Abstract
Objective:
To evaluate the degree of expression of cyclin-D1, p27 and p63 in mild, moderate and severe dysplasia using immunohistochemical evaluation in order to illustrate their prognostic value and attempt to propose a molecular grading system for oral epithelial dysplasia.
Materials and Methods:
The analysis included thirty cases of mild, moderate and severe dysplasia from Department of Oral and Maxillofacial Pathology, Saveetha Dental College, Chennai after a critical review of the Hematoxylin and Eosin (H and E) stained sections. They were subjected to immunohistochemical evaluation using the markers cyclin-D1, p27 and p63. The assessment of the expression based on staining intensity and distribution of immunohistochemical staining of the various markers was analyzed followed by statistical analysis.
Results:
A highly significant increase in the expression of cyclin-D1 (P < 0.000) and p63 (P < 0.001) and a moderately significant decrease in the expression of p27 (P < 0.012) with the increasing severity of dysplasia was observed in our study.
Conclusions:
The result of our research affirms the fact that the increase in the expression of markers of cell cycle regulators such as cyclin D1, decrease in the expression of cell cycle inhibitors like p27 and increased expression of p63 in parallel with the increasing severity of dysplasia, emphasizes the use of immunohistochemical markers cyclin D1, p27 and p63 as prognostic markers for better understanding the behaviour of these potentially malignant disorders aiming towards proposing a molecular grading system for oral epithelial dysplasia to enable timely management prior to their possible malignant transformation.
Keywords: Cyclin-D1, immunohistochemistry, leukoplakia, oral epithelial dysplasia, p27, p63
INTRODUCTION
Oral leukoplakia being the most common and one of the major forms of potentially malignant disorders in India, has been under constant research owing to its high potency to transform to oral squamous cell carcinoma.[1] Their premalignant potential was first documented by Paget in 1870.[2] Leukoplakia was most recently defined by Warnakulasuriya et al. (2007) as a potentially malignant disorder with recognizable white plaques of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer”.[3] The annual incidence of oral leukoplakia among subjects >15 years of age was reported as 0.2% to 11.7% in different populations of India.[4,5] Histopathologically, they are designated as hyperkeratosis with or without oral epithelial dysplasia. They have been reported to show a significant tendency to malignant transformation from 0.13 to 6%, and rising to 14% or higher when dysplasia is present.[6,7] Oral epithelial dysplasia is typically subdivided into three prognostically significant categories: Mild dysplasia, moderate dysplasia and severe dysplasia.[8] Thus it is generally accepted that the appearance of squamous cell carcinoma in the oral mucosa is most often preceded by epithelial dysplasia, which is considered to satisfy the criteria of a potentially malignant disorder.[9] Clinical studies suggest that 10 ± 20% of dysplastic oral lesions progress to carcinoma and 20 ± 30% increase in severity within 10 years; in the case of severe dysplasia, the risk of progressing to carcinoma may be as high as 43%.[10] Oral epithelial dysplasia, thus generally considered as a precancerous lesion, also shows increased cell proliferation compared with non-dysplastic epithelium.[11] Furthermore, studies have also shown that there is a positive correlation between severity of dysplasia and proliferative index.[12]
Cell proliferation is one of the universal properties of malignant tumor cells. This property is invariably shared by the preceding potentially malignant disorder as well.[13] Tumor kinetics were assessed by measuring the number of cycling cells using a variety of methods, including the quantification of the Ki-67 antigen in various studies[14] which have shown that there is a positive correlation between severity of dysplasia and proliferative index. Although, the analysis of these cell kinetic markers has been considered useful in estimating the proliferative activity of an array of tumors, their clinical value for the prognosis of head and neck neoplasms is still a subject of debate.[15] In numerous studies, the proliferative activity in oral epithelial dysplasia has been evaluated by means of the mitotic index (MI). It has also been reported, that the number of mitoses in the mucosal epithelial area significantly differed among mild, moderate, and severe dysplasia, indicating that measurement of the increased number of mitotic figures was important in assessing oral epithelial dysplasia. Thus, it has been proven that the evaluation of mitotic index is a simple factor with to determine the histological severity of oral epithelial dysplasia.[16] A strong correlation between malignant clinical behavior of the oral neoplasms and high proliferation indices[13,17,18,19] elucidated using various proliferation assays, have shown that oral carcinomas have a significantly higher proliferative index than normal epithelium.
Therefore, dysregulation of cell proliferation and cell cycle progression are likely to play an important role in oral carcinogenesis. Cell cycle progression is governed by a family of cyclin-dependent kinases (CDKs), which are activated by binding to cyclin proteins and inhibited by the CDK inhibitors.[20] There are at least 11 cyclins isolated, termed as: A, B1, B2, C, D1, D2, D3, E, F, G and H. Cyclin D1-3 and cyclin E bind with CDK 4/6 and CDK 2 respectively, and regulate transition from G1 to S phase.[21] Cyclin D1 gene is the key regulator of the G1 phase of cell cycle located on chromosome 11q13. A significant proportion of dysplasias contain molecular abnormalities that may result in cyclin D1 overexpression.[22] The p27 gene is an inhibitor of the CDKs belonging to the group of kinase inhibitor proteins (Kips). p27 was first identified as a cyclin dependant kinase inhibitor due to its ability to block the activity of various cyclins in G1 phase.[23] It regulates the proliferation of cells by binding and inhibiting G1 cyclin-CDK complexes and negatively regulating progression through G1 and S phases of the cell cycle. It has been suggested that the role of p27 misregulation in tumorigenesis may extend beyond cyclin-CDK inhibition and modulation of cell proliferation. Indeed, some studies have indicated that p27 levels in tumors do not always correlate with proliferative index, and increasing evidence points to the importance of the subcellular localization of p27 in the control of its function, with cytoplasmic localization being a negative prognostic factor in certain instances.[24] Reduced levels of p27 have been reported in a number of human tumors, including breast, pituitary, colon, and gastric cancers, and loss of this inhibition has been associated with aggressive biological behavior.[23,25] Alterations in p27 expression appear to precede the invasive stages of oral tumorigenesis[26] and associated with changes in cell kinetics in epithelial dysplasia.[27]
In addition to the genes which control cell cycle, other genes such as p53 and p63 are also considered to have a pivotal role in the neoplastic transformation of the potentially malignant lesions. The p63 gene shows structural and functional homology with the p53 transcription factor family and is located on chromosome 3q27-29.[28] Upon the maturation of normal oral stratified squamous epithelium, the expression of p63 protein gets down-regulated and p63 protein is rarely detected in upper layers of the epithelium.[29] The function of p63 in oral epithelium might be to maintain stem cell function rather than the direct correlation of oncogenesis and malignant transformation.[30] It is suggested that the distributional disturbance of p63-positive cells in the upper layers in oral epithelial dysplasia varies according to the grade of the dysplasia, which might be play a role in oral tumorigenesis.[31] The architectural disorganization of proliferating cells and stem cells in oral epithelium could thus be a useful index to estimate the grading of epithelial dysplasias if added to histomorphological examinations in H and E staining sections.
