Abstract
Background:
In the traditional system of medicine, the roots and rhizomes of Glycyrrhiza glabra (Gg) (family: Leguminosae) have been studied for their ability to improve a variety of health ailments.
Aims:
The present study was designed to investigate the beneficial effects of Gg root extract on learning and memory in 1-month-old male Wistar albino rats. Four doses (75, 150, 225, and 300 mg/kg) of aqueous extract of root of Gg was administered orally for six successive weeks.
Materials and Methods:
The aqueous extracts were evaluated for their effect on spatial learning and memory in rats using the elevated plus maze, Hebb–William maze, and Morris water maze tests which served as the exteroceptive behavioral model. Diazepam-induced amnesia served as the interoceptive behavioral model.
Results:
Results showed that all the doses of aqueous root extract of Gg significantly enhanced the memory; however, in the doses of 150 and 225 mg/kg, it showed a significant (P < 0.01) enhancement in learning and memory. Furthermore, Diazepam-induced amnesia was reversed by the aqueous root extract of Gg (150 and 225 mg/kg, p.o.).
Conclusion:
These findings suggest that the memory enhancement effects of Gg may be mediated by its antioxidant and anti-inflammatory activities. Thus, Gg appears to be a promising drug for improving memory in the management of impaired learning, dementia, Alzheimer's disease, and other neurodegenerative disorders.
Keywords: Diazepam, elevated plus maze, Glycyrrhiza glabra, Hebb–William maze, learning, memory, water maze
INTRODUCTION
The hippocampus is a major component of the brain of humans and is located inside the medial temporal lobe, beneath the cortical surface. It plays an important role in spatial navigation and long-term memory, thus thousands of experiments have studied the physiology of activity-driven changes in synaptic connections in the hippocampus.[1,2]
The central cholinergic pathways play a prominent role in learning and memory processes.[3]
Memory is the ability of an individual to record sensory stimuli, events, information, etc., retain them over a short or long period of time, and recall the same at a later date when needed.[4] Learning is the process of acquiring knowledge about the world and memory could be considered as the retention of the acquired knowledge, which can be recalled as and when needed.[5] Dementia is a mental disorder characterized by loss of intellectual ability, which invariably involves impairment of memory and also other higher mental functions. The most common cause of dementia is Alzheimer's disease, which is a progressive neurodegenerative disorder associated with loss of neurons in distinct brain areas. Stressful conditions are often associated with loss of memory and cognitive functions, which may lead to threats of schizophrenia and Alzheimer's disease.
India is the largest producer of plant-based drugs in traditional medicines for treatment of various diseases and, therefore, is called as the botanical garden of the world. Traditionally, herbal drugs have been used to enhance cognitive functions and to alleviate other functions associated with Alzheimer's disease. In the traditional system of medicine, the roots and rhizomes of Glycyrrhiza glabra (Gg) (family: Leguminosae) have widely been used worldwide in food, confectionery, and pharmaceutical products, and it is cultivated in the Mediterranean basin of Africa, in southern Europe, and in India. It consists of polysaccharides, flavonoids, triterpene, saponins, pectins, simple sugars, mineral salts, amino acids, and various other substances.
Glycyrrhizin (glycyrrhizic acid or glycyrrhizinic acid), a triterpenoid saponin compound, is the main ingredient in licorice root that accounts for its sweet taste, which is 50-170 times sweeter than sucrose. Glycyrrhizin and its aglycone, glycyrrhetinic acid, are the essential active components that are believed to be responsible for its anti-inflammatory activity. Studies show that Gg has anti-ulcer, anti-inflammatory,[6] antioxidant,[7] antimalarial, expectorant, antispasmodic, diuretic, laxative, and sedative properties. It also possesses antifungal, antihepatotoxic, antipyretic,[8] anti-herpes,[9] antiviral,[10] antimicrobial, and anxiolytic[11] activities.
In the present study, the effects of aqueous root extract of Gg were evaluated experimentally with regard to learning and memory processing in 1-month-old male Wistar albino rats.
MATERIALS AND METHODS
Plant material
The roots of Gg were purchased from a local ayuredic store in Udupi, Karnataka, India on 02/04/2012. The material was authenticated by Dr. Krishna Kumar, Chairman, Department of Applied Botany, Mangalore University.
