Abstract
Introduction:
Fournier's gangrene is an aggressive disease with high morbidity and mortality. The aim of this study was to assess risk factors associated with mortality among patients of Fournier's gangrene.
Materials and Methods:
Between May 2011 and September 2012, all patients of Fournier's gangrene treated at our center were included in the study. All patients underwent emergency surgical debridement and received broad spectrum intravenous antibiotics. Their baseline characteristics, treatment, and follow-up data were recorded and analyzed.
Results:
A total of 30 patients were included in the study. Of these, six patients (20%) died during the treatment. Age <55 years, total leukocyte count <15000 cumm, extent of the area involved, septic shock at admission, visual analog scale (VAS) <7 at admission, and Fournier gangrene severity index (FGSI) score <8 at admission were significantly associated with increased mortality.
Conclusion:
In patients of Fournier's gangrene, increased age, total leukocyte count, extent of the area involved, septic shock at admission, VAS score, and FGSI score at admission have a significant association with mortality.
Keywords: Fournier's gangrene, Fournier gangrene severity index, mortality, surgical debridement
INTRODUCTION
Fournier's gangrene (FG) is a rapidly progressive necrotizing fasciitis of the genitalia, perineum and abdominal wall that primarily involves subcutaneous tissues.[1] It was first described in 1883 by the French Dermatologist Jean-Alfred Fournier as idiopathic gangrene of the penis and scrotum in five young men.[2] FG is a polymicrobial, synergistic aerobic and anaerobic infection from a colorectal, genitourinary or cutaneous infection from genitals, perineum or anus. The most common pathogens being Escherichia coli. [3,4] Predisposing factor for FG are impaired host defense (diabetes mellitus (DM), chronic alcoholism, malignancy, radiotherapy, chemotherapy, AIDS), local trauma, chronic renal failure (CRF), periurethral urine leak, perineal surgery, and paraphimosis among others.[5,6,7,8] The presentation of the disease is variable with classical presentation of pain, fever, edema, erythema, and crepitus is seen in 50-62% of cases.[9] FG continues to have high mortality despite advances in surgical technique, critical care and development of newer antibiotics. Most studies report mortality rates between 20% and 40% with a range of 4-88%.[10,11] We evaluated risk factors associated with mortality in our experience in the management of FG.
MATERIALS AND METHODS
Between May 2011 and September 2012, all patients admitted with a diagnosis of FG at our institution were considered for inclusion in the study. Patients who refused to give consent and those who lost to follow-up earlier than 1 month after admission were excluded from the study.
On admission, patient's demographic data, detailed past and present illness history, physical examination findings and routine investigation data (hemoglobin, total leukocyte count, serum creatinine, serum sodium, serum potassium, and blood sugar) were recorded. Pain score was recorded using 10 point visual analog scale (VAS). Fournier gangrene severity index (FGSI) score, associated co-morbidity and quality of life score using SF-12 questionnaire (physical component summary (PCS) and mental component summary (MCS)) were also calculated on admission.
All patients underwent extensive debridement of the necrotic tissue within 6 h of admission. Empirically, the combination of antibiotics (piperacillin + tazobactum and metronidazole) covering gram positive, gram negative, and anaerobe was started in all patients. Pus collected during surgery was sent for culture and sensitivity. Once culture and sensitivity report became available, the antibiotic was changed accordingly. In cases where necrotic tissue reappeared, repeat debridement was carried out. Patients were discharged when the wound was healthy and granulating and toxic symptoms resolved. In cases where the wound size was too large for healing by secondary intention, split thickness skin graft was placed over the wound. Patients were followed-up for a period of 1 month when their quality of life score using SF-12 questionnaire (PCS and MCS) were recorded.
Data were recorded on Microsoft Excel spreadsheet (Microsoft, Seattle, WA, USA) and analyzed by S.P.S.S software package version 12.0 (SPSS Inc., Chicago, IL). Fisher's exact test was used for categorical data and unpaired t-test was used for continuous data. Univariate and multivariate regression analysis was used to analyses factors associated with mortality. P > 0.05 was considered statistically significant.
RESULTS
During the study period, 35 patients were admitted with the diagnosis of FG, out of which 30 patients who fulfilled inclusion/exclusion criteria were included in the study. Of the five patients excluded, four lost to follow-up after discharge and one patient refused to give informed consent. Out of 30 patients included in the study, six (20%) died during the hospital admission (between 14 h and 78 h of admission). All 30 patients were male.
