Abstract
Background: Dietary saffron and photobiomodulation (low-level infrared radiation, PBM) are emerging as therapeutically promising protectants for neurodegenerative conditions, such as the retinal dystrophies. In animal models, saffron and PBM, given in limited daily doses, protect retina and brain from toxin- or light-induced stress. This study addresses the rate at which saffron and PBM, given in daily doses, induce neuroprotection, using a light damage model of photoreceptor degeneration in Sprague Dawley (SD) rats. Results: Rats were raised in dim cyclic (12 h 5 lux, 12 h dark) illumination, treated with saffron or PBM for 2-10 d, and then exposed to bright damaging light (1,000 lux for 24 h). After 1 week survival, the retina was assessed for photoreceptor death (using the TUNEL reaction), for surviving photoreceptor damage (thickness of the outer nuclear layer) and for the expression of a stress-related protein GFAP, using immunohistochemistry. Preconditioning the retina with saffron or PBM reduced photoreceptor death, preserved the population of surviving photoreceptors and reduced the upregulation of GFAP in Müller cells. At the daily dose of saffron used (1 mg/kg), protection was detectable at 2 d, increasing to 10 d. At the daily dose of PBM used (5 J/cm2 at 670 nm) protection was detectable at 5 d, increasing to 7-10 d. Conclusions: The results provide time parameters for exploration of the mechanisms and durability of the protection provided by saffron and PBM.
Keywords: Saffron, photobiomodulation, retinal degeneration, neuroprotection, light damage
Background
The idea that central nervous tissue can be protected from stresses which cause it to degenerate has arisen from several lines of work in the retina and brain. For degenerations of retinal photoreceptors, protection has been reported in animal models by trophic factors [1,2], acute hyperoxia [3], light restriction [4-6], light conditioning [7-9], photobiomodulation [10-12], dietary saffron [13] and gamma radiation [14]. Stabilisation of human retinal degenerations has been reported using hyperoxia [15], photobiomodulation [16], dietary anti-oxidants (reviewed [17]) and dietary saffron [18,19]. For brain tissue, protection from ischemic or toxic damage has been reported in animals for photobiomodulation [20,21], saffron or its extracts [22-25], ischaemic preconditioning [26], remote ischemic preconditioning [27], exercise conditioning [28], low dose plant toxins [29], caloric restriction [30] and, in humans, for photobiomodulation [31,32].
These sources of protection are diverse, yet converging in that each approach seems to activate endogenous protective systems. Much work is being directed to the mechanisms of neuroprotection (for example [33,34]), and to testing whether protective agents which are effective against acute stress (such as light damage in the retina or ischemic damage to brain tissue) will be effective in the slow, age-related degenerations that afflict the long-lived (dementia, Parkinsonism, macular degeneration). Schwartz and colleagues have developed evidence that some of the neuroprotective effects are mediated by cells (T-cells, monocytes) normally associated with the immune system [35-37].
This study traces the onset of the protection induced by two neuroprotectants that we have explored in recent studies of photoreceptor degenerations, dietary saffron [13,18,25,34] and photobiomodulation [12,34,38].
Materials and methods
Rats raised in controlled, low level ambient illumination were conditioned either with dietary saffron or with PBM, for up to 10 d, and then exposed to bright light, sufficient to cause photoreceptor degeneration. We then assessed the level of damage cause by the bright light, and compared that level with the damage caused in unconditioned control animals.
Rearing and light damage conditions
Following our previous work [39], albino Sprague–Dawley rats were raised in dim cyclic light (12 h light, 12 h dark) with the light level at 5 lux, to 60-70 d age. Immediately after conditioning with either saffron or PBM (below), the rats, housed individually in standard rat boxes, were placed in a clear plexiglass chamber, and exposed to bright continuous light (BCL) from a fluorescent light placed above the chamber. Measured as incident light by a photometer held at the floor of the cage, the brightness of the illumination was 1000 lux. Animals were exposed to BCL for 24 h beginning immediately after the 12-hour period in darkness. Food and water were available to the animals from containers kept on the floor of the cage. The animals showed an initial tendency to hide from the bright light but adapted to it within the first 12 hours, resuming feeding and grooming behavior. Persistent eyelid closure was not observed. The structure of the retina was examined at 7 d following the end of bright light exposure.
Dose series for saffron
In our previous work on photoreceptor degeneration in rats, a dose of 1 mg/kg per day was effective in limiting the damage caused by bright light [13,34], when given over periods from days to weeks. We followed this daily dose, for 2 d, 5 d and 10 d, with 5 animals in each group. Saffron (stigmata of Crocus sativus, from the Abruzzo region in Italy) was soaked in water (at 2 mg/ml H2O) and 12 h was allowed for the major bioactive components, which are water-soluble [40], to dissolve. The solute was then fed to the rats by injecting a small volume into a piece of the vegetable matrix, which the animal readily ingested. The volume for each daily feed was calculated to provide the solutes from 1 mg of saffron/kg bodyweight. Saffron feeding was done at 9.00 am each morning.
Dose series for PBM
In previous work on photoreceptor degeneration in rats [10,11,34,38], and in many other studies of PBM (for example [41-43]), a low dose of infrared (670 nm) radiation was given, once daily. In the present work, the dose was 5 J/cm2, delivered from a Quantum Devices WARP 75 device. The rat was placed in a plastic cage and a plexiglass layer was lowered over the rat to within 10 cm of the floor of the cage. This confined the rat to 10 cm, which is comfortable standing height. The WARP 75’s emission plate was placed on the ‘ceiling’, 1-2 cm from the animal’s eye.
Exposure time was 3 minutes per day and the mild physical restraint of the animal was limited to this period. The exposure was done at 9.00 am each morning, for 2 d, 5 d, 7d and 10 d, in groups of 5 animals.
