Abstract
Introduction:
One of the target organs of heavy metals is testis and many authors proposed that oxidative stress could be responsible to induce their toxicity. An experimental study was conducted to evaluate the molecular mechanisms of lead (Pb) and cadmium (Cd) toxicity, their toxicodynamic interaction and to evaluate therapeutic potential of N-Acetyl L-cysteine (NAC) against the reproductive toxicity in male Wistar rats.
Material and methods:
rats were randomly divided into 8 groups comprising of 6 rats in each. Group 1 and 2 were syam and NAC control, Group 3, 4 and 5 were kept as toxic control groups such as lead, cadmium and lead + cadmium respectively, where as Group 6, 7 and 8 were therapeutic groups with NAC. The experiment scheduled for 3 months. Body weights, anti-oxidant profile (GSH, GST, TBARS and protein carbonyls) in testis, testis weight, testicular LDH, sperm count and histopathology were conducted. And also, interaction of Pb and Cd with zinc (Zn) and copper (Cu) in testis was assessed.
Results:
The present study revealed significant alterations in body weights, anti-oxidant profile, weights of testes, testicular LDH, sperm count, and concentration of Zn and Cu in toxic control groups 3, 4 and 5 as compared to control and NAC-treated groups. The toxic combination (Pb+Cd) group 5 showed significant alterations in protein carbonyls, GST levels and testicular LDH as compared to Pb and Cd alone administered groups and these results are substantiated with marked changes in the histopathology. All the NAC-treated groups revealed significant improvement in all the parameters.
Conclusion:
The results of the investigation revealed that Pb, Cd and their combination induces toxicity to the biological system due to the excess generation of free radicals and impairment of anti-oxidant defenses. Toxic effects were more pronounced in the group that received a combination of Pb and Cd, suggesting positive toxicodynamic interaction. Use of NAC countered the adverse effects of Pb and Cd induced toxicity to a major extent suggesting its anti-oxidant potential owing to replenishment of tissue pool of GSH. Further, NAC administration reduced the extent of accumulation of Pb and Cd in various tissues.
Keywords: Cadmium, glutathione, GST, lead, male Wistar rats, TBARS and protein carbonyl, testis
INTRODUCTION
The toxic effects of lead (Pb) and cadmium (Cd) can manifest in various organs and the male reproductive organ is clearly an important target. Reproductive dysfunction of these metals have distinct morphological and biochemical features such as disorganized epithelia, decreased sperm quality, altered sperm morphology and low androgen levels. Cadmium is a non-biodegradable metal and lead is environmental pollutant are known directly to cause destruction to the testicular organs and hypothalamus-pituitary-gonadal axis.[1,2] These heavy metals induce testicular injury, highlighting the disruption of the blood-testis-barrier (BTB), which is a major target in the testis.
Effects of lead on male reproductive functions include abnormalities in sperm counts, motility, morphology[3] and spermatogenesis.[4] Toxicity is manifested in male reproductive system by deposition of lead in testes, epididymis, vas deferens, seminal vesicle and seminal ejaculate. Pb has an adverse effect on sperm count and retarded the activity of a live sperm. Moreover, motility as well as prolonged latency of sperm melting both, in exposed person and experimental animals was observed after Pb exposure. Cd also causes dose- and time-dependent inhibition of the serum hormonal levels of FSH and LH, which induce the signals for testosterone synthesis. An inhibition of these signals results in the time-dependent monophasic serum inhibition in testosterone levels showing reproductive dysfunction, cell death and apoptosis by Cd.[5,6] Exact mechanism of action of Pb and Cd induced reproductive toxicity is still unknown, but oxidative stress could response for their production of toxicity.[2,7] The heavy metal-induced decrease in the activities of anti-oxidant enzymes might result from its ability to remove biometals from active centers of these enzymes and binding to their -SH groups.[8] There is negative correlation between concentration of metals and activities of anti-oxidant enzymes in the tissues. Moreover, these metals might influence the enzymatic anti-oxidant system indirectly via disorders in the body status and depletion of bioelements necessary for proper function of these enzymes such as selenium (Se), zinc (Zn), copper (Cu), manganese (Mn) and iron (Fe). GPx is a Se-dependent enzyme; CAT contains Fe in its active centre, whereas SOD activity is dependent on Zn, Cu and Mn.[8] Most of the studies were carried out following exposure to a single metal and some studies on co-exposure to Pb and Cd showed that these metals had additive effects on anti-oxidants system in the biological system.[9]
N-acetyl L-cysteine (NAC), a potent anti-oxidant, is a sulfur-based amino acid needed to make reduced glutathione (GSH), a natural non-enzymatic anti-oxidant produced in the body to fight free-radical activity. It is being employed clinically for the treatment of many diseases. It is an excellent scavenger of free radicals and has been also used as a chelator of heavy metals to protect against oxidative stress and prevent damage to cells.[10,11] Therefore, the present study was undertaken to explore the toxicodynamic interaction of Pb with Cd and to evaluate the protective role of NAC in rats.
