Abstract
Mycobacterium tuberculosis 6-kDa early secretory antigenic target (ESAT-6) is a dominant target antigen for cell-mediated immunity in the early phase of tuberculosis. The fms-like tyrosine kinase 3 ligand (FL) that induces potent immune response has been used as an adjuvant in vaccine development. In this study, a new recombinant plasmid (pIRES-epitope-peptides-FL) encoding three T cell epitopes of ESAT-6 and FL was constructed, and the immunogenicity of the DNA vaccine was assessed in C57BL/6 mice immunized with the plasmid DNA vaccine. Additionally, a strategy of intramuscular injection with the DNA vaccine (prime) and intranasal administration of the epitope peptides (boost) was employed to induce higher immune reaction of the mice. The results showed that mice vaccinated with the recombinant plasmid DNA vaccine and boosted with the peptides not only increased the levels of Th1 cytokines (IFN-γ and IL-12), the number of IFN-γ+ T cells and activities of cytotoxic T lymphocytes as well as IgG, but also enhanced protection against Mycobacterium tuberculosis challenge. In conclusion, these data indicate that the novel recombinant pIRES-epitope-peptides-FL plasmid is a useful DNA vaccine for preventing Mycobacterium tuberculosis infection.
Keywords: early secretory antigenic target-6 (ESAT-6), fms-like tyrosine kinase 3 ligand (FL), Mycobacterium tuberculosis, recombinant plasmid, T cell epitopes
INTRODUCTION
Tuberculosis is a re-emerging disease that represents a major public health problem[1]. The World Health Organization (WHO) estimates that tuberculosis causes three million deaths and over eight million new cases annually[2],[3]. At present, the only available tuberculosis vaccine is the attenuated strain of Mycobacterium (M.) bovis bacillus Calmette-Guérin (BCG). Although effective in preventing M. tuberculosis infection in newborns and toddlers, BCG provides poor protection in adults with variable efficacies between 0 to 80%[4]. Thus, more efficient vaccines and vaccination strategies against tuberculosis are urgently needed.
In order to control M. tuberculosis infection, numerous efforts have been made in preparing new vaccines, including DNA and subunit protein vaccines[5],[6]. Reportedly, M. tuberculosis 6-kDa early secretory antigenic target (ESAT-6) has been evaluated as DNA vaccines in several models[7]–[9]. Previous studies have demonstrated that ESAT-6 contains antigen epitopes recognized by T and B cells in patients and experimental animals with active tuberculosis[6],[10]–[14]. Besides T cell responses, enhanced antibody response against ESAT-6 has also been displayed in M. tuberculosis-infected individuals[12]–[14]. Hence, many investigators have shown great interests in exploring the roles of ESAT-6. However, comparative genomics reveal that ESAT-6 is only located in virulent M. tuberculosis[15],[16], and it may also contribute to cellular invasion, phagolysosome escape and M. tuberculosis dissemination as a cytolytic toxin[17]–[22]. Therefore, when a new tuberculosis vaccine is developed, it is important to avoid adverse reaction.
As we know, vaccines based on T cell antigen epitopes can result in effective immune reactions because these epitopes have highly conservative sequences and relatively higher safety[23]–[25]. Moreover, experiments on mucosal immunization have displayed that antigen-specific T cells in the mucosa play a key role in robust immune protection[26]–[27]. Given that strategies involved in prime-boost have achieved variable successes against M. tuberculosis infections[28]–[30], immunization of mice with DNA vaccines containing T cell epitopes and vaccinated with prime-boost strategy may be an ideal approach to induce effective protection.
Many studies have demonstrated that DNA vaccines encoding single or multi-T cell epitopes could obviously induce potent T cell responses[23]–[25], and combination of DNA vaccines and some adjuvants could enhance their immunogenicity, including elevating cell-mediated immunity (CMI)[31]. The fms-like tyrosine kinase 3 ligand (FL) is a growth factor that influences the development of multiple hematopoietic lineages[31]. FL has been found to promote the growth of T cells, B cells and dendritic cells (DCs)[32],[33] and augment immuno-stimulatory responses to some antigens[34]. Therefore, co-delivery of FL for DNA vaccine may be a feasible design.
To prepare a novel and effective recombinant DNA vaccine, in the current study, the gene fragments encoding the three T cell epitopes of M. tuberculosis ESAT-6 were selected and cloned into a pIRES plasmid together with the FL gene (pIRES-epitope-peptides-FL). Thereafter, the immune responses and protective effects in C57BL/6 mice immunized with the plasmid DNA vaccine, including the effects of prime-boost strategy were evaluated.
MATERIALS AND METHODS
T cell epitope prediction and plasmid construction
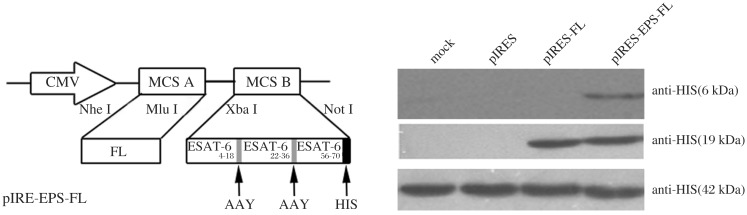
The construction of pIRES-ESAT-6-FL plasmid containing the ESAT-6 and FL genes has been previously described[35]. The primary structure of ESAT-6 protein that contains potential MHC I and MHC П -binding T cell epitopes was analyzed using epitope prediction software (http://www.syfpeithi.com/scripts/MHCSr.dll/home.htm; http://tools.immuneepitope.org). Thereafter, the three T cell epitopes, including ESAT-64-18 (QQWNFAGIEAAASAI), ESAT-622-36 (VTSIHSLLDEGKQSL) and ESAT-656-70 (QKWDATATELNNALQ) were selected based on higher scores via the prediction software. The three epitope-peptides with HIS-tag and Ala-Ala-Tyr (AAY) linker were synthesized and inserted into pIRES vector or pIRES-FL plasmid, termed pIRES-epitope-peptides and pIRES-epitope-peptides-FL (Fig. 1A). Thereafter, these recombinant plasmids were identified by sequencing and expressing HIS and FL proteins (Fig. 1B). The peptides of three T cell epitopes of ESAT-6 were synthesized by Shanghai Shangon Biological Engineering Technology and Services Co. (purity > 95%). BCG (Denmark strain 1331) was provided by the Center for Disease Control of Jiangsu Province in China.
Fig. 1. Construction and identification of pIRES-epitope-peptides-FL plasmid.
A: Schematic representation of pIRES-epitope-peptides-FL. B: The expression of different plasmids was detected by using Western blotting assays. 293T cells were transfected with the indicated plasmids, and the cell lysate was subjected to Western blotting assays with anti-His or anti-FL antibody. Individual experiments were conducted three times, with one representative photo for each group.
