Abstract
Background:
Gestational diabetes mellitus (GDM) is the most common metabolic disorder during pregnancy. GDM causes substantial morbidity and mortality and long- term complications. GDM-related risk factors have not been completely identified yet. Some studies have found relationship between increased serum ferritin and impaired oral glucose tolerance test but the relationship between serum ferritin and risk of GDM has been controversial. The aim of the study was to determine serum iron and ferritin levels and total iron binding capacity (TIBC) in women with GDM and comparison with normal pregnant women.
Materials and Methods:
This case-control study was performed among 200 pregnant women (case = 100, control = 100) who were referred to Yahya-Nejad Hospital in the second trimester in Babol from 2008 to 2009. GDM was diagnosed by impaired OGTT based on Carpenter and Coustan criteria. The 2 groups were matched in age, gestational age and parity.
Results:
High serum ferritin level increased the risk of gestational diabetes to 2.4-fold [OR = 2.4 (0.83-6.9) CI = 95% (P = 0.10)], while in those with low ferritin levels, the risk of developing gestational diabetes was reduced to 82% [OR = 0.8 with (0.08-0.37) CI = 95% (P = 0.001)]. Using the logistic regression model, after adjustment for BMI, the OR was 2.37 [(0.80-7.01) CI = 95% (P = 0.11)] for low ferritin level and OR = 0.20 [(0.09-0.44) CI = 95% (P = 0.0001)] for high ferritin level, which was statistically significant.
Conclusion:
The serum ferritin level was markedly higher in women with gestational diabetes than in normal pregnant women; therefore, high ferritin can be regarded as a significant risk factor for the development of gestational diabetes.
Keywords: Ferritin, gestational diabetes mellitus, serum iron, total iron binding capacity
INTRODUCTION
Today, diabetes mellitus is one of the major health problems affecting millions of people worldwide.[1] Gestational diabetes is considered as a type of diabetes, which is the most common metabolic disorder during pregnancy.[2] Gestational diabetes refers to carbohydrate intolerance with different degrees of severity that begins or is diagnosed during pregnancy [gestational diabetes mellitus (GDM)].[3] Nearly 90% cases of diabetes during pregnancy are due to gestational diabetes; the prevalence is varied from 1% to 14%, and the related frequency has been reported from 2.3% to 8.6% in studies conducted in Iran.[4,5,6,7] Several investigations have shown that women with a history of gestational diabetes have higher risk for developing diabetes, especially type II diabetes.[8] The disease is associated with severe fetal and maternal consequences, and thus, early diagnosis of this common metabolic disorder is of high importance for the prevention of maternal and prenatal complications.[9,10] Gestational diabetes-related risk factors have not been completely identified yet. However, family history of type II diabetes in first-degree relatives, older age, obesity, hypertension, elevated blood platelet count, and increased hemoglobin and ferritin levels can be enumerated as potential risk factors for GDM.[1,11] In some studies, a statistically significant relationship has been found between increased serum ferritin, decreased serum TIBC (total iron binding capacity) and impaired oral glucose tolerance test (OGTT).[12,13] Studies revealed that increased body iron storage plays a role in impaired glucose tolerance in type II diabetes and gestational diabetes.[14,15,16] There is evidence of a link between insulin metabolism and excess body iron, since in frequent blood donors who have iron deficiency postprandial hyperinsulinemia is reduced, leading to enhanced insulin sensitivity and diabetes prevention.[17,18] Increased serum ferritin concentration accompanied by insulin resistance and diabetes in general population has recently been reported in gestational diabetes.[19,20,21] Nevertheless, the relationship between serum ferritin and insulin resistance or risk of diabetes have been announced controversially in cross-sectional and case-control studies, therefore, further investigations are required.[22] Uncertain relationship between stored iron status in mothers’ serum and gestational diabetes has been reported. The present study has been carried out to compare serum iron and ferritin levels and TIBC to transferrin between women with and without GDM.