MATERIALS AND METHODS
Chemical reagents
Lead acetate, Cadmium chloride and all other chemicals used in our experiments were purchased from SRL Pvt. Ltd., Mumbai.
Animals
Male Albino rats of Wistar strain weighing about 200-250 g were procured from National Institute of Nutrition, Hyderabad, Andhra Pradesh. The animals used in this study were approved by Committee for the Purpose of Control and Supervision of Experiments on Animals (approval no. 5/I/10/2010).
Experimental design
The study was carried out on 48 male Wistar rats randomly divided into 8 groups with six rats in each group. All the groups were maintained as per the following schedule for 3 months.
Group 1: Normal control.
Group 2: N-acetyl-L-cysteine (NAC) @ 300 mg/kg body weight.
Group 3: Lead toxic control (lead acetate @ 1000 ppm in feed).
Group 4: Cadmium toxic control (cadmium chloride @ 300 ppm in feed).
Group 5: Lead and cadmium toxic control @1000 and 300 ppm, respectively, in feed.
Group 6: Lead and NAC, respectively @ 1000 ppm in feed and @ 300 mg/kg body weight.
Group 7: Cadmium and NAC, respectively @ 300 ppm in feed and @ 300 mg/kg body weight.
Group 8: Lead, cadmium and NAC, respectively @1000, 300 ppm in feed and @ 300 mg/kg body weight.
Lead and cadmium were used in the form of lead acetate and cadmium chloride given in the form of mash feed, NAC administered by oral gavage by diluting in the distilled water.
Biochemical assays
Rat testis tissues were collected at the end of 3rd month. Rats were fasted overnight and sacrificed by cervical decapitation; testes were removed, dissected out, weighted, and washed in ice-cold saline. Some pieces were immediately homogenized (1:10, w/v) in a cold (4°C) buffer containing Tris base (20 mmol/L), EDTA (1 mmol/L) and sucrose (0.5 mmol/L), KCl (150 mmol/L) with the pH adjusted to 7.4. Homogenates were centrifuged at 4000 g for 20 min at 4°C, and clear supernatant collected for estimation of lipid peroxidation levels i.e., TBARS,[12] GSH,[13] GST,[14] Protein carbonyls,[15] testicular LDH (kit procured from SRL Pvt. Ltd., Mumbai.), Epididymal sperm count,[16] Zn and Cu[17] and lead and cadmium[18] and protein levels[19] using serum bovine albumin as the standard. Histopathology of testis was done by standard procedure.[20]
The data were analyzed using one way ANOVA by Statistical Package for Social Sciences (version 10.00) and results were expressed as mean ± standard error.