Animal vaccination
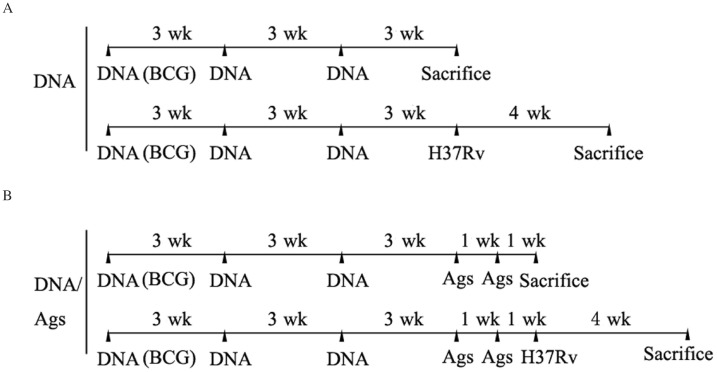
Six to eight week-old female C57BL/6 mice (H-2b) were purchased from the Experimental Animal Centre of Chinese Academy of Science (Shanghai, China). Animal welfare and the experimental procedures were carried out strictly in accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Health, China, 1998). The study protocol was approved by the local institutional review board at the authors' affiliated institutions (Permit No.: 2010254). Endotoxin-free plasmids were prepared using the of EndoFree plasmid purification kit (Qiagen, USA). C57BL/6 mice were injected intramuscularly at 3-week intervals in both the quadriceps muscles with 100 µg plasmid DNA (the DNA group), including pIRES (vector), pIRES-epitope-peptides, pIRES-FL, pIRES-epitope-peptides-FL, pIRES-ESAT-6 (containing full-length ESAT-6), pIRES-ESAT-6-FL plasmids, or phosphate buffered saline (PBS), respectively. Moreover, 3 weeks after vaccination, some mice were intranasally treated with 2.5 µg mixed ESAT-6 peptides once a week for 2 weeks (the DNA/Ag group). All mice were sacrificed at 1 week after the last epitope-peptides boost or 3 weeks after the last plasmid DNA vaccination alone. In the BCG group, mice were injected subcutaneously with 106 colony forming units (CFUs) of freshly prepared BCG only once at equal pace. The whole schedule of vaccination is displayed in Fig. 2.
Fig. 2. Entire immunization schedule.
A: C57BL/6 mice were injected intramuscularly three times at 3-week intervals in both quadriceps muscles with 100 µg of plasmid DNA and sacrificed at 3 weeks after the last plasmids DNA vaccination alone or at 4 weeks after infection with M. tuberculosis H37Rv via the airway (the DNA group). B: The mice at 3 weeks after the last plasmids DNA injection were conduced through intranasal administration of the mixed ESAT-6 epitope-peptides (Ags) once a week for 2 weeks, and then parts of the mice were sacrificed or infected with M. tuberculosis H37Rv via the airway the DNA/Ags group. Moreover, these mice post M. tuberculosis H37Rv challenge for 4 weeks were sacrificed.
Western blotting assays
293T cells (American Tissue Culture Collection, USA) were transfected with pIRES-epitope-peptides or pIRES-epitope-peptides-FL using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and cultured for 48 hours. The lysate was electrophoresed on SDS-PAGE gels. Thereafter, the transferred membrane was probed with anti-HIS or anti-FL antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by horseradish peroxidase (HRP) labeled goat anti-mouse antibody (Invitrogen, Carlsbad, CA, USA), and the signals were detected by enhanced chemiluminescence (Pierce, Rockford, IL, USA).
ELISA
For mice immunized with plasmid DNA (the DNA group), spleen cells were isolated 3 weeks after the last treatment. For mice treated with plasmid DNA plus the epitope-peptide boost (the DNA/Ag group), spleen cells were taken 1 week after the last peptide boost. Then, the cells were cultured and stimulated with the mixture of 3 peptides (10 µg/mL) for 72 hours. The levels of IFN-γ, IL-12, IL-4 and IL-10 in the supernatants were determined by ELISA (Biolegend, San Diego, CA, USA). Besides, for cytokine detection in vivo, bronchoalveolar lavage (BAL) fluids were collected, and the contents of the same cytokines were also detected by ELISA.
The level of ESAT-6-peptide-specific antibody in the sera of mice was measured by ELISA. First, the microtiter plates were coated with the mixed epitope-peptides (10 µg/mL). Then, 1:100 diluted sera were added into the coated plates. The bound antibodies were detected with HRP-conjugated goat anti-mouse IgG.
MTT and ELISPOT assays
Spleen cells from immunized mice were harvested and incubated with the epitope-peptides or medium. Splenocyte proliferation was detected by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays[36],[37]. The results were expressed as stimulation index (SI). SI was calculated using the formula: SI = [OD value (stimulated culture)-OD value (control culture)] / OD value (control culture). Moreover, IFN-γ+ T cells were examined using the ELISPOT assay kit. Briefly, plates were coated with anti-IFN-γ antibodies overnight. Splenocytes (5×105 cells/well) were cultured upon the epitope-peptide stimulation for 48 hours. Thereafter, the biotinylated anti-IFN-γ antibodies were added and incubated for 2 hours, and the plate was supplemented with streptavidin-alkaline phosphatase for 1 hour, and then the positive spots were counted.
In vivo CTL assay
CTL target cells were prepared from the spleen of näive mice and pulsed with the mixture of the epitope-peptides (10 µg/mL) or without the peptides overnight. The peptide-pulsed splenocytes were then labeled with a high concentration of 5-(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) (5 µmol/L) or non-pulsed splenocytes with a low concentration of CFSE (0.5 µmol/L). Then, 2.5×106 pulsed and 2.5×106 non-pulsed splenocytes were mixed and adoptively transferred to the immunized mice. The splenocytes at 18 hours after treatment were isolated for acquisition on a FACScalibur instrument (BD Biosciences, San Jose, CA, USA). To evaluate the percentage of specific lysis, the ratio of CFSEhigh/CFSElow in the vaccinated mice was compared[37].
M. tuberculosis H37Rv challenge
Mice immunized with the plasmid DNA vaccine alone or in combination with the last epitope-peptide boost again were infected by intratracheal instillation with 5×105 CFUs of M. tuberculosis H37Rv (ATCC 27294, USA) for 4 weeks. For the bacterial burden, the lung and spleen of the mice were examined 4 weeks post M. tuberculosis challenge by plating serial dilutions of tissue homogenates onto Middlebrook 7H10 agar plates, and then the plates were cultured for 4 weeks. M. tuberculosis colonies were calculated and displayed as log10 CFU per organ. For histological changes, paraffin sections of lung tissues were stained with hematoxylin and eosin (H&E) and observed by a pathologist who was blind to treatment. To score lung inflammation and damage, entire lung sections were analyzed with inflammatory infiltrations, which were quantified and expressed as a percentage of the lung surface. Furthermore, to determine infiltration of T cells and macrophages, the sections were first stained with rabbit anti-mouse CD3 antibodies (Bioworld, Dublin, OH, USA) or F4/80 antibodies (Abcam, Cambridge, MA, USA), and then incubated with HRP-conjugated secondary antibody and counterstained with hematoxylin.
