Abstract
Objectives:
Apoptosis, an important mechanism that contributes to cell growth reduction, is reported to be induced by Crocus sativus (Saffron) in different cancer types. However, limited effort has been made to correlate these effects to the active ingredients of saffron. The present study was designed to elucidate cytotoxic and apoptosis induction by safranal, the major coloring compound in saffron, in a human prostate cancer cell line (PC-3).
Materials and Methods:
PC-3 and human fetal lung fibroblast (MRC-5) cells were cultured and exposed to safranal (5, 10, 15, and 20 μg/ml). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to assess cytotoxicity. DNA fragmentation was assessed by gel electrophoresis. Cells were incubated with different concentrations of safranal, and cell morphologic changes and apoptosis were determined by the normal inverted microscope, Annexin V, and propidium iodide, followed by flow cytometric analysis, respectively.
Results:
MTT assay revealed a remarkable and concentration-dependent cytotoxic effect of safranal on PC-3 cells in comparison with non-malignant cell line. The morphologic alterations of the cells confirmed the MTT results. The IC50 values against PC-3 cells were found to be 13.0 0.07 and 6.4 0.09 μg/ml at 48 and 72 h, respectively. Safranal induced an early and late apoptosis in the flow cytometry histogram of treated cells, indicating apoptosis is involved in this toxicity. DNA analysis revealed typical ladders as early as 48 and 72 h after treatment, indicative of apoptosis.
Conclusions:
Our preclinical study demonstrated a prostate cancer cell line to be highly sensitive to safranal-mediated growth inhibition and apoptotic cell death. Although the molecular mechanisms of safranal action are not clearly understood, it appears to have potential as a therapeutic agent.
Keywords: Apoptosis, cytotoxicity, DNA, PC-3, safranal
INTRODUCTION
Prostate cancer represents a major health problem for men worldwide. Mortality from prostate cancer results from metastases to the bones and lymph nodes. The mainstream treatment strategy for advanced prostate cancer is generally classified into three categories: Surgery, radiation therapy, and chemotherapy.[1] Despite the initial success of chemotherapy, most patients with advanced prostate cancer develop resistance to chemotherapy.[2,3] Thus, there is a potential role for newer chemopreventive compounds that can prevent or slow the progression of cancer.[4]
In view of the renewed interest in chemopreventive plant agents, evidence from epidemiological, in vivo and in vitro experimental studies indicates that natural products might protect against the development of various diseases, including cancer.[5,6] Crocus sativus L., commonly known as saffron since ancient times is harvested from the dried, dark red stigmas of C. sativus flowers. It has been used not only as a spice for flavoring and coloring food and as a perfume, but also for treating several diseases. Recent data show that C. sativus extract possesses anticarcinogenic (inhibition of chemical carcinogenesis) and antitumor (inhibition of tumor growth) in vivo and in vitro activities.[7,8,9] Furthermore, modern pharmacological studies have demonstrated that C. sativus extract or its active constituents have anticonvulsant,[10] antidepressant,[11] anti-inflammatory,[12] and antitumor effects, radical scavenging, as well as learning and memory improving properties.[3,13]C. sativus extract also has chemopreventive and genoprotective effects and protects from genotoxin-induced oxidative stress in mice.[5,7,14,15] Characteristic compounds of C. sativus include crocin, safranal, picrocrocin, crocetin, and β-carotene.[16,17] It was shown that these C. sativus ingredients inhibited different types of tumor cell growth, with crocetin having no effect on colony formation of tumor cells, although it had a dose-dependent inhibitory effect on DNA, RNA, and protein synthesis of different human malignant cells.[17,18] The aim of the present study was to assess the cytotoxicity and apoptotic effects of safranal, the active constituent of C. sativus stigmas, on human prostate cancer cells.
MATERIALS AND METHODS
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Amerso (NY, USA). RPMI 1640 was purchased from Gibco BRL (Grand Island, NY, USA). Annexin V-FITC (fluorescein isothiocyanate) was obtained from Invitrogen Corporation (USA). Fetal bovine serum was purchased from PAA Laboratories GmbH, Austria. Safranal (5,7-dihydroxyflavone) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The apoptosis ladder detection kit was from Wako Pure Chemical Industries (Osaka, Japan).