Thus the overexpression of cyclin D1 and p63 with increasing severity of dysplasia and a decrease in p27 expression can be attributed to the possible neoplastic transformation of the potentially malignant disorders. In spite of the plenteous studies performed earlier, the parameters to classify oral epithelial dysplasia have been a subject under constant review and revision due to the dilemma in establishing their grades, among various researchers. Hence, it has become mandatory to reconsider their classification and develop a more reliable system at the molecular level.
This retrospective preliminary study, being the first of its kind in the Indian population was designed to evaluate the degree of expression of cyclin D1 and p27 and p63 in epithelial dysplasia to illustrate their prognostic value and suggest a classification of the various grades of epithelial dysplasia at a molecular level, which would provide an evidence for their progression towards oral squamous cell carcinoma.
MATERIALS AND METHODS
Tissue samples: The analysis included thirty biopsy cases of mild, moderate and severe dysplasia from patients aged 45-65 years (mean 50 years) selected from pathology files in the Department of Oral and Maxillofacial Pathology, Saveetha Dental College, Chennai, from 2000 to 2010 after a critical review of the Hematoxylin and Eosin (H and E) stained sections. The cases were classified as, mild dysplasia, moderate dysplasia and severe dysplasia based on the grading criteria in the latest WHO classification of head and neck tumors.[32] These cases were evaluated immunohistochemically for cyclin D1, p27 and p63 expression.
Immunohistochemistry: Paraffin embedded tissues were sectioned at three micron thickness (Leica Semi automated microtome) and immunohistochemistry was performed using the standardized technique. The sections were incubated with precisely diluted mouse monoclonal primary antibodies against cyclin D1 (Biogenex, USA), p27 (Biogenex, USA) and p63 (Biogenex, USA) at 37˚C temperature for 60 min respectively for all the cases in a humid chamber. Subsequently, the sections were incubated with a secondary antibody conjugated with peroxidase-labeled dextran polymers (Super Sensitive Polymer-HRP Detection system, Biogenex) at room temperature and then they were treated with 0.5 mg/ml DAB solution containing 0.001% hydrogen peroxide to visualize brown coloured reaction products, and counterstained with Harris hematoxylin.
Microscopic evaluation
The nuclear expression of Cyclin-D1, p27 and p63 was counted according to epithelial layers as the basal layer, nuclei positive just above the basement membrane; parabasal layer, nuclei positive within two layers above the basement membrane and next to the basal layer; and suprabasal layer, nuclei positive in a more upper layer above the parabasal layer, using a microscope at 40 × magnification. The grading was based on the intensity of the brown color and the area of positive staining.
The cells from the highly positive stained area were selected and scored. The areas were then graded based on the staining intensity as grade-0, total absence of staining (− =0-5% cells stained); grade-1, mild to moderate nuclear staining (+ = 5 ± 50% cells stained); grade-2, strong nuclear staining (++ = >50% cells stained). Cytoplasmic staining was not regarded as positive expression. Most cases displayed uniform intensity of staining, and in such cases the quantitation was straight forward. For the occasional cases with heterogenous expression, we graded the immunoreactivity according to the intensity displayed by the majority of the epithelial cells. For the purposes of statistical analysis, the cases were divided into three groups of mild, moderate and severe dysplasia for each of the variables of cyclin-D1, p27 and p63 expression. Univariate analysis was performed on all parameters using the Chi-square test. Statistical significance was ascribed at P < 0.05.
RESULTS
Cyclin D1 and p63 expression was assessed based on both the intensity of nuclear staining within the basal, parabasal and suprabasal layers of the epithelial cells and the percentage of cells that were positive [Table 1]. Epithelium with mild and moderate dysplasia, the cells showed weak to moderately strong expression of cyclin D1 [Figures 1-4] and p63 [Figures 5 and 6] mostly restricted to the middle third of the epithelium. While in cases with severe dysplasia, an intense positivity for the dysplastic cells in all the layers of the epithelium, using cyclin D1 and p63 was obtained. Intensity of cyclin D1 expression in the basal and parabasal layers of the epithelium also significantly increased according to the grade of dysplasia. Similarly the distribution and intensity of p63 expression was also found to significantly increase with the increasing severity of dysplasia among the dysplastic cells. p27 expression was also assessed based on the intensity of nuclear staining of the epithelial cells and cases of mild dysplasia showed strong reactivity for p27 while dysplastic cells in moderate and severe dysplasia cases showed infrequent expression of p27. While comparing the expression of the three markers in mild dysplasia cases, a significant difference (P < 0.001) was obtained. p27 showed a significantly increased expression, compared to cyclin D1 and p63. Similarly in cases with severe dysplasia, a significant difference in expression was obtained (P < 0.001) where p27 showed a significant decrease in expression compared to cyclin D1 and p63, while in case of moderate dysplasia, a significant difference in the expression (P < 0.312) among the markers was not obtained. Three cases which were graded as moderate dysplasia based on the histopathological grading showed significantly increased expression of cyclin-D1 and p63 compared to the other cases, hence they were considered to be cases of severe dysplasia, while four cases of mild dysplasia were graded as moderate due to decreased expression of p27 and significantly increased expression of p63, following the study. In comparison with the other cases, two cases of severe dysplasia did not show a strong positivity for cyclin D1, hence they were categorized as moderate dysplasia. Assessment of immunoreactivity was done jointly by three of the authors and kappa analysis was done to assess the inter-observer agreement (κ > 0.8). Thus, the overexpression of cyclin D1 (P < 0.000) and p63 (P < 0.001) and the decreased expression of p27 (P < 0.012) with the increasing grades of dysplasia may prove to be of a predictive value to assess the malignant transformation in comparison with histopathological grading.
Table 1.
Of cases showing of cyclin D1, p27 and p63 immunohistochemical expression based on staining intensity and distribution in mild moderate and severe dysplasia cases
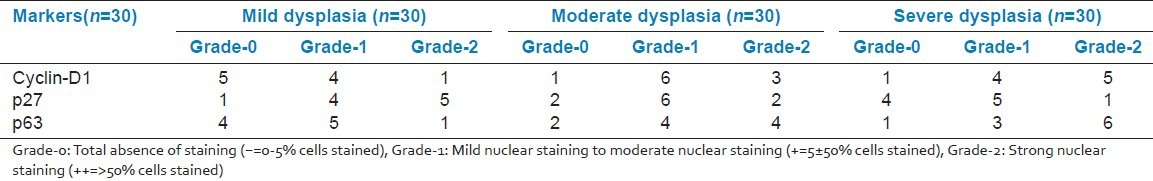
Figure 1.