MATERIALS AND METHODS
This case-control study was performed among pregnant women referred to prenatal care clinic affiliated to Shahid Yahya-Nejad Hospital in Babol from 2008 to 2009. Regarding the existing analytical studies in this field, with 95% confidence interval, 80% statistical test power, and the percentage difference between case and control exposure to 15%, the study samples were estimated to be 200 (100 participants in each group). Data-collecting instrument was a questionnaire including the information on demographic and fertility characteristics, ferritin, TIBC, and serum iron levels and the OGTT test. Convenience sampling method was used in the study. In this research, for each case who was diagnosed as GDM by impaired OGTT based on Carpenter and Coustan criteria after 26 weeks of pregnancy, one sample was selected from the routine prenatal care clinic of the same center as the control. The two groups were matched in terms of age, gestational age and parity. For selecting the control group, in 24 to 28 weeks of gestational age an initial screening was done with one-hour 50-gram glucose challenge test, regardless of the last meal. If patients’ glucose was higher than the threshold of 130 mg/dl, three-hour 100-g glucose tolerance test (OGTT) would be undertaken for more evaluation. To perform the mentioned test, three days of preparation (including use of at least 150 grams of carbohydrate per day) was recommended, and the test was performed on the fourth day after 8 to 12 hours of fasting period. Subjects with normal glucose tolerance test were considered as the control group. GDM was diagnosed if two out of four times blood glucose measurements were higher than Carpenter and Coustan criteria's cut-off level such as 95 mg/dl fasting blood glucose, 180, 155 and 140 mg/dl blood glucose at one, two and three hours after 100 g oral glucose intake, respectively.
Subjects with a history of gestational diabetes, recurrent miscarriages (three consecutive abortions), previous child with congenital abnormalities and dead-born baby, smoking before and during pregnancy, preterm delivery and medical diseases such as hemoglobinopathies, infections, and other chronic disorders were excluded. After selecting the samples and obtaining the consent letter, individual's personal characteristics were recorded in the questionnaire by a trained midwife, and 5 ml venous blood samples were collected from all participants and were subsequently sent to Pasteur Laboratory of Babol for ferritin, TIBC and serum iron measurements. Serum iron and TIBC were measured by RA-1000 auto-analyzer using the biochemistry kits and the serum ferritin by IRMA ferritin kit from Kavoshyar Iran Company and Gamma Counter. The study was approved by Medical Ethics Committee of University of Medical Sciences of Babol. Data were analyzed by SPSS statistical software using t-test, Chi-square and Man-Whitney for quantitative, qualitative and ordinal variables, respectively to examine differences in the case and the control group. To determine the risk of diabetes based on the amount of body iron, logistic regression was used and was considered as statistically significant at P < 0.05 level.
Finding
The findings showed no significant difference between the 2 groups in terms of the number of parities and educational level. The mean (SD) of maternal age, gestational age, body mass index, serum iron, total iron binding capacity to ferritin and transferrin is presented in Table 1. As it can be seen from the table there was a significant difference in body mass index and serum ferritin levels between the 2 groups (P < 0.05).
Table 1.
Comparison of the mean and standard deviation of relevant variables in pregnant women with and without GDM
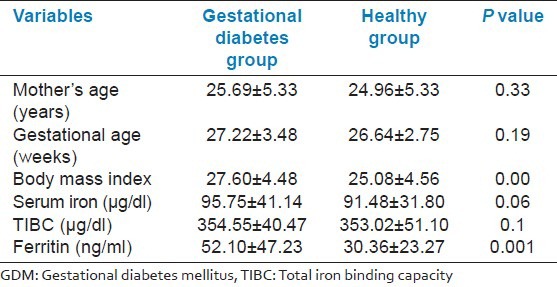
The mean serum iron concentration was 95.75 ± 41.14 in the case and 91.48 ± 31.80 in the control group; no significant difference was found. Total iron binding capacity to transferrin was 354.55 ± 40.47 in the control and 353.02 ± 51.10 in the case group, which was not statistically significant. The serum ferritin level was found to be higher in pregnant women with gestational diabetes in comparison with normal pregnant women, and it was statistically significant (P < 0.001). Moreover, high ferritin levels (greater than 80 ng/ml) increased the risk of gestational diabetes to 2.4-fold [OR = 2.4 (0.83-6.9) CI = 95% (P = 0.10)], while in those with low ferritin levels (less than 20 ng/ml), the risk of developing gestational diabetes was reduced to 82% [OR = 0.8 with (0.08-0.37) CI = 95% (P = 0.001)]. Using the logistic regression model, after removing the effects of BMI, the adjusted OR was 2.37 [(0.80-7.01) CI = 95% (P = 0.11)] for low ferritin level (less than 20 ng/ml) and OR = 0.20 [(0.09-0.44) CI = 95% (P = 0.0001)] for high ferritin level, which was statistically significant. For per-unit increase in BMI, the risk of developing gestational diabetes was significantly increased 1 percent after neutralizing effect of ferritin [OR = 1.1 (1.03-1.18) CI = 95% (P = 0.01)].
