Abstract
A simple, sensitive method for the determination of meta-chlorobenzoic acid in bupropion hydrochloride is described. Chromatographic separation of m-chlorobenzoic acid is achieved using a mobile phase consisting of n-hexane and ethanol (1000:50, v/v) at a flow rate of 1.0 ml/min on a Chiralpak ADH (250 × 4.6 mm). Absorbance is monitored at 235 nm. The method is linear for m-chlorobenzoic acid over concentration range of LOQ, 5.0 μg/ml to 15.0 μg/ml for m-chlorobenzoic acid with correlation coefficient greater than 0.99. This method is more selective and accurate than United States Pharmacopoeia method for the determination of m-chlorobenzoic acid in bupropion hydrochloride.
Keywords: 1-(3-Chlorophenyl)-1,2-propanedione; meta-chlorobenzoic acid; bupropion Hcl; HPLC method; method validation
INTRODUCTION
Bupropion hydrochloride (Bup-HCl) is described chemically as shown in Figure 1 as (±)-2-(tert-butyl amino)-1-(3-chlorophenyl) propan-1-one.[1] The drug is an anti-depressant that acts as norepinephrine, dopamine reuptake inhibitor, and nicotine antagonist. Bupropion hydrochloride is used also for depression, smoking cessation, obesity, and attention deficit hyperactivity disorder.[2] It is a water-soluble salt of an aminoketone,[3] with a pKa of 7.9,[4] and it is also known with the generic name of amfebutamone hydrochloride. Bupropion is structurally related to phenylethylamines, cathinone (a CNS stimulant from leaves of Catha edulis), and to the anorectic drug diethylpropion.[5,6] Various methods were reported for the determination of Bup-HCl including spectrophotometry,[7,8,9] potentiometry,[7] conductometry,[7] titrimetry,[9] and polarography.[10] HPLC methods were used for the determination of Bup-HCl.[11,12,13]
Figure 1.
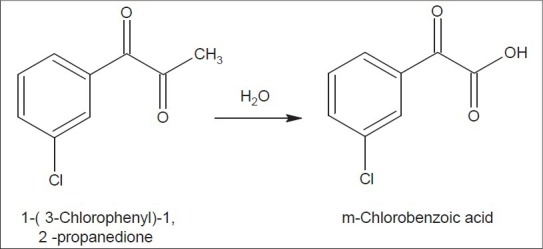
Reaction Scheme for the degradation of 1-(3-Chlorophenyl)-1,2-propanedione to m- chlorobenzoic acid
Bupropion monograph is official in USP.[14] Under organic impurities (Test 1) by HPLC, meta-chlorobenzoic acid is controlled at a limit of NMT 0.2%. The verification of bupropion hydrochloride is performed as per organic impurities in Bup-HCl API following USP[14] verification of compendial procedures. During verification of organic impurities (Test 1) By HPLC as per USP,[14] it is found that impurity at relative retention time (RRT) 0.58 i.e., 1-(3-Chlorophenyl)-1,2-propanedione of organic impurities of Test 2 appeared at the same retention time (RT) as that of meta-chlorobenzoic acid peak. Therefore, it is need to develop an accurate method for the determination of meta-chlorobenzoic acid inbupropion hydrochloride. Thus, the paper reports a normal phase method for the determinations of meta-chlorobenzoic acid in bupropion hydrochloride.
EXPERIMENTAL
Reagents and chemicals
Standard m-chloro benzoic acid and bupropion are obtained from Glenmark Generics Limited, Navi Mumbai, India. n - hexane, ethanol, and acetonitrile used were of HPLC grade and purchased from RFCL Limited, New Delhi, India.
Analytical mode high–performance liquid chromatography
A waters (USA) 2695 series HPLC system equipped with 2695 series quaternary gradient pump, auto sampler with cooler and PDA system, and a Shimadzu LC 2010 CHT (Japan) HPLC module equipped with quaternary gradient pump, column oven, auto sampler, and DAD system were used for the analysis and validation of the proposed method. The data were recording using Empower 2 and LC solution software for Waters and Shimadzu system.
