Abstract
Introduction:
Reduction of functioning minor salivary glands may contribute to emergence of mucosal infections, mucosal ulceration, and possibly dental caries. A study was, therefore, designed to understand the exact role of minor salivary gland secretions over dental caries.
Methodology:
We studied the average labial distribution of functional minor salivary glands using various pre-defined locations, counted the minor salivary gland secretion imprints, and correlated the decayed missing filledlevels in subjects. The functional level and amount of secretion of minor salivary gland were evaluated. The radial immunodiffusion was performed by Diffu-Plate kit and the dimensions of the ring were correlated with the amount of immunoglobulin A in saliva.
Results:
The mean number of functional labial minor salivary glands, amount of secretion, level of glycoprotein secretion, and immunoglobulin A secretion levels could very well dictate the functional status and role of minor salivary glands over caries assessment.
Conclusion:
The above-mentioned tests could be of major significance in routine diagnosis of the most common oral disease, i.e., dental caries.
Keywords: Radial immunodiffusion, salivary immunoglobulin A, unstimulated whole saliva
INTRODUCTION
Saliva is commonly referred to as the blood stream of oral cavity. It has many functions, one of the major functions being protection of teeth against dental caries. There are many components in saliva, each one having a specific role in the prevention of dental caries.[1] Whole saliva is a product of secretion of three major glands (parotid, submandibular, and sublingual) and many minor glands (labial, buccal, palatal).[2] Minor salivary glands are distributed throughout the human oral cavity. These glands can be found on the lower and upper lips, the cheeks, much of the palate, and the tongue.[3] The minor salivary glands contribute 0-8% of the total daily volume of saliva and produce four times as much immunoglobulin as other glands.[4] For example, over one-third of the secretary immunoglobulin A (IgA) in whole saliva is secreted by the minor salivary glands.[5] These are the main source of glycoproteins and their protective mechanisms involve mechanical, biochemical, and immunological reactions.
Streptococcusmutans has been implicated as the principal causative agent of human dental caries and salivary antibodies enhance antibacterial mechanisms by potentiating innate defenses.[3] Several laboratories have shown that salivary immunoglobulin A (SIgA) and serum antibodies are important in preventing dental caries in humans and animals; however, some studies show the protective effects of SIgA antibodies, whereas others support serum antibody-mediated control of carious lesions.[3] SIgA is presumed to prevent the adherence of cariogenic microorganisms to hard surfaces and are also supposed to inhibit the activity of glucosyltransferases.[6]
SIgA is a secretory immunoglobulin and is different from the serum IgA by having a dimeric structure compared to the monomeric structure of the latter. SIgA contributes 60% of the total immunoglobulin count in the saliva and helps in the antibacterial action of the saliva by neutralizing the bacterial toxins and enzymes, and preventing the adherence of the bacteria to the tooth surface by blockage of bacterial adhesions, reduction of hydrophobicity, and agglutination of the bacteria.[1,7] Previous investigations on the role of IgA in dental caries have reported contradictory results. Some authors reported higher levels of SIgA in caries-resistant individuals in relation to caries-susceptible ones, suggesting an effective protective function.[1] Radial immunodiffusionis the most applicable method for detecting SIgA as reported in literature. However, there are more sensitive and automatic methods such as nephelometry and Enzyme Linked Immuno Sorbent Essay ELISA.[2]
This study was therefore planned to ascertain the relationship of dental caries with: (1) Average number of functional labial minor salivary glands, (2) amount of secretion from labial minor salivary glands, (3) functional levels in terms of glycoprotein secretion of labial minor salivary glands, and also (4) correlationof the IgA estimations from whole saliva using radial immunodiffusion was performed in relation to dental caries.
METHODOLOGY
The average numbers of functional labial minor salivary glands were counted using the locations suggested by Shern et al.[5] The numbers of active minor salivary glands were counted after 20 s of drying the mucosa. The droplets were colored using 1% toluidine blue for ease of identification.
The amount of secretion was ascertained using the method suggested by Gaubenstock.[8] The average of the difference of post-weight and pre-weight of 4 mm diameter chromatography paper disc suggests the amount of secretion. The 6 × 16 mm chromatography strip was placed 4 mm from bottom of the vestibule.
The level of glycoprotein secretion was ascertained using the same chromatography paper strip which was stained with periodic acid 0.104 mol/l for 3 min and the diameter of the ring was calculated.