RESULTS
The concentration of GSH (μ moles/mg protein) and the activity of GST (μ moles/min/mg protein) in testis revealed a significant (P < 0.05) decrease in toxic control groups 3, 4 and 5 as compared to groups 1 and 2 and the lowest concentration of GSH (significantly 5% level) and GST (non significant at 5% level) were found in the combination group 5, which was significantly (P < 0.05) different when compared to groups 3 and 4. The GSH and GST concentrations in NAC treated groups 6, 7 and 8 showed a significant (P < 0.05) increase as comparable to the toxic control groups and it was comparable to groups 1 and 2.
The concentrations of TBARS (n moles of MDA released/mg protein) and protein carbonyls (n moles/mg protein) in testis revealed a significant (P < 0.05) increase in toxic control groups 3, 4 and 5 as compared to groups 1 and 2 and the highest concentration of TBARS (non significant at 5% level) and protein carbonyls (significant at 5% level) were found in combination group 5, when compared to groups 3 and 4. The TBARS and protein carbonyls concentrations in NAC treated groups 6, 7 and 8 showed a significant (P < 0.05) decrease as compared to that of toxic control groups and were comparable to groups 1 and 2 [depicted in Table 1].
Table 1.
Concentrations of oxidative biomarkers, Pb, Cd, Zn and Cu in different groups of rats
The activity of LDH (IU/L) in testis revealed a significant (P < 0.05) increase in toxic control group 3, 4 and 5 as compared to groups 1 and 2 and the highest concentration was found in combination group 5, which was significantly (P < 0.05) different when compared to groups 3 and 4. The LDH activity in NAC treated groups 6, 7 and 8 showed a significant (P < 0.05) decrease in the activity as compared to that of toxic control groups.