Preparation of retinal sections
The retina was examined in sections labelled for cell death, GFAP expression and structure. After the animal was euthanized (sodium pentobarbitone, 60 mg/kg i.p.), each eye was dissected free. The superior aspect of the eye was marked with a pen and eyes were fixed by immersion in 4% paraformaldehyde fixative buffer at 4°C for 3 h. The eyes were rinsed three times in 0.1 M phosphate-buffered saline (PBS) and then were left overnight in a 15% sucrose solution, to provide cryoprotection. They were embedded in mounting medium (Tissue-Tek OCT compound; Sakura Finetek, Torrance, CA) by snap freezing in liquid nitrogen. Sections were cut at 20 μm along the vertical meridian of the eye to produce sections that extended from the superior to the inferior edge. Sections were mounted on gelatin and poly-L-lysine-coated slides, dried overnight in 50°C oven and stored at -20°C until processed.
Detection of cell death (TUNEL)
Sections were labelled for apoptotic cell death using the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) technique [44], following protocols published previously [3]. To demonstrate cellular layers, sections were also labelled with the DNA-specific dye bisbenzimide (Calbiochem, La Jolla, CA), by incubating them for 2 min in a 1:10,000 solution in 0.1 M PBS. Sections cut adjacent to or through the optic nerve head were chosen, to minimise variations in retinal length and position. Counts of TUNEL+ profiles (apoptotic cells) were made using a calibrated 20 x objective and an eyepiece graticule. Each section was scanned from the superior to inferior edge, and the number of TUNEL+ profiles was recorded for each 400 μm length of the section. Counts were averaged from at least four sections per animal and were recorded separately for the outer nuclear layer (ONL) and inner nuclear layer (INL).
Measurement of photoreceptor survival (ONL/retina ratio)
The thickness of the outer nuclear layer (ONL) and of the retina (from inner to outer limiting membranes) was measured at 400 μm intervals from the superior to the inferior edge of the retina. The ratio of ONL to retinal thickness was used as a measure of ONL thickness; the use of the ratio was adopted to compensate for oblique sectioning. Measurements were made in two sections from one eye of each animal.
Immunohistochemistry for GFAP
Retinal sections were washed in 0.1 M PBS (3 x 5 min), and then incubated in 10% normal goat serum in 0.1 M PBS for 1 hour at room temperature to block non-specific binding. Sections were then incubated overnight at 4°C in rabbit polyclonal anti-GFAP (1:700; Dako Cytomation, Campbellfield, Australia). After three 10 min rinses in PBS, sections were incubated with an appropriate secondary antibody (1:1000 ALEXA Fluor 594, Molecular Probes, Invitrogen Carlsbad, CA), for 1 hour at room temperature or overnight at 4°C, before being coverslipped with glycerol–gelatin.
Assessment of GFAP expression
It is long established that the intermediate filament protein GFAP is expressed by astrocytes in retina and brain [45], but is expressed at much lower levels by Müller cells, particularly where the retina is unstressed, for example where its exposure to light is minimized [39,46]. All forms of stress examined (ambient light [39], intense focused light [47], hypoxia [39], genetically-induced stress [46,48], mechanical injury [49,50], edge-related stress [51], and ischaemia [52]) upregulate GFAP expression by Müller cells. We noted that, when GFAP expression is upregulated, the expression spreads from the inner to the outer feet of the Müller cells so that the length of Müller cell along which expression can be detected increases with stress. We therefore measured the length of Müller cells showing GFAP expression, normalizing the measure as a proportion of the thickness of the retina (the distance from the inner to the outer limiting membrane).
Statistical tests
The significance of differences in the frequency of TUNEL+ cells, ONL thickness and GFAP labelling associated with conditioning time were assessed using both ANOVA, followed by a Tukey test. The Tukey test was used for all pairwise comparisons of the mean responses of the different treatment groups. The ANOVA outcomes are indicated in Figures 2, 3 and 5.
Figure 2.
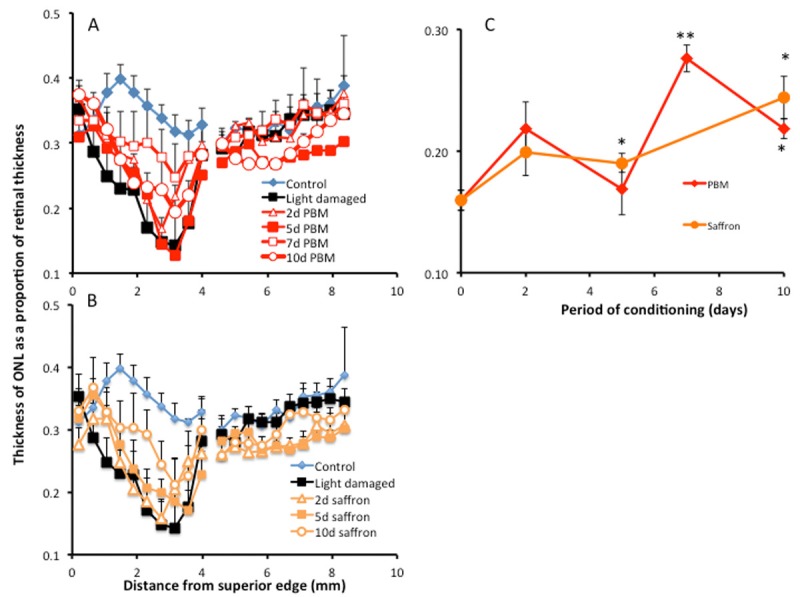
Impact of conditioning on the thickness of the ONL. A, B: Thickness of the ONL as a function of distance from superior to inferior edge of the retina. Data are shown for control and light-damaged groups, and for groups preconditioned with PBM for 2, 5, 7 and 10 d (A), or with dietary saffron for 2, 5 and 10 d (B). The thinning of the ONL is more marked in the sensitive region of superior retina and is reduced by conditioning. C: Summary graph, for values averaged over superior retina. The thickness of the ONL after light damage tended to increase with the period of conditioning. The increase was significant after 7 d conditioning with PBM and 5 d with saffron. After 7 d conditioning with PBM and 10 d conditioning with saffron, the greater thickness of ONL (compared to unconditioned light damage group) was statistically significant. One asterisk indicates P < 0.05; two indicate P < 0.01. The error bars show standard errors.