Statistical analysis
Data analyses were carried out by using the SPSS 16.0 software. All values were expressed as the mean±SEM. Analysis was performed by using two-way ANOVA. Multiple comparisons were performed with LSD test. P < 0.05 was considered statistically significant.
RESULTS
pIRES-epitope-peptide-FL vaccination and epitope-peptide boost potentiate Th1-biased cellular immune response
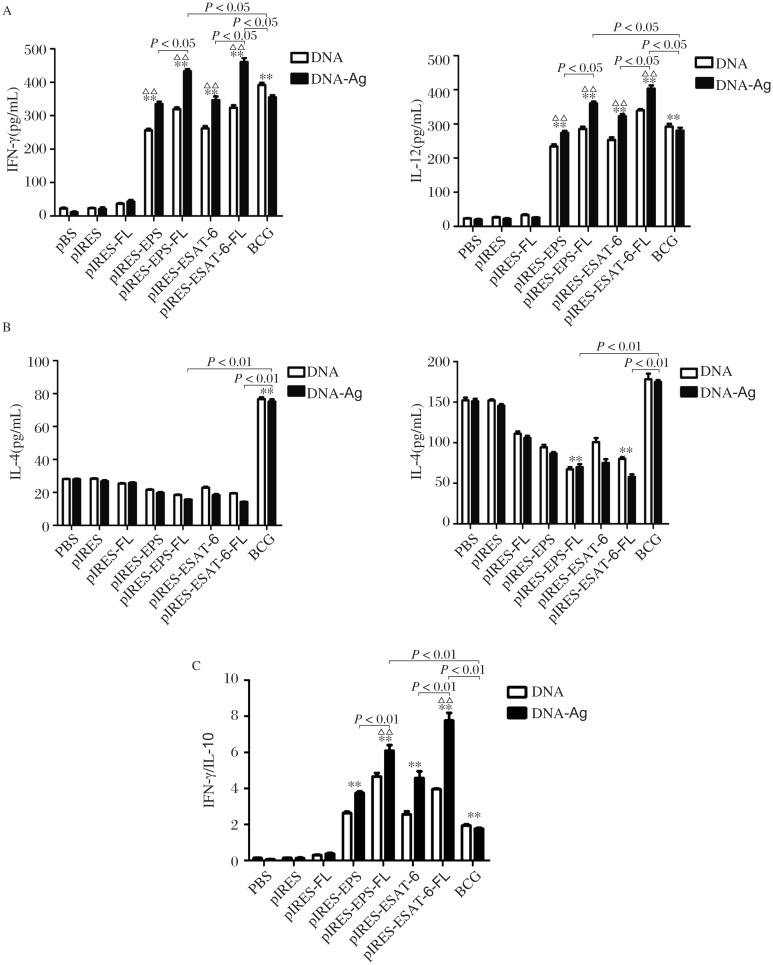
To investigate the changes of cytokine profile induced by vaccinations, we measured the levels of Th1/Th2 cytokines secreted by spleen cells from the mice. The levels of IFN-γ and IL-12 (Fig. 3A) in the supernatants of mice vaccinated with pIRES-epitope-peptides, pIRES-epitope-peptide-FL, pIRES-ESAT-6 and pIRES-ESAT-6-FL plasmids were significantly increased, compared with mice treated with PBS, pIRES, pIRES-FL and BCG, respectively. While mice immunized with pIRES-epitope-peptides-FL and pIRES-ESAT-6-FL plasmids showed markedly higher levels than those of mice treated with other plasmids, especially in mice vaccinated with the two plasmids plus the ESAT-6 peptide boost (the DNA/Ag groups). Meanwhile, the levels of IL-4 and IL-10 were relatively lower in mice treated with the same plasmids except for BCG, and a marked reduction of IL-10 was found in mice immunized with pIRES-epitope-peptide-FL and pIRES-ESAT-6-FL (Fig. 3B). Additionally, the ratios of IFN-γ and IL-10 average values were also calculated to represent Th1 and Th2 reaction. As shown in Fig. 3C, mice immunized with pIRES-epitope-peptide-FL and pIRES-ESAT-6-FL plasmids manifested obviously higher Th1/Th2 ratios than those of mice treated with other constructs. The changes of Th1 and Th2 cytokine profile mentioned previously were more significant in the corresponding DNA/Ag treatment (Fig. 3A-C).
Fig. 3. Th1 immune responses of mice immunized with different plasmids.
The concentrations of Th1/Th2 cytokines in the culture supernatant of splenocyte from the mice were measured by ELISA. The levels of IFN-γ and IL-12 represented Th1 immune response A: while levels of IL-4 and IL-10 standed for Th2 immune response B: The ratio of IFN-γ/IL-10 was calculated to indicate Th1/Th2 reaction (C). **P < 0.01, indicating the levels of these cytokines in corresponding mice were markedly increased compared with those of the mice treated with PBS, pIRES or pIRES-FL; ΔΔP < 0.01, indicating the levels of these cytokines in the mice of DNA/Ags group significantly higher than those of the mice with other corresponding DNA treatment. Other difference (such as lower than the BCG group) between the groups are presented in the figure directly. Data are shown as mean ± SEM (n = 10), done in triplicate.
Besides, to further evaluate the boost effects of the ESAT-6 epitope-peptides (Ag) after intranasal administration, we collected BAL fluids of the immunized mice and measured the levels of Th1/Th2 cytokines. The results showed a significantly enhanced IFN-γ and IL-12 production in mice vaccinated with pIRES-epitope-peptides, pIRES-epitope-peptide-FL, pIRES-ESAT-6 and pIRES-ESAT-6-FL plus the peptide boost. Furthermore, changes of cytokine profile were similar to those in the splenocyte supernatants of mice only treated with different plasmids (data not shown). The results indicated that effective Th1 responses were elicited in mice immunized with plasmid DNA vaccines, especially by using the strategy of DNA prime and the peptide boost.