The weight of testes (g) and the Epididymal sperm count (million/ml) of testis revealed a significant (P < 0.05) decrease in the toxic control groups 3, 4 and 5 as compared to groups 1 and 2. The testicular weight in NAC treated groups 6, 7 and 8 showed a significant (P < 0.05) improvement as compared to that of toxic control groups.
The concentration of Zn and Cu (ppm) in the testis revealed a significant (P < 0.05) increase in the toxic control groups 3, 4 and 5 as compared to groups 1 and 2. The concentration in NAC treated groups 6, 7 and 8 was comparable to groups 1 and 2 [Table 1].
The concentration of Pb (ppm) in the testis revealed a significant (P < 0.05) increase in the toxic control groups 3 and 5, as compared to NAC treated groups 6 and 8, while it was not detectable in groups 1, 2, 4 and 7. Whereas the concentration of Cd (ppm) revealed a significant (P < 0.05) increase in the toxic control groups 4 and 5 as compared to in NAC treated groups 7 and 8, while it was not detectable in groups 1, 2, 3 and 6.
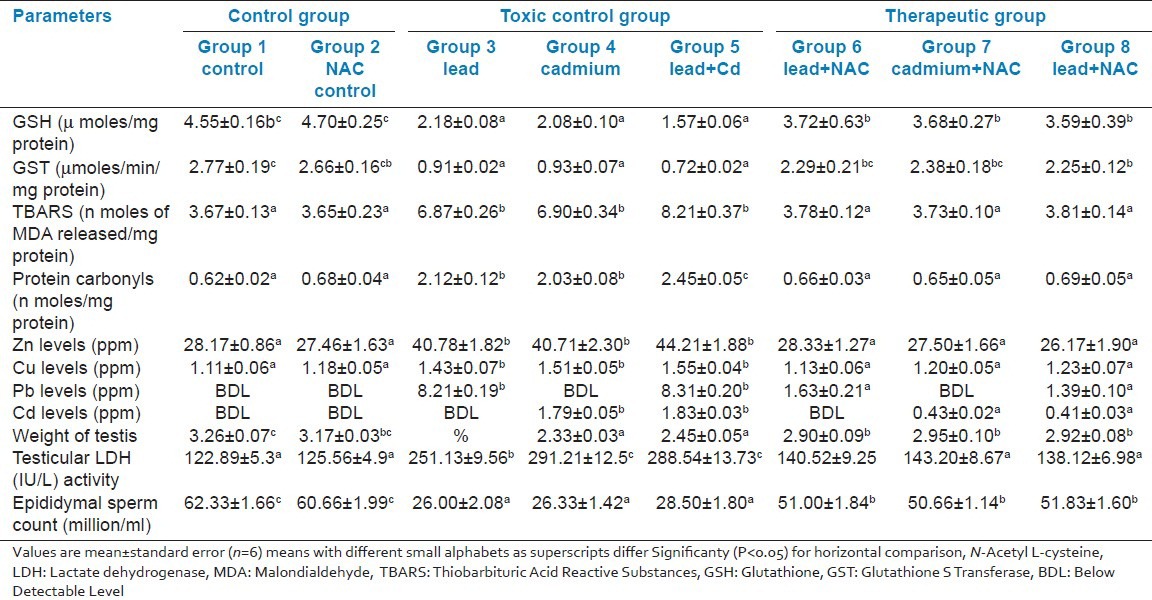
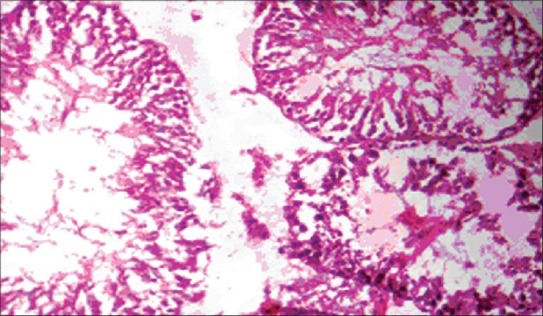
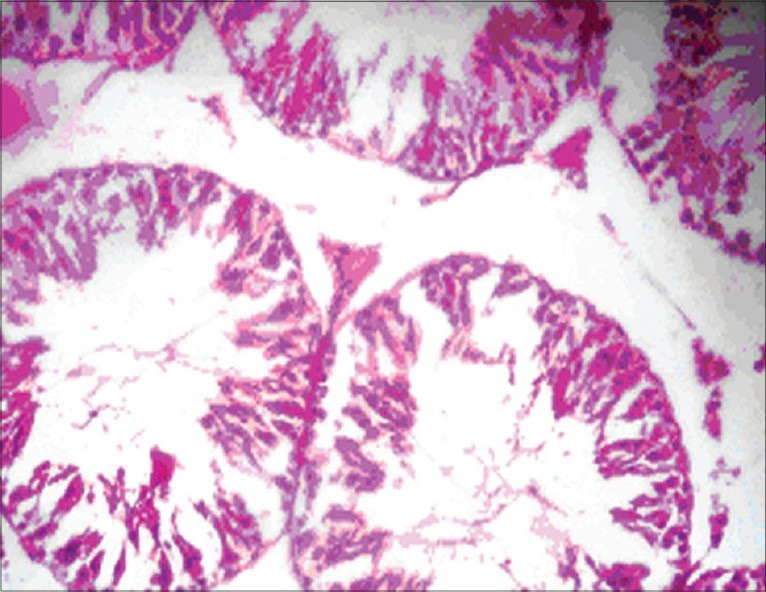
The histopathology of testis in group 3 showed degenerated seminiferous tubules and reduced number of leydig cells [Figure 1], group 4 revealed degenerated seminiferous tubules and very few number of leydig cells [Figure 2], group 5 revealed hemorrhages, interstitial edema, degeneration of tubules and few tubules have full spermatozoa and some are lacking [Figure 3]. The NAC treated group 6 showed mild changes in and around tubules [Figure 4], 7 revealed mild degeneration and separation of tubules [Figure 5] and 8 showed almost normal architecture [Figure 6] when compared with their respective toxic control groups at the end of the 3rd month.
Figure 1.
Photomicrograph of testis showing degenerated seminiferous tubules and reduced number of leydig cells (H and E ×200) (Group 3)
Figure 2.
Photomicrograph of testis degenerated seminiferous tubules and very few number of leydig cells (H and E ×200) (Group 4)
Figure 3.