Figure 3.
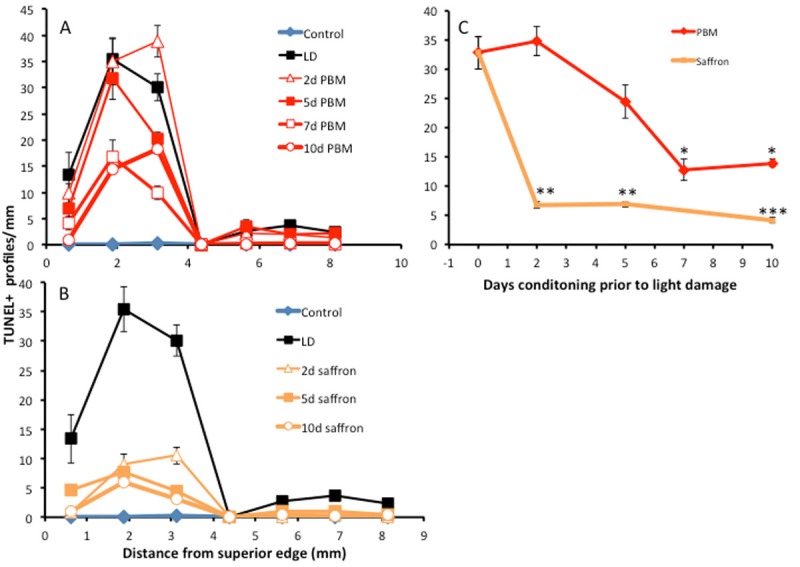
Impact of conditioning on photoreceptor death, assessed by TUNEL-labelling of photoreceptors, 7 d after light damage. A: TUNEL-labelling in the ONL as a function of distance from the superior to the inferior edge of the retina, for control and light-damaged groups, and for groups preconditioned with PBM for 2, 5, 7 and 10 d. B: TUNEL-labelling in the ONL as a function of distance from the superior to the inferior edge of the retina, for control and light-damaged groups, and for groups preconditioned with dietary saffron for 2, 5 and 10 d. C: Summary diagram, averaging labelling over superior retina. The reductions in labelling were statistically significant for 7 d and 10 d PBM conditioning, and for 2, 5 and 10 d saffron conditioning. The asterisks indicate points at which the TUNEL count was significantly less than in the unconditioned, light-damaged retina; one asterisk indicates P < 0.05; two indicate P < 0.01; three indicate P < 0.005. The error bars show standard errors.
Figure 5.
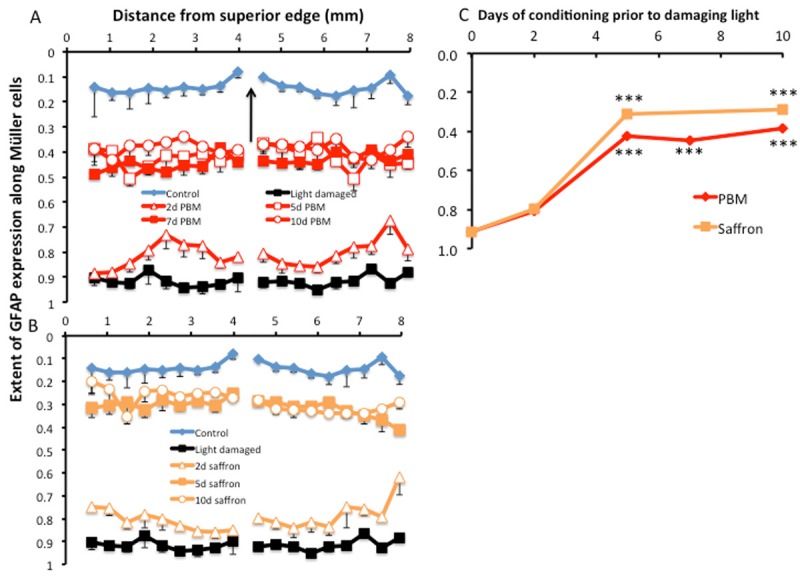
The impact of conditioning on GFAP labelling in Müller cells. This response to stress was distinct from photoreceptor apoptosis and survival in being fairly constant across the retina. Error bars represent standard errors. A: Labelling of Müller cells was least in control retinas and greatest in unconditioned light damaged retina. Preconditioning by PBM for 2, 5, 7 and 10 d progressively reduced the length of Müller cell which labelled for GFAP. B: Preconditioning with dietary saffron fro 2, 5 and 10 d also reduced the length of Müller cell that labelled for GFAP. C: A summary graph, for each of the 2 experimental groups. The reduction in length labelled reached statistical significance (P < 0.001) at 5 d and 10 d preconditioning with saffron and at 5 d, 7 d and 10 d preconditioning with PBM.
Results
Measures of neuroprotection
Three measures of neuroprotection were used, the surviving population of photoreceptors, the rate of photoreceptor death, and the expression of the stress-inducible protein GFAP in Müller cells. All were assessed 1w after exposure to damaging light.