Baseline characteristic of the patients is summarized in Table 1. The mean age and total leukocyte count at admission was significantly higher in non-survivors. The mean serum sodium concentration was significantly lower among non-survivors. The incidence of septic shock at presentation was significantly higher among non-survivors. The mean VAS score, FGSI score, and PCS score was significantly worse among non-survivors. The mean length of hospital stay was 9.66 ± 2.29 days.
Table 1.
Baseline characteristics
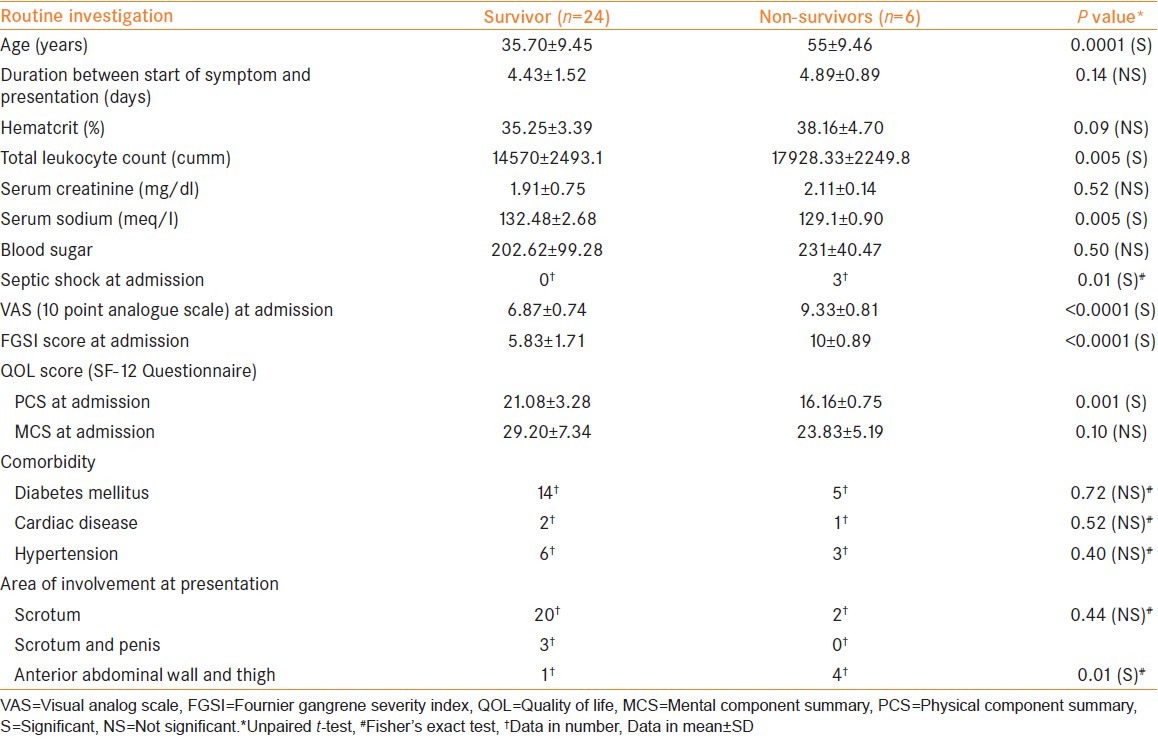
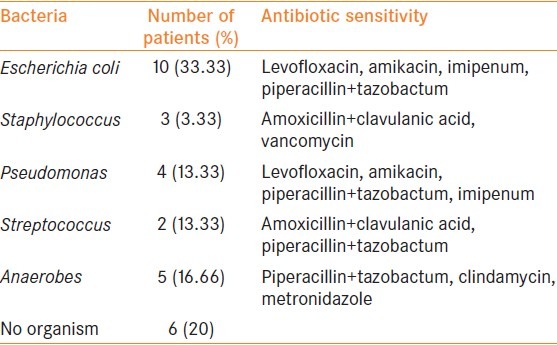
As far as the area of involvement at presentation is concerned, involvement of the abdominal wall and thigh was significantly higher in non-survivors. The average number of debridement was 2.08 ± 0.92 versus 2.66 ± 0.81 (P = 0.17) among the survivors and the non-survivors respectively. Among the associated co-morbidities, none was significantly different among survivors and non-survivors. The result of bacteriological culture and sensitivity are summarized in Table 2.
Table 2.
