Abstract
Organophosphorous (OP) compound poisoning is one of the most common causes for admission to the Medical Intensive Care Unit. The morbidity and mortality associated with OP poisoning is due to the action of the compound at the muscarinic, nicotinic receptors, and the central nervous system. Here is a rare case of extrapyramidal manifestations occurring in the intermediate phase of OP poisoning, use of amantidine led to subsiding of the symptoms.
Keywords: Amantidine, rigidity, extrapyramidal signs, organophosphorous poisoning
INTRODUCTION
Organophosphorous (OP) compounds continue to be an important and common cause of self-poisoning in rural areas of India.[1] OP compound causes phosphorylation of the serine hydroxyl residue of acetylcholinesterase enzyme (AChE). There is a covalent phophorous-enzyme bond formation that is extremely stable, and its hydrolysis in water occurs at a slow rate. This phosphorylated enzyme complex may undergo a process called as “aging,” which further strengthens the phosphorous-enzyme bond, leading to inactivation of AChE and so there will be accumulation of acetylcholine (ACh). This results in overstimulation of muscarinic and nicotinic synaptic junctions, which is often characterized by muscarinic effects (with symptoms of miosis, bradycardia, hypotension, dyspnoea, cyanosis, salivation, vomiting, and diarrhea), nicotinic effects (with fasciculations, cramps and paralysis), and central symptoms like headache, anxiety, generalized weakness, confusion, convulsions, and coma. Three distinct phases of neurological illness following OP poisoning are observed. Phase I is an acute paralysis phase secondary to continued depolarization at the neuromuscular junction. Phase II (intermediate syndrome) develops 24-96 h after resolution of the initial phase and is characterized by weakness of respiratory muscles, proximal muscles, and cranial nerve palsies. Extrapyramidal symptoms are a rare occurrence in OP poisoning in the intermediate phase.[2] Phase III or organophosphate-induced delayed polyneuropathy (OPIDP) occurs 2-3 weeks after exposure.[3] Presentation with symptoms of severe parkinsonism such as bradyphrenia, rigidity, resting tremors, akinesia, and impairment of speech is uncommon after acute organophosphate intoxication. As per literature search there have been reports of 21 patients with extrapyramidal symptoms in OP poisoning, of which six are from India. Here, we would like to report a rare case of extrapyramidal complications and its response to amantidine therapy.
CASE REPORT
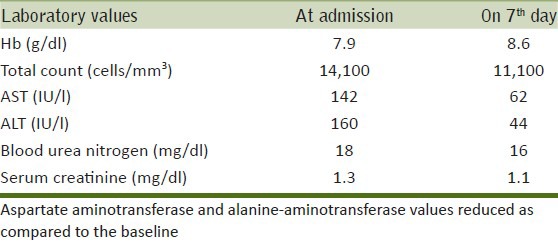
A 30-year-old female patient ingested an unknown dose of Triazophos 40% (Hostathion) in a suicide attempt. As per the history revealed by her parents, she had consumed the above mentioned compound 8 h prior to hospitalization. At the time of admission to the ICU, the patient was drowsy with a Glasgow Coma Scale score of 10/15 and had bilateral pinpoint pupils reactive to light, blood pressure measured 140/70 mmHg. Her pseudocholinesterase level was 869 U/l (Normal: 4852-12000 U/l). Table 1 depicts other laboratory parameters. She was treated with pralidoxime 24 g infusion over 48 h and atropine 120 mg infused over 4 days. Thereafter, the patient regained consciousness, but was immobile. On day 6, neurological examination revealed that the patient had coarse resting tremors with cog-wheel rigidity and signs of dyskinesia affecting both the upper and lower limbs, more predominant on the right side. Cerebral spinal fluid analysis following a lumbar puncture was normal. Magnetic resonance imaging (MRI) showed no focal abnormality in the brain parenchyma.
Table 1.
Laboratory parameters of the patient
The patient's mobility did not improve despite complete recovery from respiratory and cardiovascular distress. It was noticed that even on the 10th day, she was unable to speak and turn around on the bed. In view of the above signs, a provisional diagnosis of OP-induced Parkinsonism was made. Treatment with tablet amantadine 100 mg/day thrice daily was started orally. After receiving therapy for a day, there was marked improvement in orientation, mobility, and speech. By the 4th day of amantadine treatment, cog-wheel rigidity, tremors, and bradykinesia improved. After 14 days of treatment with the same dose of amantidine, the patient recovered completely and the therapy was discontinued. She did not develop any adverse effects to amantidine. The patient was discharged on day 26 and subsequent follow-up revealed no signs of relapse.
DISCUSSION
In India, easy accessibility of pesticides results in consumption of these compounds for suicidal attempts. OP compounds inactivate AChE by phosphorylating the enzyme. In the present case, the patient had manifestations of parkinsonism by day 6 of alleged OP consumption. She did not have a past history of parkinsonism nor had she received any drugs that induced parkinsonism. As per review of literature, there were a few cases that reported extrapyramidal features by day 4,[4] 5,[5] and 40.[2] The causative agents reported so far are dimethoate,[3,5] fenitrothion,[6] dichlorvos,[7] chlorpyrifos,[8] and fenthion,[2] and, in our case, it was hostathion.