Chromatographic conditions
The analysis was carried out on amylase-based chiralpak AD-H (250 mm × 4.6 mm, 5 μm, Daicel Chemical Industries Limited, Japan) using a mobile phase consisting of n-hexane/ethanol (1000/50, vol/vol) and diluents (Acetonitrile: Ethanol = 9:1) with UV detector at 235 nm at a flow rate of 1.0 ml/min. The column was maintained at 25°C throughout the analysis. A 20-μl sample of concentration was injected, and chromatogram was recorded for 60 minutes.
METHODS
Preparation of blank solution
Into a 50 ml separating funnel, transferred 20 ml diluents and added 20 ml n-hexane to it and shaked well for 5 minutes. Hold the separating funnel for 2 minutes to separate the 2 layers. Discard the bottom layer, and use the upper hexane layer for analysis.
Preparation of test solution
About 200.0 mg of sample is accurately weighed into 20 ml volumetric flask. 10-15 ml of diluents is added and sonicated to dissolve. Volume was made up with diluent and mixed well. Into a 50 ml separating funnel transferred this solution and added 20 ml n-hexane to it and shaked well for 5 minutes. Hold the separating funnel for 2 minutes to separate the 2 layers. Discard the bottom layer and use the upper layer for analysis.
Preparation of reference solution
About 5.0 mg of m-chlrobenzoic acid standard is accurately weighed into 50 mL volumetric flask. 25-30 mL of diluents is added and sonicated to dissolve. Volume was made up with diluent and mixed well. Further dilute 2 ml of this solution to 20 ml diluents and mixed. Into a 50 ml separating funnel transferred this solution and added 20 ml n-hexane to it and shaked well for 5 minutes. Hold the separating funnel for 2 minutes to separate the 2 layers. Discard the bottom layer, and use the upper hexane layer for analysis.
System suitability test
The relative standard deviation of 6 replicate injections of reference solution is not more than 5%, set as the system suitability criteria of the proposed method.
Validation
Specificity and selectivity
The ability of the method to determine accurately and specifically the analyte of interests in the presence of other components in a sample matrix (that may be expected to be present in the sample matrix) under the stated conditions of the test (specificity = 100% selectivity).
Linearity
The ability of the method to obtain test results proportional to the concentration of analyte. The linearity was determined by dividing the response with the respective concentrations and to plot these ‘relative responses’ as a function of the concentration, on a log scale. The line obtained should be horizontal over the full linear range, with a positive deviation at low concentrations and a negative deviation at high concentrations.
Accuracy and precision
The accuracy of an analytical method is the extent to which test results generated by the method and the true value agree. The precision of a method is the closeness of agreement between independent test results obtained under stipulated conditions.
Limit of detection (LOD)
The limit of detection is the point at which a measured value is larger than the uncertainty associated with it. It is the lowest concentration of analyte in a sample that can be detected but not necessary quantified.
LOD = 3.3 × SO/b
Where SO and b are standard deviation and slope of the calibration line, respectively.
Limit of quantitation (LOQ)
The lowest concentration or amount of analyte that can be determined quantitatively with an acceptable level of repeatability precision and trueness.
LOQ = 10.0 × SO/b
Ruggedness
The (intra – laboratory tested) behavior of an analytical process when small changes in the environmental and/or operating conditions are made.
Robustness
A measure of the capacity of the analytical procedure to remain unaffected by small but deliberate variations in method – performance parameters, which provides an indication of its reliability during normal usage.
RESULTS AND DISCUSSION
On studying the structural similarity of 1-(3-Chlorophenyl)-1,2-propanedione (Impurity at RRT 0.58) and m-Chlorobenzoic acid, it was observed that m-chlorobenzoic acid was the degradation product of 1-(3-Chlorophenyl)-1,2-propanedione. During analysis on contact with water, 1-(3-Chlorophenyl)-1,2-propanedione undergoes degradation to give m-chlorobenzoic acid. The reaction is shown in Figure 1.
System suitability
The relative standard deviation of 6 replicate injections of reference solution was found to be 2.8%.
