Abstract
A rapid, sensitive, selective and validated reverse phase high-performance liquid chromatography (RP-HPLC) method for the estimation of paclitaxel in micro-sample of rat plasma and in culture of cancer cells was performed in this study. The mobile phase consisted of an optimized mixture of methanol:water: trifluroacetic acid (80: 20: 0.1, v/v/v). Column elution at a flow rate of 1 mL/minute with UV detection at 225 nm at room temperature was used. The RP-HPLC method was successfully applied for the determination of paclitaxel in plasma samples and in culture of cancer cells with nano-quantity of estimation. The validation studies were performed in accordance with the International Conference on Harmonization (ICH) guidelines. The intra- and inter-day precision showed that the coefficients of variation ranged from 1.07% to 4.27% at different levels of concentrations. To the best of our knowledge, this study also reported for the first time the optimization of different solvents for effective extraction of paclitaxel wherein tert.-butyl methyl ether (TBME): diethyl ether (DEE) in 50: 50 v/v composition was found most efficient with extraction efficiency ranging between 77.99% and 91.74% and between 76.14 and 93.66% in the plasma and cell culture, respectively. This proposed method was successfully applied to study the pharmacokinetics of paclitaxel and the influence of verapamil and all-trans retinoic acid (atRA) on paclitaxel pharmacokinetics in rat models. This proposed method might emerge as a valuable aid in the laboratory monitoring of paclitaxel in a variety of in vitro as well as in vivo scenarios.
Keywords: high-performance liquid chromatography (HPLC), paclitaxel, extraction optimization, micro-sample rat plasma, plasma profile
INTRODUCTION
Paclitaxel (Fig. 1, Taxol) is a semi-synthetic antineoplastic agent isolated from the Pacific yew tree, Taxus brevifolia, and it acts by stabilizing the microtubules[1]. It is a hydrophobic Biopharmaceutics Classification System (BCS) class IV drug with extremely low water solubility of 4 µg/mL. Paclitaxel is one of the key drugs used against a variety of human tumors such as ovarian cancer, cancer of the breast and the lungs, head and neck cancers and melanoma[2]–[4]. It was found to be a potent drug with significantly high cytotoxicity at concentrations as low as 50 nmol/L[5]. The development of a sensitive assay for the drug is warranted to acquire a comprehensive profile of its pharmacokinetic performance, as it exerts its cytotoxic activity at comparatively lower concentrations (≈50 nmol/L)[4].
Fig. 1. Chemical structure of paclitaxel.
To date, several approaches have been used to develop assays for the determination of paclitaxel in biological fluids. These include capillary electrophoresis[6], chromatography-mass spectrometry (LCMS)[7], immunoassay[8] and high-performance liquid chromatography (HPLC)[9]–[11]. Among these methods, HPLC methods have been most frequently used in the pharmacokinetic and toxicokinetic studies of paclitaxel due to their simplicity and sensitivity. Most of these HPLC methods utilize solid-phase extraction (SPE)[12]–[13], protein precipitation and SPE[14], liquid-liquid extraction (LLE)[10]–[11],[13],[15] or a combination of SPE and solvent extraction[9]. Some methods utilize complex extraction process while others showed inadequate sensitivity or were not validated according to the International Conference on Harmonization (ICH) guidelines[12]–[14].
HPLC methods have been widely used for studies of paclitaxel since the 1980's[11]–[13]. LC-MS was verified to be more responsive and explicit than HPLC-UV, but it is not reasonable to be used at most laboratories due to its high equipment cost. This report describes a simple, robust and rapid RP-HPLC method for routine quantitation of paclitaxel in standard quality control (QC) samples, rat plasma sample as well as in cancer cell lines. This method has been validated for its accuracy, precision, limit of detection (LOD) and limit of quantitation (LOQ) as per ICH guideline. The method is selective, sensitive and suitable for quantitation of paclitaxel in the plasma and cancer cells to support a pharmacokinetics, toxicokinetics and cancer cell uptake study. The validated method has been administered to investigate the systemic exposure of paclitaxel following i.v. administrations in Sprague-Dawley rats.
MATERIALS AND METHODS
Instruments
HPLC quantification was carried out using a HP Agilent 1200 series (Agilent Technologies, CA, USA), containing Agilent Eclipse XDB-C18 column, particle size 5 µm, length 150 mm, equipped with Agilent auto injector and UV-Vis absorbance detector. HP ChemStation software (CA, USA) was used for data analysis.
Chemicals and reagents
Paclitaxel was purchased from ChemieTek®, USA. Trifluoro acetic acid and methanol (all HPLC grade) were obtained from Sigma (Germany) while acetonitrile and HPLC grade water were obtained from Fischer Scientific (UK) and Alfa Aesar (UK), respectively. Deionized water was obtained from an in-house Milli-Q system (Millipore, Milford, MA, USA). All other analytical-grade chemicals and HPLC-grade solvents were obtained from commercial sources.
Chromatographic conditions
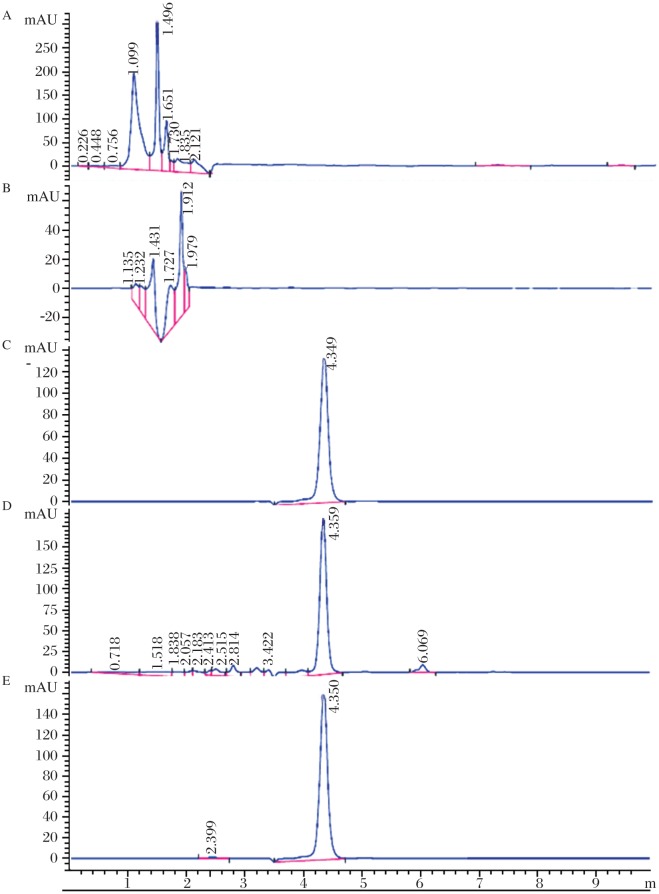
The HPLC column was an Agilent EclipseXDB-C18 column, particle size 5 µm, length 150 mm (Agilent Technologies, CA, USA). The optimized HPLC mobile phase consisted of a mixture of methanol and water (80: 20, v/v) containing trifluoroacetic (0.1% v/v) to maintain the pH≈4.5. Prior to usage, the mobile phase was filtered by passing through a 0.45 µm pore size membrane filter (Millipore, MA, USA). The injection volume was 20 µL and elution was carried out at a flow rate of 1.0 mL/minute, with UV detection at 225 nm. All separations were executed at ambient temperature under all optimized conditions (Fig. 2A-E).