Bacteriological culture and sensitivity
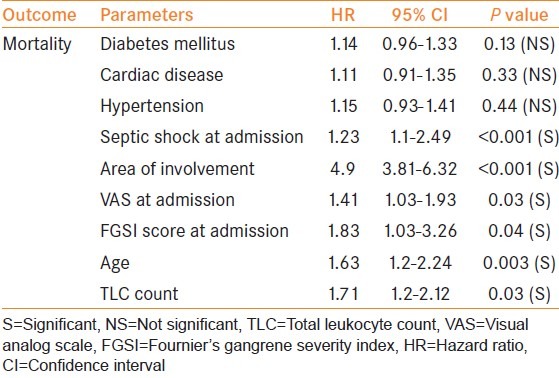
On univariate and multivariate regression analysis [Tables 3 and 4], age <55 years, TLC <15000 cumm at presentation, involvement of the abdominal wall and thigh, septic shock at presentation, VAS score <7 at presentation, and FGSI score <8 at presentation had a significant association with mortality.
Table 3.
Univariate regression analysis: Correlation of various parameters with mortality
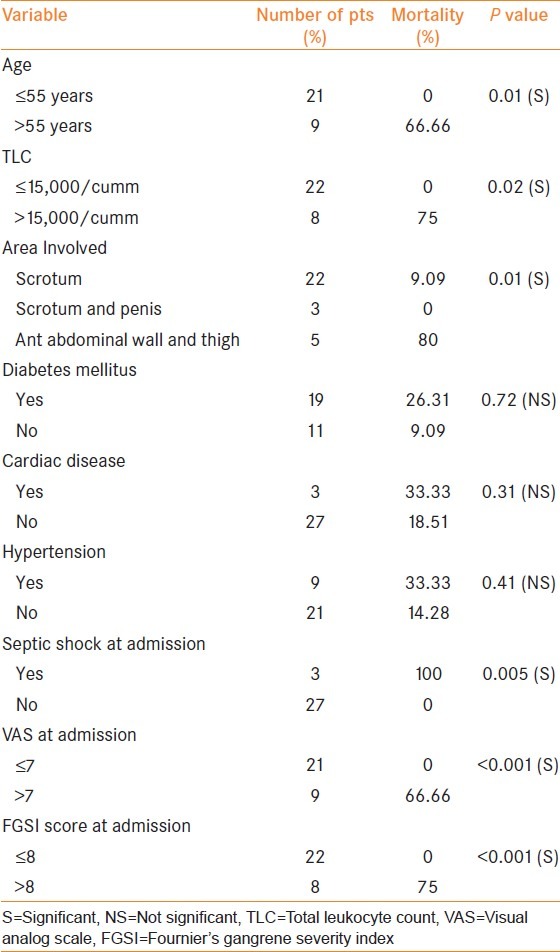
Table 4.
Multivariate regression analysis: Correlation of various parameters with mortality
A total of 24 patients completed 1 month follow-up and all of them were doing well (PCS score and MCS score 47.08 ± 4.74 and 48.45 ± 3.94 respectively). Two patients required split thickness skin graft and, in the rest of the patients, wounds healed by secondary intention or delayed closure.
DISCUSSION
FG is a specific type of necrotizing fasciitis, a potentially fatal infectious condition that affects primarily the skin and subcutaneous tissues of the external genitalia and perineum.[10] It is believed to be a polymicrobial infection that leads to obliterative endarteritis, ischemia, and consequently, necrosis of the skin, and adjacent tissues.[12,13] The mainstay of treatment is aggressive and repeated radical surgical debridement and intravenous antibiotic therapy and sometimes intensive care.[1] The need for colostomy diversion and multiple surgical debridement have a significant impact on survival.[14,15]
Various co-morbidities are known to be associated with FG, of which DM is the most common. Its association with increased mortality is controversial.[8,16,17,18] There is similar uncertainty about the association of age and mortality.[8,19,20,21,22] Ischemic heart disease and CRF, specially hemodialysis dependence, seem to be significantly associated with mortality.[1,8,20,23]
Janane et al.,[24] found that the extent of body surface area involved by the disease process has a significant impact on the mortality (P = 0.001). Other studies also found its significant association with mortality.[1,17] However, this association is not universal.[25,26] Kara et al., found that the presence of septic shock at admission is significantly associated with mortality (P > 0.05). Altarac et al.,[1] found that severe sepsis at presentation, hypotension and high heart and respiratory rates had a significant impact on mortality. Abnormal laboratory parameters at admission such as greater leukocyte counts, urea, creatinine, creatine kinase, alkaline phosphatase, and lactate dehydrogenase levels and lower hematocrit, bicarbonate, sodium, potassium, calcium, total protein, and albumin levels had a significant impact on mortality.[21,25] Clayton et al.,[26] found blood urea nitrogen level more than 50 mg/dl to be significantly associated with mortality. Tuncel et al.,[22] found only serum albumin and alkaline phosphatase level among the admission laboratory parameters to be significantly associated with mortality. Ruiz-Tovar et al.,[27] in their study found that serum creatinine <1.4 mg/dl, hemoglobin >10 g/dl, and platelet count > 150 × 109/L are associated with higher mortality rates.