Weak to moderate immunostaining of areas of mild dysplasia with cyclin D1 in basal, parabasal and minimally in suprabasal cells under ×10 magnification
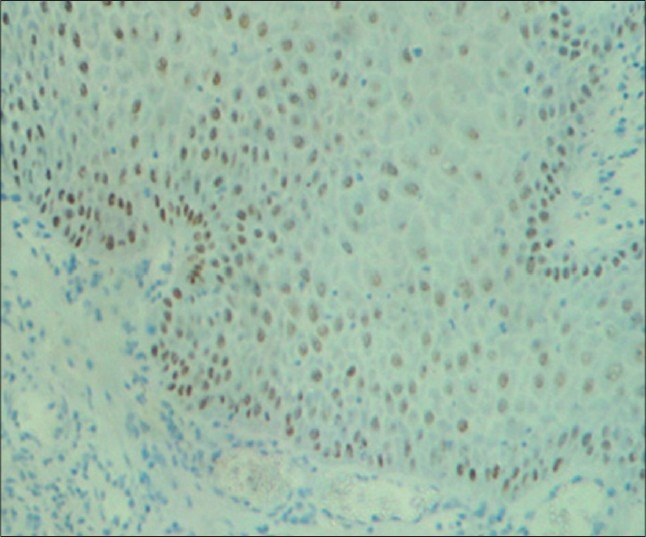
Figure 4.
Strong immunostaining of areas of moderate dysplasia with cyclin D1 in basal cells with basilar hyperplasia, parabasal and suprabasal cells under ×10 magnification
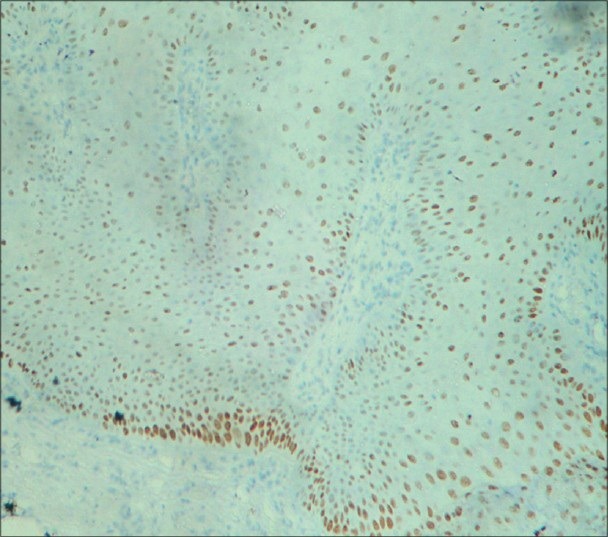
Figure 5.
Moderate to strong immunostaining of areas of moderate dysplasia with p63 predominantly in basal cells with basilar hyperplasia, parabasal and minimally in suprabasal cells along with few mitotic figures under ×10 magnification
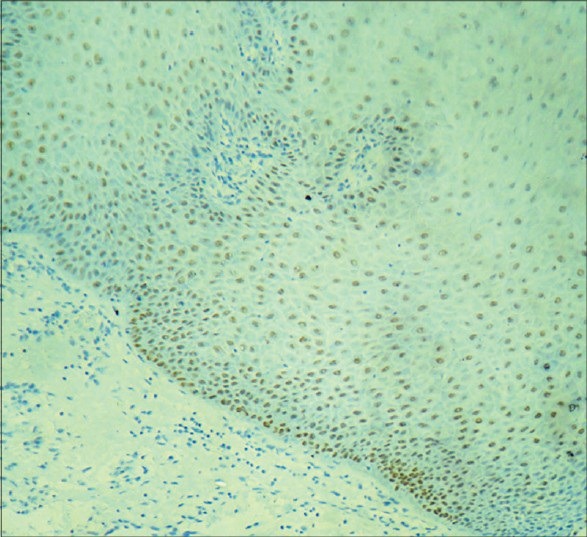
Figure 6.
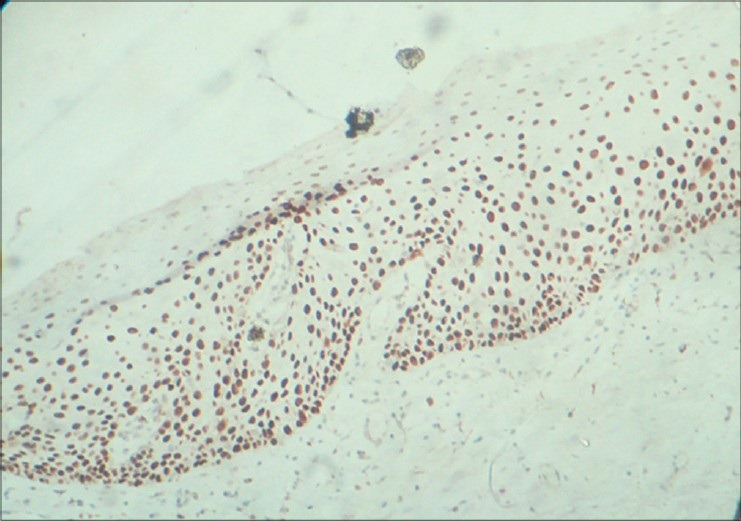
Strong immunostaining of areas of moderate dysplasia with p63 predominantly in basal, parabasal, suprabasal cells and superficial spinous layer under ×10 magnification
Figure 2.
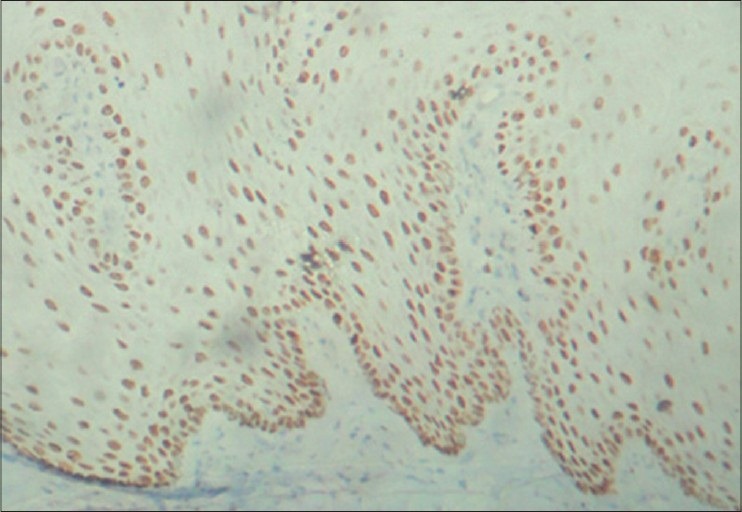
Strong immunostaining of areas of moderate dysplasia with cyclin D1 in basal, parabasal and suprabasal cells under ×10 magnification
Figure 3.