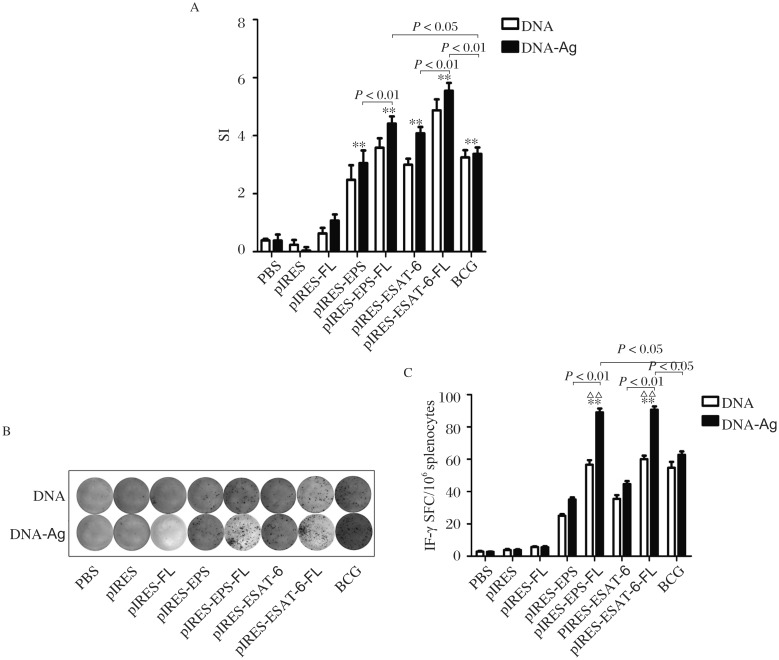
pIRES-epitope-peptide-FL vaccination and epitope-peptide boost increase the proliferation of spleen cells and IFN-γ+ T cells
As shown in Fig. 4A, splencytes from mice immunized with pIRES-epitope-peptides, pIRES-epitope-peptide-FL, pIRES-ESAT-6, pIRES-ESAT-6-FL and BCG displayed remarkable proliferation. Furthermore, SI increase in mice with pIRES-epitope-peptides-FL or pIRES-ESAT-6-FL treatment was more apparent relative to mice immunized with pIRES-epitope-peptides or pIRES-ESAT-6 plasmids. In addition, cell proliferation in mice treated with the corresponding plasmids plus the peptide boost (the DNA/Ag group) exhibited much higher than that in mice only treated with these plasmids (the DNA group). Meantime, as shown in Fig. 4B, the number of IFN-γ+ T cells in mice immunized with pIRES-epitope-peptides, pIRES-epitope-peptide-FL, pIRES-ESAT-6, pIRES-ESAT-6-FL and BCG were more notably increased than those of mice vaccinated with PBS, pIRE, or pIRES-FL, especially much more in mice treated with pIRES-epitope-peptide-FL and pIRES-ESAT-6-FL plasmids. Moreover, mice vaccinated with the same plasmids plus epitope-peptide boost (the DNA/Ag group) produced significantly greater IFN-γ+ T cells than those of mice immunized with the corresponding plasmids alone.
Fig. 4. The proliferation of spleen cells and numbers of IFN-γ+ T cells of mice vaccinated with different plasmids.
A: The spleen cells from mice at 3 weeks after the last DNA vaccination alone or at 1 week after the last epitope-peptides (Ag) boost were cultured for 72 hours upon epitope-peptides or medium stimulation alone. Then, the proliferation of spleen cells was examined using MTT, and the stimulation index (SI) in each group was calculated to determine the cell proliferation activity. B: The splenocytes as the above metioned time were collected and treated with the same method, and the nnubers of IFN-γ+T cells were quantified using ELISPOT assay. Representative images of ELISPOT response in the immunized mice and the frequency of IFN-γ+ T cells in the spleen were displayed. All data are shown as mean ± SEM (n = 10), done in triplicate. **P < 0.01, showing much higher than that of the PBS, pIRES or pIRES-FL group, and other differences between the groups were displayed in the figure directly. ΔΔP < 0.01, meaning DNA/Ags group vs the corresponding DNA group, separately.
pIRES-epitope-peptide-FL vaccination and epitope-peptide boost markedly augment CTL activity
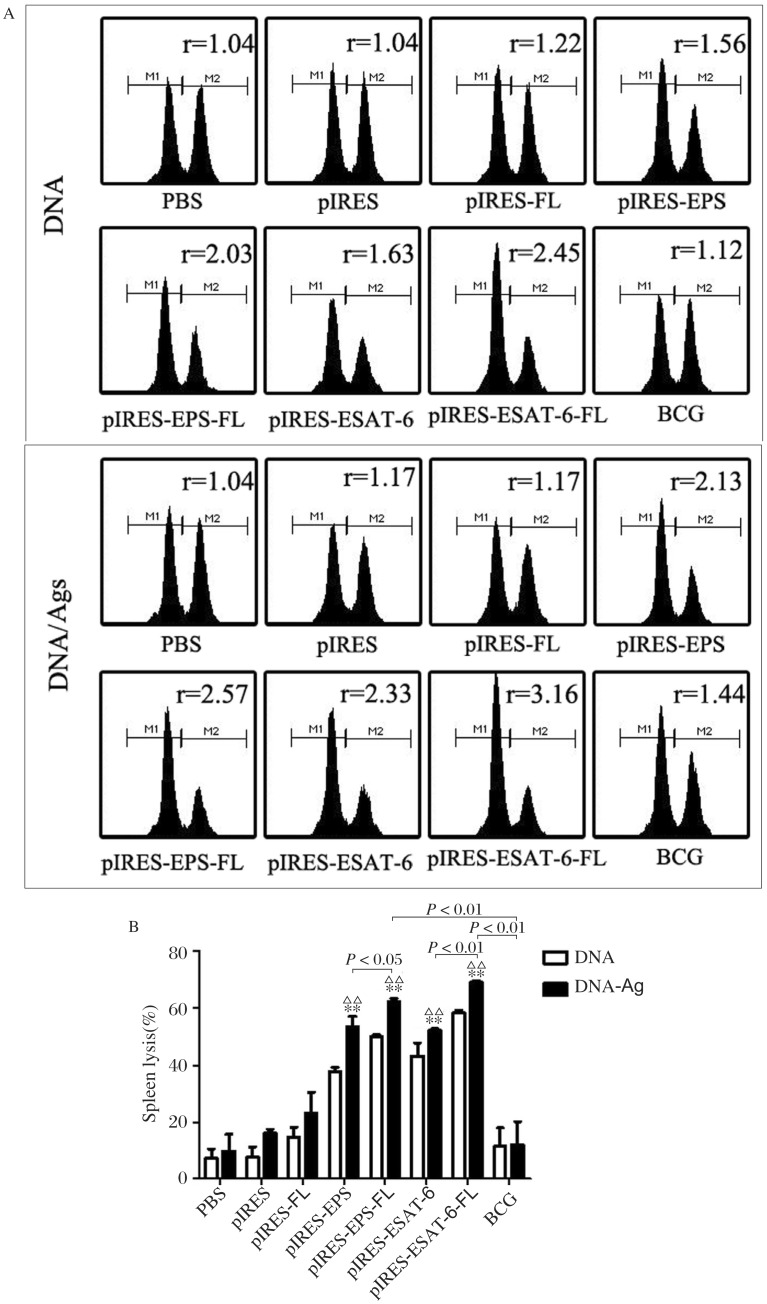
To assess the cytolytic function of T cells induced by pIRES-epitope-peptides-FL and other plasmid DNA vaccines, we examined epitope-specific lysis of CFSE-labeled transfected cells by flow cytometric analyses. When compared with the CTL activity of mice treated with PBS, pIRES, pIRES-FL and BCG, increased lyses were determined in mice vaccinated with the DNA vaccines of pIRES-epitope-peptides, pIRES-epitope-peptide-FL, pIRES-ESAT-6 and pIRES-ESAT-6-FL, especially much higher in mice immunized with pIRES-epitope-peptide-FL and pIRES-ESAT-6-FL (Fig. 5). Besides, a significantly elevated CTL activity in mice given with these plasmid vaccinations and the peptide boost were also achieved compared with mice treated with these plasmids alone.