As for the correlation of IgA secretion with dental caries, radial immunodiffusion was chosen as a reliable method and hence 5 μl of centrifuged whole saliva was placed in the wells and incubated at 37°C for 24 h. The sharp ring of immunoprecipitation was measured.[7]
RESULTS
The mean number of minor salivary glands [Figure 1] was significantly lower in control as compared to cases (collectively) for both upper and lower lips. Comparison of control with Group I Decayed Missing Filled Teeth (1-5) did not show a significant difference for upper lip (P = 0.169) though the mean difference for lower lip was significant statistically (P = 0.025). Group II had significantly lower mean value as compared to control as well as Group I for both upper and lower lips (P < 0.001) [Table 1].
Figure 1.
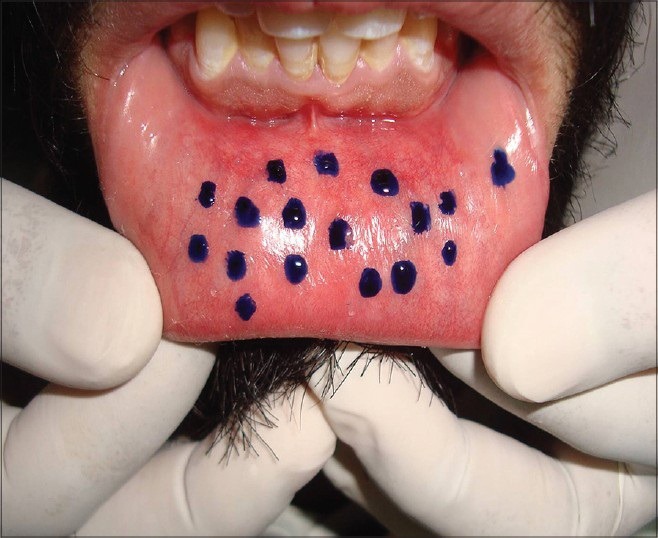
Droplets from minor salivary glands stained with 1% toluidine blue
Table 1.
Average number of labial minor salivary glands
The average amount of secretion of labial minor salivary glands and average functional levels in terms of glycoprotein secretion [Figures 2 and 3] showed a statistically significant difference between controls and cases with controls showing significantly lower grades as compared to cases (P < 0.05) for both upper and lower lips. However, on comparing the control with Group I only, no statistically significant difference between two groups was observed for either of the two lips. But comparison between control group as well as Group I with Group II showed a statistically significant difference for both upper and lower lips (P < 0.001) [Tables 2 and 3].
Figure 2.
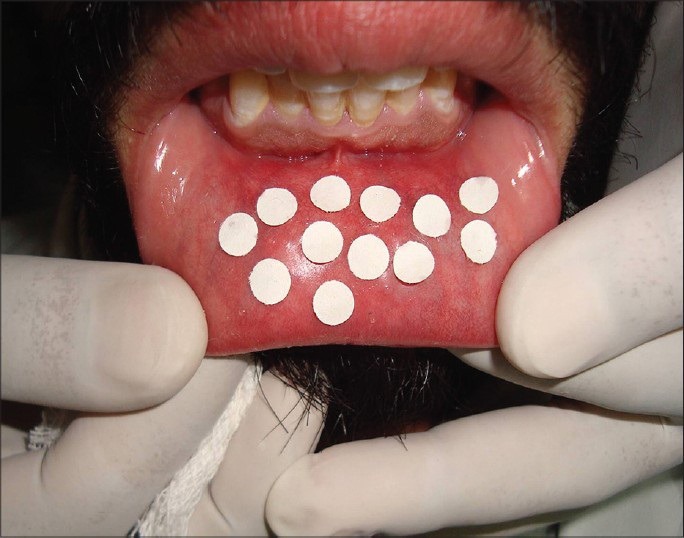
Collection of secretions using paper discs
Figure 3.