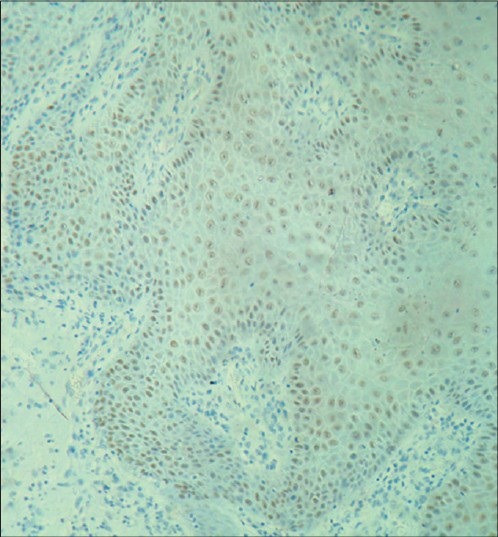
Moderate to strong immunostaining of areas of moderate dysplasia with cyclin D1 in basal, parabasal and suprabasal cells along with numerous mitotic figures in the spinous layer under ×10 magnification
DISCUSSION
Oral squamous cell carcinoma develops through a multistep process of the accumulation of genetic mutations related to cell proliferation, differentiation and tumor suppression, and the process can be morphologically recognized as cancer precursor lesions, although malignant transformation may rarely also develop directly from normal epithelium.[33] Those precursor lesions which have likelihood to progress into oral squamous cell carcinoma are termed as “potentially malignant disorders”.[3] The main purpose of identifying oral potentially malignant disorders is to prevent malignant transformation by initiating adequate intervention.[34] In order to improve treatment and enable diagnosis at an early stage, the understanding of the molecular mechanisms behind head and neck squamous cell carcinoma is of immense importance. The current study sought to formally investigate an initial observation of the possible malignant transformation of the dysplastic lesions, clinically diagnosed as leukoplakia using the degree of immunohistochemical expression of a few of the genes that have been suggested to be involved in head and neck squamous cell carcinoma namely cyclin D1, p27 and p63. Such markers have been used to predict prognosis and progression to malignancy in cases where clinical and histopathological parameters fail to determine its aggressiveness.
Another reason for the renewed focus on these potentially malignant disorders, most importantly leukoplakia is owing to the highest prevalence of leukoplakia as a potentially malignant disorder of about 0.2 to 5%[35,36,37,38] in the Indian population when compared to the other disorders such as oral sub-mucous fibrosis (0.03-3.2%)[35,37,38] and lichen planus (0.02-0.4%).[36,39] Also, higher occurrence of leukoplakia and cancer are observed in OSF patients and it is believed to be an important risk factor for oral cancer among youths.[40] In a recent study conducted in Tamilnadu population, similar results were obtained in which the prevalence of leukoplakia, OSF and oral lichen planus was found to be 0.59%, 0.55%, and 0.15%, respectively.[41]
It has been reported that the proliferating cell growth fractions in oral potentially malignant disorders were significantly higher than those in normal mucosa and suggested that these growth fractions correlated with the degree of severity of dysplasia[42] which linked to the increasing probability in the development of malignancy.[43] Assessment of histological features namely loss of polarity of the basal cells, an increased nuclear-cytoplasmic ratio, cellular pleomorphism, and enlarged nucleoli, was suggested to represent the histological criteria to determine the severity of oral epithelial dysplasia and the proliferative activity based on these features were proposed to be enhanced in relation to the severity of oral epithelial dysplasia. Furthermore, as stated in earlier studies, detailed examination of the genetic changes using makers of proliferation may aid to provide precise histopathological diagnosis.[13]
Thus, immunohistochemically detectable proliferation markers could be of great value in predicting the behavior of the potentially malignant disorders and carcinomas, serving as surrogate biomarkers in cancer chemoprevention studies to evaluate possible regression or improvement in abnormal features in the tissues of subjects at increased risk.[44] The assessment of surrogate end point biomarkers is possible because invasive carcinomas are known to be invariably preceded by potentially malignant disorders characterized by a spectrum of cellular abnormalities extending from mild dysplasia to carcinoma in situ.[45]
Nevertheless, because of the variety of methods used to assess these changes and the relatively little concern for the baseline proliferative values of the oral epithelia at different intraoral sites, numerous studies were performed to evaluate the proliferative characteristics of normal and leukoplakic epithelium using various markers of cell proliferation,[44] which have concluded that a positive balance exists between cell proliferation and cell death, which is considered to be essential for tumor growth. Wide arrays of studies have therefore determined the proliferative index (PI) in histological sections of tumors in order to determine the value of this index as a prognostic indicator.[46] A higher proliferative index was associated with a poorer prognosis in most carcinomas, with an overall increase in proliferative index with the progression from normal tissue, through dysplasia to carcinoma in cervical and oesophageal tissues.[47,48,49] Various methods have been used to determine PI, in order to examine the possible association between epithelial proliferation and disease progression in the oral mucosa.[46]
In our study, cyclin D1 was used which is the best characterized among the cyclins.[20] In view of their crucial role in cell cycle regulation and proliferation, cyclins have attracted considerable attention with regard to their putative involvement in oncogenesis. Various studies showed that cyclin D1 might be a useful prognostic factor for oral squamous cell carcinoma and also indicated that cyclin D1 might be involved in the early carcinogenesis of oral carcinoma. These data strongly supported that cyclin D1 may be involved in the initiation and development of oral squamous cell carcinoma.