Increased photoreceptor survival
The survival of photoreceptors was examined as the thickness of the ONL. Light damage reduced the thickness of the ONL, causing loss of and damage to many cells in that layer, i.e. to photoreceptors (compare Figure 1A, 1B). When the animal was preconditioned with PBM, the loss of neurones from the ONL was mitigated in a progressive way (Figure 1C, 1E, 1G, 1I). When the animal was preconditioned with dietary saffron for 2, 5 and 10 days, again the thinning of the ONL was mitigated (Figure 1D, 1F, 1H).
Figure 1.
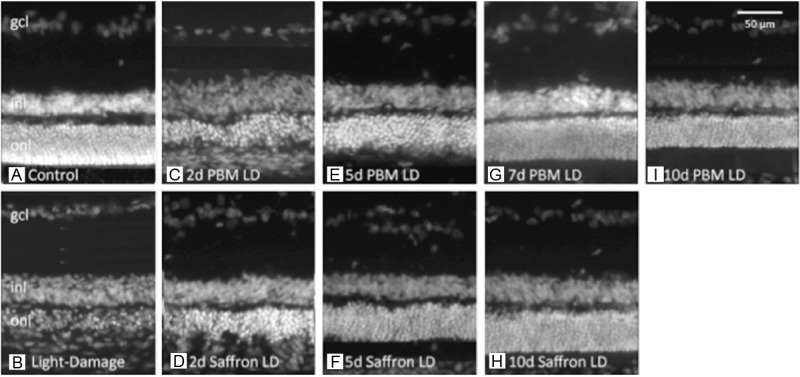
Representative retinal sections, labelled with bisbenzimide, for nuclear DNA. gcl – ganglion cell layer, inl – inner nuclear layer, onl – outer nuclear layer. All images are from the light-vulnerable region of retina, superior to the optic disc. A: Control retina. B: Light damaged retina – the ONL is thinned. C, E, G, I: Retinas exposed to damaging light after preconditioning with PBM for 2, 5, 7 and 10 d. D, F, H: Retinas exposed to damaging light after preconditioning with dietary saffron for 2, 5 and 10 d.
To quantify this effect, ONL thickness was recorded as a proportion of the thickness of the retina, measured from the ILM to the OLM, formed by the ONL. In the control (unstressed, unconditioned) retina, the thickness of the ONL was between 0.3 and 0.4, across the retina (control, Figure 2A, 2B). Exposure to damaging light caused a thinning of the ONL, most markedly in the superior retina where, approximately 3 mm from the superior edge, the layer was reduced to a localised minimum of 0.15. This localised region of sensitivity to bright light has been described in earlier studies [5,39]. Preconditioning with PBM (Figure 2A) or with saffron (Figure 2B) was associated with reduced thinning of the ONL in superior retina.
The graph in Figure 2C shows the thickness of the ONL, averaged over superior retina, as a function of days preconditioning, with PBM or saffron. Compared to the light damage control, the ONL was significantly thicker after 7 d preconditioning with PBM, and after 5 d preconditioning with saffron.
Reduced rate of photoreceptor death
The converse of photoreceptor survival is photoreceptor death. The rate of photoreceptor death in the control retina (dim-reared, not exposed to bright light, unconditioned by saffron or PBM) was very low (‘control’ in Figure 3A, 3B). Exposure to damaging light increased the count of TUNEL+ cells, most prominently in superior retina (light damaged, in Figure 3A, 3B). When the retina was preconditioned with 2, 5, 7 and 10 d PBM, or with 2 d, 5 d and 10 d saffron, the TUNEL count in superior retina was reduced. Because, in the current experiments, retinas were examined 1w after the bright light exposure that induced cell death, the numbers of TUNEL+ profiles were lower than in earlier studies (for example [39]), in which the retina was examined immediately after the damaging light exposure.
The graph in Figure 3C shows the variation in the frequency of TUNEL+ cells, average over the two positions (2 mm, 3 mm) sampled within superior retina, as a function of the period of preconditioning. For PBM, a reduction in cell death was detectable at 5 d and reached statistical significance at 7 and 10 d. For saffron, a reduction was evident and significant at 2 d and 5 d, and was increased at 10 d.
Reduced upregulation of GFAP
The impact of preconditioning by saffron and PBM on retinal stress is illustrated in Figures 4 and 5 using GFAP expression in Müller cells as a marker of stress. In the unstressed retina, GFAP expression is prominent in astrocytes, at the inner surface of the retina, but not in Müller cells (Figure 4A). The image in Figure 4A is from mid-peripheral retina, away from the anterior and disc edges of the retina, at which an edge-related stress upregulates photoreceptor death and GFAP expression in Müller cells [51,53,54].
Figure 4.
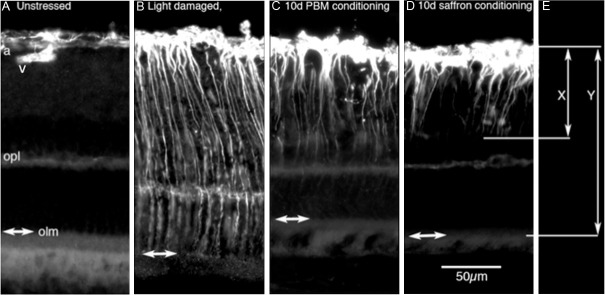
The measurement of GFAP labelling in Müller cells. All images were from inferior retina, where the ONL was not significantly thinned by the damaging light, but the expression of GFAP was nevertheless regulated. A: GFAP labelling in control (unstressed) retina was limited to astrocytes at the inner surface; these extended deep from the inner surface only along blood vessels (v). B: In the light-damaged retina, GFAP was strongly expressed in Müller cells from the inner surface to the outer limiting membrane (olm and double headed arrows in A-D). C, D: Conditioning with saffron or PBM reduced the extent of the labelling. We recorded the length of labelling as the ratio of the length of labelling along Müller cells normalized to retinal thickness (so X/Y in E).
