Abstract
Breast cancer is the foremost common malignancy among the female population around the world. Female breast cancer incidence rates have increased since 1980, slowed in 1990, the rate of increase have leveled off since 2001. In spite of the advances in the early detection, treatment, surgery and radiation support, almost 70% of the patients develop metastasis and die of the disease. Around 10% of the patients when diagnosed with breast cancer have metastases. Survival among the breast cancer patients have increased due to the introduction of novel single agent, combination of chemotherapeutic agents and targeted biologic agents, which is breast cancer specific. The staging of tumor-node-metastasis is significant for the prognosis and treatment. Predominantly the combination of chemotherapeutic regimen is given to improve the rate of clinical benefit and the overall survival rate. Novel mono-therapeutic options are being used often in metastatic setting as they will not be able to endure the toxicity of the combination regimen. Usually, endocrine therapy is recommended for hormone-responsive breast cancer due to efficacy and favorable side effect profile but chemotherapy becomes an option when endocrine therapy fails. This review summarizes the newer therapeutic options for early breast cancer and advanced breast cancer that are pretreated heavily on other chemotherapeutic agents. Further it provides monotherapies and other emerging novel combination regime which can be opted for first line or second line setting.
Keywords: Breast cancer, metastasis, novel monotherapy, combination regimen, clinical benefit rate
Breast cancer is a malignant proliferation of epithelial cell lining the ducts or lobules of the breast, which may have diverse outcomes and responses to treatment depending on the early diagnosis. This is the most common cancer in woman in many developed and developing countries. Breast cancer is the leading cause of cancer deaths in females between 20 and 59 years[1]. Early accurate diagnosis is important for optimizing the treatment and potential for cure. During the last decades, breast cancer survival has increased considerably[2,3] due to earlier diagnosis and increasing use of adjuvant and neoadjuvant therapies but around 30-70% of the patients eventually develop recurrence and die of metastasis every year. Improved treatment options offer a better prognosis for the patients with breast cancer.
The possibility of breast cancer is augmented with the increasing age, past medical history of uterus cancer, ovarian cancer, and family history of breast cancer. Postmenopausal hormone replacement therapy may also direct to breast cancer. Patients who had prior treatment for breast cancer have 30-50% chance of developing cancer in the contra lateral breast which can be reduced by adjuvant endocrine therapy for minimum 5 years. Several genetic, hormonal, and environmental factors are involved in the development of breast cancer.
Chemotherapy treatment during early stage breast cancer has shown to extend the survival. Chemotherapeutic option with anthracyclines or taxanes is considered to be the most effective in breast cancer. Almost 70% of the patients with metastatic breast cancer have human receptor (HR) positive tumors, which are responsive to adjuvant hormone therapy and helps in prolonging the disease free survival. Chemotherapy is the only treatment option for rapidly progressing metastatic breast cancer (MBC) and various classes of drugs along with their mechanism, available for the treatment as monotherapy is represented in fig. 1. Treatment failure in metastatic setting occurs due to the resistance to the chemotherapeutic agents which may be due to the prior exposure to these agents. No standard monotherapy or combination palliative chemotherapy is existing for patients who are pretreated with anthracyclines or taxanes. The median survival rate of the patient with metastasis is within the range of 3 years. When patients with metastasis are seen then the aim of the treatment is palliative care providing mental support and increasing the median survival. This article summarizes some of the newer therapeutic options currently available for the treatment of MBC.
Fig. 1.
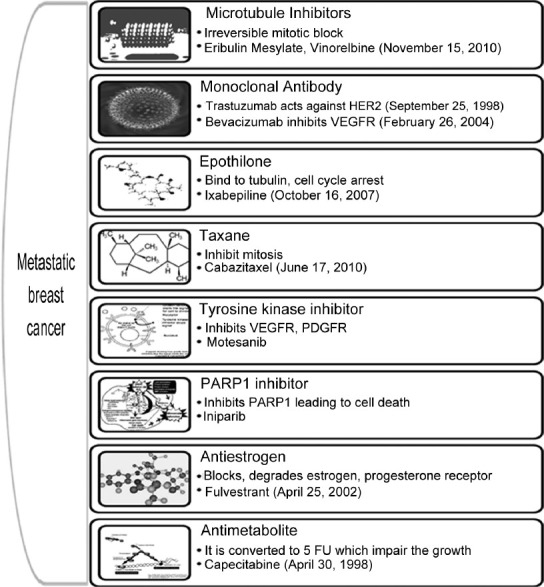
Various drugs used in the treatment of metastatic breast cancer and their mechanism of action.