Preparation of aqueous root extract
The crude aqueous extract of Gg was prepared by macerating the dried powdered root with the respective solvent for 24 h. The macerated powdered roots were then extracted by using soxhlet extractor for 36 h, 1-2 cycles per hour. The extract was dried and weighed. A brownish-black waxy residue with 16% yield was obtained. This aqueous extract of Gg was administrated orally to separate groups of 1-month-old male Wistar albino rats in four different doses of 75, 150, 225, and 300 mg/kg respectively.
Animals
The experimental protocol was approved during September 2011 by the Institutional Animals Ethics Committee (IAEC), Yenepoya University, and the laboratory animals were taken care of as per the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines. Rats were housed individually (Animal house, Yenepoya University, Reg. no. 347/CPCSEA) in polypropylene cages of standard dimensions (22.5 × 35.5 × 15 cm) and maintained at a temperature of 25°C ± 2°C with the duration of light from 08:00 to 20:00 h in a controlled room with a relative humidity of 50-55%. Food and water were provided ad libitum. Experiments were carried out between 09:00 and 14:00 h.
Drugs
Diazepam (Vishal enterprises, Mangalore, India) was used in the present study.
Treatment groups
Rats were randomly divided into eight groups.
Group I – Control (n = 6): A known volume of distilled water was administered orally every day for 6 weeks.
Group II – Diazepam control (n = 6): Diazepam 7 mg/kg was injected i.p. 20 min before the test session.
Group III (n = 6): Received 75 mg/kg aqueous extract of Gg orally every day for 6 weeks.
Group IV (n = 6): Received 150 mg/kg aqueous extract of Gg orally every day for 6 weeks.
Group V (n = 6): Received 225 mg/kg aqueous extract of Gg orally every day for 6 weeks.
Group VI (n = 6): Received 300 mg/kg aqueous extract of Gg orally every day for 6 weeks.
Group VII – Gg 150 mg + Diazepam (n = 6): Received 150 mg/kg aqueous extract of Gg orally every day for 6 weeks. Diazepam 7 mg/kg was injected i.p. 20 min before the test session.
Group VIII – Gg 225 mg + Diazepam (n = 6): Received 150 mg/kg aqueous extract of Gg orally every day for 6 weeks. Diazepam 7 mg/kg was injected i.p. 20 min before the test session.
n = number of animals
Assessment of learning and memory
All experimental animals were tested for spatial memory by elevated plus maze, Hebb–William maze, and Morris water maze tests, 90 min after the last dose.
Elevated plus maze
The elevated plus maze served as the exteroceptive behavioral model to evaluate learning and memory in rats. It is made of wood block and consists of four arms (50 cm length × 10 cm breadth × 40 cm width) fixed to a central platform (10 × 10 cm): two have 12-cm-high walls (closed arms) and the other two have no border in place of the walls (open arms). The maze was elevated to a height of 50 cm. On the first day (i.e., after the last dose of 6 weeks), each rat was placed at the end of the open arm, facing away from the central platform. Transfer latency (TL) was defined as the time (in seconds) taken by the animal to move from the open arm into one of the closed arms with its four legs. TL was recorded on the first day (training session) for each animal. Retention of this learned task (memory) was examined 24 h after the first day trial (i.e., 43rd day, 24 h after the last dose of 6 weeks duration). Significant reduction in TL value of retention indicated improvement in memory.
Hebb–William maze
The Hebb–William maze consists of completely enclosed rectangular box with an entry and a reward chamber appended at opposite ends. The box is partitioned with wooden slats into blind passages, leaving just one twisting corridor leading from the entry to the reward chamber.
The learning assessment for control and drug-treated animals was conducted at the end of drug treatment under a 0 W red-colored bulb, so as to minimize the nocturnal cycle disturbances. Before the training, all the animals were familiarized with Hebb–William maze for a period of 10 min. From the 1st to 3rd day (i.e., from 42nd to 44th day, after the last dose of 6 weeks duration), the rats received four consecutive trials of training per day in the maze. In each trial, the rat was placed in the entry chamber and the timer was activated as soon as the rat left the chamber. The time taken for the rat to reach the reward chamber (TL in minutes) was taken as the learning score of the trial. The average of four trials was taken as the learning score for the day. Lower scores of assessment indicate efficient learning while higher scores indicate poor learning in animals. Retention of this learned task (memory) was examined 96 h after the last day trial (i.e., 48th day). Significant reduction in TL value of retention indicated improvement in memory.