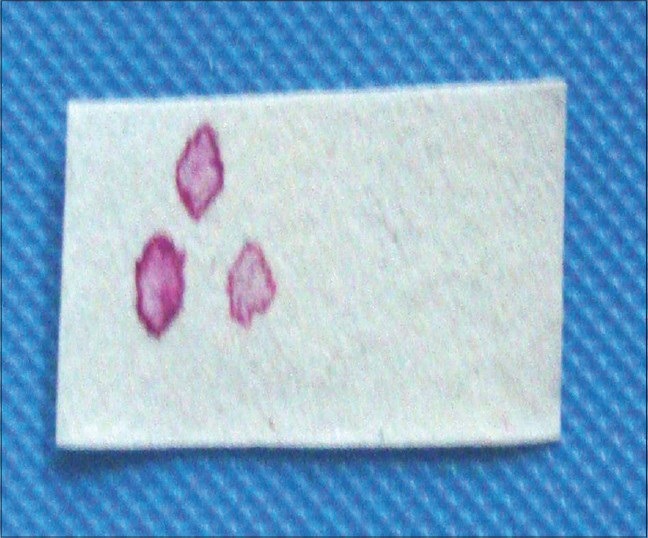
Glycoprotein estimation through diameter of droplets stained with periodic acid
Table 2.
Average functional levels in terms of glycoprotein secretion (represented by diameter of ring)
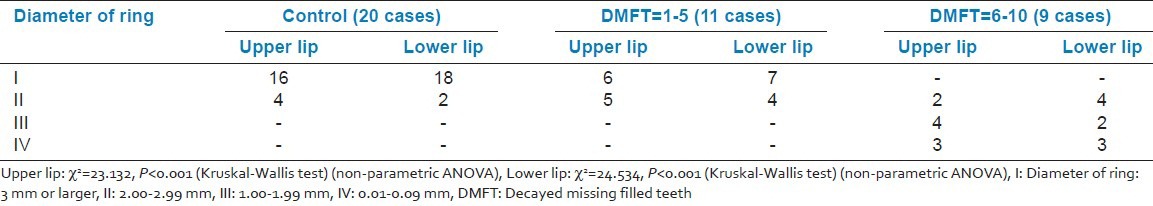
Table 3.
Average amount of secretion of labial minor salivary glands
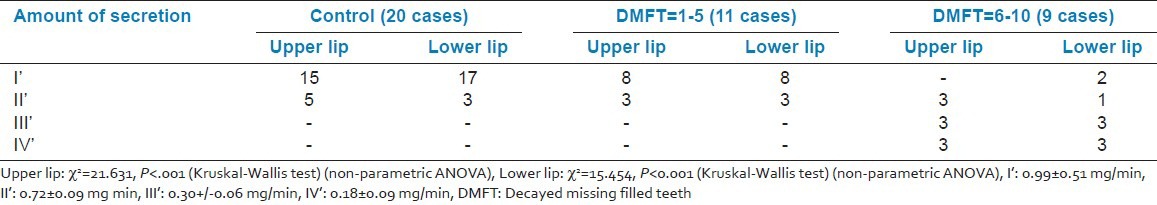
Comparison of ring diameter and IgA levels using radial immunodiffusion [Figure 4] revealed a statistically significant difference for all the comparisons. It was observed that for both the variables, the mean value of control group was significantly higher as compared to that of Group I and Group II, whereas the mean value of Group I was significantly higher as compared to Group II [Table 4] [Figures 5 and 6].
Figure 4.
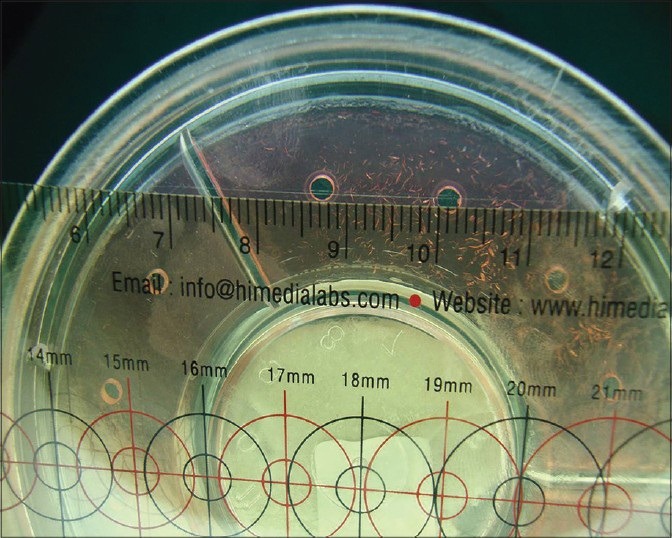
Immunoglobulin Aestimation by measuring sharp immunoprecipitation ring
Table 4.