NOVEL MONOTHERAPEUTIC OPTIONS AVAILABLE FOR THE TREATMENT
Eribulin mesylate:
Eribulin mesylate is a non-taxane, synthetic analog of halichondrin B isolated from a sea sponge. Eribulin has a mechanism of action that is distinct from those of the other tubulin targeting agents[4] and it inhibits the microtubule growth phase causing tubulin sequestration into the non-productive aggregates[5]. It causes irreversible mitotic block and apoptosis. It was hypothesized that Eribulin may be shown to have efficacy in cancer related to tubulin targeted agents. Eribulin is given to patients who are having metastatic breast cancer and pretreated with an anthracycline or a taxane. EMBRACE trial[5] was conducted to know the effect of Eribulin monotherapy versus the treatment of physician choice in patients with MBC, which concluded that the median progression free survival was 3.7 months, clinical benefit rate was 23%, overall survival rate was 58%. Cotes et al.[6] conducted a phase 2 study of the halichondrin B analog in patients with advanced MBC previously treated with an anthracyclines, taxanes, and capecitabine. The study results had reported that the objective response rate was 11.5% and clinical benefit rate of 17.2% with manageable tolerability profile (Table 1).
TABLE 1.
MONOTHERAPY USED IN VARIOUS TRIALS FOR THE TREATMENT OF METASTATIC BREAST CANCER

Fulvestrant:
It is an estrogen receptor (ER) antagonist that down regulates the circulating ERs[7]. It is currently used in the treatment of postmenopausal women with advanced breast cancer even with prior endocrine treatment[8]. It acts by ER dimerization causing rapid degradation leading to the loss of cellular ER. The drug was approved by USFDA in 2002. CONFIRM trial[9] conducted in 2010 concluded that the median progression free survival was 6.5 months in the group of patients administered with 500 mg and 5.5 months in 250 mg group. The median duration of response was 19.4 months for 500 mg and 16.4 months for 250 mg of fulvestrant (Table 1).
Trastuzumab:
It is a humanized monoclonal antibody directed against extracellular domains of the HER2 receptor and it dramatically improved the outcomes in patients with HER2 over expressing (HER2 positive) breast cancer[10]. It binds to an epitope in the HER2 receptor that cause uncoupling of HER2–HER3 heterodimers thereby inhibiting antibody dependent cell-mediated cytotoxicity[11]. It is the first antiHER2 therapy approved by USFDA in 1998. Recently concluded HERA trial (2011) reported that disease free survival in the patients treated with 1 year trastuzumab was 78.6% as compared with the observation group 72.2% while there was no significant difference in the overall survival rate in the both groups[12]. NOAH Trial reported the addition of trastuzumab as neoadjuvant significantly improved the event free survival in HER2 positive breast cancer patients by 71% versus 56% without trastuzumab[13] (Table 1).
Olaparib:
It acts by inhibiting enzyme poly ADP ribose polymerase. It is one of the first PARP inhibitor[14]. Olaparib 400 mg twice daily was found to be active in patients with BRCA1 or BRCA2 mutations, MBC which was resistant to conventional chemotherapy. Tutt et al.[15] concluded that olaparib 400 mg twice daily had better progression free survival, the objective response rate than 100 mg twice daily. The objective response rate with 400 mg olaparib group was 41% and with 100 mg group was 22% (Table 1).