Fig. 2. Representative chromatograms.
A: normal rat plasma; B: MCF-7 cell line extract; C: paclitaxel alone; D: chromatogram showing well separated peak of paclitaxel (Rt = 4.359 minutes) from the components of plasma; E: chromatogram showing well separated peak of paclitaxel (Rt = 4.350 minutes) from the components of cell culture. Chromatographic conditions are described in text.
Preparation of standard solutions
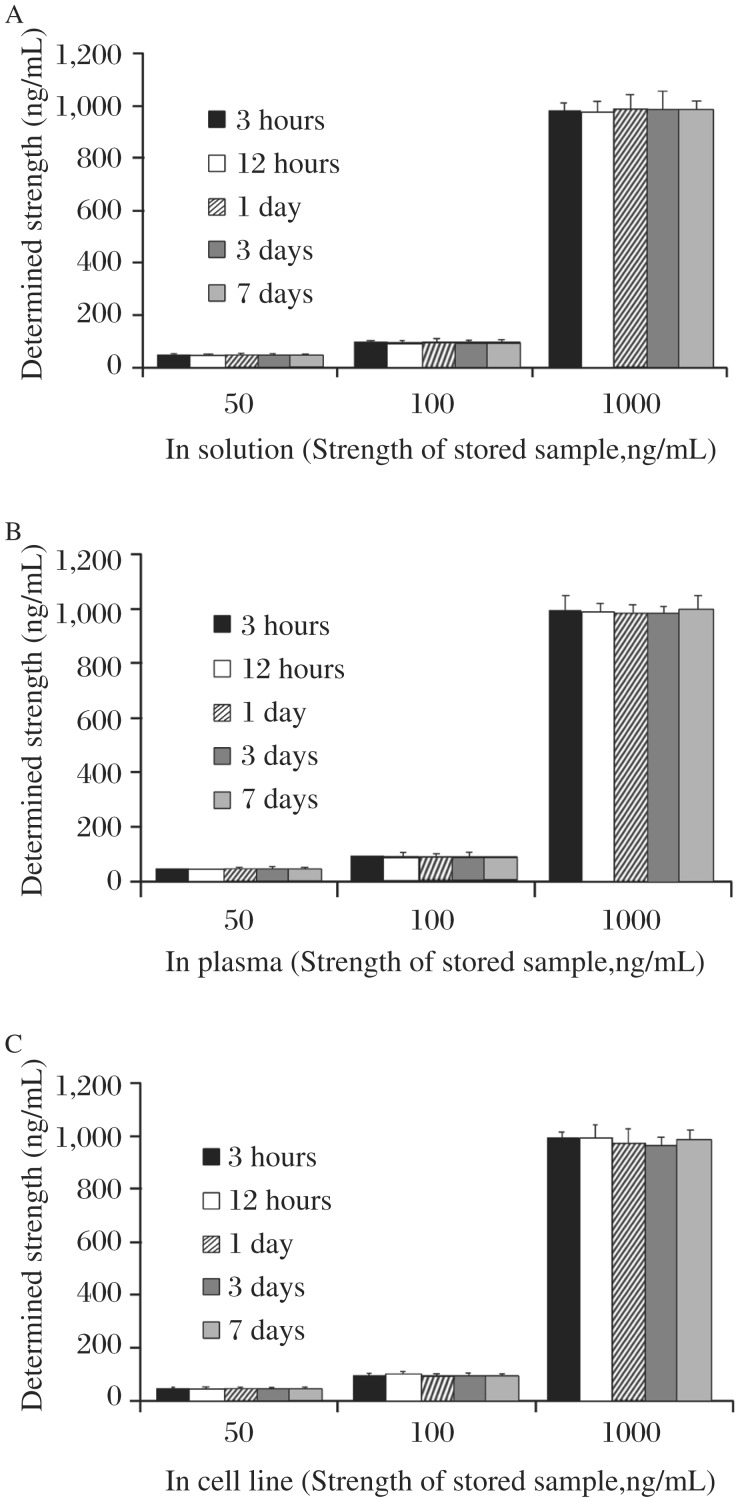
Primary standard stock solution of paclitaxel (100 µg/mL) was prepared by dissolving 10 mg of pure paclitaxel (RS) in methanol (100 mL) and this standard stock solution was further diluted appropriately with the mobile phase to obtain serial working standard solutions. Three standard solutions of 50, 100 and 1,000 ng/mL, respectively, representing low, intermediate and high strength stock were stored in well sealed amber colored volumetric flask to assess solution stability at 4±0.5°C. The samples were found stable in terms of identity, purity, strength and quality (IPQC) (Fig. 3). To assure safer storage condition, all primary/secondary working standard solutions as well as samples were stored in a freezer at -20±2°C (RB Scientific, Hampshire, UK).
Fig. 3. Stability of stock solutions of paclitaxel (A) solution, (B) plasma, (C) cell line stored at 4±0.5°C over a period of 7 days.
Chromatograms obtained by running three concentrations (50, 100 and 1000 ng/mL) on 0.125, 0.5, 1, 3 and 7th day from the preparation stock solution have been compared with those obtained initially. Given values denotes mean determined strength±SD. Recovery and SD are seen to be within statistical limits; hence, the solutions were confirmed stable over a period of 7 days under 4±0.5°C.
Preparation of analytical standard solutions of paclitaxel in spiked plasma and in cancer cell line
The primary standard stock solution as prepared in earlier section was further diluted with an appropriate volume of mobile phase methanol: water: TFA: 80: 20: 0.1, v/v/v to prepare serial calibration solutions ranging between 10 and 2,800 ng/mL. The thawed rat plasma (0.5 mL) was transferred to a centrifuge tube (Greiner Bio-One, UK) containing standard paclitaxel solution (10 to 2800 ng/mL) and 4.5 mL methanol was mixed with each sample for protein precipitation. This mixture was centrifuged for 10 minutes at 1,000 rpm (Z36HK, HERMLE Labortechnik GmbH, Germany), and after decantation, the solutions were injected into HPLC to obtain the chromatogram under conditions mentioned above. Standard dilution was prepared for cancer cell uptake study of paclitaxel in cancer cell line in concentration ranges from 10 to 2,800 ng/mL as used in spiked plasma standard solutions.