Morris water maze
It is a well-validated test for spatial learning and memory. The technique of using escape from water to motivate learning has been used for many years.[12,13,14] There are several advantages of Morris water maze over other models of learning and memory, including absence of motivational stimuli such as food and water deprivation, electrical stimulations, and buzzer sounds.
Morris water maze was used to assess learning and memory in experimental 1-month-old male albino Wistar rats. Each animal was subjected to four acquisition trials per day for four consecutive days (i.e., after the last dose of 6 weeks duration) and their memory was tested on the 5th day, during which the platform was removed (probe trial). A plastic circular swimming pool (117 cm in diameter, 60 cm high) was filled to a depth of 25 cm with water. Two hundred milliliters of evaporated milk was added to make the water opaque and prevent visualization of the platform. Four points on the rim of the pool were designated as north (N), south (S), east (E), and west (W), thus dividing the pool into four quadrants (NW, NE, SE, SW). An 8 × 8 cm Plexiglas platform, onto which the rat could escape, was positioned in the center of one of the quadrants, 1 cm below the water surface. One day before the test, each rat was placed in the pool for 60 seconds without the platform present; this free swim enabled the rat to become habituated to the training environment. The latency (TL) from immersion into the pool to escape onto the platform was recorded for each trial. If the rat did not find the platform in 120 seconds, it was manually placed on the platform for a 30-second rest. On day 5 (i.e., 46th day, 96 h after the last dose of 6 weeks duration), the platform was removed. The rat was allowed 60 seconds of free swimming. The time spent in the quadrant where the platform was previously located was measured (probe trial), which was considered to assess memory for platform location.
Statistical analysis
All the results were expressed as Mean ± Standard Error of Mean (SEM). Data were analyzed using analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. P < 0.05 were considered as statistically significant.
RESULTS
Effect on TL (by elevated plus maze)
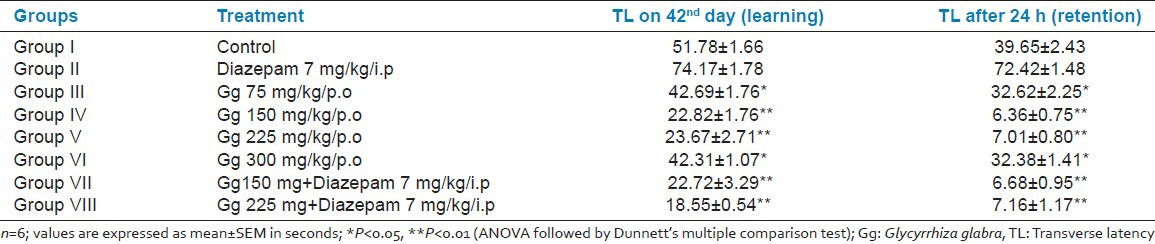
TL in seconds was defined as the time taken by the animal to move from the open arm into one of the covered arms with all its four legs. Significant reduction in TL value of retention indicated improvement of memory. Aqueous extract of Gg (75, 150, 225, and 300 mg/kg, p.o.) administered for 6 weeks showed reduction in TL of 43rd day in 1-month-old male albino Wistar rats, when compared with the respective control groups, indicating significant improvement in memory. Aqueous extract of Gg (150 and 225 mg/kg, p.o.) administered for 6 weeks showed more significant improvement in memory, when compared with all the other groups. Diazepam injected before training significantly increased the TL of 43rd day, indicating impairment of memory (amnesia). Aqueous extract of Gg (150 and 225 mg/kg, p.o.) administered for 6 weeks reversed the amnesia induced by Diazepam [Table 1].
Table 1.