DISCUSSION
In the present research, the serum ferritin level was markedly higher in women with gestational diabetes than in women in the control group; therefore, high ferritin can be considered as a significant factor for the development of gestational diabetes. Several studies have shown that increased iron stores in the general population is accompanied by elevated incidence of diabetes.[23,24] In the study by Scholl et al., women with high ferritin levels represented the risk of developing type II diabetes nearly three times within the next 10 years, without being associated with other risk factors such as body mass index, age and race.[25] In another investigation, no difference was found for the serum iron and total iron binding capacity to transferrin between women with GDM and the control group. However, ferritin level was noticeably higher in women with GDM.[16] In our study, no significant difference was observed between the 2 groups in terms of the serum iron and transferrin-iron binding capacity; nevertheless, the serum ferritin level was higher in women with gestational diabetes in comparison with normal group without being correlated with other risk factors such as body mass index, and the difference was statistically significant.
Likewise, according to other studies, ferritin level is also in consistence with body iron stores and, therefore, is the most appropriate laboratory indicator for the estimation of iron stores.[2] Hence, serum ferritin measurement is recommended for the evaluation of body iron status.
Based on other reports, high iron level in the body has been suggested as a risk factor for developing GDM.[13,19,20] Administration of iron supplements along with vitamin C in women with sufficient levels of iron stores contributes to free radical overproduction, lipid membrane damage, delayed growth and increased carcinogenesis.[26] In addition, increased iron administration affects insulin secretion and increases lipid oxidation and leads to decrement in muscle glucose uptake and consumption and increment in gluconeogenesis in liver, resulting in enhanced sensitivity to insulin and predisposition to GDM.[27]
Elevated serum ferritin concentration, which is associated with insulin resistance and diabetes in the general population, has also been recently reported in gestational diabetes.[19,20,28,29] In some studies, iron level augmentation has been identified as a harmful factor for the body through oxidative stress and free radicals.[30,31] Excess iron and oxidative stress play a role in the pathogenesis and increased risk of type II diabetes and other associated disorders. Recently, it has been clear that iron influences glucose metabolism even in the absence of excess iron. The surveys have displayed that body iron stores are involved in impaired glucose tolerance and gestational diabetes, because iron compounds can affect insulin synthesis and secretion, increased lipid oxidation and subsequent reduction in glucose transport into the muscle and elevation in gluconeogenesis, and as a result, eventuate in insulin resistance in tissues.[28,16,32] Iron has a role in diabetes development via three mechanisms: (1) decreased insulin production, (2) increased resistance to insulin and (3) causing liver dysfunction.[33]
Body mass index is another risk factor for the development of gestational diabetes; this indicator has been significantly higher in women with GMD than healthy pregnant women (P < 0.000), and for per-unit increase in BMI, the risk of gestational diabetes even after neutralizing effect of ferritin was significantly increased by 1 percent, which is consistent with other studies.[34,35] Wrede et al. also reported that serum ferritin is significantly increased in men and women with a BMI >25 kg/m2.[36]
Regarding the study findings and in comparison with similar researches, it seems that routine administration of iron supplements to all pregnant women needs more investigation, since a significant relationship has been found between diabetes and increased serum ferritin level.
ACKNOWLEDGMENT
This project has been supported by Research Deputy of Babol University of Medical Sciences; hereby, the authors would like to appreciate the reverent deputy and also the personnel of Yahya-Nejad hospital, especially Mrs. Soraya Poor-norouz, and all the participating patients for their sincere cooperation.