Correlation of immunoglobulin A estimation by radial immunodiffusion and DMFT
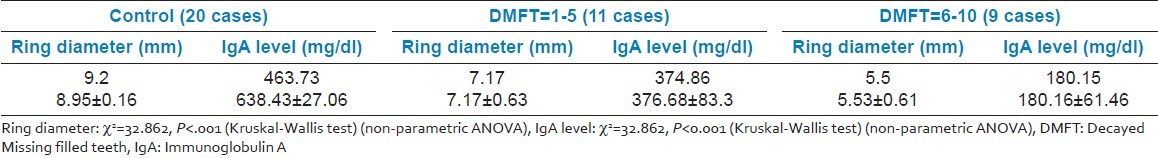
Figure 5.
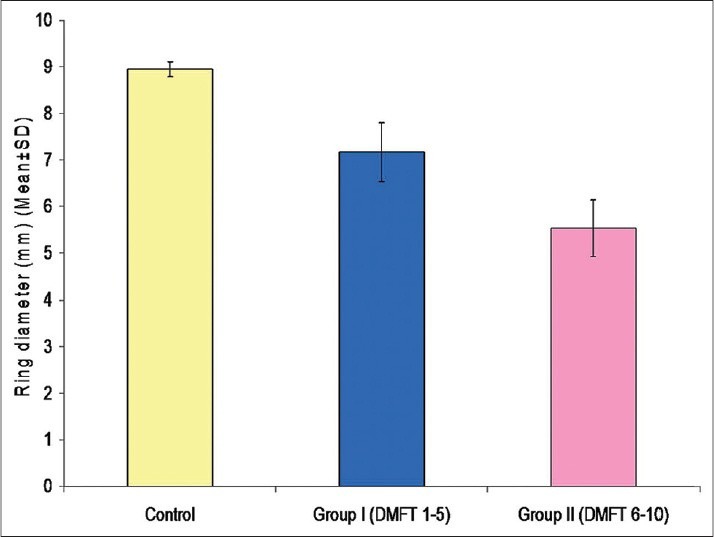
Mean ring diameters in different groups
Figure 6.
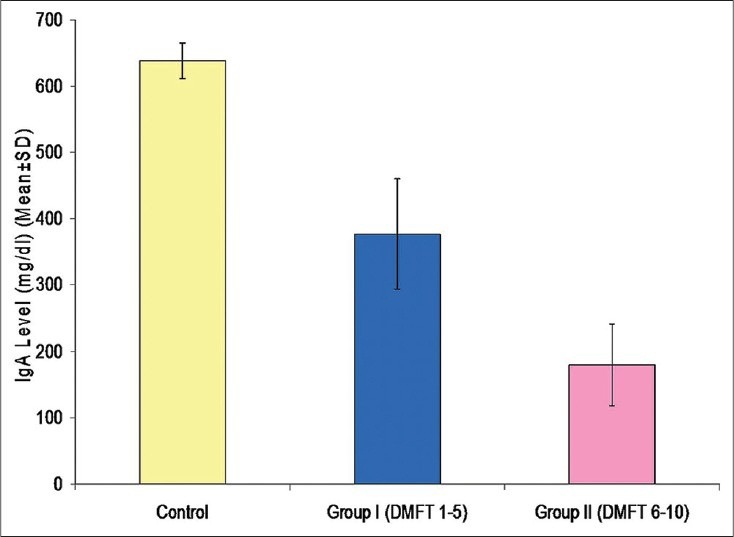
Mean immunoglobulin A levels in different groups
DISCUSSION
An inverse correlation was observed between the number, quality, and quantity of secretion of labial minor salivary glands with DMFT which were similar to the findings of Shern et al.,[5] Smith et al.,[3] and Gaubenstock.[8] However, all the afore-mentioned methods with simple techniques were used together in this study, making the study unique. Also, application of IgA estimation could help to confirm the findings of functional status of labial minor salivary glands in dental caries. The presence of significant quantities of immunoglobulin of three isotopes (IgA, IgG, and IgM) in minorgland saliva theoretically enhances the antibacterial potential within the respective secretory micro-environments.[3] The SIgA has two antigenically distinct components. One of the components appears immunochemically identical to serum IgA. The other component is immunochemically identical to a protein, the “transport piece,” found in the saliva of patients completely lacking both serum and SIgA. Thus far, the IgA component has been found only in combination with the transport piece component in the saliva of children and adults. However, the transport piece is also present in an unbound form in the saliva of most children and some adults. It may be that transport piece is present in free form in young children's saliva because of the gradual increase in IgA production during childhood. The IgA serum levels rise slowly with age and reach average adult levels around puberty.[9]
Eliasson et al.[10] stated that the major sIgA is found in high concentrations in labial than in other minor salivary glands and this saliva could be easily harvested as small droplets. SIgA represents an immune glycoprotein and is helpful in predicting caries status as also highlighted by Yoo et al.[11] The method has further advantages in that it is not susceptible to error from the fluid in the labial sulcus, it permits smaller volumes to be accurately measured, and it allows flow rates from individual glands and from defined areas of mucosa to be determined. It provides a permanent record of observations which include the distribution and the numbers of active glands in the area studied. Although it is excellent for measuring unstimulated flow, it is suitable only for measuring stimulated flow over short intervals because of the rapid increase in droplet size which leads to fusion and non-spherical drops. It should be pointed out, however, that the measurement of the drop dimensions becomes more precise as the drops increase in size. The earlier, smaller drops appear similar in shape to the larger but cannot be measured with the same precision.[12]
The selection of subjects was based on the concept that any natural immunity to caries would be best revealed by a comparison of antibody titers in subjects of low and high caries experience. For a valid comparison, these subjects should be free of carious lesions because, if caries is a bacterial infection which induces an immune response, antibody titers in subjects with carious lesions might be expected to differ from a matched group without lesions.[13]