Fig. 5. The specific CTL activity of mice triggered by various plasmid DNA vaccines.
Non-pulsed splenocytes (CFSElow) and ESAT-6 peptides-pulsed splenocytes (CFSEhigh) from naive mice were transferred to the immunized mice. The representative histograms (A) and percentages (B) of splenic ESAT-6-specific lysis in the immunized mice were compared. **P < 0.01, vs. the PBS, pIRES or pIRES-FL group, respectively; ΔΔP < 0.01, meaning the DNA/Ags group vs. the corresponding DNA group, respectively. Images indicate that one representative result from three experiments, and data present as mean ± SEM (n = 10).
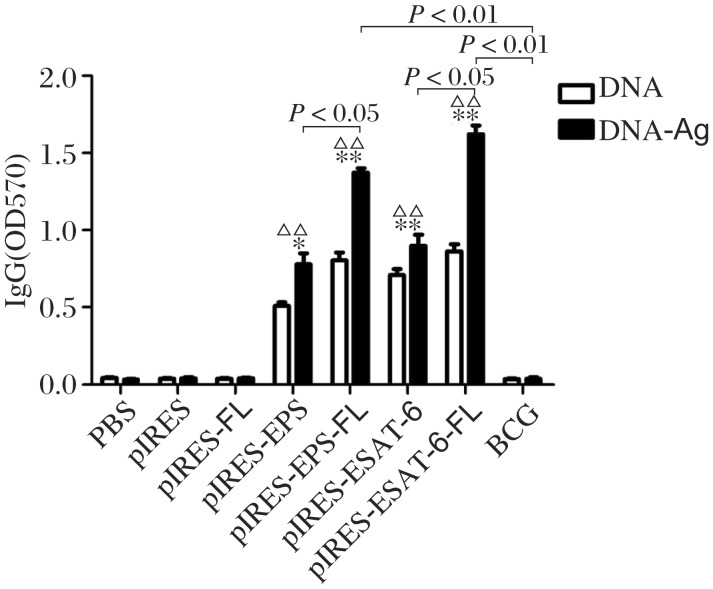
pIRES-epitope-peptide-FL vaccination and epitope-peptide boost significantly elevate the levels of ESAT-6 peptide-specific antibody
To explore whether these plasmid DNA vaccines can induce antigen-specific humoral immune responses, we collected sera from vaccinated mice and then detected ESAT-6 peptide-specific antibody with ELISA. As shown in Fig. 6, the levels of specific IgG in mice vaccinated with pIRES-epitope-peptides, pIRES-epitope-peptide-FL, pIRES-ESAT-6 and pIRES-ESAT-6-FL were higher than those of mice with PBS, pIRES or pIRES-FL treatment. The levels of antibody in mice vaccinated with pIRES-epitope-peptide-FL and pIRES-ESAT-6-FL were much higher than those of mice given with other plasmids. Additionally, mice vaccinated with pIRES-epitope-peptides, pIRES-epitope-peptide-FL, pIRES-ESAT-6 and pIRES-ESAT-6-FL plasmids and boosted with the peptides (DNA/Ag) showed a notably elevated level of IgG compared with others, but not mice immunized with BCG.
Fig. 6. Specific antibody for epitope-peptides in the sera of mice followed by immunization procedure.
The sera samples from the mice were obtained at the indicated time (see Fig.2), and the levels of anti-ESAT-6 epitope-peptides antibody are detected by ELISA. The result is representative of 3 independent experiments. The significant differences are shown in the figure directly (data showed as mean±S.E.M., n = 10, *P < 0.05, **P < 0.01 indicating the level of antibody obviously higher than that in the PBS or pIRES or pIRES-FL group, respectively; ΔΔP < 0.01 showing that the level of antibody in the DNA/Ag group is significantly higher than that in the corresponding DNA group).
pIRES-epitope-peptide-FL vaccination and epitope-peptides boost notably enhances protection from M. tuberculosis challenge
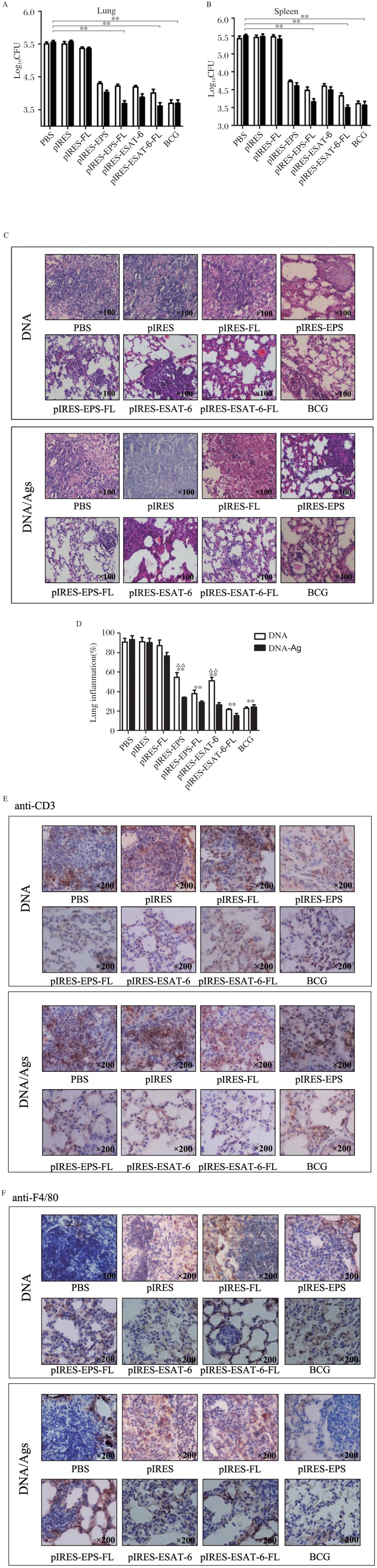
To further evaluate the protective potential of pIRES-epitope-peptide-FL DNA vaccine and the strategy of prime-boost vaccination, we challenged mice 3 weeks after the last plasmid immunization (the DNA group) or the plasmid injection (3 weeks) plus 1 week after the last peptide boost (the DNA/Ag group) intranasally with 5×105 CFUs of M. tuberculosis H37Rv (Fig. 2), and the bacterial burdens in the lungs or spleens including pulmonary injury of mice were observed 4 weeks post M. tuberculosis challenge. As shown in Fig. 7A and Fig. 7B, mice immunized with pIRES-epitope-peptides, pIRES-epitope-peptide-FL, pIRES-ESAT-6, pIRES-ESAT-6-FL and BCG displayed efficient protection from M. tuberculosis H37Rv infection, and mice treated with the corresponding DNA/Ag showed remarkably augmented protective effects.