In the light- damaged retina, GFAP expression is evident along the full length of Müller cell processes, from the ILM to the OLM (Figure 4B). This full-length upregulation was apparent throughout the retina, not just at the light-sensitive region of superior retina. After PBM and saffron preconditioning, some expression of GFAP in Müller cells is evident, but it is limited to the inner ends of the Müller cell processes (Figure 4C, 4D). That is, preconditioning limited the upregulation of GFAP in Müller cells; this limiting effect also was evident throughout the retina. Figure 4E shows how the length of Müller cell expressing GFAP was recorded – as the length of Müller cell labelled for GFAP (X), divided by the thickness of the retina from ILM to OLM (Y).
The results are summarised in Figure 5, and confirm the recent observation [48] that PBM reduces the upregulation of GFAP in Müller cells of mouse retina degenerating consequent to a complement factor knockout. In the control (unstressed, unconditioned) retina, GFAP was found only at the inner surface, in astrocytes, extending ~ 1/10th of the distance from the ILM to the OLM (control in A, B). In the light-stressed but unconditioned retina, GFAP expression extended 90% or more of that distance (light damaged in A, B). Preconditioning with PBM (A) or with saffron (B) reduced the length of expression to intermediate values. Figure 5C shows the dose-related reduction in GFAP upregulation, averaged across the length of the retinal section. The reduction was statistically significant for PBM at 5 d, 7d and 10 d, and for saffron at 5 d and 10 d.
Discussion
Present data indicate that saffron and PBM, given daily in doses, protect retinal photoreceptors, the protection increasing with the number of days of treatment, up to 7-10 d. The findings raise issues of toxicity, daily dose, mechanism and therapeutic implications.
Toxicity
Saffron has been used for millenia as a spice and traditional medicine, and is generally regarded as non-toxic. Correspondingly, the peer-reviewed literature on saffron (> 300 studies and reviews since 1990) gives little evidence of toxicity, and some compendia of toxic plants [55-57] make no mention of saffron. Nevertheless, saffron, like all plants, is toxic if ingested in sufficient quantities. LD50 levels have been reported (> 600 mg/kg [58]; 20.7 g/kg [59]) for rodents and toxicity (intestinal bleeding) has been reported in humans [60] at 5 g/day. These levels are much greater than neuroprotective doses (below), giving a large margin of safety. In the one safety trial of saffron in humans [61] that we identified, no clinically significant ill effects were reported for doses of up to 400 mg/d for 7 d.
For PBM, also known as NIR (near-infrared radiation) or LLLT (low level laser/light therapy), we have been unable to find reports of tissue damage or morbidity. Doses higher than the daily levels recommended by the World Association of Laser Therapy [62] have been explored, showing a loss of effectiveness (hormesis, below), but no toxicity.
This relative freedom from toxicity makes saffron and PBM distinctive as neuroprotectants, as many stimuli for neuroprotection (exercise [28], phytotoxins [29,63,64] gamma rays [14,65] caloric restriction [30] or conditioning by heat [66], light [7,9], hypoxia [67] and mechanical injury [49]) are stressful, or directly damaging.
Establishing an effective dose
For neuroprotectants such as saffron and PBM, whose mechanisms are not well established, the effective dose has been determined empirically. For PBM, the basis for choice is extensive [42,68] and has been codified [62]. In their meta-analysis, Tumilty and colleagues [68] conclude that the failure of 13 of the 25 trials of PBM in treating tendinopathy resulted from the use of intensities outside the range recommended by the World Association on Laser Therapy [62]. The daily dose used in this study lies within, and towards the low end of that range. High doses are associated with a reduced effect (the ‘biphasic’ response discussed by Huang and colleagues [42], which others have termed ‘hormesis’ [14,42,69], but not (yet) by evidence of toxicity.
In clinical trials of saffron, with positive outcomes for cognition in Alzheimer’s disease [70,71], premenstrual stress [72] and depression [73,74], a daily dose of 30 mg was used over days and weeks. In a clinical trial of saffron in age-related macular degeneration (AMD), with strongly positive outcomes [18,19], a daily dose of 20 mg was used, for over a year. In rodent experiments [13,34], significant protection of retinal photoreceptors was reported with doses of 1 mg/kg/d, as an aqueous extract; Hosseinzadeh and colleagues [75] reported increases in sexual potency with doses of 80-320 mg/d; Pitsikas and colleagues [76] reported improvements in memory with doses of 30-60 mg/kg; and Premkumar and colleagues [77-80] reported protection from genotoxicity with doses of 20-100 mg/kg daily.
In the present study we chose a daily dose at the low end of the range established as effective by earlier work, for both PBM and saffron, and then increased the number of days of preconditioning. This approach was chosen because it seemed possible that the actions of these therapies include the upregulation of slow-responding, but still unidentified protective systems, which take days to take effect.
Implications for mechanism
The day-by-day increase in the neuroprotective effectiveness of saffron and PBM evident in Figures 1, 2, 3, 4 and 5 is a simple but novel observation, which gives some insight into the time course of the underlying mechanisms. The build-up of neuroprotection over 7-10 d suggests that saffron and PBM slowly active a still unknown, endogenous protective mechanism. One possibility is the activation of local tissue mechanisms, such as the repair of mitochondria by PBM [42]; another is the activation of circulating immune-related cells, which migrate to sites of damage.
Saffron is the most strongly antioxidant-rich plant known [81], and might have a significant effect as a chemical antioxidant. Studies of the absorption of dietary saffron into the blood, where it is detected as crocetin [82,83], suggest that blood levels reach a peak ~ 4 h after oral administration of the precursor crocin, and then decline with a half-life of 6-7 h. Single daily dosing with saffron, as used in this study, seems therefore to generate a daily pulse of bioactive molecules in the blood. The slow build-up of the neuroprotective effect of saffron does not correlate with this daily rhythm, supporting the view [13,33,34] that saffron does not act as a direct anti-oxidant.