Evidence from animal studies suggest that OP compounds have effect on bilateral basal ganglia.[9] The observed extrapyramidal features could probably be due to the pharmacological actions of OP compound at a higher concentration in basal ganglia. As proposed by Muller et al.,[10] increased ACh concentration in the cholinergic interneuron's of the striatum has the ability to stimulate efferent enkephalin-containing GABA projections to the globus pallidus externus. This may cause glutaminergic excitation in the subthalamic nucleus and a reduction in cortical glutamate stimulation (indirect pathway of cortico-striato-pallido-thalamo-cortical circuit). Thus, we speculated that reduced striatal activity resulting in decreased cortical glutamate stimulation clinically mimicked a dopamine-deficiency state.[10] Excess of glutamate can cause neurotoxicity by increasing the influx of calcium through NMDA receptors.[10] Amantadine is a weak antagonist of the NMDA-type glutamate receptor, so it may antagonize this effect in the subthalamic nucleus and inhibit ACh release in the striatal interneurons. It can also increase dopamine release and block dopamine reuptake.
The patient completely recovered with amantidine therapy without any relapse after discontinuation. This further supports the above statement that the dopamine-deficiency state could be a transient phase. Timely diagnosis and treatment can enhance recovery. Similar result was observed in another study.[5] Some studies have shown that these manifestations have disappeared even without treatment, but the time taken for recovery was longer.[2,11,12]
CONCLUSIONS
Extrapyramidal symptoms may occur following OP poisoning, which may be a transient manifestation, and has been reported infrequently. Therapeutic intervention with amantidine may be considered as a treatment option.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
REFERENCES
- 1.Pandit V, Seshadri S, Rao SN, Samarasinghe C, Kumar A, Valsalan R. A case of organophosphate poisoning presenting with seizure and unavailable history of parenteral suicide attempt. J Emerg Trauma Shock. 2011;4:132–4. doi: 10.4103/0974-2700.76825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senanayake N, Sanmuganathan PS. Extrapyramidal manifestations complicating organophosphorous insecticide poisoning. Hum Exp Toxicol. 1995;14:600–4. doi: 10.1177/096032719501400708. [DOI] [PubMed] [Google Scholar]
- 3.Tanima D, Maisnam I, Kundu AK, Saha SP, Ghosh S, Maity A. Atypical central nervous system involvement in acute organophosphorous poisoning. J Assoc Physicians India. 2011;59:326–7. [PubMed] [Google Scholar]
- 4.Hsieh BH, Deng JF, Ger J, Tsai WJ. Acetylcholinesterase inhibition and the extrapyramidal syndrome: A review of the neurotoxicity of organophosphate. Neurotoxicology. 2001;22:423–7. doi: 10.1016/s0161-813x(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Bentur Y, Bar-Joseph G, Cahana A, Hershman E. Extrapyramidal parkinsonism complicating acute organophosphate insecticide poisoning. Pediatr Neurol. 2005;33:378–82. doi: 10.1016/j.pediatrneurol.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Nakamagoe K, Watanabe M, Takeda T, Mizutani T, Tamaoka A. Parkinsonism with organophosphate poisoning. BMJ Case Rep. 2009 doi: 10.1136/bcr.04.2009.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmi N, Gueye PN, Thabet H, Kouraichi N, Ben Salah N, Amamou M. Extrapyramidal syndrome as a delayed and reversible complication of acute dichlorvos organophosphate poisoning. Vet Hum Toxicol. 2004;46:187–9. [PubMed] [Google Scholar]
- 8.Shahar E, Andraws J. Extra-pyramidal parkinsonism complicating organophosphate insecticide poisoning. Eur J Paediatr Neurol. 2001;5:261–4. doi: 10.1053/ejpn.2001.0527. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Yang Y, Yang J, Meng L. Brain injury due to acute organophosphate poisoning: Magnetic resonance imaging manifestation and pathological characteristics. Nerve Regen Res. 2007;2:403. [Google Scholar]
- 10.Müller-Vahl KR, Kolbe H, Dengler R. Transient severe parkinsonism after acute organophosphate poisoning. J Neurol Neurosurg Psychiatry. 1999;66:253–4. doi: 10.1136/jnnp.66.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatt MH, Elias MA, Mankodi AK. Acute and reversible parkinsonism due to organophosphate pesticide intoxication: Five cases. Neurology. 1999;52:1467–71. doi: 10.1212/wnl.52.7.1467. [DOI] [PubMed] [Google Scholar]
- 12.Praveen Kumar AS, Subrahmanyam DK. Acute reversible parkinsonism following accidental exposure to organophosphate insecticide. Int J Nutr Pharmacol Neurol Dis. 2013;1:70–2. [Google Scholar]