Many studies were conducted to assess the cyclin D1 protein expression immunohistochemically in oral epithelial dysplasias and squamous cell carcinomas. Studies have demonstrated that abnormalities of cyclin D1 is an early event in oral neoplasia.[50] Cyclin D1 gene amplification and over-expression have been found in all grades of dysplasia and squamous cell carcinoma with correlation identified between amplification and over-expression of the proteins.[51] The results of our study showed a significant increase in the expression of cell cycle regulator cyclin-D1 (P < 0.000) with progression from mild to severe dysplasia. The prevalence of cyclin D1 over expression varied from weak in low-grade lesions to intense staining in higher grades of epithelial dysplasia. The cyclin D1 staining in the precursor lesions was mostly seen in suprabasal cell layers[52] and also an increased expression is observed in the parabasal and superficial layers of oral leukoplakias. Hence, this marker is considered useful and sensitive, and it is able to differentiate both normal epithelium from low grade dysplasia and low grade dysplasia from high grade dysplasia. The first study to demonstrate the association between the cyclin D1 expression and the risk of premalignant lesions was performed by Huang et al. in 2006 and they found a significant increase in the expression of cyclin D1 with progression of the potentially malignant lesions towards malignancy, which is similar to the results of our study.[53] Furthermore, because of the higher number of cells and the relatively high proliferative activity in dysplastic leukoplakias, the superficial layer and the basal layer seem to be the most adequate tissue components in which to investigate the possible modulation of cell proliferation in precursor lesions treated with chemopreventive agents.[44] It has been substantiated that the overexpression of cyclin D1 leads to shortened duration of the G1 phase and reduced growth factor dependency. Therefore, deregulated expression of the cyclin D1 can cause disturbances in cell cycle control and normal mitogenicsignaling pathways, which contribute to cell transformation and tumorigenesis.[54,55]
p27 which was first identified as a cyclin dependant kinase inhibitor due to its ability to block the activity of cyclin E/cdk2 and cyclin A/cdk2 in cells arrested in G1 phase, was the other marker used in our study. A large number of studies have examined the diagnostic and prognostic significance of p27 expression in various tumors. Low p27 expression was also associated with increasing lymph node metastasis and stage of tumor and resulted in a poor prognosis for patients with oral tongue squamous cell carcinoma. p27 is apparently a significant predictor of survival.[56] Many studies have demonstrated a decrease in the expression of p27 protein in dysplasias compared with the non-dysplastic epithelium along with a trend of decreasing p27 levels with increasing grades of dysplasia.[57] Whether the changes in p27 protein seen in epithelial dysplasias and carcinomas are a primary event or are simply a consequence of increased cell proliferation has yet to be determined.[58,59] A significant decrease in expression of p27 (P < 0.012) with the progressive grades of dysplasia was observed in our study, which suggests that reductions in p27 protein may contribute to, or reflect, the increased cell proliferation seen in any progression towards oral carcinoma and early carcinogenesis.[60] Reduced p27 protein expression in squamous cell carcinomas of the oral cavity showed that p27 was observed in oral dysplasias and carcinomas compared with that in normal squamous epithelium.[57] Expression of p27 was inversely correlated with the expression of Ki-67, which has been reported to be a good indicator of cell proliferation activity in premalignant and malignant oral lesions.[60] These findings indicate that reduced expression of p27 may play an important role in an abnormal proliferation through a loss of cell cycle regulation which may concern the cancer development directly or indirectly.[61] The down-regulation of p27 is believed to play an important role in cancer invasion process, as well as metastasis mediated by loss of cell adhesion either directly or indirectly, due to heterogeneous its expression which tends to diminish toward the invasive fronts in some early invasive OSCCs.[62] Aggressive human cancers were also found to express low levels of p27, which may be due to its decreased stability or due to an enhancement of its degradation.[63] The simultaneous involvement of cyclin-D1 and p27 genes was estimated by Pignataro et al., and an inverse correlation between p27 and cyclin D1 expression was established. It was concluded that the absence of p27 expression may be due to sequestration by cyclin D1 and that the balance of these two opposing regulators of the cell cycle may be a determinant factor in cell proliferation.[64] Similar results were obtained in our study where p27 expression was inversely related to that of cyclin-D1 owing to their functional diversity.
The other marker of interest in our study is p63, a homologue of the p53 gene which is located on chromosome 3q27-29. A dual role of p63 protein has been reported; during embryogenesis, p63 may be the molecular switch required for initiation of epithelial stratification because, if lacking p63, epithelium remains single layered; for mature epithelium, p63 needs to be switched off for terminal differentiation to take place; otherwise, p63 may maintain the proliferative potential of basal keratinocytes preventing stratification to occur. Based on immunohistochemical data, p63 protein is expressed in the proliferative layer of cells near the basement membrane of the normal oral mucosa, where it likely serves to prevent basal cells from differentiating and thereby helps to maintain their basal cell status.[29] Although, p63 gene has extensively been studied in various tumors, only some have examined p63 expression in oral squamous cell carcinoma, but the involvement of p63 in human oral potentially malignant disorders remains largely unelaborated except for a few studies. Earlier studies have reported the expression of the p63 protein and mRNA in oral epithelial dysplasia,[65] however; no attempt was made to grade degree of staining with respect to the severity of the dysplastic lesions. But later the relationship between p63 protein expression and various grades of oral epithelial dysplasia was also analyzed.[66] In our study, the expression of p63, showed a significant increase (P < 0.001), from mild to severe dysplasia, which is similar to the results of the previous studies. Normally, upon the maturation of the oral stratified squamous epithelium, the expression of p63 protein should have been downregulated and p63 protein has rarely been detected in superficial layers of the normal epithelium.[29] However, upon dysplastic change, dysplastic keratinocytes above the basal layers may shift to a status similar to the embryogenesis condition that are still able to express p63 protein producing an anti-differentiation effect, as well as maintain the proliferative capacity of dysplastic cells in oral dysplastic mucosa.[29]
Furthermore, the increased proliferating cell population in both basal and suprabasal layers of epithelial dysplasia suggest that proliferating cells might increase not only in a superficial direction but also downward to the basal layer in epithelial dysplasias.[30] Although, previous studies have described increase in the total number of proliferating cells according to the degree of epithelial dysplasia and SCC, the increased expression of p63 in the suprabasal layer may be a useful marker of high-grade dysplasia.[67] It was suggested that p63 may contribute to the development of epithelial dysplasia through the alteration of stem cell function in the basal layer, resulting in an increased number of proliferating cells, and its altered distribution in basal and suprabasal layers within oral epithelial dysplasia.[39]
According to Steeg and Abrams (1997),[68] for a new prognostic marker to enter into routine clinical use, at least three criteria must be met. (1) The marker provides information independent of any better than conventional pathological criteria. (2) The marker provides information that can alter treatment decisions. (3) Studies with the marker are reproducible. Many reports have validated the utility of p27, cyclin D1 and p63 as prognostic and/or diagnostic markers,[69] hence these markers can prove to be valuable aids in the grading of oral epithelial dysplasia.
Markers of cell proliferation have been used to predict prognosis and progression to malignancy in cases where clinical and pathological parameters fail to determine its aggressiveness[70] and the evaluation of the cell growth fraction is considered to be potentially one of the most powerful tools for predicting neoplastic behavior. The assessment of cell proliferation activity by immunohistochemistry has been extensively studied in order to find new prognostic markers for a wide variety of tumors.[71,14] Many authors have stated that one of the important characteristics of the neoplastic transformation of a steady-state epithelial system is the alteration of growth rate, commonly reflected as increased cell proliferation.[72,73]
In our study, we investigated the progression of various grades dysplasia towards malignant transformation using the comparative expression of cyclin-D1, p27 and p63 in order to categorize oral epithelial dysplasia based upon immunohistochemical expression of cyclin-D1, p27 and p63, as the histopathological classification of leukoplakia into mild moderate and severe dysplasia is not a reliable indicator to predict the progression of oral epithelial dysplasia towards malignant transformation. The present study is the first of its kind which is performed using three different immunohistochemical markers in the Indian population where oral leukoplakia is the predominant form of potentially malignant disorders.
CONCLUSION
Our study may thus prove to be a preliminary maiden approach to a disease classification based on the molecular expression of these markers, which may aid in the discovery of new knowledge relevant to diagnosis of the malignant transformation of clinically diagnosed cases of leukoplakia with mild moderate and severe dysplasia [Table 2].