Effects of aqueous root extract of Gg on transfer latency of 1-month old male Wistar albino rats by elevated plus maze
Effect on TL (by Hebb–William maze)
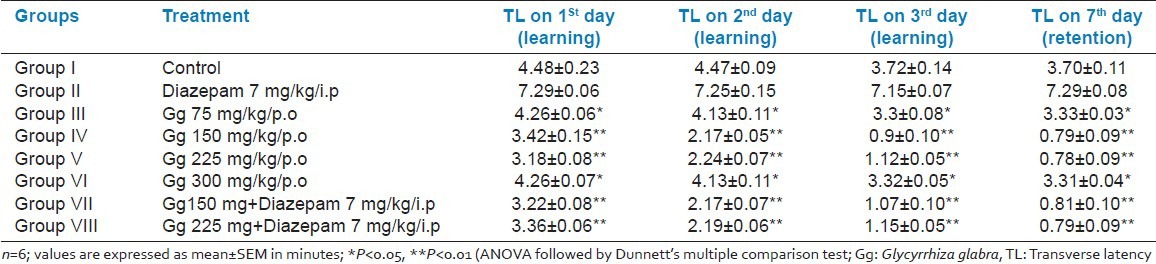
Significant reduction in the TL value of retention indicated improvement of memory. The time taken by the animals (learning score) to reach the reward chamber from the entry chamber on treatment with all the doses of aqueous root extract (75, 150, 225, and 300 mg/kg, p.o.) of Gg was reduced on day 1, 2, and 3 (i.e., from 42nd to 44th day), when compared with the respective control groups, indicating significant improvement in memory. Even there was significant reduction in the TL value of retention (memory) on day 7 (i.e., 48th day) at all the doses of aqueous root extract of Gg (75, 150, 225, and 300 mg/kg, p.o.) administered, but showed a significant result (P < 0.01) with 150 and 225 mg/kg doses [Table 2]. Diazepam injected before the training significantly increased the TL on day 1, 2, and 3, indicating impairment of memory (amnesia). Aqueous extract of Gg (150 and 225 mg/kg, p.o.) administered for 6 weeks reversed the amnesia induced by Diazepam [Table 2].
Table 2.
Effects of aqueous root extract of Gg on transfer latency of 1-month old male Wistar albino rats by Hebb-William maze
Morris water maze
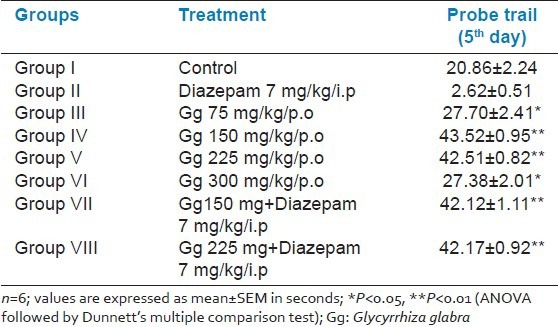
In this test, the rats that were treated with aqueous root extract of Gg (75, 150, 225, and 300 mg/kg, p.o.) for 6 weeks learned the platform location faster than the controls. Aqueous extract of Gg (150 and 225 mg/kg, p.o.) administered for 6 weeks also reversed the amnesia induced by Diazepam [Table 3]. Even in the probe trial, there was more preference for platform location in the target quadrant at all the doses of aqueous root extract of Gg (75, 150, 225, and 300 mg/kg, p.o.), but showed a significant result (P < 0.01) with 150 and 225 mg/kg [Table 4 and Graph 1].
Table 3.
Effects of aqueous root extract of Gg in 1-month old male Wistar albino rats by Morris water maze: (Four acquisition trials per day for 4 consecutive days)
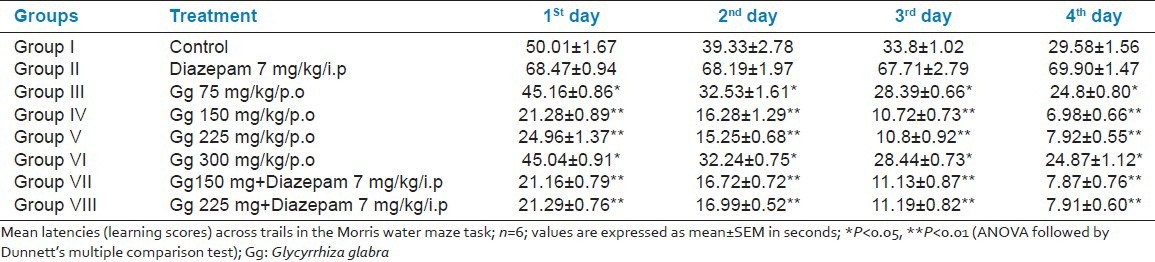
Table 4.