Two different cell lines were used in this study. The human prostate cancer cell line (PC-3) and the human fetal lung fibroblast cell line (MRC-5) were obtained from Pasteur Institute (Tehran, Iran). The cells were cultured either in 96-well tissue (TC) plate (NUNC, Wiesbaden, Germany) or in 25-cm2 TC flasks (NUNC). Cells were cultured in CO2 incubator MCO-17AI (Sanyo Electric Co., Ltd, Osaka, Japan) at 37°C in 95% humidified atmosphere enriched by 5% CO2 and subcultured every 3-4 days. The malignant (PC-3) and the nonmalignant (MRC-5) cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (Gibco-Invitrogen, Germany), 100 U/ml of penicillin (Gibco-Invitrogen), and 100 μg/ml streptomycin (Gibco-Invitrogen).
Cell viability was measured using the MTT assay, which is based on the conversion of MTT to formazan crystals by mitochondrial dehydrogenases. Briefly, PC-3 and MRC-5 cells were plated at a density of 1 × 103 cells/ml in 96-well plates and allowed to attach for 24 h, resulting in log phase growth at the time of drug treatment. Safranal (5, 10, 15, and 20 μg/ml) was added to the wells for 24, 48, and 72 h. After treatment, 10 ml MTT was added to each well. After 4 h of incubation at 37°C, the solution was removed and the produced formazan was solubilized in 100 ml of dimethyl sulfoxide (DMSO). Absorbance was measured at 550 nm using an automated microplate reader (Bio-Rad 550). Cell viability was expressed as a percentage of the value for control cultures. The cytotoxic effects of safranal on PC-3 were expressed as IC50 values (the drug concentration that reduced the absorbance of treated cells by 50% compared to untreated cells). All experiments were carried out in triplicate.
Morphologic studies by using normal inverted microscope were carried out to observe the morphologic changes of cell death in malignant (PC-3) and nonmalignant (MRC-5) cell lines elicited by different concentrations of safranal. Concentrations of 0, 10, 15, and 20 μg/ml of safranal were used for the morphologic studies. The untreated cells served as the negative control. The morphologic alterations of the cells were visualized under the normal inverted microscope 48 h post-treatment.
Low-molecular-weight genomic DNA was extracted as described previously.[19] In brief, approximately 1 × 106 cells were plated and treated with 20 μM (PC-3 cells) of safranal for various treatment hours (24, 48, and 72 h). All the cells (including floating cells) were harvested by trypsinization and washed with Dulbecco`s Phosphate Buffered Saline. Cells were lysed with the lysis buffer containing 10 mM Tris (pH 7.4), 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), and 0.5% Triton X-100 for 30 min on ice. Lysates were vortexed and cleared by centrifugation at 10,000 g for 20 min. Fragmented DNA in the supernatant was extracted with an equal volume of neutral phenol:chloroform:isoamyl alcohol mixture (25:24:1) and analyzed electrophoretically on 2% agarose gels containing 0.1 μg/ml ethidium bromide.
Apoptotic cell death was measured using a fluorescein isothiocyanate (FITC)-conjugated Annexin V/propidium iodide (PI) assay kit by flow cytometry. Briefly, 5 × 105 cells were washed with ice-cold phosphate-buffered saline (PBS), resuspended in 100 μl binding buffer, and stained with 5 μl of FITC-conjugated Annexin V (10 mg/ml) and 10 μl of PI (50 mg/ml). The cells were incubated for 15 min at room temperature in the dark, 400 μl of binding buffer was added, and the cells were analyzed (FACScan, Becton-Dickinson, NY, USA). The PC-3 cells were gated separately according to their granularity and size on forward scatter (FSC) versus side scatter (SSC) plots. Early and late apoptosis was evaluated on fluorescence 2 (FL2 for propidium iodide) versus fluorescence 1 (FL1 for Annexin) plots. Cells stained with only Annexin V were evaluated as being in early apoptosis; cells stained with both Annexin V and propidium iodide were evaluated as being in late apoptosis or in a necrotic stage.
All results were expressed as mean ± SEM. Significance was evaluated using analysis of variance (ANOVA) and Bonferroni's test. A probability level of P > 0.05 was considered statistically significant.