Photomicrograph of testis showing hemorrhagic, interstitial edema degeneration of tubules and few tubules have full spermatozoa and some are lacking. (H and E ×200) (Group 5)
Figure 4.
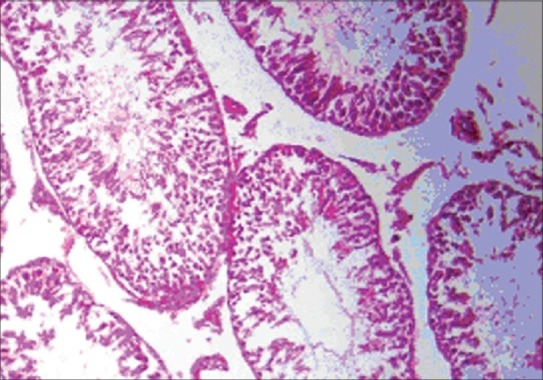
Photomicrograph of testis showing mild changes in and around tubules. (H and E ×200) (Group 6)
Figure 5.
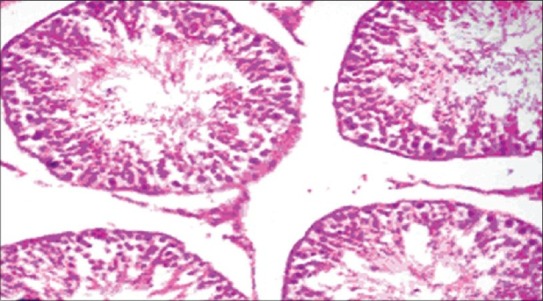
Photomicrograph of testis showing mild degeneration and separation of tubules. (H and E ×200) (Group 7)
Figure 6.
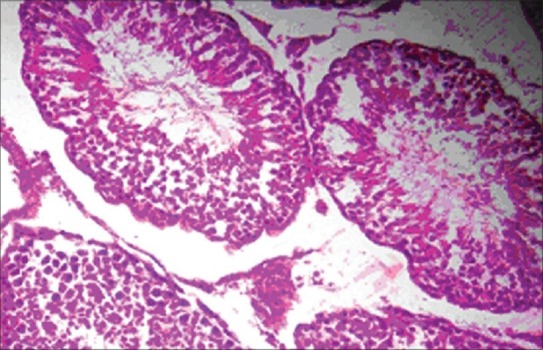
Photomicrograph of testis showing almost normal architecture. (H and E ×200) (Group 8)
DISCUSSION
The biomarkers of oxidative stress (GSH, GST, TBARS and protein carbonyls) were studied in testis to evaluate the extent of free radical-induced damage due to the toxic heavy metals. An imbalance between pro-oxidants and anti-oxidants, in favor of the pro-oxidants, results in oxidative stress associated with oxidative modification of bio-molecules such as lipids, proteins and nucleic acids.[21] The present study revealed significantly decreased (P < 0.05) concentration of GSH in toxic co exposed control group 5 as compared to individual exposed Pb and Cd groups 3 and 4. Similarly, a decreased concentration of GST was observed in Pb and Cd combination group 5, whereas the concentration of TBARS and protein carbonyls revealed a significant (P < 0.05) increase in toxic co exposed control group 5. Protein carbonyls found increased significantly (P < 0.05) when compared to individual exposed groups 3 and 4 and activity of TBARS did not show any significant change as compared to individual toxic control group. Hence, from the data it is hypothysed that there is a positive pharmacodynamic interaction exist in between these metals. The testis expresses several anti-oxidants enzymes, such as superoxide dismutase, catalase and glutathione peroxidase to counteract the oxidative stress, their levels are greatly diminished upon Cd+2 exposures.[22] As such, Cd+2 is likely to substitute Ca+2 or Zn+2 in crucial physiological processes that are mediated by these ions, resulting in the activation and/or inhibition of several signaling pathways. For instance, Cd+2 may cause an increase in oxidative stress by binding to sulfhydryl groups of proteins and by depleting glutathione.[23] Thereafter, the oxidative stress may promote alteration of DNA repair mechanisms and induction of cell proliferation, which, in turn, may lead to tumorigenesis.[24] Significant increase in the lipid peroxidation products (LPP) in lead-treated rats was found[25] when compared to controls. An increase in LPP damages various cellular components of tissues and the investigation showed a significantly increased concentration of LPP in testis, epididymis.