Footnotes
Source of Support: Babol Medical Science University, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Fernandez-Real JM, Lopez BA, Ricart W. Cross talk between iron metabolism and diabetes. Diabetes Care. 2002;51:2348–54. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Braunwald E, Kasper DI, Hauser SL, Longo DL, Jameson JL, Loscalzo J. 17th ed. New York: McGraw Hil Medical; 2008. Harrisin's principles of Internal Medicine; p. 631. [Google Scholar]
- 3.Cunningham FG, Leveno KG, Bloom SL, Hauth JC, Gilsrab LC, Wenstrom KD. 22nd ed. New York: McGraw Hill; 2005. Williams Obstetrics; pp. 1170–2. [Google Scholar]
- 4.American Diabetes Association Classification and diagnosis of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S4–7. [PubMed] [Google Scholar]
- 5.American Diabetes Association: Gestational Diabetes Mellitus (Position statement) Diabetes Care. 2006;29(Suppl 1):S46–8. [Google Scholar]
- 6.Navaiy L, Kimyagar M, Khirkhahi M, Azizi F. The survey of diabetic epidemiology in rural pregnant women Tehran province. Res Med. 2002;26:217–23. [Google Scholar]
- 7.Larijani B, Azizi F, Pajuhi M, Bastanhagh MH, Marsusi V, Hosainnejad A, et al. The incidence of gestational diabetes in pregnant women who referred to Hospital of Tehran University 1992-1993. Iran J Endocrinol Metab. 1998;1:123–5. [Google Scholar]
- 8.Hosainnejad A, Maghboli Z, Larijani B. The incidence of gestational diabetes in women with previous gestational diabetes. Iran J Diabetes Lipid. 1983;4:27–35. [Google Scholar]
- 9.Griffin ME, Coffery M, Johnson H, Scanlon P, Foley M, Stronge J, et al. Universal vs, risk factor-based screening for gestational diabetes mellitus: Detection rates, gestation at diagnosis and outcome. Diabetes Med. 2000;17:26–32. doi: 10.1046/j.1464-5491.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 10.Sayah SH, Chondra A, Eberhardt MS. Pregnancy expreince among women with and without gestational in the U.S. 1995 national survey of family growth. Diabetes Care. 2005;28:1035–40. doi: 10.2337/diacare.28.5.1035. [DOI] [PubMed] [Google Scholar]
- 11.Kale SD, Kulkarni SR, Lubree HG, Meenaku Mari K, Deshpande VU, Rege SS, et al. Characteristies of gestational diabetic mothers and their babies in an India diabetes clinic. J Assoc Physician India. 2005;53:857–63. [PubMed] [Google Scholar]
- 12.Lao TT, Pon TC. Anemia in pregnancy is the current definition meaningful. Eur J Obstet Gynecol Report Biol. 1996;68:53–8. doi: 10.1016/0301-2115(96)02479-7. [DOI] [PubMed] [Google Scholar]
- 13.Lao TT, Chan PL, Tam XF. Gestational diabetes mellitus in the last trimester: A Feature of maternal iron excess? Diabetes Metab. 2001;18:218–23. doi: 10.1046/j.1464-5491.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- 14.Medalie JH, Papier CM, Goldbourt U, Herman JB. Major factors in the development of diabetes mellitus in 10,000 men. Arch Intern Med. 1975;135:811–7. [PubMed] [Google Scholar]
- 15.Barbieri M, Ragno E, Benvenutti E, Zito GA, Corsi A, Ferrucci L. New aspects of the insulin resistance syndrome, impact on the hematological parameters. Diabetologia. 2001;44:1232–7. doi: 10.1007/s001250100634. [DOI] [PubMed] [Google Scholar]
- 16.Lao TT, Tam KF. Maternal serum ferritin and gestational impaired glucose tolerance. Diabetes Care. 1997;20:1368–9. doi: 10.2337/diacare.20.9.1368. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Real JM, Lopez BA, Wifredo R. Cross-Talk between iron metabolism and diabetes. Diabetes. 2002;51:2348–54. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- 18.Ascherio A, Rimm EB, Giovannucci E, Willett WC, Stampfer MJ. Blood donations and risk of coronary heart disease in men. Circulation. 2001;103:52–7. doi: 10.1161/01.cir.103.1.52. [DOI] [PubMed] [Google Scholar]