Several previous studies have reported neuroprotection using long-term daily doses of saffron and PBM. In the light damage/SD rat model, data from Maccarone and colleagues [13] show a neuroprotective effect of saffron given over 6w; and in a human clinical trial [19] the neuroprotective effect of saffron persisted to 1 year, provided treatment continued. Significant effects of PBM have been reported in human studies using single doses [31,43], but the use of PBM in several doses over two weeks has been reported to be effective in the treatment of AMD [16].
Contrast with biphasic dose-response relationships reported for PBM
The monotonic dose-response relationship observed here for PBM is distinct from ‘biphasic’ relationships reported for PBM in a number of models of tissue degeneration (reviewed [42]). In this context, ‘biphasic’ refers to a trend for the effectiveness of conditioning with PBM first to increase and then to reduce, as the strength of irradiation is increased. Huang and colleagues cite examples, both in vitro and in vivo. The same biphasic relationship has been reported in studies of tissue protection caused by phytotoxins [29] and gamma rays [14]; these authors used the term ‘hormesis’ to describe the shift from beneficial effects at low doses to toxicity at higher doses.
It is a feature of the studies that reported a biphasic dose-response relationship for PBM, that dose was increased by giving multiple doses within a 24 h (usually 12 h) period. Our data suggest that, when the interval between doses is 1 d, and dose is increased by increasing the number of once-a-day treatments, the dose-response relationship is monotonic.
Conclusions
The neuroprotective effects of dietary saffron and photobiomodulation, given in established daily doses, were assessed in a light damage model of photoreceptor degeneration, in the rat. The neuroprotective effects of both built up over 5-10 d of administration, suggesting that they activate endogenous protective mechanisms, possibly the same mechanism. This time course of effect does not support the possibility that saffron, although highly anti-oxidant, acts as an anti-oxidant. Possible mechanisms are discussed, and are under investigation.
Acknowledgements
This work was supported by Australian Travel Awards for L’Aquila Researchers (ARIA) to F.D.M. and S.R. and by Programma Operativo Regionale (P.O.R.) Abruzzo to F.D.M. It also received support from the ARC Centre of Excellence in Research, and from the Sir Zelman Cowen Universities Fund. JS is a director or CSCM Pty Ltd.
References
- 1.Steinberg RH. Survival factors in retinal degenerations. Curr Opin Neurobiol. 1994;4:515–524. doi: 10.1016/0959-4388(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 2.Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, Chu Y, Barnett N, Provis J, Lewis G, Fisher S, Bisti S, Gargini C, Cervetto L, Merin S, Pe’er J. Mechanisms of photoreceptor death and survival in mammalian retina. Prog Retin Eye Res. 1999;18:689–735. doi: 10.1016/s1350-9462(98)00032-9. [DOI] [PubMed] [Google Scholar]
- 3.Maslim J, Valter K, Egensperger R, Hollander H, Stone J. Tissue oxygen during a critical developmental period controls the death and survival of photoreceptors. Invest Ophthal Vis Sci. 1997;38:1667–1677. [PubMed] [Google Scholar]
- 4.Jozwick C, Valter K, Stone J. Reversal of functional loss in the P23 h-3 rat retina by management of ambient light. Exp Eye Res. 2006;83:1074–1080. doi: 10.1016/j.exer.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Chrysostomou V, Stone J, Stowe S, Barnett NL, Valter K. The status of cones in the rhodopsin mutant P23 h-3 retina: light-regulated damage and repair in parallel with rods. Invest Ophthalmol Vis Sci. 2008;49:1116–1125. doi: 10.1167/iovs.07-1158. [DOI] [PubMed] [Google Scholar]
- 6.Paskowitz DM, LaVail MM, Duncan JL. Light and inherited retinal degeneration. Br J Ophthalmol. 2006;90:1060–1066. doi: 10.1136/bjo.2006.097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Peng M, Laties AM, Wen R. Preconditioning with bright light evokes a protective response against light damage in the rat retina. J Neurosci. 1998;18:1337–1344. doi: 10.1523/JNEUROSCI.18-04-01337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Peng M, Wen R. Pre-exposure to constant light protects photoreceptor from subsequent light damage in albino rats. Invest Ophthal Vis Sci. 1997;38:S718. [Google Scholar]
- 9.Zhu Y, Valter K, Stone J. Environmental damage to the retina and preconditioning: contrasting effects of light and hyperoxic stress. Invest Ophthalmol Vis Sci. 2010;51:4821–4830. doi: 10.1167/iovs.09-5050. [DOI] [PubMed] [Google Scholar]
- 10.Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100:3439–44. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albarracin R, Eells J, Valter K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011;52:3582–3592. doi: 10.1167/iovs.10-6664. [DOI] [PubMed] [Google Scholar]
- 12.Kirk DK, Gopalakrishan S, Schmitt H, Abroe B, Stoeht M, Dubis A, Carroll J, Stone J, Valter K, Eells J. Proc. SPIE, Mechanisms for Low-Light Therapy VIII. 2013. Photobiomodulation reduces photoreceptor death and regulates cytoprotection in early states of P23 h retinal dystrophy. [Google Scholar]
- 13.Maccarone R, Di Marco S, Bisti S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Invest Ophthalmol Vis Sci. 2008;49:1254–1261. doi: 10.1167/iovs.07-0438. [DOI] [PubMed] [Google Scholar]