Pertuzumab:
It binds to HER2 at the dimerization domain[16], thereby inhibiting its ability to form dimmers, bind competitively to the intracellular adenosine tri phosphate binding site of HER[17]. Gianni et al.[18] concluded that in patients treated with loading dose of pertuzumab (arm A) the median duration of clinical benefit was 36.5 weeks and those treated with no loading dose of pertuzumab (arm B) the median duration of clinical benefit was 33-36 weeks. Median time to treatment failure was 6.3 weeks in arm A and 6 weeks in arm B (Table 1).
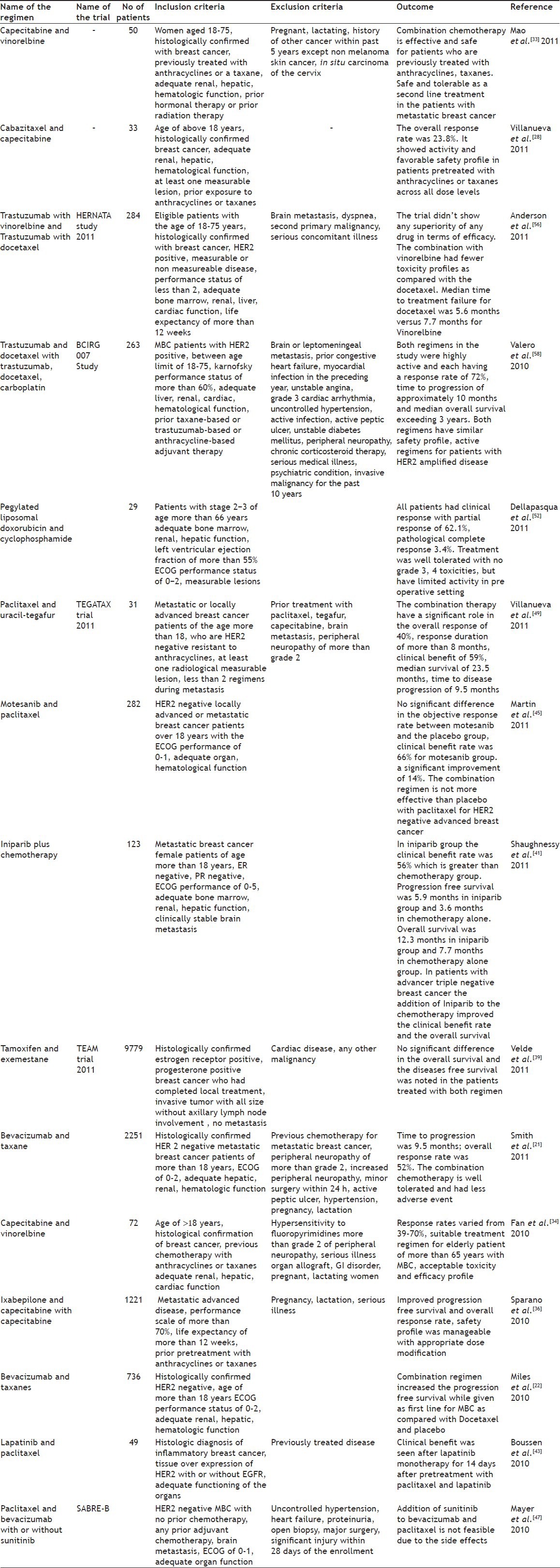
CURRENT COMBINATION REGIMEN(S) FOR THE TREATMENT OF BREAST CANCER
Bevacizumab and taxanes:
Bevacizumab is humanized monoclonal antibody that specifically inhibits vascular endothelial growth factor. It is a key mediator of angiogenesis[19,20]. Bevacizumab is used for the treatment of metastatic breast cancer, colorectal cancer, and non-small cell lung cancer. It is approved as the first line treatment in glioblastoma multiforme. It was approved by USFDA in 2004. It was also granted approval to be used in combination with interferon alfa for the treatment of metastatic renal cell carcinoma. Smith et al.[21] reported the overall response rate of 47% and median time to progression of 7.2 months with median overall survival of 19.5 months. Smith et al.[21] reported that this combination regimen has improved the time to progression, overall response rate and had less adverse event (Table 2). Miles et al.[22] reported that the regimen increased the progression free survival when combined with docetaxel.
TABLE 2.
COMBINATION REGIMENS USED IN VARIOUS TRIAL FOR THE TREATMENT OF MBC AND ITS OUTCOME
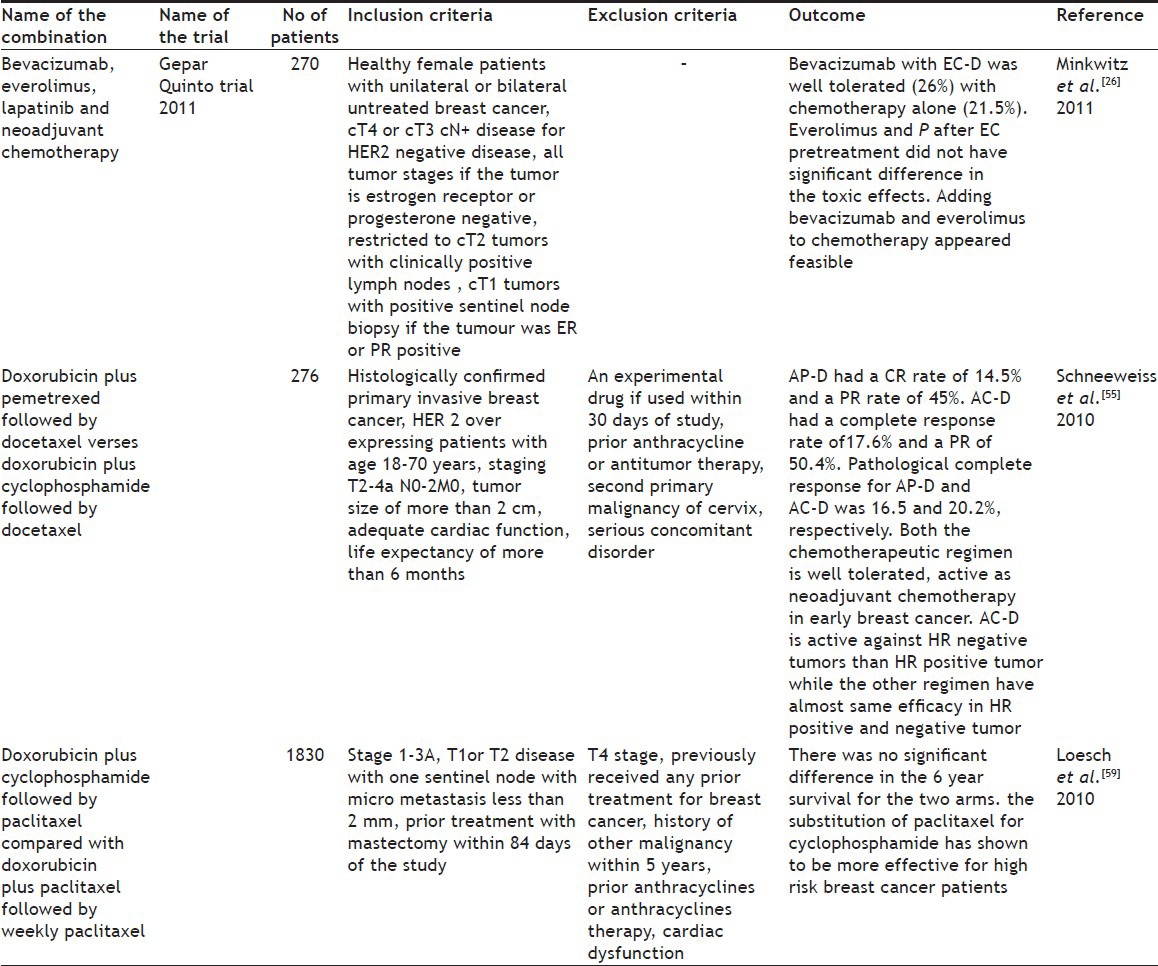
Bevacizumab, everolimus, and lapatinib:
Everolimus inhibits mammalian target of rapamycin complex 1 (mTORC1) which plays an essential role in protein synthesis thereby reducing cell proliferation, tumor growth, and angiogenesis[23]. It is also approved for treating patients with progressive neuroendocrine tumors in the pancreas, which cannot be surgically removed and in metastatic pancreatic cancer. Lapatinib is a 4-anilinoquinazoline kinase inhibitor of intracellular tyrosine kinase domains of epidermal growth factor receptor. It also inhibits HER2 and HER1 receptor tyrosine kinase[24,25] and it was approved in 2007 by USFDA. Minkwitz et al.[26] reported the treatment of chemotherapy with bevacizumab had 19.4% discontinuations with everolimus the discontinuation was 24.1% and with lapatinib the discontinuation was 34.5%. Treatment was discontinued in patients with lapatinib 1250 mg due to the side effects (Table 3).
TABLE 3.
MULTIPLE NEWER REGIMENS ON RECENT TRIALS AND ITS OUTCOME
Cabazitaxel and capecitabine:
Cabazitaxel is a novel tubulin binding taxoid. It has a safety profile in docetaxel or paclitaxel resistant breast cancer[27]. Recently in the year 2010, this drug was approved by USFDA for the treatment of metastatic breast cancer patients. It is also used in the treatment of metastatic hormone refractory prostrate cancer. Villannueva et al.[28] reported that the median duration of response was 3.1 months and the median time to progression was 4.9 months (Table 2).
Capecitabine and vinorelbine:
Vinorelbine is third generation vinca alkaloid that has significant activity against advanced breast cancer in the first line setting[29]. Capecitabine is an oral fluoropyrimidines precursor which is orally administered prodrug activated in liver and converted to 5-fluorouracil at the tumor site via a three-phase enzymatic cascade[30]. After two intermediate steps involving carboxyl esterase I the liver and cytidine deaminase in the liver/tumor tissue, the final metabolite is converted to 5-fluorouracil by thymidine phosphorylase[31]. It has shown its efficacy in patients who are heavily pretreated with anthracycline or a taxane[30]. Due to its favorable toxicity and efficacy profile it is used frequently in clinical trial of novel drugs as control treatment. Capecitabine can be used as a single agent in patients with advanced breast cancer who are pretreated with anthracycline or taxanes. Capecitabine monotherapy has time to progression (3.1-4.9 months) and the response rate of 20-28% approximately[32]. Mao et al.[33] have reported that the overall clinical benefit rate was 72% and the objective response rate was 26% with median time to progression of 5 months. Fan et al.[34] reported that median time to progression was 7.7 months, median survival of 26.1 months and response rate of 53.8% (Table 2).
Capecitabine and ixabepilone:
Ixabepilone is a semisynthetic analog of epothilone analog which acts by binding to β tubulin subunits of microtubules leading to induction of tubulin polymerization and disruption of chromosomal segregation that is needed for the completion of mitosis. It ultimately induces apoptosis[35]. It has low susceptibility to common resistance mechanism that is related to anthracyclines or taxanes. Combination of this regimen is mainly used in advanced breast cancer and those patients who are treated prior with anthracycline or taxanes. Sparano et al.[36] reported that the overall response rate of the combination regimen was 43%, time to response was 6.6 weeks, median progression free survival was 6.24 months for the combination as compared with 4.4 months for capecitabine alone (Table 2).
Exemestane and tamoxifen:
Exemestane is a third generation steroidal aromatase inhibitor used for metastatic breast cancer. Exemestane has more efficacy as compared with tamoxifen given alone[37]. Exemestane has currently emerged as the first line treatment option for metastatic breast cancer after a phase 2 randomized trial conducted by the European organization for research and treatment of cancer (EORTC)[38]. Exemestane is mainly used in postmenopausal women with early or advanced breast cancer. Velde et al.[39] reported that when 5 years data were taken, no difference was noted in the disease free survival and overall survival in patients with HR positive breast cancer treated with exemestane and tamoxifen but musculoskeletal adverse events were increased with exemestane therapy alone.