Preparation of sample solution
Rat plasma
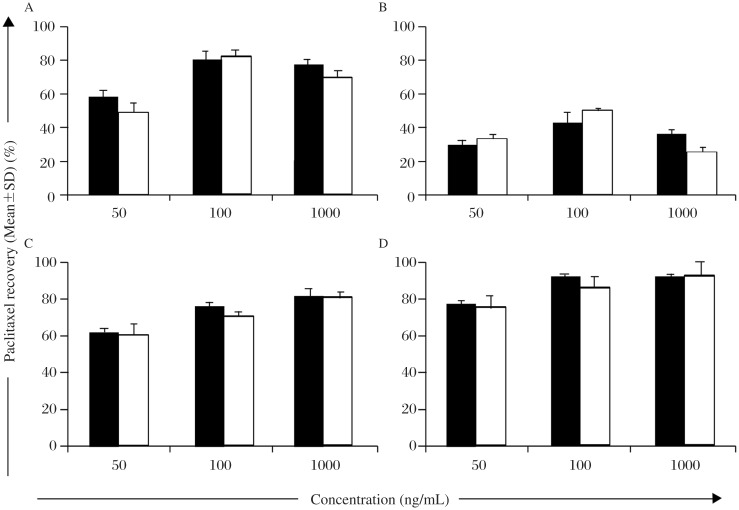
Liquid-liquid extraction method was used for the preparation of sample solution with tert.-butyl methyl ether (TBME):diethyl ether (DEE): 50:50, v/v as an extracting solvent (Fig. 4). All plasma samples and standard QC spiked plasma solutions were aliquoted (0.1 mL) into 1.5 mL microcentrifuge tubes (Sigma, UK) and then mixed with 1 mL TBME: DEE: 50: 50, v/v mixture. The tubes were vortexed in high speed mode (Labnet VX-200, UK) for 1 minute and then centrifuged at 3,000 rpm for 10 minutes at 4°C to separate aqueous and organic layers. The organic layer was transferred into a clean tube and evaporated at ambient temperature under nitrogen condition (Buchi Rotavapor R-215, Switzerland). The residue was reconstituted in 250 µL of HPLC mobile phase (methanol: water: TFA: 85: 15: 0.1, v/v/v) and vortexed for 120 seconds (Labnet VX-200, UK). A portion of the reconstituted sample (20 µL) was injected into the chromatographic system (Agilent Technologies, CA, USA).
Fig. 4. Extraction recovery (%) of paclitaxel in plasma and in cell culture (n=3) using (A) DEE, (B) chloroform, (C) TBME, (D) TBME:DEE (50:50, v/v).
Spiked samples were analyzed at three concentrations in triplicate on 3 consecutive days. The results indicate the mean±SD as calculated from the standard curves generated on the respective days.
Cancer cells
Cancer cells (MCF-7, breast cancer) containing paclitaxel were separated from the culture media and subjected to lysis by freeze thawing in liquid nitrogen (3 cycles) in order to release the amount of paclitaxel uptaken by the cancer cells[16]. After removal of dead cells by centrifugation at 3,000 rpm for 10 minutes, the solution were aliquoted (0.2 mL) into 1.5 mL microcentrifuge tubes (Sigma) and then mixed with 0.5 mL of extraction solution (3X 0.5 mL portions). After vortexing, the samples were centrifuged for approximately 5 minutes at 3,000 rpm. The supernatant was removed and 20 µL was injected in to the chromatographic system.
Validation criteria
Specificity
The specificity is the ability of the analytical method to accurately and specifically measure the analyte of interest in the presence of other components that might be expected to be present in the sample matrix. We assessed the method specificity through the peak purity curves by comparing the chromatograms obtained from drugs alone and of those obtained from the spiked samples.
Linearity
Standard calibration curves were prepared by appropriate dilution of primary stock in order to generate working standard solution of concentrations ranging from 10 to 2,800 ng/mL. The primary plasma standard (100 µg/mL) was further diluted with blank rat plasma to prepare plasma standards of concentrations ranging between 10 to 2,800 ng/mL. These plasma samples were extracted by the liquid-liquid extraction method as stated earlier. Calibration curves of paclitaxel were plotted by taking concentration (ng/mL) and corresponding area under curve (AUC, mAU) at x and y-axis, respectively. Similar protocol was followed for cancer cell culture spiked samples.
Stability of stock solutions
Three standard solutions of 50, 100 and 1,000 ng/mL were arbitrarily selected to assure sample stability under storage conditions. The chosen samples were stored in well sealed amber colored volumetric flasks at 4±0.5°C to study solution stability. The stored samples were monitored for their identity, purity, strength and quality (IPQC) and assayed for determining changes in the standard QC labeled strength of paclitaxel in the presence of plasma as well as cell culture extract during the storage.
Extraction recovery
To determine the extraction efficiency, 20 µL of the different working solutions of paclitaxel of 0.1, 0.5, 1.5, 3, and 14 µg/mL were separately added to 0.2 mL of control plasma to yield concentrations of 10, 50, 150, 300, and 1400 ng/mL, respectively. The samples were subjected to extraction using TBME: DEE: 50: 50 v/v by the method described above. Samples were run in HPLC and peak areas of the extracted samples were compared with non-extracted samples to determine the recovery (%). Similar protocol was followed for cell culture samples. Percentage extraction recovery was calculated [Eqn. 1] and presented as the mean (±SD) of five samples in triplicate (n = 3).
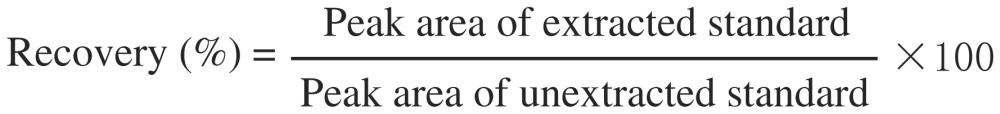 |
[Eqn. 1] |
Accuracy and precision
The magnitude of the deviation (or error) between two measurements was determined by equation [Eqn. 2]. Precision is reported as coefficient of variation (COV) and was determined by analyzing QC samples at five different concentrations within the calibration range in triplicate (n = 3). The accuracy and precision of the analytical procedure were evaluated by determining the intraday and interday COV and percent deviation for a statistically significant number of replicate measurements. The intraday precision of the selected method was estimated by the analysis of five different concentrations of the drug in triplicate on the same day using the same calibration curve. The interday precision was assessed by analyzing samples in the same way as for intraday precision assay, and was repeated for 5 consecutive days. For establishing interday precision, a new calibration curve was constructed every day.
Determination of the limit of detection (LOD) and lower limit of quantitation (LLOQ)
The LOD and LLOQ were measured according to the FDA guidance[9],[17]. The LOD can be defined as the lowest concentration of paclitaxel that this assay can reliably differentiate from background noise (Signal/Noise≥3). The LLOQ was determined by spiking an aliquot of blank rat plasma with paclitaxel at the concentration of the lowest calibrator with a precision of 20% and accuracy of 80%-120%. The LLOQ assay was performed on 5 different days.