Validation
Specificity/selectivity
Specificity of the method was evidenced by comparing blank, meta-chlorobenzoic acid, bupropion, impurity at different relative retention time (RRT) and spiking with meta-chlorobenzoic acid with these impurities. From the experimental data [Table 1], there are no interfering peaks at retention time of meta-chlorobenzoic acid from the chromatogram [Figures 2-4]. In the modified method, all the impurities of bupropion hydrochloride are well separated from m-chlorobenzoic, i.e., method is selective for determination of content of m-chlorobenzoic acid.
Table 1.
Selectivity of the proposed method
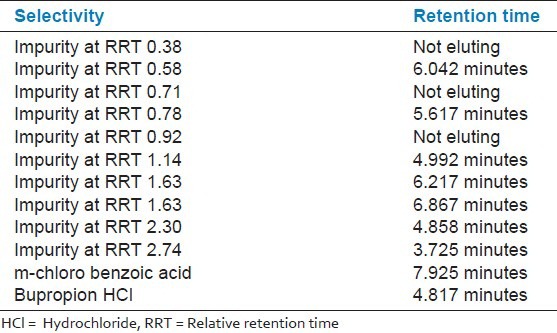
Figure 2.
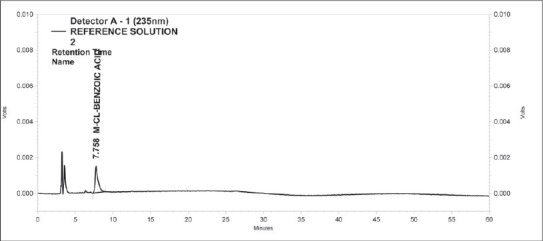
Standard Chromatogram of m-chlorobenzoic acid
Figure 4.
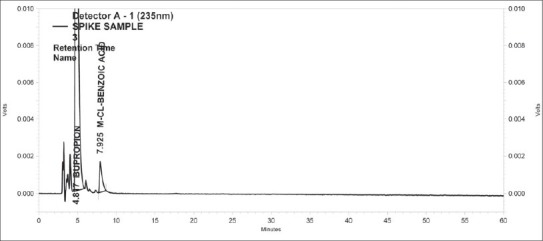
Standard Chromatogram of Bupropion and m-chlorobenzoic acid
Figure 3.
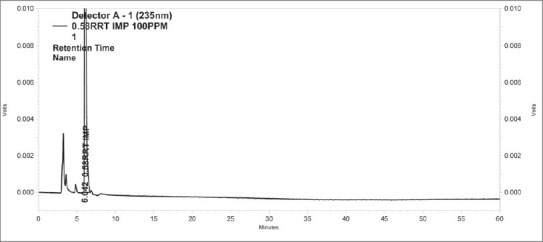
Standard Chromatogram of 1-(3-Chlorophenyl)-1,2-propanedione
Solution stability
The study reveals that m-chlorobenzoic acid in bupropion hydrochloride is stable in the diluents for 48 hours when stored at room temperature under laboratory light conditions. There were no significant changes with initial data up to 48 h.
Linearity
The linearity regression studies demonstrated the acceptability of the method for the quantitative determination of m-chlorobenzoic acid. For linearity range determination, the data generated was analyzed by linear regression analysis to calculate the slope, intercept, and the correlation coefficient. The method follows a linear range over 5 μg/ml to 15 μg/ml for m-chlorobenzoic acid (i.e., 50% to 150%) [Table 2] with a correlation coefficient greater than 0.99 [Figure 5].
Table 2.
Linearity of the proposed method
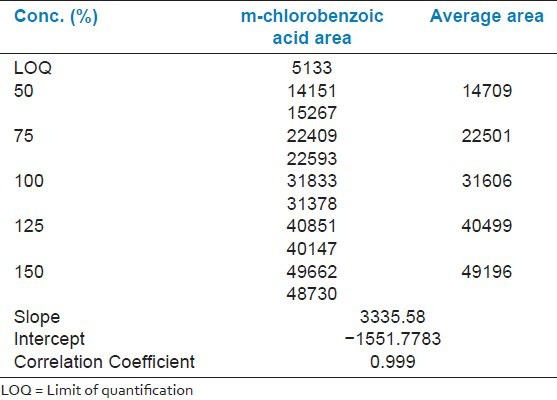
Figure 5.