Iniparib plus chemotherapy:
Iniparib is a poly adenosine diphosphate-ribose polymerase 1 (PARP1) inhibitor. PARP1 regulates the DNA base-excision repair. Now iniparib is recommended for triple negative breast cancer. Iniparib synergize the cytotoxic and antiproliferative effects of gemcitabine and carboplatin[40]. Shaughnessy et al.[41] reported that when iniparib was added to gemcitabine and carboplatin the clinical benefit rate improved from 34 to 56%, median overall survival from 7.7 months to 12.3 months with median progression free survival from 3.6 to 5.9 months (Table 2).
Lapatinib and paclitaxel:
It is a tyrosine kinase inhibitor and inhibits the cellular proliferation which is overexpressed with HER1 or HER2. It acts intracellularly, binds with cytoplasmic ATP binding site thereby blocking the phosphorylation and the activation leading to apoptosis[42]. Boussen, et al.[43] concluded that clinical response rate was 78.6% in cohort A (tumors overexpressing with HER2 without coexpression of EGFR) patients. In cohort B (tumors expressing EGFR without HER2 overexpression) the clinical response rate was 71.4%.
Motesanib and paclitaxel:
Motesanib is a vascular endothelial growth factor tyrosine kinase inhibitor (VEGFRTKI). VEGF plays an important role in angiogenesis. It is also acting as an antagonist at VEGFR1, VEGFR2, and VEGFR3. The inhibition of kinase is different from that of other VEGFR inhibitors[44]. Martin et al.[45] has reported the overall response rate for motesanib group was 49% and for placebo was 41%, the median progression free survival for motesanib group was 9.5 months versus 9.0 months, clinical benefit rate for motesanib was 66%, and for placebo was 18% (Table 2).
Paclitaxel, bevacizumab, and sunitinib:
Sunitinib is an oral multi kinase inhibitor that blocks multiple molecular targets that is affecting the growth, proliferation, and metastatic progression of cancer. It increases the circulating vascular endothelial growth factor[46]. Mayer et al.[47] concluded that patients receiving the three drug regimen had unacceptable high level toxicity with discontinuations. Poor tolerability was noted in the regimen with paclitaxel, bevacizumab, and sunitinib.
Paclitaxel and tegafur:
Tegafur is a derivative of 5-fluorouracil that inhibits dihydropyrimidine dehydrogenase. Combination therapy or monotherapy with tegafur have a response rate of 6-28%[48]. Villanueva et al.[49] reported that the response duration was 8.4 months, median time to progression was 9.5 months, median overall survival was 23.5 months (Table 2).
Pegylated liposomal doxorubicin and cyclophosphamide:
Pegylated liposomal doxorubicin is a formulation of doxorubicin in polyethylene glycol coated liposome. Pegylated formulation prolongs the circulation time and accumulation in the tumor tissues. It helps in the reduction of the side effects as compared with free doxorubicin[50]. Metronomic chemotherapy is the chronic administration of low doses of chemotherapeutic drugs at regular intervals[51]. Dellapasqua et al.[52] have reported that the partial response rate was 62.1% and no grade four toxicity was reported (Table 2).
Pemetrexed plus doxorubicin followed by docetaxel verses doxorubicin plus cyclophosphamide followed by docetaxel:
Pemetrexed is a folate antimetabolite, which inhibits thymidylate synthase and dihydrofolate reductase. In pretreated metastatic breast cancer single agent pemetrexed with or without vitamin supplementation provided the response rate of 8-28%[53]. Pemetrexed is transported into the cell by the reduced folate carrier and membrane folate binding protein transport systems. In the cell it is converted into the polyglutamated forms which are then retained in the cell[54]. The polyglutamated metabolites have prolonged action in the malignant cells. The drug was approved by USFDA in the year 2010. It is also approved by FDA for maintenance treatment of patients with nonsquamous non-small cell lung cancer whose disease has not progressed after four cycle of first line chemotherapy with platinum based agents. Schneeweiss et al.[55] reported that the pathological complete response with the regimen doxorubicin, pemetrexed, docetaxel was 16.5% and with the regimen doxorubicin, cyclophosphamide, docetaxel was 20.2%. Response rate of doxorubicin, cyclophosphamide was 43.7%, and with doxorubicin, pemetrexed was 40.5% which shows almost similar activity (Table 3).
Trastuzumab plus docetaxel with trastuzumab plus vinorelbine:
Vinorelbine is a vinca alkaloid chemotherapeutic drug. It acts synergistically when given in combination with trastuzumab. The antitumor activity of vinorelbine is due to the inhibition of mitosis at the metaphase. It may also interfere with the cyclic AMP, nucleic acid biosynthesis, and inhibit mitotic microtubule formation. Trastuzumab is now approved to be used in combination with cisplatin and fluoropyrimidines for treatment of HER2 over expressing metastatic gastric or gastroesophageal junction adenocarcinoma, who have not received prior treatment for metastatic disease. HERNATA trial concluded in 2011 reported that there was not much difference on overall survival and median time to progression, response rate between the two treatment arms[56] (Table 2).