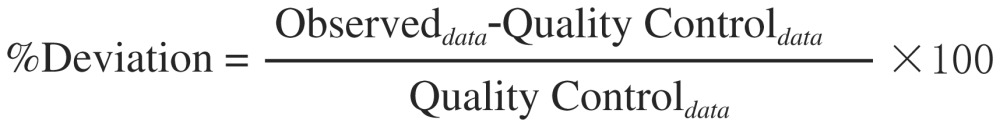 |
[Eqn. 2] |
Method application: Pharmacokinetic drug interaction study
The illustrated method was used to quantitate paclitaxel concentrations in micro sample rat plasma (100 µL) in a pharmacokinetic study to investigate drug interaction of paclitaxel with verapamil[18], cytochrome P450 substrate[19] and P-glycoprotein inhibitor[20]. The effect of atRA, a differentiation inducer, plus mild anticancer agent on paclitaxel pharmacokinetics was assessed. All experiments were performed on Sprague-Dawley rats (male, 200±40 g) as previously described with minor modification[23]. The protocols for animal experiments were approved by the Institutional Animal Ethics Committee (IAEC) of B. R. Nahata College of Pharmacy and BRNSS Contract Research Centre, Mandsaur, India (Regd No: 918/ac/05/CPCSEA, vide Proposal No: 122/PhD/09/IAEC/BRNCP/09-10/Mandsaur). Animals were housed and handled in accordance with the institutional guidelines. The rats were housed in laminar flow cages with temperature at 22±2°C, relative humidity at 50-60% and a 12:12 hours light/dark cycle. The rats were maintained in these facilities for at least 1 week before the experiment. Four rats per plastic cage were housed and allowed to acclimatize in standard conditions for 1 week. The rats were permitted free access to tap water and commercialized food (Jae II Chow, Korea), throughout the experiment.
Rats were divided in 3 groups of 6 each: Group I (the control group) was given paclitaxel with saline at a dose of 10 mg/kg, group II was given paclitaxel (10 mg/kg) to rats pre-treated with verapamil (2 mg/kg) for 2 hours, while group III received paclitaxel (10 mg/kg) pretreated with atRA (at 5 mg/kg twelve hours before paclitaxel treatment). Each rat was anaesthetized with ether and blood sampling (0.25-0.5 mL), performed at 0, 0.25, 1, 2, 4, 6, 8 and 12 hours after i.v. administration. For blood collection, rats anaesthetized with DEE and blood samples (0.25-0.3 mL) were obtained in glass tubes from the retro-orbital sinus (kept frozen at -20±2°C until analysis). The samples were subjected to extraction by method described in section 2.6. Paclitaxel levels in rats were plotted versus time for control as well as the treatment group.
Statistical analysis
All the means were expressed with their standard deviation (mean±SD). An unpaired Student's t-test was used to test significant difference between the controls and paclitaxel treated with verapamil or atRA. The differences were considered to be significant at P < 0.05. All the statistical calculations were performed with Graph Pad Instat Software (Version 3.0, Graph Pad Software, California, USA) using either unpaired t test or one-way ANOVA followed by Tukey-Kramer multiple comparison test. P < 0.05 was considered as extremely significant.
RESULTS
Assay specificity
Assay specificity was established in standard QC solutions as well as in plasma and cancer cell culture samples. Fig. 2 illustrates representative chromatograms of blank rat plasma, blank cell culture, plasma and cell line spiked with pure paclitaxel solutions. Plasma as well as cell line components were eluted well before 2.5 minutes (Fig. 2a and b) while paclitaxel was eluted at 4.349 minutes with high peak purity (Fig. 2c). No significant interference was observed at or near the retention times of paclitaxel. All the obtained chromatograms were satisfactory in peak shape, sharpness and separation from solvent front. Plasma as well as cell culture spiking showed no interference with the elution of paclitaxel as evinced from practically the same retention times of 4.359 and 4.35 minutes in case of plasma and cell culture spiked samples, respectively (Fig. 2d-e). This retention time (Rt) of paclitaxel observed with this method was significantly lower than that previously reported (8.5[9], 10[10], 10.5[24] and 7.7 minutes[25]).
Linearity
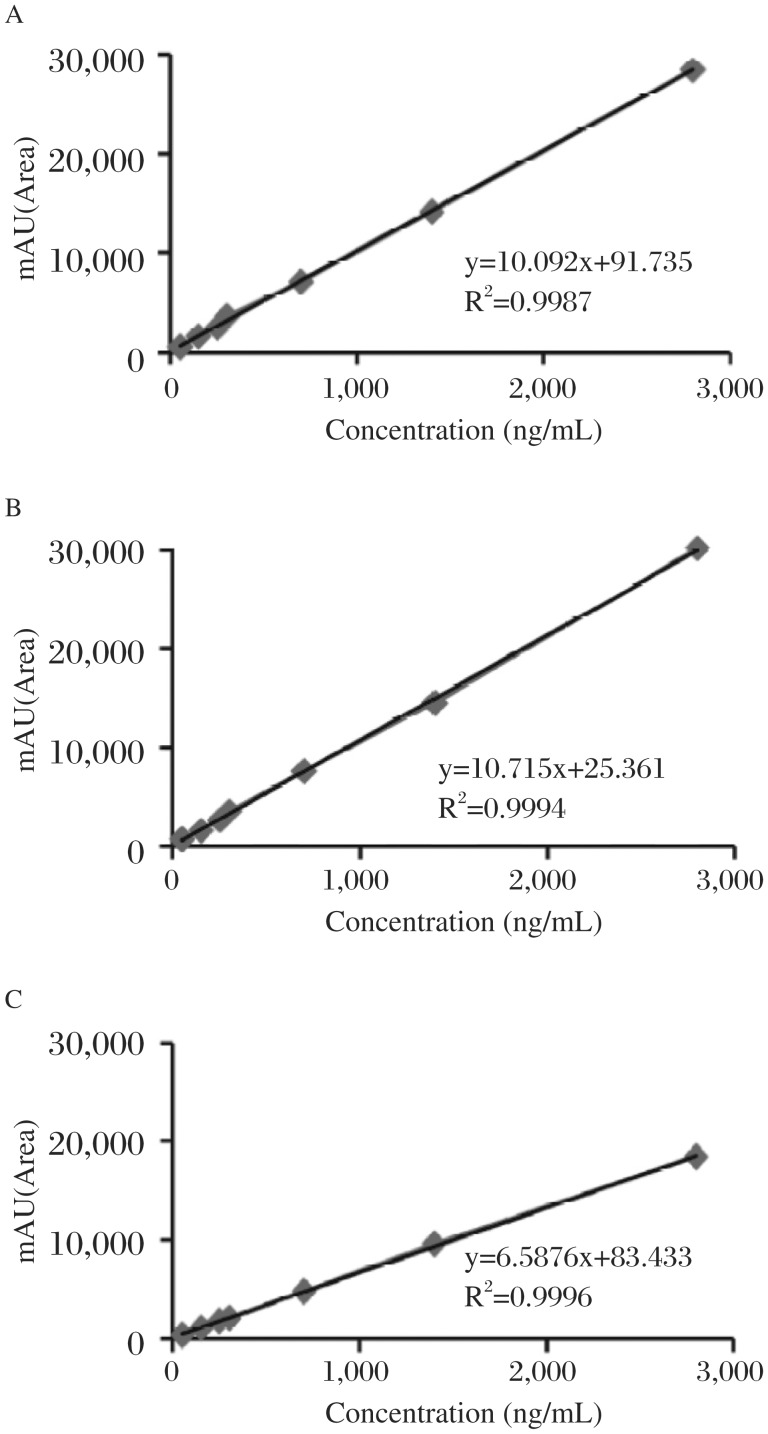
The linearity of curve in solution was in the concentration range from 25 to 2800 ng/mL with a typical linearity line equation of y=10.715x+25.361 and an average correlation coefficient (r2) of 0.9994. In plasma and cell line culture, linearity of calibration curves was found in the same range. The typical straight line equations for plasma and cell culture spiked samples were y=10.092x+91.735 (r2=0.9987) and y=6.5876x+83.433 (r2=0.996), respectively (n = 5) (Table 1, Fig. 5).
