario observed in this arsenic paradigm, in which exposure during development induces effects on hippocampal structure which could get expressed as functional deficits in later life.
ACKNOWLEDGMENTS
We acknowledge the financial and technical support provided by the Department of Anatomy, All India Institute of Medical Sciences (AIIMS), New Delhi, India.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jarup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–82. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez VM, Carrizales L, Jiménez-Capdeville ME, Dufour L, Giordano M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol. 2002;24:743–50. doi: 10.1016/s0892-0362(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 3.Tchounwou PB, Patlolla AK, Centeno JA. Carcinogenic and systemic health effects associated with arsenic exposure-a critical review. Toxicol Pathol. 2003;31:575–88. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- 4.Flora SJ, Bhadauria S, Kannan GM, Singh N. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: A review. J Environ Biol. 2007;28:333–47. [PubMed] [Google Scholar]
- 5.Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176:127–44. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Arsenic; Environmental Health Criteria. 2001;224:1–144. [Google Scholar]
- 7.Toxicological profile for arsenic. Atlanta, GA, USA: U.S. Department of Health and Human Services; 2007. Agency for Toxic Substances and Disease Registry (ATSDR) [PubMed] [Google Scholar]
- 8.Anawar HM, Akai J, Mostofa KM, Safiullah S, Tareq SM. Arsenic poisoning in groundwater: Health risk and geochemical sources in Bangladesh. Environ Int. 2002;27:597–604. doi: 10.1016/s0160-4120(01)00116-7. [DOI] [PubMed] [Google Scholar]
- 9.Del Razo LM, Garcia-Vargas GG, Garcia-Salcedo J, Sanmiguel MF, Rivera M, Hernandez MC, et al. Arsenic levels in cooked food and assessment of adult dietary intake of arsenic in the region lagunera, Mexico. Food Chem Toxicol. 2002;40:1423–31. doi: 10.1016/s0278-6915(02)00074-1. [DOI] [PubMed] [Google Scholar]
- 10.Santra A, Maiti A, Das S, Lahiri S, Charkaborty SK, Guha Mazumder DN. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J Toxicol Clin Toxicol. 2000;38:395–405. doi: 10.1081/clt-100100949. [DOI] [PubMed] [Google Scholar]
- 11.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 12.Shila S, Kokilavani V, Subathra M, Panneerselvam C. Brain regional responses in antioxidant system to α-lipoic acid in arsenic intoxicated rat. Toxicology. 2005;210:25–36. doi: 10.1016/j.tox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Bhadauria S, Flora SJ. Response of arsenic-induced oxidative stress, DNA damage, and metal imbalance to combined administration of DMSA and monoisoamyl-DMSA during chronic arsenic poisoning in rats. Cell Biol Toxicol. 2007;23:91–104. doi: 10.1007/s10565-006-0135-8. [DOI] [PubMed] [Google Scholar]
- 14.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 15.Mendola P, Selevan SG, Gutter S, Rice D. Environmental factors associated with a spectrum of neurodevelopment deficits. Ment Retard Dev Disabil Res Rev. 2002;8:188–97. doi: 10.1002/mrdd.10033. [DOI] [PubMed] [Google Scholar]
- 16.Rodier PM. Environmental causes of central nervous system maldevelopment. Pediatrics. 2004;113:1076–83. [PubMed] [Google Scholar]
- 17.Rodier PM. Vulnerable periods and processes during central nervous system development. Environ Health Perspect. 1994;102:121–4. doi: 10.1289/ehp.94102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miki T, Harris SJ, Wilce P, Takeuchi Y, Bedi KS. Neurons in the hilus region of the rat hippocampus are depleted in number by exposure to alcohol during early postnatal life. Hippocampus. 2000;10:284–95. doi: 10.1002/1098-1063(2000)10:3<284::AID-HIPO9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Miki T, Harris SJ, Wilce PA, Takeuchi Y, Bedi KS. The effects of alcohol exposure during early life on neuron numbers in the rat hippocampus hilus neurons and granule cells. Hippocampus. 2003;13:346–56. doi: 10.1002/hipo.10072. [DOI] [PubMed] [Google Scholar]