The present study also exhibited the increased testicular LDH activity, the highest concentration was found in combination group 5, which was significantly (P < 0.05) different when compared to individual exposed groups 3 and 4 and significant reduction in epididymal sperm count in toxic control groups 3, 4 and 5. Lactate Dehydrogenase (LDH) is a marker cytoplasmic enzyme known to be present only in the primary spermatocyte and spermatids, is the most active form of enzyme present in the mature sperm. Whereas LDH is affected by these metals.[26] The spermatozoa require LDH for necessary metabolic activity during passage from testis to the site of fertilization in the oviduct.[27] As the enzyme appears to be the reliable marker for metabolic abnormalities.[28] Various studies suggest an interaction of heavy metals with the hypothalamo-hypophysis axis controlling spermatogenesis.[29] Some authors have reported that male rats exposed to lead acetate showed a significant decrease in the weight of the testes[25] and epididymis.[2,7] This reduction in weight of sex glands was accompanied by an alteration of the normal histological structure and also exhibited disordered arrangement of germ cells, a decrease spermatogenic cell layer in the seminiferous tubules. The present study also revealed significant reduction on the weight of the testes in the toxic control group. Further, these results were well substantiated by marked alteration in the histopathological examination of testis. NAC prevented these changes at the end of the experiment owing to its anti-oxidant potential by replenishing GSH pool in the tissues.
Present study also aimed to evaluate the correlation between micro minerals (Zn and Cu) with bio metals (Pb and Cd), results of the data concluded that there was a positive correlation exist among Zn, Cu concentration and accumulation of biometals in respective toxic control group. Interactions between trace elements in this study were similar to those found in polluted areas or in experimental studies in animals receiving diets containing high levels of toxic metals or inadequate levels of nutritional essential elements.[30,31] These interactions probably indicate that mineral balance in the body is regulated by important homeostatic mechanisms in which toxic elements compete with the essential metals, even at low levels of metal exposure. The knowledge of these correlations may be essential to understand the kinetic interactions of metals and their implications in the trace metal metabolism. Zn redistribution in the body is under the influence of Cd. The Cd-induced Zn redistribution in the body and kidney Cu deposition were a result of Cd accumulation and its ability to introduce metallothionein synthesis in the liver and kidney. The metallothionein is important in homeostasis and storage of Zn and Cu.[32] Lead has also been shown to induce the synthesis of metallothionein in several instances.[33] Both Zn and Cu have also been shown to bind to metallothioneins.[34] Besides, inhibition of micronutrient utilization by several metabolizing enzymes in the presence of lead and cadmium also contributes to the accumulation of these micronutrients in tissues. As Pb and Cd replace them from anti-oxidant enzymes by false substitution, the anti-oxidant enzymes fail to function properly, which results in free radical-induced tissue damage. This is evident from the results of the present study, which revealed oxidative stress in various organs studied.