- 14.Otani A, Kojima H, Guo C, Oishi A, Yoshimura N. Low-Dose-Rate, Low-Dose Irradiation Delays Neurodegeneration in a Model of Retinitis Pigmentosa. Am J Pathol. 2012;180:328–36. doi: 10.1016/j.ajpath.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Vingolo EM, Rocco M, Grenga P, Salvatore S, Pelaia P. Slowing the degenerative process, long lasting effect of hyperbaric oxygen therapy in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2008;246:93–98. doi: 10.1007/s00417-007-0652-z. [DOI] [PubMed] [Google Scholar]
- 16.Ivandic BT, Ivandic T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed Laser Surg. 2008;26:241–245. doi: 10.1089/pho.2007.2132. [DOI] [PubMed] [Google Scholar]
- 17.Chew EY, Lindblad AS, Clemons T. Summary results and recommendations from the age-related eye disease study. Arch Ophthalmol. 2009;127:1678–1679. doi: 10.1001/archophthalmol.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsini B, Piccardi M, Minnella A, Savastano C, Capoluongo E, Fadda A, Balestrazzi E, Maccarone R, Bisti S. Saffron Supplementation Improves Retinal Flicker Sensitivity in Early Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2010;51:6118–6124. doi: 10.1167/iovs.09-4995. [DOI] [PubMed] [Google Scholar]
- 19.Piccardi M, Marangoni D, Minnella AM, Savastano MC, Valentini P, Ambrosio L, Capoluongo E, Maccarone R, Bisti S, Falsini B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid Based Complement Alternat Med. 2012;2012:429124. doi: 10.1155/2012/429124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, Lampl Y, Streeter J, DeTaboada L, Chopp M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37:2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 21.Shaw VE, Spana S, Ashkan K, Benabid AL, Stone J, Baker GE, Mitrofanis J. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J Comp Neurol. 2010;518:25–40. doi: 10.1002/cne.22207. [DOI] [PubMed] [Google Scholar]
- 22.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8:394–399. [PubMed] [Google Scholar]
- 23.Saleem S, Ahmad M, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Islam F. Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J Med Food. 2006;9:246–253. doi: 10.1089/jmf.2006.9.246. [DOI] [PubMed] [Google Scholar]
- 24.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, Lamari FN. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;54:8762–8768. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 25.Purushothuman S, Nandasena C, Peoples CL, El Masri N, Johnstone DM, Mitrofanis J, Stone J. Saffron pre-treatment offers neuroprotection to nigral and retinal dopaminergic cells of MPTP-treated mice. J Parkinsons Dis. 2013;3:77–83. doi: 10.3233/JPD-130173. [DOI] [PubMed] [Google Scholar]
- 26.Bhuiyan MI, Kim YJ. Mechanisms and prospects of ischemic tolerance induced by cerebral preconditioning. Int Neurourol J. 2011;14:203–212. doi: 10.5213/inj.2010.14.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen HA, Loukogeorgakis S, Yannopoulos F, Rimpilainen E, Petzold A, Tuominen H, Lepola P, Macallister RJ, Deanfield JE, Makela T, Alestalo K, Kiviluoma K, Anttila V, Tsang V, Juvonen T. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123:714–721. doi: 10.1161/CIRCULATIONAHA.110.986497. [DOI] [PubMed] [Google Scholar]
- 28.Ding Y, Li J, Luan X, Ding YH, Lai Q, Rafols JA, Phillis JW, Clark JC, Diaz FG. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004;124:583–591. doi: 10.1016/j.neuroscience.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–260. doi: 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- 31.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 32.Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J, Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M, Streeter J. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40:1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- 33.Ordoudi SA, Befani CD, Nenadis N, Koliakos GG, Tsimidou MZ. Further examination of antiradical properties of Crocus sativus stigmas extract rich in crocins. J Agric Food Chem. 2009;57:3080–3086. doi: 10.1021/jf804041g. [DOI] [PubMed] [Google Scholar]
- 34.Natoli R, Zhu Y, Valter K, Bisti S, Eells J, Stone J. Gene and noncoding RNA regulation underlying photoreceptor protection: microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol Vis. 2010;16:1801–1822. [PMC free article] [PubMed] [Google Scholar]
- 35.Hauben E, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Akselrod S, Neeman M, Cohen IR, Schwartz M. Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet. 2000;355:286–287. doi: 10.1016/s0140-6736(99)05140-5. [DOI] [PubMed] [Google Scholar]
- 36.Hauben E, Schwartz M. Therapeutic vaccination for spinal cord injury: helping the body to cure itself. Trends Pharmacol Sci. 2003;24:7–12. doi: 10.1016/s0165-6147(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 37.London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, Schwartz M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte- derived macrophages. J Exp Med. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eells J, DeSmet K, Kirk D, Wong-Riley M, Whelan H, Ver Hoeve J, Nork T, Stone J, Valter K. Photobiomodulation for the Treatment of Retinal Injury and Retinal Degenerative Diseases. In: Waynant R, Tata D, editors. Proceeding of Light- Assisted Tissue Regeneration and Therapy Conference. New York: Springer; 2008. pp. 39–51. [Google Scholar]
- 39.Bowers F, Valter K, Chan S, Walsh N, Maslim J, Stone J. Effects of Oxygen and bFGF on the Vulnerability of Photoreceptors to Light Damage. Invest Ophthalmol Vis Sci. 2001;42:804–815. [PubMed] [Google Scholar]
- 40.Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food Sci Nutr. 2004;44:155–172. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- 41.Hamblin M, Demidova N. Mechanisms of low-light therapy. In: Hamblin M, Waynant R, Anders J, editors. Mechanisms for Low-Light Therapy. Bellingham, WA: The International Society for Optical Engineering; 2006. Proc SPIE. [Google Scholar]