Fig. 7. Protection against M.tb H37Rv infection induced by different plasmid DNA including DNA/Ags vaccination.
The different vaccinated mice received an intratracheal 5×105 CFU of M.tb H37Rv challenge for 4 weeks, and the bacterial loads in the lungs (A) and spleens (B) of the mice were determined. Data were shown as mean ± SEM (n = 10), performed in triplicate. C: The sections from lung tissues were stained with HE, and evaluated for the lung inflammation (100×). **P < 0.01, vs. PBS, pIRES or pIRES-FL group, separately. Other significant differences were displayed in the figures directly (A, B). D: Representative sections of the lung tissue from mice immunized with different plasmids (100×). E: The infiltration of CD3+ T cells was observed by immunohistochemistry staining so as to evaluate the inflammation response of the lung. **P < 0.01, vs. PBS, pIRES or pIRES-FL group, separately; ΔΔP < 0.01, indicating DNA/Ags vs. the corresponding DNA group. F: The infiltration of F4/80+ macrophages was observed by immunohistochemistry in order to evaluate the inflammation response of the lung. Individual experiments were conducted 3 times, with one representation shown (200×).
For histological changes, the results revealed that the lung tissues of mice treated with PBS, pIRES, and pIRES-FL exhibited widespread and severe interstitial pneumonia, intense inflammation and diffuse granuloma, as well as increased infiltration of T cells (Fig. 7E) and macrophages (Fig. 7F) after M. tuberculosis challenge. However, mice immunized with pIRES-epitope-peptides, pIRES-epitope-peptide-FL, pIRES-ESAT-6 or pIRES-ESAT-6-FL plasmids showed moderate damages in the alveolar tissues accompanied with relatively fewer numbers of infiltrated T cells and macrophages, as those of mice immunized with BCG (Fig. 7C and 7D). Furthermore, mice in the DNA/Ag group displayed much stronger protection, particularly mice with pIRES-epitope-peptide-FL plus the peptides boost (Fig. 7C and 7D), indicating that this novel plasmid DNA vaccine (pIRES-epitope-peptide-FL) can indeed provide efficient protection against M. tuberculosis infection.
DISCUSSION
CMI is crucial for protection against M. tuberculosis infection in both humans and animasl[38]–[43]. The future success of DNA vaccines for tuberculosis depends on inducing stronger CMI such as producing more IFN-γ-secreting cells or Th1 cytokines. Early studies confirmed that DNA vaccines, which contained single or multiple epitopes of pathogen protein, could trigger efficient immune responses[44], and possess better advantages i.e. greater stability, safety and standardization. Thus, the use of DNA vaccines encoding pathogen epitopes may be an effective approach to promote CMI. Reportedly, ESAT-6 is an effective antigen, and is strongly involved in anti-mycobacterial T cell immune reaction[10],[11]. However, ESAT-6 has been found to cause apoptosis of macrophages and lysis of erythrocytes as well as injury of epithelial cells[17],[20],[45]. Therefore, in order to avoid ESAT-6 toxic effects, in the study, three T cell epitopes of ESAT-6 (ESAT-64-18, ESAT-622-36 and ESAT-656-70) were selected based on higher scores via prediction software and ESAT-6 functions described previously[9]. Thereafter, a recombinant DNA vaccine (pIRES-epitope-peptide plasmid) was first constructed. It is worth noting that these ESAT-6 T cell epitopes not only contain dominant Th1 (CD4+ T cell) epitopes, but also include CTL epitopes[46].
As we know, the drawback of DNA vaccine is its relatively low immunogenicity. Antigen presenting cells (APCs), especially DCs, is an important inducer of immune response[31]–[33]. It has been demonstrated that FL can elevate CMI through increasing the number of DCs, and the maturation and function of DCs[31],[33]. Since FL has been reported as a good adjuvant in DNA vaccine, in the experiment, some recombinant plasmid DNA vaccines such as pIRES-epitope-peptide-FL (encoding the three T cell epitopes of ESAT-6 and FL protein), pIRES (blank vector), pIRES-epitope-peptides (only containing the three T cell epitopes of ESAT-6), pIRES-ESAT-6 (containing full length ESAT-6) and pIRES-ESAT-6-FL (containing full length ESAT-6 and FL) were successfully constructed for the first time. Besides, in our previous study, a DNA vaccine expressing a fusion protein of ESAT-6-FL (pIRES-ESAT-6-FL), which contains full length ESAT-6 and FL, had been developed. The vaccine could induce effective immune responses from in mice[35], but protection against M. tuberculosis challenge remained to be demonstrated.
Our results from the present experiment revealed that mice immunized with pIRES-epitope-peptide-FL plasmids markedly enhanced the levels of Th1 cytokine (IFN-γ and IL-12), the proliferation of spleen cells, and the number of IFN-γ+ T cells and activity of CTLs including the content of anti-ESAT-6 antibody. Meanwhile, mice treated with pIRES-epitope-peptide-FL plasmids displayed remarkably enhanced protection against M. tuberculosis challenge, e.g. reducing bacterial burden in the lung and spleen or pathological lesions of the lungs. For mice immunized with pIRES-epitope-peptide-FL or pIRES-ESAT-6-FL plasmids, although similar effects on immunity and protection from mice were obtained, we considered the pIRES-epitope-peptides-FL plasmid as a relatively better vaccine because the epitope vaccine could avoid the toxicity of ESAT-6 whole protein as shown by other investigators[9],[16],[23].
Recent studies confirmed that mucosal vaccination could trigger greater protection against M. tuberculosis infection[47]–[50]. Besides, airway deposition of soluble mycobacterial antigens in previously immunized mice could effectively mobilize systemic T cells into the airway lumen and provide marked immune protection upon airway M. tuberculosis challenge[27]. This phenomenon was also achieved from histological examination in our experiment, such as a large number of CD3 positive T cells and F4/80 positive macrophages around the bronchioles and vessels of mice treated by prime-boost vaccination.
Our data also showed that mice immunized with pIRES-epitope-peptide-FL plasmids, and then boosted with the epitope-peptides elicited significantly higher levels of IFN-γ and IL-12, which was coupled with a greater number of IFN-γ+ T cells and activity of CTLs, compared to mice treated with other plasmids alone. Importantly, better protection against M. tuberculosis H37Rv challenge, i.e. reduction of bacterial loads in the lung and spleen, and decreased pulmonary damage in the mice, was observed. Overall, these findings indicated that the strategy of priming with the plasmid DNA and boosting with the peptides is very efficient on improving Th1 response and CTL activity as well as immune protection against M. tuberculosis infection.