- 19.Toumanen TR, Korpela H, Nyyssonon Salonen JT. Body iron stores are associated with serum insulin and blood glucose concentrations: Population study in 1013 eastern Finnish men. Diabetes Care. 1997;20:426–8. doi: 10.2337/diacare.20.3.426. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Real JM, Casamitijana-Abella R, Ricart-Engel W, Cabrero D, Arroyo E, Fernández-Castaner, et al. Serum ferritin as a component of the insulin resis-tancesyndrome. Diabetes Care. 1998;21:62–8. doi: 10.2337/diacare.21.1.62. [DOI] [PubMed] [Google Scholar]
- 21.Lao TT, Ho LF. Gestational diabetes and Maternal third trimester blood count. J Reported Med. 2002;47:309–12. [PubMed] [Google Scholar]
- 22.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body Iron Stores in Relation to Risk of Type 2 Diabetes in Apparently Healthy Women. JAMA. 2004;291:711–7. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- 23.Salonon JT, Tuomainen TP, Nyysso Non K, Lakka HM, Punnonen K. Relationship between iron stores and non-insulin dependent diabetes in men: Case control study. BMJ. 1998;317:727. doi: 10.1136/bmj.317.7160.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978–83. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 25.Scholl OT. Iron status during pregnancy: Setting the stage for mother and infant. Am J Clin Nutr. 2005;81(suppl):1218–22. doi: 10.1093/ajcn/81.5.1218. [DOI] [PubMed] [Google Scholar]
- 26.Lachili B, Hininger I, Faure H, Arnaud J, Richard MJ, Favier A, et al. Increased lipid proxidation in pregnant women after iron and vitamin C supplementation. Biol Trace Elem Res. 2001;83:103–10. doi: 10.1385/BTER:83:2:103. [DOI] [PubMed] [Google Scholar]
- 27.Defronzo RA. Lilly lecture 1987. The triumvirate: Beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–87. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 28.Barbieri M, Ragno E, Benvenutti E, Zito GA, Corsi A, Ferrucci L. New aspects of the insulin resistance syndrome: Impact on haematological parameters. Diabetologia. 2001;44:1232–7. doi: 10.1007/s001250100634. [DOI] [PubMed] [Google Scholar]
- 29.Lao TT, Chan LY, Tam KF, Ho LF. Maternal hemoglobin and risk of gestational diabetes mellitus in Chinese women. Obstet Gynecol. 2002;99:807–12. doi: 10.1016/s0029-7844(02)01941-5. [DOI] [PubMed] [Google Scholar]
- 30.Lund EK, Fairweather-Tait SJ, Wharf SG, Johnson IT. Chronic exposure to high levels of dietary iron fortification increases lipid peroxidation in the mucosa of the rat large intestine. J Nutr. 2001;131:2928–31. doi: 10.1093/jn/131.11.2928. [DOI] [PubMed] [Google Scholar]
- 31.Pierre JL, Fontecave M. Iron and activated oxygen species in biology: The basic chemistry. Biometals. 1999;12:195–9. doi: 10.1023/a:1009252919854. [DOI] [PubMed] [Google Scholar]
- 32.De Frozo RA. The triumvirate: β-cells, muscle, liver: A collusion responsible for NIDDM. Diabetes. 1988;37:667–87. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 33.Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. [Last accessed on 2007 Apr 11]. Available from: http://care.diabetesjournals.org . [DOI] [PubMed]
- 34.Schrauwers C, Dekker G. Maternal and perinatal outcome in obese pregnant patients. J Matern Fetal Neonatal Med. 2009;22:218–26. doi: 10.1080/14767050902801652. [DOI] [PubMed] [Google Scholar]
- 35.Madhavan A, Beena Kumari R, Sanal MG. A pilot study on the usefulness of body mass index and waist hip ratio as a predictive tool for gestational diabetes in Asian Indians. Obes Rev. 2009;10:487–90. doi: 10.1080/09513590802444134. [DOI] [PubMed] [Google Scholar]
- 36.Wrede CE, Buettner R, Bollheimer LC, Scholmerich J, Palitzsch KD, Hellerbrand C. Association between SF and the insulin resistance syndrome in a representative population. Eur J Endocrinol. 2006;154:333–40. doi: 10.1530/eje.1.02083. [DOI] [PubMed] [Google Scholar]