Probe trail (retention) of the Morris water maze task for 6 weeks duration
Graph 1.
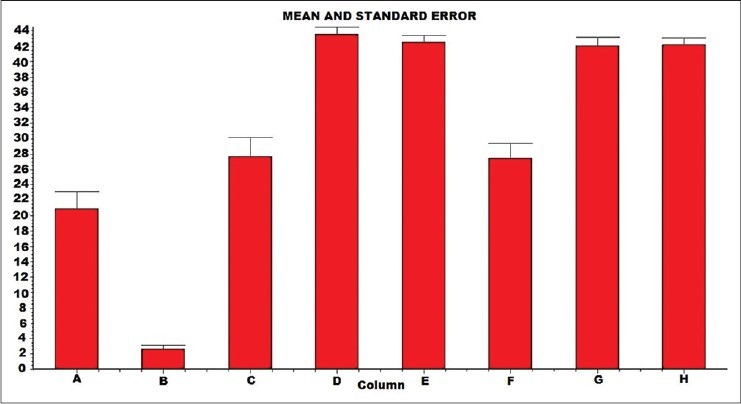
Probe trail (retention) of the Morris water maze task for 6 Weeks duration: n = 6; values are expressed as Mean ± SEM in seconds; A- Control; B- Diazepam 7 mg/kg/i.p; C- Gg 75 mg/kg/p.o; D- Gg 150 mg/kg/p.o; E- Gg 225 mg/kg/p.o; F- Gg 300 mg/kg/p.o; G- Gg150 mg + Diazepam 7 mg/kg/i.p; H- Gg 225 mg+Diazepam7 mg/kg/i.p
DISCUSSION
Age, oxidative stress, harmful free radicals, and inflammation are all the key components in the development of memory impairment, including the conditions such as dementia, schizophrenia, and Alzheimer's disease. A number of herbs employed in the traditional system of medicine have yielded positive results. Nootropics are used as smart drugs, memory enhancers, neuro enhancers, cognitive enhancers. They may act by improving the availability of neurotransmitters, enzymes, and hormones, improving brain's oxygen supply, or by stimulating nerve growth. Emblica officinalis,[15] Bacopa monnieri,[16] Evolvulus alsinoides,[17] Withania somnifera,[18] Acorus calamus,[19] Centella asiatica,[20] etc., are used as a memory enhancer drugs.
Until now, only limited herbal drugs are available with valid scientific data, especially as a monotherapy on learning and memory. An ayurvedic preparation called Abana contains various herbal ingredients, one of which is Gg. Hence, the present study was conducted to evaluate the contribution of aqueous root extract of Gg for its learning and memory enhancing activity in 1-month-old Wistar albino rats.
In exteroceptive behavior models (elevated plus maze, Hebb–William maze, and Morris water maze), the stimulus lies outside the body, whereas it lies within the body in the case of interoceptive models (Diazepam). In the present study, aqueous root extract of Gg administered orally for 6 weeks improved learning and memory of 1-month-old rats significantly in both the exteroceptive and interoceptive behavioral models employed. All the doses of aqueous root extract of Gg (75, 150, 225, and 300 mg/kg, p.o.) given for 6 weeks in 1-month-old rats improved the memory, as reflected by the diminished TL compared with control animals. Additionally, the aqueous root extract of Gg in the dose of 150 and 225 mg/kg significantly (P < 0.01) increased learning and memory in rats compared with controls. Aqueous root extract of Gg (150 and 225 mg/kg, p.o.) also reversed the amnesia caused by interoceptive stimuli (Diazepam).
Free radicals and reactive oxygen substances generated by living cells as a result of physiological and biochemical processes, and accumulation of these free radicals in the body can cause oxidative damage to lipids, proteins, and DNA, which may lead to cancer, diabetes, aging, and other neurodegenerative diseases. Thus, the memory-enhancing activity of aqueous root extract of Gg may be mediated by its antioxidant and anti-inflammatory activities, by virtue of which susceptible brain cells get exposed to less oxidative stress resulting in reduced brain damage and improved neuronal function.