Trastuzumab and docetaxel with trastuzumab, docetaxel, carboplatin:
A pivotal phase 3 study conducted by Salmon et al. demonstrated that the combination of trastuzumab and chemotherapy significantly prolonged time to progression and overall survival compared with chemotherapy alone in patients with HER2 positive MBC. Carboplatin predominantly cause interstrand DNA cross links[57]. It nonspecifically affects the cell cycle. Carboplatin is used as first line agent in recurrent ovarian carcinoma. It was approved in 2005 by USFDA. Valero et al.[58] reported that there was no significant difference between docetaxel, trastuzumab (TH) and docetaxel, trastuzumab, carboplatin (TCH). Time to progression in TH regimen was 11.1 months and in TCH was 10.4 months. Response rate in the both regimen was 72%, overall survival rate was 37.1 month in TH regimen, and 37.4 in TCH regimen (Table 2).
CONCLUSION
There are several treatment options for breast cancer like chemotherapy, hormonal therapy, targeted anti-HER2 therapy, antiangiogenic therapies are available. Now adjuvant and neoadjuvant chemotherapy is advancing as it cures the micro-metastasis leading to better outcomes. There is a significant improvement in the overall survival rate and progression free survival after the introduction of novel drugs. When selecting a treatment option, the tolerability, efficacy, and mechanism of overcoming resistance have to be considered. The single agent eribulin is a choice of monotherapy for third line monotherapy in advanced breast cancer. Certain drugs like eribulin, ixabepilone with capecitabine, vinorelbine with capecitabine are approved in breast cancer resistant to anthracyclines and taxanes. Fulvestrant a novel single agent drug have considerable progression free survival in ER positive breast cancer. Iniparib in combination with gemcitabine and carboplatin have shown the efficacy in triple negative breast cancer. In HER2 positive breast cancer addition of trastuzumab to neoadjuvant chemotherapy improved overall response rate, reduced progression, relapse and death. Several other treatment options are coming up with maximum clinical efficacy and minimum toxicity level.[59]
Footnotes
Mohan and Ponnusankar: Treatment of Metastatic Breast Cancer: Clinical Update
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun M. Cancer statistics 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Sant M, Capocaccia R, Colerman MP, Berrino F, Gatta G, Micheli A, et al. EUROCARE Working Group. Cancer survival increases in Europe, but international differences remain wide. Eur J Cancer. 2001;37:1659–67. doi: 10.1016/s0959-8049(01)00206-4. [DOI] [PubMed] [Google Scholar]
- 3.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BF. Cancer survival among US whites and minorities: A SEER (Surveillance, Epidemiology, and End Results) Program population – based study. Arch Intern Med. 2002;162:1985–93. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 4.Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, et al. The primary antimitotic mechanism of action of synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4:1086–95. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 5.Cortes J, Shaughnessy JO, Blum JL, Vahdat LT, Petrakova K, Chollet P, et al. Eribulin monotherapy versus treatment of physician choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open label randomized study. Lancet. 2011;377:914–23. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 6.Cortes J, Vahdat L, Blum JL, Twelves C, Campone M, Roche H, et al. Phase 2 study of the Halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracyclines, a taxanes, and capecitabine. J Clin Oncol. 2010;28:3922–8. doi: 10.1200/JCO.2009.25.8467. [DOI] [PubMed] [Google Scholar]
- 7.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–73. [PubMed] [Google Scholar]
- 8.Howell A. Fulvestrant (Faslodex): Current and future role in breast cancer management. Crit Rev Oncol Hematol. 2006;57:265–73. doi: 10.1016/j.critrevonc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Leo AD, Jerusalem G, Petruzelka L, Torrges R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase 3 trial comparing fulvestrant 250 mg with fulvestrant 500 mg in post menopausal women with estrogen receptor positive advanced breast cancer. J Clin Oncol. 2010;28:4594–600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 10.Hudis CA. Trastuzumab mechanism of action and use in clinical practice. N Eng J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 11.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich BO, Yang X. The therapeutic effect of anti HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–70. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch C, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow up of a randomized controlled trial. Lancet Oncol. 2011;12:236–44. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 13.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab verses neoadjuvant chemotherapy alone, in patients with HER2 positive locally advanced breast cancer (the NOAH trial): A randomized controlled superiority trial with a parallel HER2 negative cohort. Lancet. 2010;375:377–84. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 14.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic stratergy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 15.Tutt A, Robson M, Garber JE, Dornchek SM, Audeh MW, Weitzel JN, et al. Oralpoly (ADP ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof of concept trial. Lancet. 