Table 1. Linear regression and statistical parameters of paclitaxel from rats plasma and in cell line culture (n = 5).
| Linearity range (ng/mL) | Regression data |
Sa | Sb | LOD (ng/mL) | LLOQ (ng/mL) | |||
| A | b | r2 | ||||||
| Solution | 25-2800 | 10.715 | 25.361 | 0.9994 | 1.107 | 1.387 | 10 | 45 |
| Plasma | 50-2800 | 10.092 | 91.735 | 0.9996 | 0.987 | 2.193 | 10 | 45 |
| Cell culture | 50-2800 | 6.5876 | 83.433 | 0.9996 | 1.023 | 1.543 | 15 | 50 |
a= Slope; b= Intercept; r2=Correlation coefficient; Sa =Standard deviation of intercept; Sb =Standard deviation of slope; LOD = Limit of detection; LLOQ= Lower limit of quantitation. Each standard curve was generated on 3 consecutive days distributed evenly across the linearity range. Values are reported as mean±SD of three calibration curves.
Fig. 5. Calibration curves of paclitaxel in (A) solution (B) plasma (C) MCF-7 cell culture.
Stability of stock solutions
The stability profiles of the standard solutions containing paclitaxel as naked solution as well as in plasma/cell line spiked samples are shown in Fig. 3. The investigated solutions were stable at 4±0.5°C throughout the observation period and % recovery was within the statistical limits. No notable change was observed in the standard QC labeled strength of the drugs in the presence of plasma as well as cell line extract during the storage. All the standard and working solutions were stored in freezer at -20±2°C (RB Scientific, Hampshire, UK) to maintain safer storage circumstances.
Extraction Recovery
The recovery of paclitaxel from plasma or cultured cells was determined by comparing peak heights of extracted samples with those in corresponding standard QC label claims. For recovery of paclitaxel from samples, solvents viz DCM, acetonitrile, DEE and TBME were investigated on one platform. Among DEE, TBME and chloroform, solvent extraction of paclitaxel as plasma spiked solution (low strength, 50 ng/mL), TBME resulted in most effective extraction of 63.04% as against 30.57% and 58.94% in case of chloroform and DEE, respectively. DEE and acetonitrile both resulted in noisy baseline and incomplete extraction. However, during general laboratory trials (data not shown), addition of DEE to TBME mediated extraction was found to influence paclitaxel extraction to a significant level, and hence combinations of TBME and DEE were also optimized for paclitaxel recovery. The TBME: DEE in 50: 50 (v/v) combination was found to be most efficiently recovering paclitaxel by 77.99% and 76.14% in plasma and cell culture, respectively. Analogous observations were made at intermediate (100 ng/mL) and high strength (1000 ng/mL) extractions in plasma as well as cell culture samples (Fig. 4).
Extraction efficiency of this optimized extraction milieu was also studied for paclitaxel extraction from standard spiked solutions (50 to 1400 ng/mL), wherein mean extraction efficiency was found to be 88.26±1.98% and 86.76±2.56% in plasma and cancer cell culture spiked samples, respectively (n = 5) (Table 2).
Table 2. Extraction recovery (%) of paclitaxel in plasma and in cell culture (n = 3).
| Concentration (ng/mL) | Paclitaxel recovery (mean±SD) (%) |
||||||
| Plasma |
Cell culture |
||||||
| Recovery (%) | COV | Recovery (%) | COV | ||||
| 50 | 88.34±1.05 | 1.18 | 83.36±4.44 | 5.32 | |||
| 150 | 87.66±2.59 | 2.95 | 87.26±2.15 | 2.46 | |||
| 300 | 86.77±0.97 | 1.11 | 87.76±1.12 | 1.27 | |||
| 1400 | 90.26±4.78 | 5.29 | 88.66±0.97 | 1.09 | |||
| Mean | 88.26±1.98 | 86.76±2.56 | |||||
Recovery is expressed as mean±SD of five individual investigations (n = 5). SD, standard deviation; COV, coefficient of variation.
Precision and accuracy
The accuracy and precision of the assay for both plasma and culture were within acceptable limits. The between- and within-run accuracies for plasma and cell culture QC sample were between 92.1% to 115% and 89.2% to 107.1%, respectively. For all tested samples, the COV's of plasma and culture were between 1.48% to 7.36% and 0.96% to 4.27% in cases of intraday and intraday analysis in plasma and cell culture samples, respectively. The results deviated between -1.36 to 9.38% and -1.04 to +8.57% in cases of intraday and interday analysis in plasma and cell culture samples, respectively. The explicit inter- and intra-assay precision data are shown in Table 3, wherein the coefficients of variations (COVs, %) were less than 6.9% over the concentration range from 50-1400 ng/mL.
Table 3. Precision and accuracy of HPLC assay for paclitaxel from rat plasma and in cell line culture.
| Spiked conc. (ng/mL) | Precision and accuracy data of paclitaxel (mean±SD) (%) |
||||||
| Plasma |
Cell Culture |
||||||
| Measured conc.(ng/mL) | COV | %Deviation | Measured conc. (ng/mL) | COV | %Deviation | ||
| Intra-day (n = 3) | |||||||
| 50 | 49.35±0.73 | 1.48 | -1.36 | 48.24±0.52 | 1.07 | -3.52 | |
| 100 | 98.19±2.58 | 2.60 | -1.81 | 106.21±1.98 | 1.86 | +6.21 | |
| 300 | 288.71±11.21 | 3.88 | -3.74 | 284.16±8.91 | 3.13 | -5.28 | |
| 700 | 723.28±21.17 | 2.92 | +3.32 | 671.65±19.65 | 2.92 | -4.05 | |
| 1,400 | 1356.11±39.2 | 2.89 | -3.13 | 1313.92±45.52 | 3.46 | -6.14 | |
| Inter day (n = 3) | |||||||
| 50 | 48.95±0.94 | 1.92 | -2.15 | 48.55±0.47 | 0.96 | -2.91 | |
| 100 | 97.12±2.27 | 2.33 | -2.88 | 108.57±4.25 | 3.91 | +8.57 | |
| 300 | 282.71±10.11 | 3.57 | -5.76 | 296.18±7.41 | 2.50 | -1.27 | |
| 700 | 634.28±46.59 | 7.36 | -9.38 | 692.69±29.59 | 4.27 | -1.04 | |
| 1,400 | 1374.13±36.2 | 2.64 | -1.84 | 1389.94±35.26 | 2.53 | -0.71 | |
Results are represented as mean±SD (n = 3). Accuracy and precision were determined with standard quality control samples, which were analyzed on 3 consecutive days. For intraday determinations, 3 standard curves were prepared on the same day, while for interday determinations, 3 standard curves were generated on three consecutive days. The accuracy is represented by %Deviation and precision by %COV.