In subjects of low DMFT, SIgA contributes to oral clearance by agglutination of cariogenic organisms, which prevents their colonization on the tooth surfaces. Hence, the SIgA estimation was inversely related to DMFT as supported by Bolton et al.,[6] Gregory et al.,[3] and Jafarzadeh et al.[14] but was in negative concordance to Roushdy[7] who stated that higher levels of microbial antigenic loads present in individuals with high DMFT probably increases the immune reaction which leads to high levels of SIgA production. Challacomb[13] stated that SIgA is not directly related to protection against dental caries, but reflects a past exposure of the host to cariogenic microorganisms. High levels of salivary antibodies have been found to be related to dental caries. Patients with dental caries show high amounts of acidogenic microorganisms, such as S.mutans in their oral cavities. The presence of caries lesions can lead to more retentive areas for dental plaque accumulation and more difficulty in carrying out good oral hygiene. This may be the reason for the high levels of S.mutans detected in their saliva. Furthermore, higher levels of microbial antigenic loads present in the oral cavity of these individuals probably increases the immune reaction which leads to high levels of antibody production. Sroisiri et al. (2008)[15] demonstrated that, the presence of dental caries was associated with increased SIgA, S.mutans levels in the oral cavity. The adherence to oral mucosa and teeth is the first important step for bacteria in colonizing the oral cavity. SIgA may interfere with this process by blocking adhesins, reducing hydrophobicity, or aggregating bacteria. SIgA has been shown to inhibit the adherence of oral bacteria to oral epithelial cells.[16]
The proposed study proves useful as a diagnostic aid and as a research tool. For example, it can be used to monitor the moisture produced on the mucosa by the secretions of minor salivary glands in various states of health and disease. Thus, this method could provide an objective assessment of the level of dysfunction of minor salivary glands of individuals whose secretary capacity is compromised by, for example, the effects of Sjogren's syndrome or headandneck radiation. Also, because the method is non-destructive, the secretion can be eluted from the paper for microbiological, chemical, or immunological analysis. This characteristic allows the researcher to establish accurately the volume in which the constituent of interest is dissolved, and to calculate the concentrations of specific components. Despite the foregoing potential advantages, the method has limitations that should be considered. The sampling strip might pick up residual moisture remaining on the mucosa following drying procedures, in addition to secretions at the orifice of the minor salivary glands. Furthermore, the number of glands under the sampling strip is unknown. Hence, the method provides a flow rate per unit area of the mucosa, not a flow rate per gland. Preliminary findings indicate the necessity of standardization of the tissue drying procedure and the exact repositioning of the paper strip.[5]
CONCLUSION
Dental caries is considered the most common infectious disease of oral cavity. Estimation of number, quality, and quantity of minor salivary gland secretion due to its easy identifiability, simplicity, and reproducibility proves to have a strong predictive value in identifying caries susceptible population in routine diagnostics. These can therefore be employed in mass screening under limited resources and low technical expertise.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shifa S, Muthu MS, Amarlal D, Rathna Prabhu V. Quantitative assessment of IgA levels in the unstimulated whole saliva of caries-free and caries-active children. J Indian Soc Pedod Prev Dent. 2008;26:158–61. doi: 10.4103/0970-4388.44031. [DOI] [PubMed] [Google Scholar]