2010;376:235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 16.Franklin MC, Carey KD, Vajdos FF, Leahy DF, Devos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2 pertuzumab complex. Cancer Cell. 2004;5:317–28. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 17.Arteaga CL, Baselga J. Tyrosine kinase inhibitor: Why does the current process of clinical development not apply to them? Cancer Cell. 2004;5:525–31. doi: 10.1016/j.ccr.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Gianni L, Llado A, Bianchi G, Cortes J, Lehtinen PL, Cameron DA, et al. Open label, phase 2, multicenter, randomised study of efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:1131–7. doi: 10.1200/JCO.2009.24.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieves BJ, D’Amore PA, Bryan BA. The function of vascular endothelial growth factor. Biofactors. 2009;35:332–7. doi: 10.1002/biof.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara N, Gerber HP, Le Couter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 21.Smith IE, Pierga JY, Biganzoli L, Funes HC, Thomssen C, Pivot X, et al. First line bevacizumab plus taxanes based chemotherapy for locally recurrent or metastatic breast cancer: Safety and efficacy in an open label study in 2251 patients. Ann Oncol. 2011;22:595–602. doi: 10.1093/annonc/mdq430. [DOI] [PubMed] [Google Scholar]
- 22.Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczat P, et al. Phase 3 study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–47. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 23.Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase 2 randomized study of neoadjuvant Everolimus plus letrozole in patients with estrogen receptor positive breast cancer. J Clin Oncol. 2009;27:2630–7. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 24.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 25.Johnston S, Pippen J, Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–46. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 26.Minkwitz GV, Eidtmann H, Loibl S, Blohmer JU, Costa SD, Fasching PA, et al. Integrating bevacizumab, everolimus and lapatinib into current neoadjuvant chemotherapy regimen for primary breast cancer. Safety results of the Gepar Quinto trial. Ann Oncol. 2011;22:301–6. doi: 10.1093/annonc/mdq350. [DOI] [PubMed] [Google Scholar]
- 27.Pivot X, Asmar L, Buzdar AU, Valero V, Hortobagyi G. A unified definition of clinical anthracyclines resistance breast cancer. Br J Cancer. 2000;82:529–34. doi: 10.1054/bjoc.1999.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villanueva C, Awada A, Campone M, Machiels JP, Besse T, Magherini E, et al. A multicenterd dose escalating study of cabazitaxel in combination with capecitabine in patients with metastatic breast cancer progressing after anthracyclines and taxanes treatment: A phase 1/2 study. Eur J Cancer. 2011;47:1037–45. doi: 10.1016/j.ejca.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Fumoleau P, Delgado FM, Delozier T, Monnier A, Gil DM, Kerbreat P, et al. Phase 2 trial of weekly intravenous vinorelbine in first line advanced breast cancer chemotherapy. J Clin Oncol. 1993;11:1245–52. doi: 10.1200/JCO.1993.11.7.1245. [DOI] [PubMed] [Google Scholar]
- 30.Gelmon K, Chan A, Harbeck N. The role of capecitabine in first line treatment for patients with metastatic breast cancer. Oncologist. 2006;11:42–51. doi: 10.1634/theoncologist.11-90001-42. [DOI] [PubMed] [Google Scholar]
- 31.Bedard PL, Chantal BM, Raimondi C, Cardoso F. The role of capecitabine in the management of breast cancer in elderly patients. J Geriatr Oncol. 2011;2:72–81. [Google Scholar]
- 32.Thomas ES, Gomez HL, Li RK, Chung HC, Fein LE, Chan VF, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–7. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 33.Mao W, Guan X, Tucker S, Li F, He Z, Wang J, et al. Second line combination chemotherapy with vinorelbine and capecitabine in patients with advanced breast cancer previously treated with anthracyclines and/or taxanes. Chemotherapy. 2011;57:71–6. doi: 10.1159/000321489. [DOI] [PubMed] [Google Scholar]
- 34.Fan Y, Xu B, Yuan P, Wang J, Ma F, Li Q, et al. Prospective study of vinorelbine and capecitabine combination therapy in Chinese patients with metastatic breast cancer pretreated with anthracyclines and taxanes. Chemotherapy. 2010;56:340–7. doi: 10.1159/000320186. [DOI] [PubMed] [Google Scholar]
- 35.Lee FY, Borzilleri R, Fairchild CR, Kim SH, Long BH, Reventos-Suarez C, et al. BMS-247550. A novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–37. [PubMed] [Google Scholar]
- 36.Sparano JA, Vrdoljak E, Rixe O, Xu B, Manikhas A, Medina C, et al. Randomized phase 3 trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracyclines and taxanes. J Clin Oncol. 2010;28:3256–63. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thürlimann B, von Euler M. Anastosole is superior to tamoxifen as first line therapy in hormone receptor positive advanced breast carcinoma. Cancer. 2001;92:2247–58. doi: 10.1002/1097-0142(20011101)92:9<2247::aid-cncr1570>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 38.Pairdaens R, Dirix L, Lohrisch C, Beex L, Nooij M, Cameron D, et al. Mature results of randomized phase 2 multicentre study of exemestane versus tamoxifen as first line hormonal therapy for postmenopausal women with metastatic breast cancer. Ann Oncol. 2003;14:1391–8. doi: 10.1093/annonc/mdg362. [DOI] [PubMed] [Google Scholar]