Limit of detection and limit of quantification
The LOD for paclitaxel in plasma was 10 ng/mL (signal-to-noise of 3). The LOQ is the lowest concentration that can be measured accurately within precision. The LOQ for paclitaxel in plasma was 45 ng/mL. In culture of cancer cell line, the LOD and LOQ were 15 ng/mL and 50 ng/mL, respectively (n = 5).
Method application
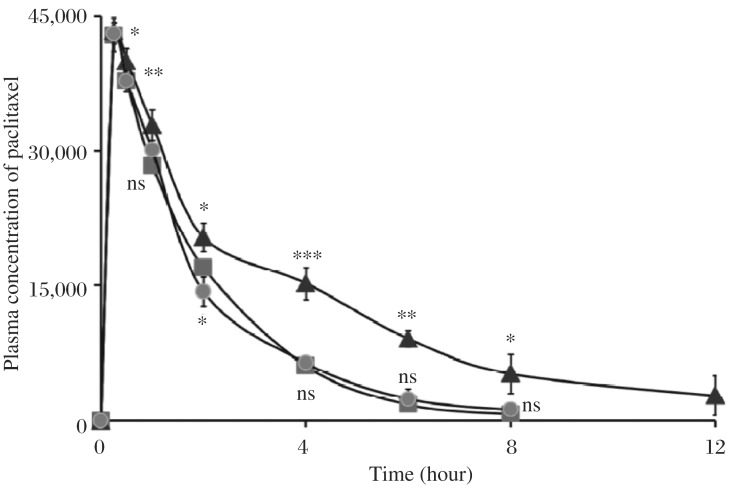
The applicability of the validated method was demonstrated in the pharmacokinetic drug interaction study of paclitaxel with verapamil and atRA in male Sprague-Dawley rats. The assay was sensitive enough to measure the plasma drug level after i.v. administration of 10 mg/kg paclitaxel. The observed plasma concentration-time profile of paclitaxel is shown in Fig. 6.
Fig. 6. Mean plasma concentration-time profiles of paclitaxel (20 mg/kg) following i.v. administration in Sprague-Dawley rats (200±40 g).
(-▪-▪-) control; (-▴-▴-) virapamil (a known substrate of CYP 450 3A and inhibitor of P-gp) pretreated group, (-•-•-) atRA pretreated rats. Each point represents the mean±SD (n = 6).
After the administration of paclitaxel to verapamil pre-treated rats, the AUC of paclitaxel was observed to be significantly higher than that of the control (P < 0.05). The plasma concentration profiles of paclitaxel after i.v injection of paclitaxel as well as a conclusive account of the pharmacokinetic parameters is shown in Table 4. The paclitaxel injection in efflux pump blocker (VPML) pretreated rats resulted in a Cmax of 43327±1017 ng/mL, which was significantly higher (P < 0.05) as compared to paclitaxel treatment in controls (42875±1922 ng/mL) and atRA pretreated rats (43072±1237ng/mL) determined 15 minutes post injection. After 4 hours of injection, paclitaxel levels exhibited by in efflux pump blocker pretreated rats was higher than those in atRA pretreated as well as control paclitaxel-treated rats by a factor of 138.30% and 150.17%, respectively. Similar trend was observed 6 and 8 hours post injection. It should be noted that 12 hours after injection, paclitaxel was not detected in rats given paclitaxel alone as well as paclitaxel to atRA pretreated rats; however, plasma level at 2802±853 was found in cases when paclitaxel was given to rats pretreated with the efflux pump blocker (Table 4, Fig. 6).
Table 4. Blood plasma levels and pharmacokinetic parameters of paclitaxel following i.v administration in Spra-gue-Dawley rats.
| Time(hour) | Blood plasma level (ng/mL) |
||
| Paclitaxel alone | Efflux pump inhibitor → paclitaxel | atRA → paclitaxel | |
| 0.25 | 42,875±1,922 | 43,327±1,017 | 43,072±1,237 |
| 0.5 | 37,756±1,219 | 39,975±1,429 | 37,756±602 |
| 1 | 28,363±836 | 32,874±1,761 | 30,078±1,576 |
| 2 | 17,041±875 | 20,387±1,635 | 14,358±1,415 |
| 4 | 6,103±786 | 15,268±811 | 6,407±752 |
| 6 | 1,824±232 | 9,127±916 | 2,456±897 |
| 8 | 736±168 | 5,222±1,038 | 1,247±72 |
| 12 | 2,802±853 | ||
| Pharmacokinetic parameters | |||
| Cmax (ng/mL) | 42,875±1,905 | 43,327±1,017 | 43,072±1,237 |
| Kelim (hour-1) | 0.524±0.016 | 0.273±0.011 | 0.457±0.005 |
| t1/2 (hour) | 1.32±0.025 | 2.54±0.102*** | 1.52±0.075 |
Pharmacokinetic parameters in male Sprague-Dawley rats (200±40 g) following i.v. administration. An unpaired Student's t-test was used to detect significant difference between the naked and corresponding dendrimeric formulations. The differences were considered to be extremely significant at P < 0.001 (***). All the statistical examinations were performed with Graph Pad Instat Software (Version 3.0, Graph Pad Software, California, USA) using either unpaired t test or one-way ANOVA followed by Tukey-Kramer multiple comparison test. Difference P < 0.001 was considered as extremely significant. Cmax, t1/2, and Kelim refer to maximum concentration, half life and elimination constant, respectively.
Results are represented as mean±S.D (n = 3)
The t1/2 of paclitaxel with verapamil was also prolonged significantly compared to the control. The half life of naked paclitaxel injection was 1.32±0.025 hours, while upon administration of paclitaxel in atRA and efflux pump blocker, its half life was 1.52±0.075 and 2.54±0.102 hours, respectively. This infers that the half life of paclitaxel extends in case when it was given after treatment of efflux pump blocker, which was almost 92.42±2.35% higher compared to paclitaxel alone treated rats (P < 0.01) (Table 4).