- 2.Smith DJ, Taubman MA, Ali-Salaam P. Immunoglobulin isotypes in human minor gland saliva. J Dent Res. 1991;70:167–70. doi: 10.1177/00220345910700030201. [DOI] [PubMed] [Google Scholar]
- 3.Gregory RL, Filler SJ, Michalek SM, McGhee JR. Salivary immunoglobulin A and serum antibodies to Streptococcus mutans ribosomal preparations in dental caries-free and caries-susceptible human subjects. Infect Immun. 1986;51:348–51. doi: 10.1128/iai.51.1.348-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford JM, Taubman MA, Smith DJ. Minor salivary glands as a major source of secretory immunoglobin A in the human oral cavity. Science. 1975;190:1206–9. doi: 10.1126/science.1198107. [DOI] [PubMed] [Google Scholar]
- 5.Shern RJ, Fox PC, Cain JL, Li SH. A method for measuring the flow of saliva from the minor salivary glands. J Dent Res. 1990;69:1146–9. doi: 10.1177/00220345900690050501. [DOI] [PubMed] [Google Scholar]
- 6.Bolton RW, Hlava GL. Evaluation of salivary IgA antibodies to cariogenic microorganisms in children. Correlation with dental caries activity. J Dent Res. 1982;61:1225–8. doi: 10.1177/00220345820610110201. [DOI] [PubMed] [Google Scholar]
- 7.Roushdy MM. Association of dental caries, Streptococcus mutans counts and secretory IgA with tobacco smoking. Australian Journal of Basic and Applied Sciences. 2009;3:3224–9. [Google Scholar]
- 8.Gaubenstock LM. Dental caries and the secretory activity of human labial minor salivary glands. Arch Oral Biol. 1995;40:525–8. doi: 10.1016/0003-9969(94)00198-k. [DOI] [PubMed] [Google Scholar]
- 9.South MA, Cooper MD, Wollheim FA, Hong R, Good RA. The IgA system. I. Studies of the transport and immunochemistry of IgA in the saliva. J Exp Med. 1966;123:615–27. doi: 10.1084/jem.123.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliasson L, Birkhed D, Osterberg T, Carlén A. Minor salivary gland secretion rates and immunoglobulin A in adults and the elderly. Eur J Oral Sci. 2006;114:494–9. doi: 10.1111/j.1600-0722.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 11.Yoo EM, Morrison SL. IgA: An immune glycoprotein. Clin Immunol. 2005;116:3–10. doi: 10.1016/j.clim.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson DB. The flow rate of unstimulated human labial gland saliva. J Dent Res. 1996;75:980–5. doi: 10.1177/00220345960750041301. [DOI] [PubMed] [Google Scholar]
- 13.Challacombe SJ. Serum and salivary antibodies to Streptococcus mutans in relation to the development and treatment of human dental caries. Arch Oral Biol. 1980;25:495–502. doi: 10.1016/0003-9969(80)90058-8. [DOI] [PubMed] [Google Scholar]
- 14.Jafarzadeh A, Hassanshahi GH, Kazemi-Arababadi M, Mostafaee A, Sadeghi M, Nematollahi MA, et al. The comparison of salivary IgA and IgE levels in children with breast- and formula-feeding during infancy period. Dent Res J. 2007;4:11–7. [Google Scholar]
- 15.Thaweboon S, Thaweboon B, Nakornchai S, Jitmaitree S. Salivary secretory IgA, pH, flow rates, mutans streptococci and Candida in children with rampant caries. Southeast Asian J Trop Med Public Health. 2008;39:893–9. [PubMed] [Google Scholar]
- 16.Marcotte H, Lavoie MC. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62:71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