- 20.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 21.Belton JC, Benson NC, Hanna ML, Taylor RT. Growth inhibition and cytotoxic effects of three arsenic compounds on cultured Chinese hampster ovary cells. J Environ Sci Health. 1985;20:37–72. [Google Scholar]
- 22.Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990;301:325–42. doi: 10.1002/cne.903010302. [DOI] [PubMed] [Google Scholar]
- 23.Dhar P, Mohari N, Mehra RD. Cerebellum following sodium arsenite exposure during rapid brain growth (RBG) period. Toxicology. 2007;234:10–20. doi: 10.1016/j.tox.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya A, Dhar P, Mehra RD. Preliminary morphological and biochemical changes in rat liver following postnatal exposure to sodium arsenite. Anat Cell Biol. 2012;45:229–40. doi: 10.5115/acb.2012.45.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization: The WHO guidelines to classification of Pesticides by Hazard. 1996 [Google Scholar]
- 26.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–49. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 27.Miki T, Satriotomo I, Li HP, Matsumoto Y, Gu H, Yokoyama T, Lee KY, Bedi KS, Takeuchi Y. Application of the physical dissector to the central nervous system: Estimation of the total number of neurons in subdivisions of the rat hippocampus. Anat Sci Int. 2005;80:153–62. doi: 10.1111/j.1447-073x.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- 28.Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- 29.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 30.Gundersen HJ. Notes on the estimation of the numerical density of arbitrary particles: The edge effect. J Microsc. 1977;111:219–23. [Google Scholar]
- 31.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 32.Jordan TC, Howells KF, McNaughton N, Heatlie PL. Effects of early undernutrition on hippocampal development and function. Res Exp Med (Berl) 1982;180:201–7. doi: 10.1007/BF01852291. [DOI] [PubMed] [Google Scholar]
- 33.Sarsilmaz M, Kaplan S, Songur A, Colakoglu S, Aslan H, Tunc AT, et al. Effects of postnatal formaldehyde exposure on pyramidal cell number, volume of cell layer in hippocampus and hemisphere in the rat: A stereological study. Brain Res. 2007:157–67. doi: 10.1016/j.brainres.2007.01.139. 1145. [DOI] [PubMed] [Google Scholar]
- 34.Pliss L, Balcar VJ, Bubeníková V, Pokorný J, Fitzgibbon T, St’astný F. Morphology and ultrastructure of rat hippocampal formation after I.C.V. administration. Neuroscience. 2003;122:93–101. doi: 10.1016/s0306-4522(03)00550-5. [DOI] [PubMed] [Google Scholar]
- 35.Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- 36.Edmondson JC, Liem RK, Kuster JE, Hatten ME. Astrotactin: A novel neuronal cell surface antigen that mediates neuronastroglial intractions in cerebellar mictrocultures. J cell Biol. 1988;106:505–17. doi: 10.1083/jcb.106.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falluel-Morel A, Sokolowski K, Sisti HM, Zhou X, Shors TJ, DiCicco-Bloom E. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem. 2007;103:1968–81. doi: 10.1111/j.1471-4159.2007.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi SS, Hwang IK, Choi JW, Won MH, Seong JK, Yoon YS. Effects of hypothyroidism on cell proliferation and neuroblasts in the hippocampal dentate gyrus in a rat model of type 2 diabetes. Anat Cell Biol. 2010;43:185–93. doi: 10.5115/acb.2010.43.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vahidnia A, Van der Voet GB, de Wolff FA. Arsenic neurotoxicity-a review. Hum Exp Toxicol. 2007;26:823–32. doi: 10.1177/0960327107084539. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez VM, Jiménez-Capdeville ME, Giordano M. Mini review: The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145:1–18. doi: 10.1016/s0378-4274(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 41.Aposhian HV, Aposhian MM. Arsenic toxicology: Five questions. Chem Res Toxicol. 2006;19:1–15. doi: 10.1021/tx050106d. [DOI] [PubMed] [Google Scholar]