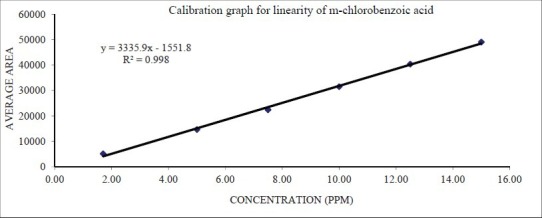
Linearity graph of proposed method
Accuracy
The accuracy of the method was determined by spiking the known amount of m-chlorobenzoic acid at LOQ, 50%, 100%, and 150% w/w of the specified limit. The accuracy in determination was checked at 3 different concentration levels, and it was observed that the recovery of the m-chlorobenzoic acid is within the prescribed range of 80-120%.
Precision
The % RSD for 6 replicate injections of standard solution of m-chlorobenzoic acid is found to be 1.31% for system precision. Method precision is performed by estimating the m-chlorobenzoic acid in control samples and 6 spiked samples on the same day. The % RSD for m-chlorobenzoic acid from 6 samples was calculated and was found to be well within the desired limits.
Limit of detection
The limit of detection is determined from the linearity experiment wherein a lower concentration of m-chlorobenzoic acid is analyzed. The LOD concentration is found to be 0.6 ppm i.e., 0.01% of test concentration for m-chlorobenzoic acid. The RSD for 6 replicate injections of m-chlorobenzoic acid is found to be 13.24%. The signal to noise ratio is above 3. The results are reported in Table 3.
Table 3.
Data sheet for limit of detection and limit of quantitation
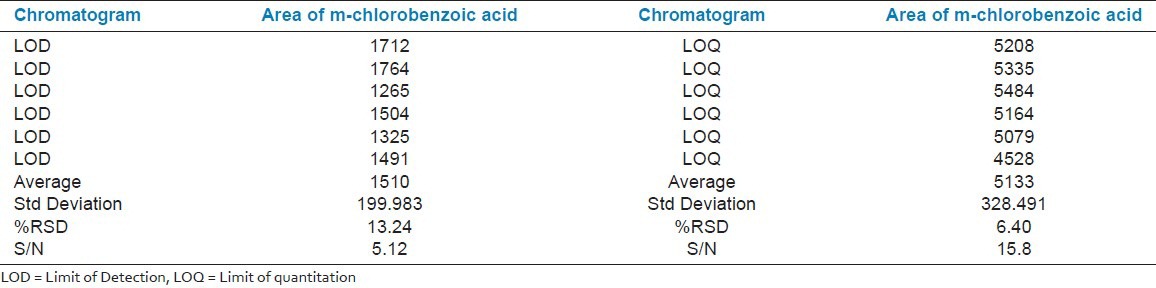
Limit of quantitation
The limit of quantification is determined from the linearity experiment wherein a lower concentration of m-chlorobenzoic acid is analyzed. The LOQ concentration is found to be 1.7 ppm i.e., 0.02% of test concentration for m-chlorobenzoic acid. The RSD for 6 replicate injections of m-chlorobenzoic acid is found to be 6.40%. The signal to noise ratio is above 10. The results are reported in Table 3.
Ruggedness
Ruggedness is performed by estimating the m-chlorobenzoic acid in 3 control samples and 6 spiked samples by 2 different analysts, using different HPLC columns on 2 different days. The % RSD for m-chlorobenzoic acid from these 12 samples is estimated and found to be well within the desired limits.
Robustness
The robustness of the method was tested by making deliberate changes in the chromatographic conditions such as flow rate, temperature, and change in the ratio of mobile phase. It provides an indication of its reliability during normal usage. It was found that the results are comparable with that under normal condition, hence minimizing the errors arising due to these changes. The experiment is carried out at a temperature of 20°C the m-chlorobenzoic acid peak appears at 7.858 minute. When the experiment is carried out at a temperature of 30°C, the m-chlorobenzoic acid peak appears at 7.650 minute. When the experiment is carried out at a flow rate of 0.8 ml/min and 1.2 ml/min, the m-chlorobenzoic acid peak appears at about 9.508 minutes and 6.783 minutes, respectively. The effect of mobile phase composition n-hexane: Ethanol (1000: 45 and 995: 55), then m-chlorobenzoic acid peak appears at about 8.467 minutes and 6.942 minutes, respectively. The results of the study performed showed that there was no difference in retention time found, and separation was also not affected.