DISCUSSION
The results of our studies are also consistent with these reported by Choi and Kim (2003) and Choi and Lee (2004) in which calcium channel blockers such as diltiazem and nifedipine increased the bioavailability of doxorubicin and vincristine[26]–[27]. Paclitaxel has been reported to be metabolized by cytochrome P-450[28], and in another report, p-glycoprotein efflux pump also inhibits the retention of paclitaxel[29]. This effect of verapamil to extend the plasma profile of paclitaxel is ascribed to its cytochrome p-450 inhibition as well as efflux pump blocking ability. This report is also concordant with the outcome of an investigation wherein the AUC of doxorubicin in plasma was considerably increased by pretreatment of verapamil in mouse models[30]. In a parallel experimentation, we tried to explore the possible interaction between paclitaxel and atRA for the first time. We clearly ruled out any pharmacokinetic drug interaction between paclitaxel and atRA as evinced from insignificant change in plasma profile of paclitaxel in atRA pretreated rats as compared to control (P > 0.05). In addition to outcomes observed in rat model, supplementary data are warranted in human subjects. These results are additionally needed to be confirmed in a clinical setting, wherein the paclitaxel dose should have to be adjusted when it is given concomitantly with verapamil. Our work defining pharmacokinetic interactions of paclitaxel with multi drug resistance (MDR) modulators is also sought to further establish interactions of other MDR modulators with paclitaxel as well as a variety of other leading cytotoxic drugs like etoposide, doxorubicin, docetaxel etc[31]–[32].
In summary, a reproducible, accurate and precise isocratic HPLC assay was developed and applied to quantify paclitaxel in rat plasma and cell culture samples. The method utilizes single stage extraction with tert-butyl methyl ether and diethyl ether at 50: 50 (v/v) compositions, which was found even better than using TBME and DEE individually as extraction media. This assay provided excellent extraction recovery, sensitivity, accuracy and precision, with relatively shorter assay run time. The HPLC method was successfully applied to quantify total paclitaxel in a pharmacokinetic study in rats and culture samples of cell line. The method was selective, sensitive and suitable for quantitation of paclitaxel during release profiling, cancer cell line uptake study, pharmacokinetic disposition and toxicokinetics. This method is promising as a valuable aid in the laboratory monitoring of the toxicity of anticancer therapy of paclitaxel[33]–[37]. It is anticipated that this method will be of significant aid while developing nanotech based therapeutic approaches to deliver paclitaxel[38]–[42]. This method may prove to be of value in all allied investigations involving laboratory monitoring of paclitaxel.
Footnotes
This work was supported by Senior Research Fellowship and Commonwealth Split Site Fellowship awards from the Council for Scientific Industrial Research (CSIR), New Delhi (India) and the Association of Commonwealth Universities, UK, respectively.
References
- 1.Wang F, Chen Y, Zhang D, Zhang Q, Zheng D, Hao L, et al. Folate-mediated targeted and intracellular delivery of paclitaxel using a novel deoxycholic acid-O-carboxymethylated chitosan-folic acid micelles. Int J Nanomed. 2012;7:325–37. doi: 10.2147/IJN.S27823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donehower RC, Rowinsky EK. An overview of experience with TAXOL (paclitaxel) in the U.S.A. Can Treat Rev. 1993;19:63–78. doi: 10.1016/0305-7372(93)90049-w. [DOI] [PubMed] [Google Scholar]
- 3.Yared JA, Tkaczuk KH. Update on taxane development: new analogs and new formulations. Drug Des Devel Ther. 2012;6:371–84. doi: 10.2147/DDDT.S28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohler DR, Goldspiel BR. Paclitaxel (taxol) Pharmacother. 1994;14:3–34. doi: 10.1002/j.1875-9114.1994.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 5.Liebmann JE, Cook JA, Lipschultz C, Teague D, Fisher J, Mitchell JB. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. J Can. 1993;68:1104–9. doi: 10.1038/bjc.1993.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hempel G, Lehmkuhl D, Krumpelmann S, Blaschke G, Boos J. Determination of paclitaxel in biological fluids by micellar electrokinetic chromatography. J Chromatogr A. 1996;745:173–9. doi: 10.1016/0021-9673(96)00351-2. [DOI] [PubMed] [Google Scholar]
- 7.Parise RA, Ramanathan RK, Zamboni WC, Egorin MJ. Sensitive liquid chromatography-mass spectrometry assay for quantitation of docetaxel and paclitaxel in human plasma. J Chromatogr B. 2003;5:231–6. doi: 10.1016/s1570-0232(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 8.Leu JG, Chen BX, Schiff PB, Erlanger BF. Characterization of polyclonal and monoclonal anti-taxol antibodies and measurement of taxol in serum. Can Res. 1993;53:1388–1391. [PubMed] [Google Scholar]
- 9.Wang LZ, Ho PC, Lee HS, Vaddi HK, Chan YW, Yung CS. Quantitation of paclitaxel in micro-sample rat plasma by a sensitive reversed-phase HPLC assay. J Pharm Biomed Anal. 2003;31:283–9. doi: 10.1016/s0731-7085(02)00611-8. [DOI] [PubMed] [Google Scholar]
- 10.Coudore F, Authier N, Guillaume D, Beal A, Duroux E, Fialip JJ. High-performance liquid chromatographic determination of paclitaxel in rat serum: application to a toxicokinetic study. J Chromatogr B. 1999;721:317–20. doi: 10.1016/s0378-4347(98)00465-4. [DOI] [PubMed] [Google Scholar]
- 11.Watchueng J, Kamnaing P, Gao JM, Kiyota T, Yeboah F, Konishi Y. Efficient purification of paclitaxel from yews using high-performance displacement chromatography technique. J Chromatogr A. 2011;20:2929–35. doi: 10.1016/j.chroma.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Hynning PA, Anderson P, Bondesson U, Boreus LO. Liquid chromatographic quantitation compared with gas chromatographic mass spectrophotometric determination of verapamil and nor verapamil in plasma. Clin Chem. 1998;34:2502–3. [PubMed] [Google Scholar]
- 13.Gaspar JR, Qu J, Straubinger NL, Straubinger RM. Highly selective and sensitive assay for paclitaxel accumulation by tumor cells based on selective solid phase extraction and micro-flow liquid chromatography coupled to mass spectrometry. Analyst. 2008;133:1742–8. doi: 10.1039/b806856a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grem JL, Tutsch KD, Simon KJ, Alberti D, Willson JK, Tormey DC, et al. Phase I study of taxol administered as a short i.v. infusion daily for 5 days. Can Treat Rep. 1987;71:1179–84. [PubMed] [Google Scholar]
- 15.Hendrikx JJ, Hillebrand MJ, Thijssen B, Rosing H, Schinkel AH, Schellens JH, et al. A sensitive combined assay for the quantification of paclitaxel, docetaxel and ritonavir in human plasma using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B. 2011;15:2984–90. doi: 10.1016/j.jchromb.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 16.Shorrocks J, Paul ND, McMillan TJ. The dose rate of UVA treatment influences the cellular response of HaCaT keratinocytes. J Invest Dermatol. 2008;128:685–93. doi: 10.1038/sj.jid.5701037. [DOI] [PubMed] [Google Scholar]
- 17. http://www.fda.gov/cder/guidance/index.htm.