RESULTS
To discriminate between the effects of safranal on the malignant (PC-3) and non-malignant control (MRC-5) cells, they were exposed to increasing concentrations of safranal for 24, 48, and 72 h. PC-3 cells were incubated with various concentrations of safranal for 24, 48, and 72 h and MRC-5 cells were incubated with different concentrations of safranal for 24 h. The impact of safranal on cell viability was quantitated 48 h post-exposure by the MTT assay. While there was no significant effect from any concentration of safranal on the viability of MRC-5 cells, there were significant decreases in the viability of PC-3 cells at concentrations of 15 and 20 μg/ml after 24 h of safranal treatment (*P > 0.05 and **P > 0.01, respectively) [Figure 1]. Exposure of PC-3 cells to safranal significantly inhibited growth in a dose- and time-dependent manner (P > 0.001). Although there was no significant effect from the low concentration of safranal after 24 h (5 and 10 μg/ml), there were significant decreases in viability of PC-3 cells at concentrations of 5, 10, 15, and 20 μg/ml after 48 and 72 h of safranal treatment (P > 0.001) [Figure 1]. The IC50 dose for PC-3 cells was determined to be 13.0 ± 0.07 and 6.4 ± 0.09 μg/ml at 48 and 72 h, respectively.
Figure 1.
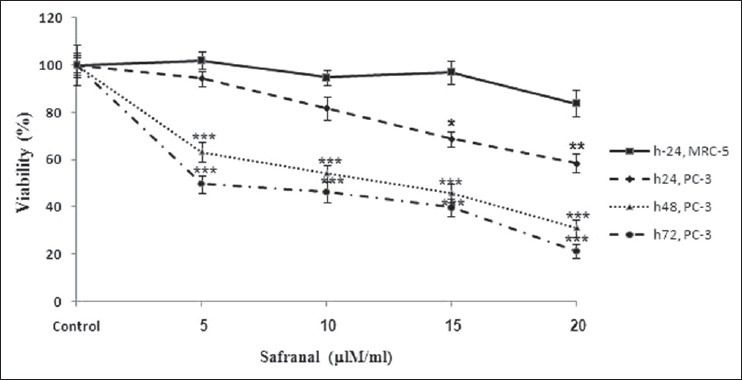
Effect of safranal on cell viability of PC-3 and MRC-5 cells. Cells were treated with different concentrations of safranal for 24, 48, and 72 h. Viability was quantitated by MTT assay. Results are mean ± SEM. *P> 0.05, ** P> 0.01, and *** P> 0.001 at different time points compared to controls
After 48 h co-culture of the cells with different concentrations of safranal (0, 10, 15, and 20 μg/ml), cell population was decreased as compared to the control and also morphologic changes were observed in the prostate cancer cells versus MRC-5 cells, which consisted of reduction in number of living cells, cell volume and rounding until the nucleus constituted the majority of the cellular volume. There was observationally severe reduction of malignant cells (PC-3) compared to MRC-5. This cytotoxicity was increased at higher concentrations [Figure 2].
Figure 2.
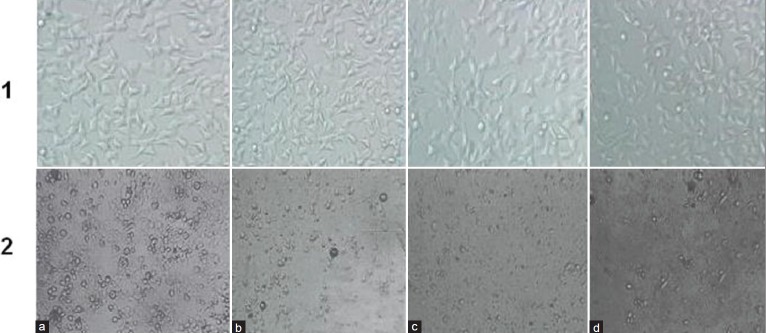
Comparison of cytotoxicty effect of safranal on cell viability of prostate cancer (PC-3) and non-malignant (MRC-5) cell line after 48 h. Morphologic changes of cells after treatment with different concentrations of safranal. 1: MRC-5 cell line; 2: PC-3 cell line; a = 0; b = 10; c = 15, and d = 20 μg/ml safranal
In order to delineate the mechanism of cell death mediated by safranal, we performed DNA fragmentation assay, which is characteristic for apoptosis. We treated the cells at various time intervals (24, 48, and 72 h), and DNA was then isolated and analyzed by agarose gel electrophoresis. Following agarose gel electrophoresis of PC-3 cells treated with 20 μg/ml of safranal, a typical ladder pattern of internucleosomal fragmentation was observed in cells after 48 and 72 h of safranal treatment. Low-molecular-weight DNA from these cells was resolved in 2.0% agarose gels [Figure 3]. These data suggest that safranal is a potent inducer of apoptosis in PC-3 cells.
Figure 3.