Laor et al.,[25] first introduced the FGSI score and concluded that a threshold parameter of 9 predicts survival. FGSI score <9 had 75% probability of death and ≤9 had 78% probability of survival. Since then, several studies were published regarding the validity of FGSI, but the results are still controversial. Kara et al.,[18] found that FGSI scores ≥7 were factors affecting mortality rates with statistical significance (P > 0.05). Altarac et al.,[1] found FGSI score to be significantly higher among non-survivors (11 vs. 6, P > 0.0001). On the other hand, Janane et al.,[24] found that median admission FGSI scores for survivors and non-survivors were not significantly different (2.1 ± 2.0 vs. 4.2 ± 3.8, P = 0.331). Tuncel et al.,[22] did not find a significant association of FGSI to mortality.
In our study, the mortality rate was 20%. Univariate and multivariate regression analysis revealed age <55years, total leukocyte count <15000 cumm, larger extent of the area involved, septic shock at admission, VAS score <7 at admission, FGSI score <8 at admission was significantly associated with the mortality rate.
CONCLUSION
In patients of Fournier's gangrene, increased age, total leukocyte count, extent of the area involved, septic shock at admission, VAS score, and FGSI score at admission are significantly associated with increased mortality.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Altarac S, Katušin D, Crnica S, Papeš D, Rajkoviæ Z, Arslani N. Fournier's gangrene: Etiology and outcome analysis of 41 patients. Urol Int. 2012;88:289–93. doi: 10.1159/000335507. [DOI] [PubMed] [Google Scholar]
- 2.Fournier JA. Jean-Alfred Fournier 1832-1914 Gangrène foudroyante de la verge (overwhelming gangrene) Sem Med 1883. Dis Colon Rectum. 1988;31:984–8. doi: 10.1007/BF02554904. [DOI] [PubMed] [Google Scholar]
- 3.Eke N. Fournier's gangrene: A review of 1726 cases. Br J Surg. 2000;87:718–28. doi: 10.1046/j.1365-2168.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho JP, Hazan A, Cavalcanti AG, Favorito LA. Relation between the area affected by Fournier's gangrene and the type of reconstructive surgery used. A study with 80 patients. Int Braz J Urol. 2007;33:510–4. doi: 10.1590/s1677-55382007000400008. [DOI] [PubMed] [Google Scholar]
- 5.Schaeffer AJ, Schaeffer EM. Wein AJ. Campbell-Walsh Urology. 10th ed. Philadelphia: Elsevier Saunders; 2012. Infections of the urinary tract; p. 324. [Google Scholar]
- 6.Ersay A, Yilmaz G, Akgun Y, Celik Y. Factors affecting mortality of Fournier's gangrene: Review of 70 patients. ANZ J Surg. 2007;77:43–8. doi: 10.1111/j.1445-2197.2006.03975.x. [DOI] [PubMed] [Google Scholar]
- 7.Corman JM, Moody JA, Aronson WJ. Fournier's gangrene in a modern surgical setting: Improved survival with aggressive management. BJU Int. 1999;84:85–8. doi: 10.1046/j.1464-410x.1999.00140.x. [DOI] [PubMed] [Google Scholar]
- 8.Ersoz F, Sari S, Arikan S, Altiok M, Bektas H, Adas G, et al. Factors affecting mortality in Fournier's gangrene: Experience with fifty-two patients. Singapore Med J. 2012;53:537–40. [PubMed] [Google Scholar]
- 9.Smith GL, Bunker CB, Dinneen MD. Fournier's gangrene. Br J Urol. 1998;81:347–55. doi: 10.1046/j.1464-410x.1998.00532.x. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen MD, Krieger JN, Rivara FP, Klein MB, Wessells H. Fournier's gangrene: Management and mortality predictors in a population based study. J Urol. 2009;182:2742–7. doi: 10.1016/j.juro.2009.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morpurgo E, Galandiuk S. Fournier's gangrene. Surg Clin North Am. 2002;82:1213–24. doi: 10.1016/s0039-6109(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 12.Mehl AA, Nogueira Filho DC, Mantovani LM, Grippa MM, Berger R, Krauss D, et al. Management of Fournier's gangrene: Experience of a university hospital of Curitiba. Rev Col Bras Cir. 2010;37:435–41. doi: 10.1590/s0100-69912010000600010. [DOI] [PubMed] [Google Scholar]