Notably, mice immunized with pIRES-epitope-peptide and pIRES-ESAT-6 plasmids alone or these plasmids plus the epitope-peptide boost or only BCG also exhibited a significant increase of Th1 cytokines, IFN-γ+ T cells, CTL activity and protective effects at different extents. However, these parameters from our study were remarkably lower than those of mice treated with pIRES-epitope-peptide-FL and pIRES-ESAT-6-FL immunization, implicating that FL played a synergistic role in inducing T cell response and protection against M. tuberculosis infection. Moreover, we noted that mice immunized with BCG could produce higher levels of Th2 cytokines (e.g. IL-4 and IL-10) besides higher levels of IFN-γ and IL-12, which resulted in a lower ratio of IFN-γ/IL-10, and the results are similar to the findings from previous studies[51],[52]. Additionally, mice treated with BCG showed that the number of IFN-γ+ T cells and bacterial loads were increased, and lung injuries were reduced, but these effects were generally less than those of mice vaccinated with pIRES-epitope-peptide-FL or pIRES-ESAT-6-FL, further implying that the two plasmid DNA vaccines may be relatively better than BCG. Although DNA vaccines ESAT-6-epitope-peptides-FL or ESAT-6-FL and BCG also elicited an effective immune response and protective effect, and the efficacy was greater in mice treated with plasmid DNA vaccines, indicating that the DNA vaccine induces more effective protection than BCG. However, because the pIRES-epitope-peptide-FL plasmid was a DNA vaccine encoding ESAT-6 T cell epitopes and FL, and had the same effects as the vaccine containing full length of ESAT-6 plus FL (pIRES-ESAT-6-FL); therefore, the novel recombinant plasmid (pIRES-epitope-peptide-FL) may be a safer and more useful vaccine in the future.
In conclusion, the data from the present study revealed that mice vaccinated with the plasmid DNA vaccine (pIRES-epitope-peptide-FL) indeed induced higher CMI than the other DNA vaccines. Mice treated with the DNA vaccine or the strategy of DNA prime and the peptide boost also efficiently led to significantly augmented protection against M. tuberculosis challenge, suggesting that the novel recombinant plasmid encoding the ESAT-6 T cell epitopes and FL is a useful DNA vaccine for preventing M. tuberculosis infection.
Acknowledgments
We thank professor Jun Dou (Department of Microbiology and Immunology, Medical College of Southeast University, Jiangsu Province, China) for providing the pIRES plasmid, and professor Zuhu Huang (Division of Infectious Diseases of People's Hospital, Jiangsu Province, China) for offering T-FL plasmid.
Footnotes
This study was supported by the Natural Scientific Fund (09KJA310002, DG216D5016, 2011NJMU263 and 11JC005) of China.
References
- 1.Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nat Rev Immunol. 2005;5:819–26. doi: 10.1038/nri1704. [DOI] [PubMed] [Google Scholar]
- 2.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.WHO WHO report 2009: Global tuberculosis control-epidemiology, strategy, financing. DocumentWHO/HTM/TUBERCULOSIS/2009.411. Available: http://www.who.int/b/publications/global_report/2009/en/ via the Internet. Accessed 25 Nov. 2010.
- 4.Young D, Perkins M, Duncan K, Barry C., III Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008;118:1255–65. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ying X, Yinlan B, Xue G, Hong J, Limei W, Hui G, Zhikai X. Expression, purification and characterization of Mycobacterium tuberculosis RpfE protein. J Biomed Res. 2012;26:17–23. doi: 10.1016/S1674-8301(12)60003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veeky B, Utpal R. Mycobacterium tuberculosis using statistical coupling analysis of the esterase family proteins. J Biomed Res. 2011;25:165–9. doi: 10.1016/S1674-8301(11)60021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lozes E, Huygen K. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997;15:830–3. doi: 10.1016/s0264-410x(96)00274-5. [DOI] [PubMed] [Google Scholar]
- 8.Tascon R, Colston M, Ragno S, Stavropoulos E, Gregory D, Lowrie DB. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–92. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 9.Brandt L, Elhay M, Rosenkrands I, Lindblad E, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun. 2000;68:791–5. doi: 10.1128/iai.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu F, Wang J, Dou J, Yang H, He X, Xu W, et al. Nanoparticle-based adjuvant for enhanced protective efficacy of DNA vaccine Ag85A-ESAT-6-IL-21 against Mycobacterium tuberculosis infection. Nanomedicine. 2012 Mar 7; doi: 10.1016/j.nano.2012.02.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy HA, et al. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–45. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 12.Brusasca P, Peters R, Motzel S, Klein H, Gennaro M. Antigen recognition by serum antibodies in non-human primates experimentally infected with Mycobacterium tuberculosis. Comparative Med. 2003;53:165–72. [PubMed] [Google Scholar]
- 13.Lyashchenko K, Colangeli R, Houde M, Al Jahdali H, Menzies D, Gennaro ML. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998;66:3936–40. doi: 10.1128/iai.66.8.3936-3940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trajkovic V, Natarajan K, Sharma P. Immunomodulatory action of mycobacterial secretory proteins. Microbes Infect. 2004;6:513–9. doi: 10.1016/j.micinf.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Behr M, Wilson M, Gill W, Salamon H, Schoolnik G, Rane S, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 16.Brodin P, Majlessi L, Marsollier L, de Jonge MI, Bottai D, Demangel C, et al. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun. 2006;74:88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derrick S, Morris S. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell Microbiol. 2007;9:1547–55. doi: 10.1111/j.1462-5822.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 18.Hsu T, Hingley-Wilson S, Chen B, Chen M, Dai AZ, Morin PM, et al. The primary mechanism of attenuation of bacillus Calmette-Guérin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci. 2003;100:12420–5. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Laine O, Masciocchi M, Manoranjan J, Smith J, Du SJ, et al. A unique Mycobacterium ESX-1 protein co-secretes with CFP-10/ESAT-6 and is necessary for inhibiting phagosome maturation. Mol Microbiol. 2007;66:787–800. doi: 10.1111/j.1365-2958.2007.05959.x. [DOI] [PubMed] [Google Scholar]
- 20.Kinhikar AG, Verma I, Chandra D, Singh KK, Weldingh K, Andersen P, et al. Potential role for ESAT6 in dissemination of M. tuberculosis via human lung epithelial cells. Mol Microbiol. 2010;75:92–106. doi: 10.1111/j.1365-2958.2009.06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith J, Manoranjan J, Pan M, et al. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun. 2008;76:5478–87. doi: 10.1128/IAI.00614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dan der Wel N, Hava D, Houben D, Fluitsma D, Zon van M, Pierson J, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–98. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 23.Ciernik I, Berzofsky J, Carbone D. Induction of cytotoxic T lymphocytes and antitumor immunity with DNA vaccines expressing single T cell epitopes. J Immunol. 1996;156:2369–75. [PubMed] [Google Scholar]