- 39.van de Velde CJ, Rea D, Seynaeve C, Putter H, Hasenbrg A, Vannetzel JM, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): A randomized phase 3 trial. Lancet. 2011;377:321–31. doi: 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 40.Allie E, Sharma VB, Sunderesakumar P, Ford JM. Defective repair of oxidative DNA damage in triple negative breast cancer confers sensitivity to inhibition of poly (ADP-ribose) polymerase. Cancer Res. 2009;69:3589–96. doi: 10.1158/0008-5472.CAN-08-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaughnessy JO, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple negative breast cancer. N Engl J Med. 2011;364:205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 42.Spector NL, Xia W, Burris H, Hurwitz H, Dees EC, Dowlati A, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23:2502–12. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 43.Boussen H, Cristofanilli M, Zaks DeSilvio M, Salazar V, Spetor N. Phase 2 study to evaluate the efficacy and safety of neoadjuvant lapatinib plus paclitaxel in patients with inflammatory breast cancer. J Clin Oncol. 2010;28:3248–55. doi: 10.1200/JCO.2009.21.8594. [DOI] [PubMed] [Google Scholar]
- 44.Karaman MW, Hergard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 45.Martin M, Roche H, Pinter T, Crown J, Kennedy MJ, Provencher L, et al. Motesanib, or open – label bevacizumab, in combination with paclitaxel, as first line treatment for HER2 negative locally recurrent or metastatic breast cancer: A phase 2, randomized, double-blind, placebo-controlled study. Lancet Oncol. 2011;12:369–76. doi: 10.1016/S1470-2045(11)70037-7. [DOI] [PubMed] [Google Scholar]
- 46.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase 2 study of sunitinib malate an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with anthracycline and a taxane. J Clin Oncol. 2008;26:1810–6. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 47.Mayer El, Dhakil S, Patel T, Sundaram S, Fabian C, Kozloff M, et al. SABRE-B: An evaluation of paclitaxel and bevacizumab with or without sunitinib as first line treatment of metastatic breast cancer. Ann Oncol. 2010;21:2370–6. doi: 10.1093/annonc/mdq260. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa Y, Ishikawa T, Chung SH, Lkeda K, Takashima T, Onoda N, et al. Oral UFT and cyclophosphamide combination chemotherapy for metastatic breast cancer. Anticancer Res. 2003;23:3453–7. [PubMed] [Google Scholar]
- 49.Villanueva C, Chaigneau L, Dufresne A, Vuillemin T, Stein U, Demarchi M, et al. Phase 2 trial of paclitaxel and uracil–tegafur in metastatic breast cancer. TEGATAX trial. Breast. 2011;30:1–5. doi: 10.1016/j.breast.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Rivera E. Liposomal anthracyclines in metastatic breast cancer: Clinical update. Oncologist. 2003;8:3–9. doi: 10.1634/theoncologist.8-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 51.Kerbel RS, Kamen BA. Antiangiogenic basis of low dose metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 52.Dellapasqua S, Mazza M, Rosa D, Ghisini R, Scarano E, Torrisi R, et al. Pegylated liposomal doxorubicin in combination with low dose metronomic cyclophosphamide as preoperative treatment for patients with locally advanced breast cancer. Breast. 2011;30:1–5. doi: 10.1016/j.breast.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Maletmartino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): A review. Oncologist. 2002;7:288–323. doi: 10.1634/theoncologist.7-4-288. [DOI] [PubMed] [Google Scholar]
- 54.Liombart CA, Martin M, Harbeck N, Anghel RM, Eniu AE, Verrill MW, et al. A randomized double blind, phase 2 study of two doses of pemetrexed as first line chemotherapy for advanced breast cancer. Clin Cancer Res. 2001;13:3652–9. doi: 10.1158/1078-0432.CCR-06-2377. [DOI] [PubMed] [Google Scholar]
- 55.Schneeweiss A, Marme F, Ruiz A, Manikhas AG, Bottini A, Wolf M, et al. A randomized phase 2 trial of doxorubicin plus pemetrexed followed by docetaxel versus doxorubicin plus cyclophosphamide followed by docetaxel as neoadjuvant treatment of early breast cancer. Ann Oncol. 2011;22:609–17. doi: 10.1093/annonc/mdq400. [DOI] [PubMed] [Google Scholar]
- 56.Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase 3 randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first line therapy of metastatic or locally advanced human epidermal growth factor receptor 2 positive breast cancer: The HERNATA study. J Clin Oncol. 2011;29:264–71. doi: 10.1200/JCO.2010.30.8213. [DOI] [PubMed] [Google Scholar]
- 57.Carter P, Prestal L, Gorman CM, Ridgway JB, Henner D, Wong WL. Humanization of an anti-p 185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valero V, Forbes J, Pegram MD, Pienkowski T, Eiermann W, von Minckwitz G, et al. Multicentre phase three randomized trial comparing docetaxel and trastuzumab as first line chemotherapy for patients with HER2 gene amplified metastatic breast cancer (BCIRG 007 study): Two highly active therapeutic regimens. J Clin Oncol. 2010;29:149–56. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 59.Loesch D, Greco FA, Senzer NN, Burris HA, Hainsworth JD, Jones S, et al. Phase 3 multicentre trial of doxorubicin plus cyclophosphamide followed by paclitaxel compared with doxorubicin plus paclitaxel followed by weekly paclitaxel as adjuvant therapy for women with high risk breast cancer. J Clin Oncol. 2010;28:2958–65. doi: 10.1200/JCO.2009.24.1000. [DOI] [PubMed] [Google Scholar]