CONCLUSION
The present investigation enunciated that Pb, Cd and their combination induced reproductive toxicity due to the excess generation of free radicals and impairment of anti-oxidant defenses. Toxic effects were more pronounced in the group that received a combination of Pb and Cd suggesting positive toxicodynamic interaction. Use of NAC countered the adverse effects of Pb and Cd induced toxicity to a major extent suggesting its anti-oxidant potential owing to replenishment of tissue pool of GSH as NAC is an excellent scavenger of free radicals and chelator of heavy metal.[11] Further, NAC administration reduced the extent of accumulation of Pb and Cd. Therefore, it is useful to administer N-acetyl L-cysteine to counter the adverse effects of these toxic metals in the biological system.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Obianime AW, Roberts II. Antioxidants, cadmium-induced toxicity, serum biochemical and the histological abnormalities of the kidney and testes of the male Wistar rats. Niger J Physiol Sci. 2009;2:177–85. doi: 10.4314/njps.v24i2.52910. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Xun P, Zhao Y, Wang X, Qian L, Chen F. Effects of lead exposure on sperm concentrations and testes weight in male rats: A meta-regression analysis. J Toxicol Environ Health A. 2008;7:454–63. doi: 10.1080/15287390701839331. [DOI] [PubMed] [Google Scholar]
- 3.Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, et al. Cadmium, lead and other metals in relation to semen quality: Human evidence for Molybdenum as a male reproductive toxicant. Environ Health Perspect. 2008;116:1473–9. doi: 10.1289/ehp.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Telisman S, Colak B, Pizent A, Jurasovic J, Cvitkovic P. Reproductive toxicity of low-level lead exposure in men. Environ Res. 2007;105:256–66. doi: 10.1016/j.envres.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Stohs SJ, Bagchi D, Hassoun E, Bagchi M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol. 2001;20:77–88. [PubMed] [Google Scholar]
- 6.Massanyi P, Lukac N, Slivkova J, Kovacik J, Makarevich AV, Chrenek P, et al. Mercury-induced alterations in rat kidneys and testes in vivo. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;7:865–70. doi: 10.1080/10934520701370410. [DOI] [PubMed] [Google Scholar]
- 7.Smith DM, Mielke HW, Heneghan JB. Sub chronic lead feeding study in male rats. Arch Environ Contam Toxicol. 2008;3:518–28. doi: 10.1007/s00244-008-9138-1. [DOI] [PubMed] [Google Scholar]
- 8.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Whittaker MH, Wang G, Chen XQ, Lipsky M, Smith D, Gwiazda R, et al. Exposure to Pb Cd, and As mixtures potentates the production of oxidative stress precursors: 30-day, 90-day and 180-day drinking water studies in rats. Toxicol Appl Pharmacol. 2010;23:1–13. doi: 10.1016/j.taap.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Blanusa M, Varnai VD, Piasek M, Kostial K. Chelators as antidotes of metal toxicity: Therapeutic and experimental aspects. Curr Med Chem. 2005;12:2271–794. doi: 10.2174/092986705774462987. [DOI] [PubMed] [Google Scholar]
- 11.Clement SL, Hellier BC, Elberson LR, Staska RT, Evans AA. Flies (Diptera: Muscidae: Calliphoridae) are efficient pollinators of Allium ampeloprasum L.(Alliaceae) in field cages. J Econ Entomol. 2007;100:131–5. doi: 10.1603/0022-0493(2007)100[131:fdmcae]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian KA, Manohar M, Mathan VI. An unidentified inhibitor of lipid peroxidation in intestinal mucosa. Biochim Biophys Acta. 1988;962:51–8. doi: 10.1016/0005-2760(88)90094-x. [DOI] [PubMed] [Google Scholar]
- 13.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S transferase in rat lung and liver. Biochim Biophys Acta. 1979;582:67–8. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 14.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 15.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 16.Belsey MA, Moshissi KS, Eliasson R, Paulsen CA, Callegos AJ, Prasad MR. Laboratory manual for the examination of human semen and Semen Cervical Mucus Interaction. 1980 Press concern. [Google Scholar]