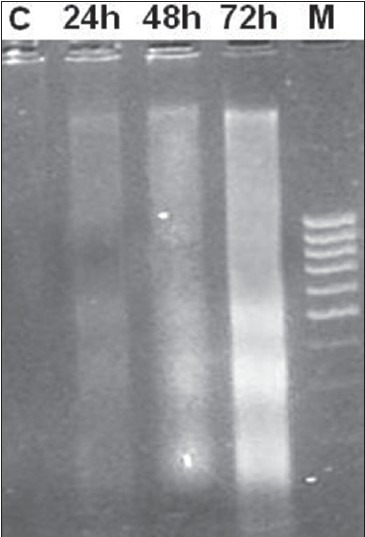
DNA fragmentation of PC-3 exposed to safranal. Fragmentations of genomic DNA in PC-3 cells were treated with 20 μg/ml of safranal for 24, 48, and 72 h. DNA laddering formation was viewed on ethidium bromide-stained gel (2%) and photographed by UV illumination. M, molecular weight marker; C, DMSO control
PC-3 cells were treated with 10 and 20 μg/ml safranal for 48 h to determine whether safranal induced apoptosis. After treatment, the cells were harvested and apoptosis was examined by flow cytometry [Figure 4]. Quantitative analysis using the Annexin V/PI assay showed that the proportion of early stage apoptotic cells (Annexin V+/PI−) increased significantly from 1.6% to 28.32% and the proportion of late stage apoptotic cells (Annexin V+/PI+) increased significantly from 1.5% to 36.7% [Figure 5]. Apoptosis induced by 10 and 20 μg/ml of safranal was significantly greater than in untreated cells and the percentage of early and late apoptotic cells significantly increased with safranal concentrations (***P > 0.001).
Figure 4.
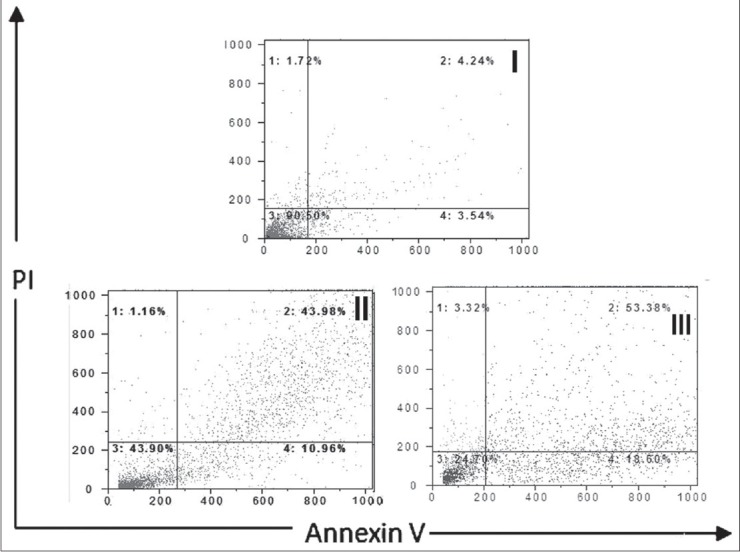
Assessment of apoptosis by Annexin V/PI on human prostate cancer cells (PC-3). The cells were treated with 10 and 20 ƒÝg/ml safranal for 48 h (symbol II, III) or media only (control symbol I), and apoptosis was examined by flow cytometry after Annexin V/PI double staining. Necrotic cells lose membrane integrity, permitting PI entry. Viable cells exhibit Annexin V (−)/PI (−); early apoptotic cells exhibit Annexin (+)/PI (−); late apoptotic cells or necrotic cells exhibit Annexin V (+)/PI (+)
Figure 5.