Table 2.
Proposed molecular grading system of oral epithelial dysplasia using the prognostic markers cyclin D1, p27 and p63
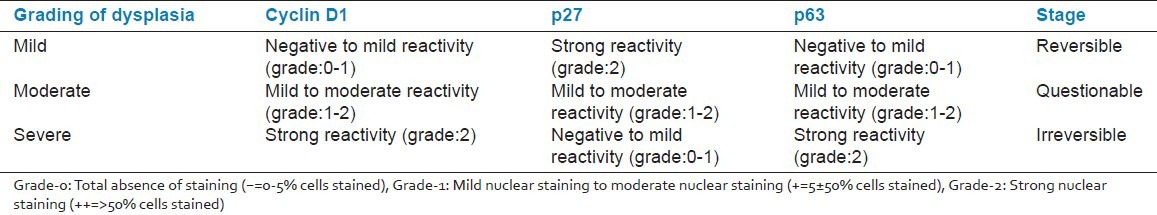
In order to reduce the apparent deficit in the early identification of potentially malignant disorders, it is imperative that such assays have to be performed to eliminate any shortfall in the treatment to be determined and addressed. Currently, there is no substantial body of strong evidence for the use of these biomarkers in the prognosis of oral dysplasia. If the present findings are reproducible and reliable, and done in a greater population, they have potential to change the present scenario in terms of establishing a novel classification of dysplasia at a molecular level, which would aid in targeting of treatment and follow-up.
Thus from this preliminary study we can hypothesise that an increase in expression of the Cyclin-D1, up-regulation of p63 expression and an inverse expression of p27 with increasing severity of dysplasia may be a prognostic indicator of any preceding malignant transformation as proved by the previous studies performed using these markers and may hence serve as biomarkers for oral cancer progression. We anticipate that this study will serve as a scaffold for further breakthrough in the classification of oral epithelial dysplasia, which has remained a topic of controversy for years and also prove to be of enormous importance in the practice of pathology and cancer research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jayant K, Notani P. Epidemiology of oral cancer. In: Rao RS, Desai PB, editors. Oral Cancer. India: Tata Press; 1991. pp. 1–17. [Google Scholar]
- 2.Paget J. Cancer following ichthyosis of the tongue. Trans Clin Soc Lond. 1870;3:88. [Google Scholar]
- 3.Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36:575–80. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 4.Gupta PC, Mehta FS, Daftary DK, Pindborg JJ, Bhonsle RB, Jalnawalla PN, et al. Incidence rates of oral cancer and natural history of oral precancerous lesions in a 10-year follow-up study of Indian villagers. Community Dent Oral Epidemiol. 1980;8:287–333. doi: 10.1111/j.1600-0528.1980.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 5.Nair UJ, Obe G, Friesen M, Goldberg MT, Bartsch H. Role of lime in the generation of reactive oxygen species from betel quid ingredients. Environ Health Perspect. 1997;98:203–5. doi: 10.1289/ehp.9298203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman S, Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer. 1984;53:56–8. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Tradati N, Grigolat R, Calabrese L, Costa L, Giugliano G, Morelli F, et al. Oral leukoplakias: To treat or not? Oral Oncol. 1997;33:317–21. doi: 10.1016/s1368-8375(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 8.Bouquot JE, Whitaker SB. Oral leukoplakia: Rationale for diagnosis and prognosis of its clinical subtypes or “phases.”. Quintessence Int. 1994;25:133–40. [PubMed] [Google Scholar]
- 9.Regezi JA, Sciubba JJ. Regezi JA, Sciubba JJ. Oral Pathology. Philadelphia PA: Saunders; 1993. White lesion; pp. 93–135. [Google Scholar]
- 10.Speight PM, Morgan PR. The natural history and pathology of oral cancer and precancer. Community Dent Health. 1993;10:31–41. [PubMed] [Google Scholar]
- 11.Girod SC, Krueger G, Pape HD. p53 and Ki 67 expression in preneoplastic and neoplastic lesions of the oral mucosa. Int J Oral Maxillofac Surg. 1993;22:285–8. doi: 10.1016/s0901-5027(05)80517-x. [DOI] [PubMed] [Google Scholar]
- 12.Coltrera MD, Zarbo RJ, Sakr WA, Gown AM. Markers for dysplasia of the upper aerodigestive tract: Suprabasal expression of PCNA, p53, and CK19 in alcohol-fixed, embedded tissue. Am J Pathol. 1992;141:817–25. [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi I, Matsuo K, Ozeki S, Ohishi M, Ishibashi Y, Sakai H. The proliferative activity in oral epithelial dysplasia analyzed by proliferating cell nuclear antigen immunostaining and argyrophilicnucleolar organizer region staining. Hum Pathol. 1995;26:907–13. doi: 10.1016/0046-8177(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzato M, Abboud P, Lechki C, Browarnyj F, O’Donohue MF, Ploton D, et al. Proliferation assessment in breast cancer: A double-staining technique for AgNOR quantification in MIB-1 positive cells especially adapted for image cytometry. Micron. 2000;31:151–9. doi: 10.1016/s0968-4328(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 15.Sittel C, Ruiz S, Volling P, Kvasnicka HM, Jungehülsing M, Eckel HE. Prognostic significance of Ki-67 (MIB1), PCNA and p53 in cancer of the oropharynx and oral cavity. Oral Oncol. 1999;35:583–9. doi: 10.1016/s1368-8375(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 16.Sano K, Takahashi H, Fujita S, Inokuchi T, Pe MB, Okabe H, Tsuda N. Prognostic implication of silver-binding nucleolar organizer regions (AgNORs) in oral squamous cell carcinoma. J Oral Pathol Med. 1991;20:53–6. doi: 10.1111/j.1600-0714.1991.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 17.Tipoe GL, Jin Y, White FH. The relationship between vascularity and cell proliferation in human normal and pathological lesions of the oral cheek epithelium. Eur J Cancer B Oral Oncol. 1996;32B:24–31. doi: 10.1016/0964-1955(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji T, Mimura Y, Wen S, Li X, Kanekawa A, Sasaki K, et al. The significance of PCNA and P53 protein in some oral tumors. Int J Oral Maxillofac Surg. 1995;24:221–5. doi: 10.1016/s0901-5027(06)80132-3. [DOI] [PubMed] [Google Scholar]
- 19.Teresa DB, Neves KA, Neto CB, Fregonezi PA, de Oliveira MR, Zuanon JA, et al. Computer-assisted analysis of cell proliferation markers in oral lesions. Acta Histochem. 2007;109:377–87. doi: 10.1016/j.acthis.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Sherr CJ. G1 phase progression: Cycling on cue. Cell. 1994;79:551–5. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 21.Poon RY. “Cell cycle control”. In: Encyclopedia of Cancer. In: Bertino JR, editor. San Diego: Academic Press; 1997. pp. 246–55. [Google Scholar]