- 17.Sawhney SK, Singh R. Analysis of plant tissues introductory practical biochemistry. Narosh Publishing House Private Limited. 2007;2:141–3. [Google Scholar]
- 18.Szkoda J, Zmudzki J. Determination of lead and cadmium in biological material by graphite furnace atomic absorption spectrometry method. Bull Vet Inst Pulawy. 2005;49:89–92. http://www.piwet.pulawy.pl/bulletin/images/stories/pdf/20051/20051089092.pdf . [Google Scholar]
- 19.Lowry OH, Rosenbrough MJ, Farr AL, Rawdell RA. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 20.Singh UB, Sulochana S. 2nd ed. Hyderabad, India: Premier Publishing House; 1997. Handbook of histological histochemical techniques. [Google Scholar]
- 21.Maybauer DM, Maybauer MO, Traber LD, Westphal M, Nakano YY, Enkhbaatar P, et al. Effects of severe smoke inhalation injury and septic shock on global hemodynamics and microvascular blood flow in sheep. Shock. 2006;26:489–95. doi: 10.1097/01.shk.0000230300.78515.ed. [DOI] [PubMed] [Google Scholar]
- 22.Gupta SR, Gupta SE, Dhakal BK, Thakur AR, Ahnn J. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cells. 2004;17:132–9. [PubMed] [Google Scholar]
- 23.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 24.Beyersmann D, Hartwig A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch Toxicol. 2008;82:493–512. doi: 10.1007/s00204-008-0313-y. [DOI] [PubMed] [Google Scholar]
- 25.Hamadouche AN, Slimani M, Merad-Boudia B, Zaoui C. Reproductive toxicity of lead acetate in adult male rats. American Journal of Scientific Research. 2009;3:38–50. [Google Scholar]
- 26.Xu HH, Chen ZP, Shen Y, Wu X, He F. Meta analysis for effect of lead on male productive function. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2006;10:634–6. [PubMed] [Google Scholar]
- 27.Tohami EM, Nattat EW. Effect of antioxidants on lead induced oxidative damage on reproductive dysfunction in male rabbits. J Anim Sci. 2010;6:14–7. [Google Scholar]
- 28.Karthikeyan J, Bavani G. Effect of cadmium on lactate dehyrogenase isoenzyme, succinate dehydrogenase and NA+-K+-ATPase in liver tissue of rat. J Environ Biol. 2009;30:895–8. [PubMed] [Google Scholar]
- 29.Sokol RZ, Wang S, Wan YJ, Stanczyk FZ, Gentzschein E, Chapin RE. Long-term, low-dose lead exposure alters the gonadotropin-releasing hormone system in the male rat. Environ Health Perspect. 2002;110:871–4. doi: 10.1289/ehp.02110871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez AM, Montana PF, Miranda M, Castillo C, Hernandez J, Benedito LJ. Interactions between toxic (As, Cd, Hg and Pb) and nutritional essential (Ca, Co, Cr, Cu, Fe, Mn, Mo, Ni, Se, Zn) elements in the tissues of cattle from NW Spain. Biometals. 2004;17:389–97. doi: 10.1023/b:biom.0000029434.89679.a2. [DOI] [PubMed] [Google Scholar]
- 31.Skoczynska A, Smolik R, Milian A. The effect of combined exposure to lead and cadmium on the concentration of zinc and copper in rat tissues. Int J Occup Med Environ Health. 1994;7:41–9. [PubMed] [Google Scholar]
- 32.Bizoska MM, Monuszko JJ, Jurczuk M, Galazyn SM, Bogalska J. The effect of zinc supply on cadmium influenced changes in the tibia of rats. Food Chem Toxicol. 2001;39:729–37. doi: 10.1016/s0278-6915(01)00007-2. [DOI] [PubMed] [Google Scholar]
- 33.Qu W, Diwan BA, Liu J, Goyer RA, Dawson T, Horton JL, et al. The metallothionein-null phenotype is associated with heightened sensitivity to lead toxicity and an inability to form inclusion bodies. Am J Pathol. 2002;160:1047–56. doi: 10.1016/S0002-9440(10)64925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goyer RA, Clarkson TW. Toxic effects of metals. In: Klaassen CD, Watkins JB III, editors. Casarett and Doull's essentials of TOXICOLOGY. New York: McGraw- Hill Company; 2003. pp. 348–59. [Google Scholar]