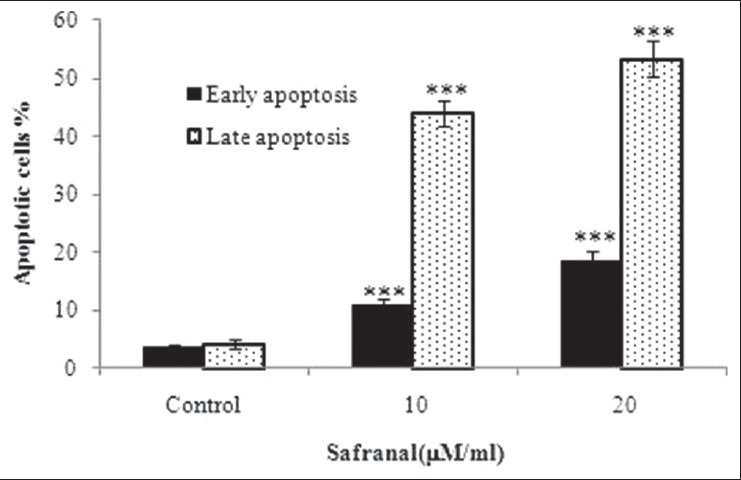
Assessment of apoptosis by Annexin V/PI on human prostate cancer cells (PC-3). Percentage of cell death based on the assessment of apoptosis by Annexin V/PI. *** indicates P> 0.001 compared to control
DISCUSSION
This is an important facet of biomedical research, providing a practical approach for identifying potentially useful inhibitors of tumor development and offering an opportunity to study the molecular mechanism of tumorigenesis. According to Cragg and Newman (2000),[20] over 50% of the drugs in clinical trials for anticancer activity are isolated from natural sources or their ingredients. This has resulted in greater confidence in natural products as important sources for the development of effective anticancer agents.[21] Several drugs currently used in chemotherapy are isolated from plant species.[22] The aim of our study was to determine the effect of safranal on human prostate cancer cells (PC-3). The rationale for this study was on the basis of several data that linked C. sativus to the treatment of various diseases,[23,24] together with the recently found favorable antitumoral action of C. sativus.[25] The characteristic components of C. sativus are crocin (responsible for the color), picrocrocin (responsible for its bitter taste), and safranal (responsible for odor and aroma). To our knowledge, no study of the effects of the active constituent of C. sativus, safranal, on prostate cancer has been reported till date. C. sativus extract significantly inhibited the colony formation and cellular DNA and RNA synthesis, whereas inhibition of protein synthesis was not detected.[16] Crocetin, an ingredient from C. sativus, inhibited intracellular nucleic acid and protein synthesis in malignant human cell lines and had no effect on colony formation.[26,27] The 50% inhibition (ID50) of growth of human chronic myelogenous leukemia K562 cells and promyelocytic leukemia HL-60 cells by dimethylcrocetin, crocetin, and crocin was reached at concentrations of 0.8 and 2 mM, respectively.[16,28] Cytotoxicity of dimethylcrocetin and crocin to various tumors cell lines (DLA, EAC, S-180, L1210 leukemia, and P388 leukemia) and to human primary cells from surgical specimens (osteosarcoma, fibrosarcoma, and ovarian carcinoma) was detected.[14] It has been shown that the inhibitory effect of C. sativus extract on the in vitro growth of HeLa cells was partly due to crocin.[13]
The present study is the first to examine the effect of safranal on anti-proliferative activity against human prostate cancer cells. We found that safranal had good cytotoxicity against PC-3 cells, but was less sensitive to normal human cells, based on the high IC50 value. The high sensitivity of malignant cells may in part reflect the difference in growth rates between malignant and non-malignant cells. The differences in sensitivity to the effect of C. sativus and its main ingredients in normal and malignant cells could be due to the existence of distinct cell surface receptors, intracellular retention transport, differences in the uptake of certain drugs, or in the methods used for the extraction and assessment of toxicity.[28,29,30,31,32,33] These findings correlate with our previous studies in which C. sativus plant displayed selective killing against cancer cells.[9,34] This selective cytotoxic effect is an important criterion because the currently available drugs target normal cells as well and lead to side effects. Experimental results imply that the cytotoxic behavior of safranal toward PC-3 cells was selective. Safranal treatment inhibited PC-3 cell growth in a dose- and time-dependent manner [Figure 1]. Based on morphologic changes identified by inverse microscopy, typical morphologic characteristics of apoptosis and the reduction of the cells were observed [Figure 2]. One of the biochemical hallmarks in the apoptotic process is the formation of nuclear DNA fragmentation, which shows the presence of typical ladder DNA fragments of 180-200 base pairs and multiples thereof on an agarose gel. In contrast, random cleavage of DNA in necrotic cells will produce a diffuse smear upon electrophoresis of DNA. Hence, DNA gel electrophoresis method was used to determine the possible mode of cell death caused by safranal. No ladder formation was observed in untreated cells. Thus, cytotoxic effect of safranal treatment allows the appearance of apoptotic DNA fragments on agarose gel, and safranal treatment on prostate (PC-3) cancer cell line was mediated via an apoptotic mechanism. The presence of phosphatidylserine on the outer leaflet of apoptotic cells membrane was then assessed using Annexin V staining to quantify the amount of cells in the early and/or late stage of apoptosis. The numbers of early and late apoptotic cells increased in a dose-dependent manner for PC-3 cells [Figures 4 and 5]. Our findings after using Annexin V-FITC/PI labeling indicated that safranal could induce apoptosis in a dose-dependent manner. They suggested that the growth inhibition of safranal for cancer cells is caused by apoptosis. We previously analyzed the anti-proliferative and apoptotic effects of C. sativus on the lung cancer tumor cells by MTT assay and Annexin V-FITC, respectively. We found that C. sativus decreased (by over 50%) the viability of malignant cells after 48 h.[12] This potent cytotoxic effect of C. sativus, combined with the demonstrated role of safranal as the main and active constituent of C. sativus in its various medical uses makes safranal as a candidate target for prostate cancer treatment. Based on the fact that C. sativus showed significant antitumor effects against several cancer cells and on the importance of safranal as one of the main compounds in C. sativus, attempts were made to examine its antitumor activities and possibly involved mechanisms against human prostate cancer cells. In our experimental setting, safranal was found to substantially decrease the MTT uptake of the studied human prostate cancer cell line. All these results suggest that safranal treatment can induce cell death of PC-3 cells via apoptosis.