- 24.Hanke T, Schneider J, Gilbert S, Hill A, McMichael A. DNA multi-CTL epitope vaccines for HIV and Plasmodium falciparum: immunogenicity in mice. Vaccine. 1998;16:426–35. doi: 10.1016/s0264-410x(97)00296-x. [DOI] [PubMed] [Google Scholar]
- 25.Olsen A, Hansen P, Holm A, Andersen P. Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. Eur J Immunol. 2000;30:1724–32. doi: 10.1002/1521-4141(200006)30:6<1724::AID-IMMU1724>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Anderson P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006;177:6353–60. doi: 10.4049/jimmunol.177.9.6353. [DOI] [PubMed] [Google Scholar]
- 27.Jeyanathan M, Mu J, Kugathasan K, Zhang X, Damjanovic D, Small C, et al. Airway delivery of soluble mycobacterial antigens restores protective mucosal immunity by single intramuscular plasmid DNA tuberculosis vaccination: role of proinflammatory signals in the lung. J Immunol. 2008;181:5618–26. doi: 10.4049/jimmunol.181.8.5618. [DOI] [PubMed] [Google Scholar]
- 28.Thomson S, Sherritt M, Medveczky J, Elliott S, Moss D, Fernando GJP, et al. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717–23. [PubMed] [Google Scholar]
- 29.Wang C, Chen Z, Fu R, Zhang Y, Chen L, Huang L, et al. A DNA vaccine expressing CFP21 and MPT64 fusion protein enhances BCG-induced protective immunity against Mycobacterium tuberculosis infection in mice. Med Microbiol Immunol. 2011;200:165–75. doi: 10.1007/s00430-011-0188-z. [DOI] [PubMed] [Google Scholar]
- 30.Radosević K, Rodriguez A, Lemckert A, Goudsmit J. Heterologous prime-boost vaccinations for poverty-related diseases: advantages and future prospects. Expert Rev Vaccines. 2009;8:577–92. doi: 10.1586/erv.09.14. [DOI] [PubMed] [Google Scholar]
- 31.McKenna H. Role of hematopoietic growth factors/flt3 ligand in expansion and regulation of dendritic cells. Curr Opin Hematol. 2001;8:149–54. doi: 10.1097/00062752-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Maraskovsky E, Brasel K, Teepe M, Roux E, Lyman SD, Shortman K, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–62. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, et al. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96:878–84. [PubMed] [Google Scholar]
- 34.Antonysamy MA, Thomson AW. Flt3 ligand (Fl) and its influence on immune reactivity. Cytokine. 2000;12:87–100. doi: 10.1006/cyto.1999.0540. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Xu W, Chen X, Zhao D, Wang Y. Recombinant DNA vaccine of the early secreted antigen ESAT-6 by Mycobacterium tuberculosis and Flt3 ligand enhanced the cell-mediated immunity in mice. Vaccine. 2008;26:4519–25. doi: 10.1016/j.vaccine.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Borsuk S, Newcombe J, Mendum AT, Dellagostin AO, McFadden J. Identification of proteins from tuberculin purified protein derivative (PPD) by LC-MS/MS. Tuberculosis. 2009;89:423–30. doi: 10.1016/j.tube.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 38.Zhu X, Venkataprasad N, Thangaraj H, Hill M, Singh M, Ivanyi J, et al. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–6. [PubMed] [Google Scholar]
- 39.Kamath A, Feng C, Macdonald M, Briscoe H, Britton W. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–7. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao H, Li K, Yu S, Xiong S. A novel DNA vaccine containing multiple TUBERCULOSIS-specific epitopes cast in a natural structure elicits enhanced Th1 immunity compared with BCG. Microbiol Immunol. 2009;53:541–9. doi: 10.1111/j.1348-0421.2009.00157.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Xu M, Wang ZY, Chen BW, Du WX, Su C, et al. The development and preliminary evaluation of a new Mycobacterium tuberculosis vaccine comprising Ag85b, HspX and CFP-10: ESAT-6 fusion protein with CpG DNA and aluminum hydroxide adjuvants. FEMS Immunol Med Microbiol. 2010;59:42–52. doi: 10.1111/j.1574-695X.2010.00660.x. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson R, Zhu X, Wilkinson K, Lalvani A, Ivanyi J. 38000 MW antigen-specific major histocompatibility complex class I restricted interferon-gamma-secreting CD8+ T cells in healthy contacts of tuberculosis. Immunology. 1998;95:585–90. doi: 10.1046/j.1365-2567.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dou J, Tang Q, Zhao F, Chu L, Chen J, Cao M, et al. Comparison of immune responses induced in mice by vaccination with DNA vaccine constructs expressing mycobacterial antigen 85A and interleukin-21 and Bacillus Galmette-Guérin. Immunol Invest. 2008;37(2):113–27. doi: 10.1080/08820130701690741. [DOI] [PubMed] [Google Scholar]
- 44.Pirson C, Vipond J, Hall Y, Williams A, Vordermeier HM. Vaccines designed to protect against Mycobacterium tuberculosis infection may aid the identification of novel vaccine constructs and diagnostic antigens for bovine tuberculosis. Vet Microbiol. 2011;148:232–7. doi: 10.1016/j.vetmic.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Brodin P, Rosenkrands I, Andersen P, Cole S, Brosch R. ESAT-6 proteins: protective antigens and virulence factors? Trends. Microbiol. 2004;12:500–8. doi: 10.1016/j.tim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Brandt L, Oettinger T, Holm A, Andersen A, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–33. [PubMed] [Google Scholar]
- 47.Chen L, Wang J, Zganiacz A, Xing Z. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect Immun. 2004;72:238–46. doi: 10.1128/IAI.72.1.238-246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giri P, Sable S, Verma I, Khuller G. Comparative evaluation of intranasal and subcutaneous route of immunization for development of mucosal vaccine against experimental tuberculosis. FEMS Immunol Med Mic. 2005;45:87–93. doi: 10.1016/j.femsim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 49.McCormick S, Santosuosso M, Small C, Shaler C, Zhang X, Jeyanathan M, et al. Mucosally delivered dendritic cells activate T cells independently of IL-12 and endogenous APCs. J Immunol. 2008;181:2356–67. doi: 10.4049/jimmunol.181.4.2356. [DOI] [PubMed] [Google Scholar]
- 50.Badell E, Nicolle F, Clark S, Majlessi L, Boudou F, Martinoa A, et al. Protection against tuberculosis induced by oral prime with Mycobacterium bovis BCG and intranasal subunit boost based on the vaccine candidate Ag85B-ESAT-6 does not correlate with circulating IFN-gamma producing T-cells. Vaccine. 2009;27:28–37. doi: 10.1016/j.vaccine.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 51.Djuardi Y, Sartono E, Wibowo H, Supali T, Yazdanbakhsh M. A longitudinal study of BCG vaccination in early childhood: the development of innate and adaptive immune responses. PloS one. 2010;5:e14066. doi: 10.1371/journal.pone.0014066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martino A, Sacchi A, Sanarico N, Spadaro F, Ramoni C, Ciaramella A, et al. Dendritic cells derived from BCG-infected precursors induce Th2-like immune response. J Leukocyte Biol. 2004;76:827–34. doi: 10.1189/jlb.0703313. [DOI] [PubMed] [Google Scholar]