CONCLUSION
In conclusion, based on our results obtained in the present study, the aqueous root extract Gg has shown spatial learning and memory-enhancing activities in all the selected doses, but it was more significant in the doses of 150 and 225 mg/kg. However, further extensive studies are needed to know its exact mechanism of action as a potent and efficacious nootropic agent.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 2.Eichenbaum H, Cohen NJ. United States: MIT Press; 1993. Memory, amnesia, and the hippocampal system. [Google Scholar]
- 3.Nabeshima T. Behavioral aspects of cholinergic transmission: role of basal forebrain cholinergic system in learning and memory. Prog Brain Res. 1993;98:405–11. doi: 10.1016/s0079-6123(08)62424-3. [DOI] [PubMed] [Google Scholar]
- 4.Nadkarni AK. Bombay: Popular Prakashan; 1998. Indian materia medica. [Google Scholar]
- 5.Hikino H. Recent Research on Oriental Medicinal Plants. In: Wagner H, Hikino H, Farnsworth NR, editors. Economic and medicinal plant research. London: Academic Press; 1985. p. 53. [Google Scholar]
- 6.Yokota T, Nishio H, Kubota Y, Mizoguchi M. The inhibitory effect of glabridin from liquorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998;11:355–61. doi: 10.1111/j.1600-0749.1998.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 7.Ju HS, Li XJ, Zhao BL, Han ZW, Xin WJ. Effects of Glycyrrhiza flavonoid on lipid peroxidation and active oxygen radicals. Yao Xue Xue Bao. 1989;24:807–12. [PubMed] [Google Scholar]
- 8.Lata S, Saxena RS, Kumar A, Kakkar S, Srivastava VK, Saxena KK. Comparative antipyretic activity of Ocimum sanctum, Glycyrrhiza glabra and aspirin in experimentally induced pyrexia in rats. Indian J Pharmacol. 1999;31:71–5. [Google Scholar]
- 9.Ceremelli C, Portolani M, Cotombari B, Castelli M, Baggio G, Galatulas I, et al. Activity of glycyrrhizin and its diasterioisomers against two new human herpes virus: HHV-6 and HHV-7. Phytother Res. 1996;10:527–8. [Google Scholar]
- 10.Hirabayashi K, Iwata S, Matsumoto H, Mori T, Shibata S, Baba M, et al. Antiviral activity of glycyrrhizin and its modofied compounds against human immunodeficiency virus type 1 and herpes simplex type 1 in vitro. Chem Pharm Bull. 1991;39:112–5. doi: 10.1248/cpb.39.112. [DOI] [PubMed] [Google Scholar]
- 11.Ambawade SD, Kasture VS, Kasture SB. Anxiolytic activity of Glycyrrhiza glabra Linn. J Nat Remedies. 2001;2:130–4. [Google Scholar]
- 12.Glaser OC. The formation of habits at high speed. J Comp Neurol. 1910;20:165–84. [Google Scholar]
- 13.Wever EG. Water temperature as an incentive to swimming activity in the rat. J Comp Psychol. 1932;14:219–24. [Google Scholar]
- 14.Waller MB, Waller PF, Brewster LA. A water maze for use in studies of drive and learning. Psychol Rep. 1960;7:99–102. [Google Scholar]
- 15.Vasudevan M, Parle M. Memory enhancing activity of Anwala churna (Emblica officinalis Gaertn.): An Ayurvedic preparation. Physiol Behav. 2007;91:46–54. doi: 10.1016/j.physbeh.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Singh HK, Dhawan BN. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi) Indian J Pharmacol. 1997;29:359–65. [Google Scholar]
- 17.Nahata A, Patil UK, Dixit VK. Effect of Evolvulus alsinoides Linn. On learning behavior and memory enhancement activity in rodents. Phytother Res. 2010;24:486–93. doi: 10.1002/ptr.2932. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni SK, Dhir A. Withania somnifera: An Indian ginseng. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1093–105. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Girish Achliya S, Barabde U, Wadodkar S, Dorle A. Effect of Bramhi Ghrita, an polyherbal formulation on learning and memory paradigms in experimental animals. Indian J Pharmacol. 2004;36:159–62. [Google Scholar]
- 20.Singh S, Gautam A, Sharma A, Batra A. Centella asiatica: a plant with immense medicinal potential but threatened. [Last accessed on 7-4-2012];Int J Pharm Sci Rev Res. 2010 4:003. Available from: http://www.globalresearchonline.net . [Google Scholar]