- 22.Huang S, Chen CS, Ingber DE. Control of Cyclin D1, p27Kip1, and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. MolBiol Cell. 1998;9:3179–93. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, et al. p27Kipl, a Cyclin-Cdk inhibitor, links transforming growth factor beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 24.Singh SP, Lipman J, Goldman H, Ellis FH, Jr, Aizenman L, Cangi MG, et al. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998;58:1730–5. [PubMed] [Google Scholar]
- 25.Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, et al. Expression of cell-cycle regulators p27Kipl and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–5. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 26.Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, et al. The cell cycle inhibitor p27 is an independent prognostic marker in small invasive breast carcinomas. Cancer Res. 1997;57:1259–63. [PubMed] [Google Scholar]
- 27.Jordan R, Bradley G, Slingerland JM. Reduced levels of the cell-cycle inhibitor p27Kipl in epithelial dysplasia and carcinoma of the oral cavity. Am J Pathol. 1998;152:585–90. [PMC free article] [PubMed] [Google Scholar]
- 28.Ou H, Löhr F, Vogel V, Mäntele W, Dötsch V. Structural evolution of C-terminal domains in the p53 family. EMBO J. 2007;26:3463–73. doi: 10.1038/sj.emboj.7601764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang A, Parsa R, McKeon F, Green H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol. 1999;113:1099–105. doi: 10.1046/j.1523-1747.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 30.Takeda T, Sugihara K, Hirayama Y, Hirano M, Tanuma JI, Semba I. Immunohistological evaluation of Ki-67, p63, CK19 and p53 expression in oral epithelial dysplasias. J Oral Pathol Med. 2006;35:369–75. doi: 10.1111/j.1600-0714.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 31.Glickman JN, Yang A, Shahsafaei A, McKeon F, Odze RD. Expression of p53-related protein p63 in the gastrointestinal tract and in esophagealmetaplastic and neoplastic disorders. Hum Pathol. 2001;32:1157–65. doi: 10.1053/hupa.2001.28951. [DOI] [PubMed] [Google Scholar]
- 32.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. International agency for research on cancer (IARC) Lyon: IARC Press; 2005. World health organization classification of tumours. Pathology and genetics of head and neck tumours. [Google Scholar]
- 33.Silverman S., Jr Demographics and occurrence of oral and pharyngeal cancers: The outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132(Suppl):7S–11S. doi: 10.14219/jada.archive.2001.0382. [DOI] [PubMed] [Google Scholar]
- 34.Reibel J. Prognosis of oral pre-malignant lesions: Significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14:47–62. doi: 10.1177/154411130301400105. [DOI] [PubMed] [Google Scholar]
- 35.Mehta FS, Gupta PC, Daftary DK, Pindborg JJ, Choksi SK. An epidemiologic study of oral cancer and precancerous conditions among 101,761 villagers in Maharashtra, India. Int J Cancer. 1972;10:134–41. doi: 10.1002/ijc.2910100118. [DOI] [PubMed] [Google Scholar]
- 36.Pindborg JJ, Chawla TN, Misra RK, Nagpaul RK, Gupta VK. Frequency of oral carcinoma, leukokeratosis, leukoedema, submucous fibrosis and lichen planus in 10,000 Indians in Lucknow, Uttar Pradesh, India Preliminary Report. J Dent Res. 1965;44:61. doi: 10.1177/00220345650440032901. [DOI] [PubMed] [Google Scholar]
- 37.Pindborg JJ, Mehta FS, Gupta PC, Daftary DK. Prevalence of oral submucous fibrosis among 50,915 Indian villagers. Brit J Cancer. 1968;22:646–54. doi: 10.1038/bjc.1968.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta FS, Gupta PC, Daftary DK, Pindborg JJ, Choksi SK. Epidemiological study of precancerous lesions of the oral cavity: A preliminary report. Ind J Med Res. 1970;50:1361–91. [PubMed] [Google Scholar]
- 39.Pindborg JJ, Kalapesi IL, Kale SA, Singh B, Taleyarkhan BN. Frequency of oral leukoplakia and related conditions among 10,000 Bombayites. Preliminary report. J All India Dent Assoc. 1965;37:228–9. [PubMed] [Google Scholar]
- 40.Gupta PC, Sinor PN, Bhonsle RB, Pawar VS, Mehta HC, Mehta H. Oral submucous fibrosis in India: A new epidemic? Natl Med J India. 1998;11:113–6. [PubMed] [Google Scholar]
- 41.Saraswathi TR, Ranganathan K, Shanmugam S, Sowmya R, Narasimhan PD, Gunaseelan R. Prevalence of oral lesions in relation to habits: Cross-sectional study in South India. Indian J Dent Res. 2006;17:121–5. doi: 10.4103/0970-9290.29877. [DOI] [PubMed] [Google Scholar]
- 42.Tsuji T, Shrestha P, Yamada K, Takagi H, Shinozaki F, Sasaki K, et al. Proliferating cell nuclear antigen in malignant and pre-malignant lesions of epithelial origin in the oral cavity and the skin: An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1992;420:377–83. doi: 10.1007/BF01600508. [DOI] [PubMed] [Google Scholar]
- 43.WHO Collaborating Centre for Oral Precancerous Lesions. Definition of leukoplakia and related lesions: An aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46:518–39. [PubMed] [Google Scholar]
- 44.Liu SC, Sauter ER, Clapper ML, Feldman RS, Levin L, Chen SY, et al. Markers of cell proliferation in normal epithelia and dysplastic leukoplakias of the oral cavity. Cancer Epidemiol Biomarkers Prev. 1998;7:597–603. [PubMed] [Google Scholar]
- 45.Crissman JD. Upper aerodigestive tract. In: Henson DE, Albores-Saavedra J, editors. Pathology of incipient neoplasia. Philadelphia: W. B. Saunders; 1986. pp. 44–63. [Google Scholar]
- 46.Macluskey M, Ogden GR, Green M, Chisholm DM, Schor SL, Schor AM. The association between epithelial prolife ration and disease progressionin theoral mucosa. OralOncol. 1999;35:409–14. doi: 10.1016/s1368-8375(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 47.Linder S, Parrado C, Falkmer UG, Blåsjö M, Sundelin P, von Rosen A. Prognostic significance of Ki-67 antigen and p53 protein expression in pancreatic duct carcinoma: A study of the monoclonal antibodies MIB-1 and DO-7 in formalin-fixed paraffin-embedded tumour material. Br J Cancer. 1997;76:54–9. doi: 10.1038/bjc.1997.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avall-Lundqvist EH, Silfversward C, Aspenblad U, Nilsson BR, Auer GU. The impact of tumour angiogenesis, p53 over expression and proliferative activity (MIB-1) on survival in squamous cervical carcinoma. Eur J Cancer. 1997;33:1799–804. doi: 10.1016/s0959-8049(97)00161-5. [DOI] [PubMed] [Google Scholar]