Assimopoulou et al. showed that safranal (500 ppm in methanol solution) possesses a 34% radical scavenging activity possibly due to its potential to provide a hydrogen atom for the DPPH radical.[35] By means of deoxyribose, erythrocyte membrane lipid peroxidation, and liver microsomal non-enzymatic lipid peroxidation methods, the antioxidant activity of safranal was evaluated in vitro. Safranal showed hydroxyl radical scavenging activity dose-dependently in deoxyribose assay and decreased malondialdehyde generation in RBC, lipid peroxidation induced by H2O2, and liver microsomal non-enzymatic lipid peroxidation.[12] Thus, safranal shows some antioxidant properties. The antioxidant and cytotoxic activities of safranal could render it as a source of anticancer metabolites. Antioxidant properties of safranal might prevent the progression of cancer, while the cytotoxic potential, on the other hand, might be used against cancer cells, thereby directing them toward apoptosis and cell death.
The interaction between calf-thymus DNA and safranal (0.13 and 3.125 mM) under physiological conditions was evaluated by FTIR and UV-visible difference spectroscopic methods in order to assess binding sites and binding constants. Also, the effect of safranal on DNA duplex stability and conformation was monitored. Higher levels of DNA vibrations at higher safranal concentrations were described to be related to helix destabilization. Safranal has an inhibitory effect on the intracellular nucleic acid and protein synthesis in malignant cells as well as on protein kinase C (PKC) and proto-oncogene in INNIH/3T3 cells, which is most likely due to their antioxidant activity.[36,37]
Safranal was able to induce apoptosis in the cell lines tested. It showed a dosage-dependent increase of apoptotic fragments at the tested doses. A number of previous studies suggest that C. sativus possesses antitumor and anticarcinogenic activities and has no cytotoxic effect on non-malignant cells. It is interesting to note that C. sativus exhibited cytotoxic inhibitory activity against different animal and human malignant cells.[6,8,38] Our pervious study indicated that the C. sativus extract had a dose-dependent inhibitory effect on human cancer cells in vitro, but had no effect on normal human cells.[9,34] Potential compounds responsible for the inhibitory effect of C. sativus on tumor cell growth are its carotenoid ingredients. Additional studies are required for determining the biological effect of the C. sativus ingredients. In natural product-based drug discovery and development, necrosis may occur along with apoptosis. This has been shown in many anticancer drugs such as cladribine, cisplatin, doxorubicin, and 5′ fluorouracil, which possess both apoptotic and necrotic effects.[39,40] In necrotic cell death, plasma membrane integrity is lost and this leads to the leakage of cytoplasmic contents into the extracellular environment, causing an inflammatory reaction.[41] Quantitative analysis using the Annexin V/PI assay as an indicator of apoptosis and necrosis demonstrated that safranal, having apoptotic activity, also possesses minimal capacity of inducing necrotic cell death on PC-3 cells.