- 18.Leveque D, Jehl F. P-glycoprotein and pharmacokinetics. Antican Res. 1995;15:331–336. [PubMed] [Google Scholar]
- 19.Benet LZ, Wu CY, Hebert MF, Wacher VJ. Intestinal drug metabolism and antitransport processes: A potential paradigm shift in oral drug delivery. J Cont Rel. 1996;39:139–43. [Google Scholar]
- 20.Sikic BI, Fisher GA, Joanne Halsey BL, Beketic-Oreskovic L, Chen G. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Can Chem Pharmacol. 1997;40(S):13–9. doi: 10.1007/s002800051055. [DOI] [PubMed] [Google Scholar]
- 21.Fenaux P, Chevret S, Botton S. Treatment of older adults with acute promyelocytic leukaemia. Best Pract Res Cli Haem. 2003;16:495–501. doi: 10.1016/s1521-6926(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 22.Cassinat B, Chevret S, Zassadowski F. In vitro all-trans retinoic acid sensitivity of acute promyelocytic leukemia blasts: a novel indicator of poor patient outcome. Blood. 2001;98:2862–4. doi: 10.1182/blood.v98.9.2862. [DOI] [PubMed] [Google Scholar]
- 23.Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK, et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 2007;338:317–26. doi: 10.1016/j.ijpharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Mittal A, Chitkara D, Kumar N. HPLC method for the determination of carboplatin and paclitaxel with. cremophorEL in an amphiphilic polymer matrix. J Chromatogr B. 2007;855:211–9. doi: 10.1016/j.jchromb.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Martin N, Catalin J, Blachon MF, Durand A. Assay of paclitaxel (Taxol) in plasma and urine by high-performance liquid chromatography. J Chromatogr B. 1998;709:281–8. doi: 10.1016/s0378-4347(98)00060-7. [DOI] [PubMed] [Google Scholar]
- 26.Choi JS, Jo BW, Kim YC. Enhanced paclitaxel bioavailability after oral administration of paclitaxel or prodrug to rats pretreated with quercetin. Eur J Pharm Biopharm. 2004;57:313–8. doi: 10.1016/j.ejpb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Woo JS, Lee CH, Shim CK, Hwang SJ. Enhanced oral bioavailability of paclitaxel by coadministration of the P-glycoprotein inhibitor KR30031. Pharm Res. 2003;20:24–30. doi: 10.1023/a:1022286422439. [DOI] [PubMed] [Google Scholar]
- 28.Cresteil T, Monsarrat B, Alvinerie P, Treluyer JM, Vieira I, Wright M. Taxol metabolism by human liver microsomes: identification of cytochrome P450 isozymes involved in its biotransformation. Can Res. 1994;54:386–92. [PubMed] [Google Scholar]
- 29.Sparreboom A, Van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci USA. 1997;94:2031–5. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Candussio L, Decorti G, Crivellato E, Granzotto M, Rosati A, Giraldi T, et al. Toxicologic and pharmacokinetic study of low doses of verapamil combined with doxorubicin. Life Sci. 2002;71:3109–19. doi: 10.1016/s0024-3205(02)02175-6. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett NL, Lum BL, Fisher GA, Brophy NA, Ehsan MN, Halsey J, et al. Phase I trial of doxorubicin with cyclosporine as a modulator of multidrug resistance. J Clin Oncol. 1994;12:835–42. doi: 10.1200/JCO.1994.12.4.835. [DOI] [PubMed] [Google Scholar]
- 32.Lum BL, Kaubisch S, Yahanda AM, Adler KM, Jew L, Ehsan MN, et al. Alteration of etoposide pharmacokinetics and pharmacodynamics by cyclosporine in a phase I trial to modulate multidrug resistance. J Clin Oncol. 1992;10:1635–42. doi: 10.1200/JCO.1992.10.10.1635. [DOI] [PubMed] [Google Scholar]
- 33.Tekade RK, Dutta T, Tyagi A, Bharti AC, Das BC, Jain NK. Surface-engineered dendrimers for dual drug delivery: a receptor up-regulation and enhanced cancer targeting strategy. J Drug Target. 2008;16:758–72. doi: 10.1080/10611860802473154. [DOI] [PubMed] [Google Scholar]
- 34.Tekade RK, Vijayarajkumar P, Jain NK. Dendrimers in oncology: An expanding horizon. Chemical Reviews. 2009;109:49–87. doi: 10.1021/cr068212n. [DOI] [PubMed] [Google Scholar]
- 35.Dhakad RS, Tekade RK, Jain NK. Cancer Targeting Potential of Folate Targeted Nanocarrier under Comparative Influence of Tretinoin and Dexamethasone. Current Drug Delivery. 2012;10 doi: 10.2174/1567201811310040012. [DOI] [PubMed] [Google Scholar]
- 36.Tekade RK, Dutta T, Gajbhiye V, Jain NK. Exploring Dendrimers towards Dual-Drug Delivery: pH Responsive Simultaneous Kinetics. J Microencapsulation. 2009;26:287–96. doi: 10.1080/02652040802312572. [DOI] [PubMed] [Google Scholar]
- 37.Dwivedi P, Tekade RK, Jain NK. Nanoparticulate Carrier Mediated Intranasal Delivery of Insulin for the Restoration of Memory Signaling in Alzheimer's Disease. Current Nanoscience. 2013;9:46–55. [Google Scholar]
- 38.Thakur S, Tekade RK, Kesharwaniet P, Jain NK. The effect of polyethylene glycol spacer chain length on the tumor-targeting potential of folate-modified PPI dendrimers. J Nanoparticle Research. 2013;15:1625. [Google Scholar]
- 39.Maheshwari RGS, Tekade RK, Sharma PA, Darwhekar G, Tyagy A, Patel RP, et al. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: A comparative assessment. Saudi Pharmaceutical J. 2012;20:161–70. doi: 10.1016/j.jsps.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kesharwani P, Tekade RK, Gajbhiye V, Jain NK. Cancer targeting potential of some ligand-anchored poly(propylene imine) dendrimers: a comparison. Nanomedicine. 2011;7:295–304. doi: 10.1016/j.nano.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Tekade RK, Dutta T, Gajbhiye V, Jain N, Jain NK. Simultaneous Spectrophotometric Estimation of Methotrexate and All trans Retinoic acid in Mixture. Asian J Chemistry. 2009;21:5145–50. [Google Scholar]
- 42.Prajapati RN, Tekade RK, Gupta U, Gajbhiye V, Jain NK. Dendimer-Mediated Solubilization, Formulation Development and in Vitro-in Vivo Assessment of Piroxicam. Molecular Pharmaceutics. 2009;6:940–50. doi: 10.1021/mp8002489. [DOI] [PubMed] [Google Scholar]