- 42.Piao F, Ma N, Hiraku Y, Murata M, Oikawa S, Cheng F. Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J Occup Health. 2005;47:445–9. doi: 10.1539/joh.47.445. [DOI] [PubMed] [Google Scholar]
- 43.Ramos O, Carrizales L, Yanez L, Mejia J, Batres L, Ortiz D, et al. Arsenic increased lipid peroxidation in rat tissues by a mechanism independent of glutathione levels. Environ Health Perspect. 1995;103:85–8. doi: 10.1289/ehp.95103s185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyake H, Kadoya A, Ohyashiki T. Increase in molecular rigidity of the protein conformation of brain Na-K-ATPase by modification with 4-hydroxy-2-nonenal. Biol Pharm Bull. 2003;12:1652–56. doi: 10.1248/bpb.26.1652. [DOI] [PubMed] [Google Scholar]
- 45.Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17:610–22. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 46.Wilde J, Pringle AK, Wright P, Iannotti F. Differential vulnerability of the CA1 and CA3 subfields of the hippocampus to superoxide and hydroxyl radicals in vitro. J Neurochem. 1997;69:883–6. doi: 10.1046/j.1471-4159.1997.69020883.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Palb R, Chenc X, Limpeanchobb N, Keshava N, Kumara B, et al. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res. 2005;140:120–6. doi: 10.1016/j.molbrainres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 48.Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol Teratol. 2003;25:519–28. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 49.Miki T, Harris SJ, Wilce PA, Takeuchi Y, Bedi KS. Effects of age and alcohol exposure during early life on pyramidal cell numbers in the CA1-CA3 region of the rat hippocampus. Hippocampus. 2004;14:124–34. doi: 10.1002/hipo.10155. [DOI] [PubMed] [Google Scholar]
- 50.Mattiasson G, Friberg H, Hansson M, Elmer E, Wieloch T. Flow cytometric analysis of mitochondria from CA1 and CA3 region of rat hippocampus reveals differences in permeability transition pore activation. J Neurochem. 2003;87:532–44. doi: 10.1046/j.1471-4159.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 51.Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- 52.Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. J Comp Neurol. 1980;190:115–34. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- 53.Guidi S, Ciani E, Severi S, Contestabile A, Bartesaghi R. Postnatal neurogenesis in the dentate gyrus of the guinea pig. Hippocampus. 2005;15:285–301. doi: 10.1002/hipo.20050. [DOI] [PubMed] [Google Scholar]
- 54.Roy TS, Sharma V, Seidler FJ, Slotkin TA. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Brain Res Dev Brain Res. 2005;155:71–80. doi: 10.1016/j.devbrainres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Bayer SA. Changes in the total number of granule cells in the juvenile and adult rats. A correlated volumetric and 3H-thymidine autoradiographic study. Exp Brain Res. 1982;46:315–23. doi: 10.1007/BF00238626. [DOI] [PubMed] [Google Scholar]
- 56.Harry GJ, Lefebvre d’Hellencourt C. Dentate gyrus: Alterations that occur with hippocampal injury. Neurotoxicology. 2003;24:343–56. doi: 10.1016/S0161-813X(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 57.Hossain K, Akhand AA, Kato M, Du J, Takeda K, Wu J, et al. Arsenic induces apoptosis of murine T lymphocytes through membrane raft-linked signaling for activation of c-Jun amino-terminal kinase. J Immunol. 2000;165:4290–7. doi: 10.4049/jimmunol.165.8.4290. [DOI] [PubMed] [Google Scholar]
- 58.Chattopadhyay SK, Bhaumik S, Purkayastha M, Basu S, Chaudhuri AN, Gupta SD. Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol Lett. 2002;136(15):65–76. doi: 10.1016/s0378-4274(02)00282-5. [DOI] [PubMed] [Google Scholar]