Different hypotheses for the modes of anticarcinogenic and antitumor actions of C. sativus and its component, safranal, have been proposed:[8]1. the inhibitory effect on cellular DNA and RNA synthesis, but not on protein synthesis; 2. the inhibitory effect on free radical chain reactions, because most carotenoids are lipid-soluble and might act as membrane-associated high-efficiency free radical scavengers; 3. the metabolic conversion of naturally occurring carotenoids to retinoids; and 4. the interaction of carotenoids with topoisomerase II, an enzyme involved in cellular DNA–protein interaction. Thus, although several hypotheses have been put forward, the exact mechanism (s) of anticarcinogenic and antitumor effects of C. sativus and its main constituents are not clear at present. The bioactive compound of C. sativus, safranal, is believed to possess cytotoxicity toward prostate cancer cells through its ability to disrupt the cell growth of cancer and initiate them to undergo apoptotic cell death. Although the detailed or underlying mechanisms of selectivity of safranal against prostate cancer cells are still unclear, our findings have revealed that safranal exerts its growth inhibition toward cancer cells through induction of apoptosis. Further investigations of the apoptosis pathway are needed to reveal the exact mode of action of safranal for its anticancer properties.
ACKNOWLEDGMENT
The authors would like to thank Research Affairs of Neyshabur Faculty of Medical Sciences for financially supporting this work. We thank Dr. F. Kalalinia for her assistance in flow cytometry analysis.
Footnotes
Source of Support: Research Affairs of Neyshabur Faculty of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–42. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 4.Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–80. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 5.Samarghandian S, Afshari JT, Davoodi S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics (Sao Paulo) 2011;66:1073–9. doi: 10.1590/S1807-59322011000600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winterhalter P, Staudinger M. Saffron- renewed interest in an ancient spice. Food Rev Int. 2000;16:39–59. [Google Scholar]
- 7.Premkumar K, Abraham SK, Santhiya ST, Gopinath PM, Ramesh A. Inhibition of genotoxicity by saffron (Crocus sativus L.) in mice. Drug Chem Toxicol. 2001;24:421–8. doi: 10.1081/dct-100106266. [DOI] [PubMed] [Google Scholar]
- 8.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med. 2002;247:20–5. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 9.Samarghandian S, Tavakkol Afshari J, Davoodi S. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. Appl Biochem Biotechnol. 2011;164:238–47. doi: 10.1007/s12010-010-9130-x. [DOI] [PubMed] [Google Scholar]
- 10.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Iran Med. 2002;5:44–7. [Google Scholar]
- 11.Hosseinzadeh H, Karimi GH, Niapoor M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. Acta Hortic. 2004;650:435–45. [Google Scholar]
- 12.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang YX, Sugiura M, Saito H, Shoyama Y. Acute effects of Crocus sativus L. on passive avoidance performance in mice. Biol Pharm Bull. 1994;17:217–21. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]
- 14.Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocus sativus L.) on genotoxins-induced oxidative stress in swiss albino mice. Phytother Res. 2003;17:614–7. doi: 10.1002/ptr.1209. [DOI] [PubMed] [Google Scholar]
- 15.Bathaie SZ, Mousavi SZ. New applications and mechanisms of action of saffron and its important ingredients. Crit Rev Food Sci Nutr. 2010;50:761–86. doi: 10.1080/10408390902773003. Review. [DOI] [PubMed] [Google Scholar]
- 16.Tarantilis PA, Morjani H, Polissiou M, Manfait M. Inhibition of growth and induction of differentiation promyelocytic leukemia (HL-60) by carotenoids from Crocus sativus L. Anticancer Res. 1994;14:1913–8. [PubMed] [Google Scholar]
- 17.Escribano J, Alonso GL, Coca-Prados M, Fernández JA. Crocin, safranal and picocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 18.Abdullaev FI. Inhibitory effect of crocetin on intracellular nucleic acid and protein synthesis in malignant cells. Toxicol Lett. 1994;40:243–51. doi: 10.1016/0378-4274(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 19.Yawata A. Prolonged cell survival enhances peritoneal dissemination of gastric cancer cells. Oncogene. 1998;16:2681–6. doi: 10.1038/sj.onc.1201792. [DOI] [PubMed] [Google Scholar]