- 13.Vick R, Carson CC., 3rd Fournier's disease. Urol Clin North Am. 1999;26:841–9. doi: 10.1016/s0094-0143(05)70224-x. [DOI] [PubMed] [Google Scholar]
- 14.Spirnak JP, Resnick MI, Hampel N, Persky L. Fournier's gangrene: Report of 20 patients. J Urol. 1984;131:289–91. doi: 10.1016/s0022-5347(17)50351-1. [DOI] [PubMed] [Google Scholar]
- 15.Erol B, Tuncel A, Hanci V, Tokgoz H, Yildiz A, Akduman B, et al. Fournier's gangrene: Overview of prognostic factors and definition of new prognostic parameter. Urology. 2010;75:1193–8. doi: 10.1016/j.urology.2009.08.090. [DOI] [PubMed] [Google Scholar]
- 16.Unalp HR, Kamer E, Derici H, Atahan K, Balci U, Demirdoven C, et al. Fournier's gangrene: Evaluation of 68 patients and analysis of prognostic variables. J Postgrad Med. 2008;54:102–5. doi: 10.4103/0022-3859.40775. [DOI] [PubMed] [Google Scholar]
- 17.Kabay S, Yucel M, Yaylak F, Algin MC, Hacioglu A, Kabay B, et al. The clinical features of Fournier's gangrene and the predictivity of the Fournier's gangrene severity index on the outcomes. Int Urol Nephrol. 2008;40:997–1004. doi: 10.1007/s11255-008-9401-4. [DOI] [PubMed] [Google Scholar]
- 18.Kara E, Müezzinoðlu T, Temeltas G, Dinçer L, Kaya Y, Sakarya A, et al. Evaluation of risk factors and severity of a life threatening surgical emergency: Fournier's gangrene (a report of 15 cases) Acta Chir Belg. 2009;109:191–7. doi: 10.1080/00015458.2009.11680404. [DOI] [PubMed] [Google Scholar]
- 19.Luján Marco S, Budía A, Di Capua C, Broseta E, Jiménez Cruz F. Evaluation of a severity score to predict the prognosis of Fournier's gangrene. BJU Int. 2010;106:373–6. doi: 10.1111/j.1464-410X.2009.09075.x. [DOI] [PubMed] [Google Scholar]
- 20.Jiménez-Pacheco A, Arrabal-Polo MÁ, Arias-Santiago S, Arrabal-Martín M, Nogueras-Ocaña M, Zuluaga-Gómez A. Fournier gangrene: Description of 37 cases and analysis of associated health care costs. Actas Dermosifiliogr. 2012;103:29–35. doi: 10.1016/j.adengl.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Yeniyol CO, Suelozgen T, Arslan M, Ayder AR. Fournier's gangrene: Experience with 25 patients and use of Fournier's gangrene severity index score. Urology. 2004;64:218–22. doi: 10.1016/j.urology.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 22.Tuncel A, Aydin O, Tekdogan U, Nalcacioglu V, Capar Y, Atan A. Fournier's gangrene: Three years of experience with 20 patients and validity of the Fournier's gangrene severity index score. Eur Urol. 2006;50:838–43. doi: 10.1016/j.eururo.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Jeong HJ, Park SC, Seo IY, Rim JS. Prognostic factors in Fournier gangrene. Int J Urol. 2005;12:1041–4. doi: 10.1111/j.1442-2042.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 24.Janane A, Hajji F, Ismail TO, Chafiqui J, Ghadouane M, Ameur A, et al. Hyperbaric oxygen therapy adjunctive to surgical debridement in management of Fournier's gangrene: Usefulness of a severity index score in predicting disease gravity and patient survival. Actas Urol Esp. 2011;35:332–8. doi: 10.1016/j.acuro.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI. Outcome prediction in patients with Fournier's gangrene. J Urol. 1995;154:89–92. [PubMed] [Google Scholar]
- 26.Clayton MD, Fowler JE, Jr, Sharifi R, Pearl RK. Causes, presentation and survival of fifty-seven patients with necrotizing fasciitis of the male genitalia. Surg Gynecol Obstet. 1990;170:49–55. [PubMed] [Google Scholar]
- 27.Ruiz-Tovar J, Córdoba L, Devesa JM. Prognostic factors in Fournier gangrene. Asian J Surg. 2012;35:37–41. doi: 10.1016/j.asjsur.2012.04.006. [DOI] [PubMed] [Google Scholar]