CONCLUSIONS
M-chlorobenzoic acid is the degradation product of 1-(3-Chlorophenyl)-1,2-propanedione. During analysis on contact with water 1-(3-Chlorophenyl)-1, 2-propanedione undergoes degradation to give m-chlorobenzoic acid. In the present method, all the impurities of bupropion hydrochloride are well separated from m-chlorobenzoic, i.e., method is selective for determination of content of m-chlorobenzoic acid. In addition, the proposed HPLC method is a simple, precise, accurate, and rapid as evidenced from the validation data, and the method has wider linear dynamic range with good accuracy and precision. Therefore, the method can be used routinely for regular analysis of bupropion for the determination of m-chlorobenzoic acid.
ACKNOWLEDGMENTS
The authors wish to thank Mr. Glenn Saldanha, Chairman and MD, Glenmark Pharmaceuticals Limited for his constant encouragement and the management of the Glenmark Generics Limited for supporting this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.O’Neil JM. 14th ed. USA: Whitehouse Station NJ; 2006. Merck index; p. 246. [Google Scholar]
- 2.Sweetman SC. 36th ed. London: The Pharmaceutical Press; 2009. Martindale - the complete drug reference; pp. 383–5. [Google Scholar]
- 3.Schroeder DH. Metabolism and kinetics of bupropion. J Clin Psychiatry. 1983;44:79–81. [PubMed] [Google Scholar]
- 4.Bryant SG, Guernsey BG, Ingrim NB. Review of bupropion. Clin Pharm. 1983;2:525–37. [PubMed] [Google Scholar]
- 5.Lane RM, Baker GB. Chirality and drugs used in psychiatry: Nice to know or need to know. Cell Mol Neurobiol. 1999;19:355–72. doi: 10.1023/A:1006997731966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinnell HG. Bupropion for smokers, Drug is almost identical in structure to diethylpropion, a controlled drug. BMJ. 2001;322:431–2. [PubMed] [Google Scholar]
- 7.Yeniceli D, Dogrukal AK. The determination of bupropion hydrochloride in pharmaceutical dosage forms by original UV and second derivative UV spectrophotometry, potentiometric and conductometric methods. Turk J Pharm Sci. 2010;7:99–110. [Google Scholar]
- 8.Basavaiah K, Abdulrahman SA. Use of charge transfer complexation reaction for the spectrophotometric determination of bupropion in pharmaceuticals and spiked human urine. Thai J Pharm Sci. 2010;34:134–45. [Google Scholar]
- 9.Abdulaziz MA, Basavaiah K, Vinay KB. Titrimetric and spectrophotometric assay of bupropion hydrochloride in pharmaceuticals using mercury (II) nitrate. Ecletica Quimica. 2010;35:9–16. [Google Scholar]
- 10.Samota S, Garg A, Pandey R. The polarographic reduction and electrode kinetics of antidepressant drug bupropion hydrochloride. Port Electrochim Acta. 2010;28:87–94. [Google Scholar]
- 11.Delazzeri L, Borba SB, Bergold AM. Development and validation of a chromatographic method for the determination of bupropion hydrochloride. Journal of Basic and Applied Pharmaceutical Sciences. 2005;26:211–16. [Google Scholar]
- 12.Zhang DD, Yuan B, Qiao M, Li F. HPLC determination and pharmacokinetics of sustained-release bupropion tablets in dogs. J Pharm Biomed Anal. 2003;33:287–93. doi: 10.1016/s0731-7085(03)00314-5. [DOI] [PubMed] [Google Scholar]
- 13.Loboz KK, Gross AS, Ray J, McLachlan AJ. HPLC assay for bupropion and its major metabolites in human plasma. J Chromatogr B Anal Technol Biomed Life Sci. 2005;823:115–21. doi: 10.1016/j.jchromb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 14.34th revision. Twinbrook Parkway, Rockville, MD: United States Pharmacopeial Convention, Inc; 2011. The United States Pharmacopeia; p. 4918. [Google Scholar]