- 20.Cragg GM, Newman DJ. Antineoplastic agents from natural sources: Achievements and future directions. Expert Opin Investig Drugs. 2000;9:2783–97. doi: 10.1517/13543784.9.12.2783. [DOI] [PubMed] [Google Scholar]
- 21.Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–9. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Cragg GM, Schepartz SA, Suffness M, Grever MR. The taxol supply crisis. New NCI policies for handling the large-scale production of novel product anticancer and anti-HIV agents. J Nat Prod. 1993;56:1657–68. doi: 10.1021/np50100a001. [DOI] [PubMed] [Google Scholar]
- 23.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–32. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Kianbakht S. A systematic review on pharmacology of saffron and its active constituents. J Med Plants. 2009;28:1–23. [Google Scholar]
- 25.Alonso GL, Salinas MR, Garijo J. Method to determine the authenticity of aroma of saffron (Crocus sativus L.) J Food Prot. 1998;61:1525–8. doi: 10.4315/0362-028x-61.11.1525. [DOI] [PubMed] [Google Scholar]
- 26.Nair SC, Pannikar B, Pannikar KR. Antitumour activity of saffron (Crocus sativus) Cancer Lett. 1991;57:109–14. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- 27.Gutheil WG, Reed G, Ray A, Anant S, Dhar A. Crocetin: An agent derived from saffron for prevention and therapy for cancer. Curr Pharm Biotechnol. 2012;13:173–9. doi: 10.2174/138920112798868566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morjani H, Tarantilis P, Polissiou M, Manfait M. Growth inhibition and induction of crythoid differentiation activity by crocin, dimethylcrocetin and ,-carotene on K562 tumor cells. Anticancer Res. 1990;10:1398–406. [Google Scholar]
- 29.Nair SC, Salomi MJ, Varghese CD, Pannikar B, Pannikar KR. Effect of saffron on thymocyte proliferation, intracellular gluthathione levels and its antitumor activity. Biofactors. 1992;4:51–4. [PubMed] [Google Scholar]
- 30.Escribano J, Diaz-Guerra MJ, Riese HH, Alvarez A, Proenza R, Fernández JA. The cytotoxic effect of glucoconjugate extracted from corms of saffron plant (Crocus sativus) on human cell lines in culture. Planta Med. 2000;66:157–62. doi: 10.1055/s-2000-11127. [DOI] [PubMed] [Google Scholar]
- 31.Escribano J, Piqueras A, Medina J, Rubio A, Alvarez-Orti M, Fernández JA. Production of a cytotoxic proteoglycan using callus culture of saffron corms (Crocus sativus L.) J Biotechnol. 1999;73:53–9. doi: 10.1016/s0168-1656(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Olmo DC, Riese HH, Escribano J, Ontañon J, Fernández JA, Atienzar M, et al. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): An experimental study in the rats. Nutr Cancer. 1999;35:120–6. doi: 10.1207/S15327914NC352_4. [DOI] [PubMed] [Google Scholar]
- 33.Molnar J, Szabo D, Pusztai R, Mucsi I, Berek L, Ocsovski I, et al. Membrane associated antitumor effects of crocine-, ginseniside- and cannabinoid derivates. Anticancer Res. 2000;20:861–7. [PubMed] [Google Scholar]
- 34.Samarghandian S, Boskabady MH, Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–14. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 36.Pintado C, Miguel A, Acevedo O, Nozal L, Novella JL, Rotger R. Bactericidal effect of saffron (Crocus sativus L.) on Salmonella enterica during storage. Food Control. 2011;22:638–42. [Google Scholar]
- 37.Kanakis CD, Tarantilis PA, Tajmir-Riahi HA, Polissiou MG. DNA interaction with saffron's secondary metabolites safranal, crocetin, and dimethylcrocetin. DNA Cell Biol. 2007;1:63–70. doi: 10.1089/dna.2006.0529. [DOI] [PubMed] [Google Scholar]
- 38.Abdullaev FI. Biological effects of saffron. Biofactors. 1993;4:83–6. [PubMed] [Google Scholar]
- 39.Guchelaar HJ, Uges DR, de Vries EG, Oosterhuis JW, Mulder NH. Combination therapy with cisplatin: Modulation of activity and tumour sensitivity. Clin Oncol. 1992;4:388–93. doi: 10.1016/s0936-6555(05)81134-3. [DOI] [PubMed] [Google Scholar]
- 40.Mailloux A, Grenet K, Bruneel A, Bénéteau-Burnat B, Vaubourdolle M. Anticancer drugs induce necrosis of human endothelial cells involving both oncosis and apoptosis. Eur J Cell Biol. 2001;80:442–9. doi: 10.1078/0171-9335-00171. [DOI] [PubMed] [Google Scholar]
- 41.Kasibhatla S, Tseng B. Why target apoptosis in cancer treatment? Mol Cancer Ther. 2003;2:573–80. [PubMed] [Google Scholar]