- 49.Polkowski W, Meijer GA, Baak JP, ten Kate FJ, Obertop H, Offerhaus GJ, et al. Reproducibility of p53 and Ki-67 immunoquantitation in Barrett's esophagus. Anal Quant CytolHistol. 1997;19:246–54. [PubMed] [Google Scholar]
- 50.Rousseau A, Lim MS, Lin Z, Jordan RC. Frequent CyclinD1 gene amplification and protein overexpression in oral epithelial dysplasias. Oral Oncol. 2001;37:268–75. doi: 10.1016/s1368-8375(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 51.Shintani S, Mihara M, Nakahara Y, Kiyota A, Ueyama Y, Matsumura T, et al. Expression of cell cycle control proteins in normal epithelium, premalignant and malignant lesions of oral cavity. Oral Oncol. 2002;38:235–43. doi: 10.1016/s1368-8375(01)00048-3. [DOI] [PubMed] [Google Scholar]
- 52.Uhlman DL, Adams G, Knapp D, Aeppli DM, Niehans G. Immunohistochemical staining for markers of future neoplastic progression in the larynx. Cancer Res. 1996;56:2199–205. [PubMed] [Google Scholar]
- 53.Huang M, Spitz MR, Gu J, Lee JJ, Lin J, Lippman SM, et al. Cyclin D1 gene polymorphism as a risk factor for oral premalignant lesions. Carcinogenesis. 2006;27:2034–7. doi: 10.1093/carcin/bgl048. [DOI] [PubMed] [Google Scholar]
- 54.Bartkova J, Lukas T, Staurs M, Bartek J. Cell cycle-related variation and tissue-restrictedexpressionof human Cyclin D1 protein. J Pathol. 1994;172:237–45. doi: 10.1002/path.1711720303. [DOI] [PubMed] [Google Scholar]
- 55.Cardo CC. Mutation of cell cycle regulators. Am J Pathol. 1995;147:545–60. [PMC free article] [PubMed] [Google Scholar]
- 56.Mineta H, Miura K, Suzuki I, Takebayashi S, Amano H, Araki K, et al. Low p27 expression correlates with poor prognosis for patients with oral tongue squamous cell carcinoma. Cancer. 1999;85:1011–7. doi: 10.1002/(sici)1097-0142(19990301)85:5<1011::aid-cncr1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 57.Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, et al. p27 kip1: A multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–23. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, et al. Mice lacking p27 (Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 59.Ponce-Castañeda MV, Lee MH, Latres E, Polyak K, Lacombe L, Montgomery K, et al. p27Kipl: Chromosomal mapping to 12p12- 12p13.1 and absence of mutations in human tumors. Cancer Res. 1995;55:1211–4. [PubMed] [Google Scholar]
- 60.Girod SC, Pfeiffer P, Ries J, Pape HD. Proliferative activity and loss of function of tumour suppressor genes as “biomarkers” in diagnosis and prognosis of benign and preneoplastic oral lesions and oral squamous cell cancer. Br J Oral MaxillofacSurg. 1998;36:252–60. doi: 10.1016/s0266-4356(98)90708-2. [DOI] [PubMed] [Google Scholar]
- 61.Kudo Y, Takata T, Ogawa I, Zhao M, Sato S, Takekoshi T, et al. Reduced expression of p27(Kip1) correlates with an early stage of cancer invasion in oral squamous cell carcinoma. Cancer Lett. 2000;151:217–22. doi: 10.1016/s0304-3835(99)00419-x. [DOI] [PubMed] [Google Scholar]
- 62.Kudo Y, Takata T, Yasui W, Ogawa I, Miyauchi M, Takekoshi T, et al. Reduced expression of Cyclin dependent kinase inhibitor p27Kip1 is an indicator of malignant behaviour of oral squamous cell carcinomas. Cancer. 1998;83:2447–55. doi: 10.1002/(sici)1097-0142(19981215)83:12<2447::aid-cncr7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 63.Kitajima S, Kudo Y, Ogawa I, Bashir T, Kitagawa M, Miyauchi M, et al. Role of Cks1 overexpression in oral squamous cell carcinomas: Cooperation with Skp2 inpromoting p27 degradation. Am J Pathol. 2004;165:2147–55. doi: 10.1016/S0002-9440(10)63264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pignataro L, Sambataro G, Pagani D, Pruneri G. Clinico-prognostic value of D type Cyclins and p27 in laryngeal cancer patients: A review. Acta Otorhinolaryng olItal. 2005;25:75–85. [PMC free article] [PubMed] [Google Scholar]
- 65.Choi HR, Batsakis JG, Zhan F, Sturgis E, Luna MA, El-Naggar AK. Differential expression of p53 gene family members p63 and p73 in head and neck squamous tumorigenesis. Hum Pathol. 2002;33:158–64. doi: 10.1053/hupa.2002.30722. [DOI] [PubMed] [Google Scholar]
- 66.Chen YK, Hsue SS, Lin LM. Expression of p63 protein and mRNA in oral epithelial dysplasia. J Oral Pathol Med. 2005;34:232–9. doi: 10.1111/j.1600-0714.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- 67.Liu SC, Klein-Szanto AJ. Markers of proliferation in normal and leukoplakic oral epithelia. Oral Oncol. 2000;36:145–51. doi: 10.1016/s1368-8375(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 68.Steeg PS, Abram JS. Cancer prognostics: Past, present and p 27. Nat Med. 1997;3:152–4. doi: 10.1038/nm0297-152. [DOI] [PubMed] [Google Scholar]
- 69.Kövesi G, Szende B. Prognostic value of Cyclin D1, p27, and p63 in oral leukoplakia. J Oral Pathol Med. 2006;35:274–7. doi: 10.1111/j.1600-0714.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- 70.Piattelli A, Rubini C, Fioroni M, Iezzi G, Santinelli A. Prevalence of p53, bcl-2, and Ki-67 immunoreactivity and of apoptosis in normal oral epithelium and in premalignant and malignant lesions of the oral cavity. J Oral Maxillofac Surg. 2002;60:532–40. doi: 10.1053/joms.2002.31851. [DOI] [PubMed] [Google Scholar]
- 71.Ceccarelli C, Trere D, Santini D, Taffurelli M, Chieco P, Derenzini M. AgNORs in breast tumors. Micron. 2000;31:143–9. doi: 10.1016/s0968-4328(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 72.Warnakulasuriya KA, Macdonald DG. Epithelial cell kinetics in oral leukoplakia. J Oral Pathol Med. 1995;24:165–9. doi: 10.1111/j.1600-0714.1995.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 73.Yue L, Iwai M, Furuta I. Evaluation of argyrophilicnucleolar organizer regions in tongue squamous cell carcinoma. Oral Oncol. 1999;35:70–6. doi: 10.1016/s1368-8375(98)00074-8. [DOI] [PubMed] [Google Scholar]