- 42.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct. 2009;5:46. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bignami A, Dahl D. The radial glia of Muller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979;28:63–69. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- 46.Yu DY, Cringle S, Valter K, Walsh N, Lee D, Stone J. Photoreceptor death, trophic factor expression,retinal oxygen status, and photoreceptor function in the P23 h rat. Invest Ophthalmol Vis Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- 47.Burns M, Robles M. Muller cell GFAP expression exhibits gradient from focus of photoreceptor light damage. Curr Eye Res. 1990;9:479–486. doi: 10.3109/02713689008999613. [DOI] [PubMed] [Google Scholar]
- 48.Begum R, Powner MB, Hudson N, Hogg C, Jeffery G. Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration model. PLoS One. 2013;8:e57828. doi: 10.1371/journal.pone.0057828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao W, Li F, Steinberg RH, Lavail MM. Development of Normal and Injury-induced Gene Expression of aFGF, bFGF, CNTF, BDNF, GFAP and IGF-I in the Rat Retina. Exp Eye Res. 2001;72:591–604. doi: 10.1006/exer.2001.0990. [DOI] [PubMed] [Google Scholar]
- 50.Cao W, Li F, LaVail M, Steinberg R. Development of injury-induced gene expression of bFGF, FGFR-1,CNTF and GFAP in rat retina. Invest Ophthal Vis Sci. 1997;38:S604. [Google Scholar]
- 51.Mervin K, Stone J. Developmental Death of Photoreceptors in the C57BL/6JMouse: Association with Retinal Function and Self-protection. Exp Eye Res. 2002;75:703–713. doi: 10.1006/exer.2002.2063. [DOI] [PubMed] [Google Scholar]
- 52.Barnett NL, Osborne NN. Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp Eye Res. 1995;61:83–90. doi: 10.1016/s0014-4835(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 53.Mervin K, Stone J. Regulation by Oxygen of Photoreceptor Death in the Developing and Adult C57BL/6J Mouse. Exp Eye Res. 2002;75:715–722. doi: 10.1006/exer.2002.2064. [DOI] [PubMed] [Google Scholar]
- 54.Stone J, Mervin K, Walsh N, Valter K, Provis J, Penfold P. Photoreceptor stability and degeneration in mammalian retina: lessons from the edge. In: Penfold P, Provis J, editors. Macular Degeneration: Science and Medicine in Practice. Springer Verlag; 2005. pp. 149–165. [Google Scholar]
- 55.Dorfler H, Rosell G. The Dictionary of Healing Plants. London: Blandford Press; 1989. [Google Scholar]
- 56.Bruneton J. Toxic Plans Dangerous to Humans and Animals. Secaucus, NJ: Lavoisier Publishing Inc; 1999. [Google Scholar]
- 57.Acamovic T, Stewart C, Pennycott T. Poisonous Plants and Related Toxins. Cambridge, MA USA: CABI Publishing; 2004. [Google Scholar]
- 58.Nair SC, Kurumboor SK, Hasegawa JH. Saffron chemoprevention in biology and medicine: a review. Cancer Biother. 1995;10:257–264. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- 59.WHO. World Health Organization. Geneva: WHO Press; 2007. WHO monographs on selected medicinal plants; pp. 126–135. [Google Scholar]
- 60.Wagstaff D. International Poisonous Plants Checklist. London: CRC Press; 2008. [Google Scholar]
- 61.Modaghegh MH, Shahabian M, Esmaeili HA, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Word Association for Laser Therapy Dosage Recommendations. http://waltza.co.za/wp-content/uploads/2012/08/Dose_table_780-860nm_for_Low_Level_Laser_Therapy_WALT-2010.pdf.
- 63.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 64.Kaplan S, Bisleri G, Morgan JA, Cheema FH, Oz MC. Resveratrol, a natural red wine polyphenol, reduces ischemia-reperfusion-induced spinal cord injury. Ann Thorac Surg. 2005;80:2242–2249. doi: 10.1016/j.athoracsur.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 65.Kipnis J, Avidan H, Markovich Y, Mizrahi T, Hauben E, Prigozhina TB, Slavin S, Schwartz M. Low-dose gamma-irradiation promotes survival of injured neurons in the central nervous system via homeostasis-driven proliferation of T cells. Eur J Neurosci. 2004;19:1191–1198. doi: 10.1111/j.1460-9568.2004.03207.x. [DOI] [PubMed] [Google Scholar]
- 66.Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988;241:1817–1820. doi: 10.1126/science.3175623. [DOI] [PubMed] [Google Scholar]
- 67.Grimm C, Hermann DM, Bogdanova A, Hotop S, Kilic U, Wenzel A, Kilic E, Gassmann M. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin Cell Dev Biol. 2005;16:531–538. doi: 10.1016/j.semcdb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD. Low level laser treatment of tendinopathy: a systematic review with meta-analysis. Photomed Laser Surg. 2009;28:3–16. doi: 10.1089/pho.2008.2470. [DOI] [PubMed] [Google Scholar]
- 69.Mattson MP, Son TG, Camandola S. Viewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicals. Dose Response. 2007;5:174–186. doi: 10.2203/dose-response.07-004.Mattson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akhondzadeh S, Sabet MS, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, Hejazi S, Yousefi MH, Alimardani R, Jamshidi A, Zare F, Moradi A. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther. 2010;35:581–588. doi: 10.1111/j.1365-2710.2009.01133.x. Original article. [DOI] [PubMed] [Google Scholar]
- 71.Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, Hejazi SS, Yousefi MH, Alimardani R, Jamshidi A, Rezazadeh SA, Yousefi A, Zare F, Moradi A, Vossoughi A. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology (Berl) 2009;207:637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- 72.Agha-Hosseini M, Kashani L, Aleyaseen A, Ghoreishi A, Rahmanpour H, Zarrinara AR, Akhondzadeh S. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: a double-blind, randomised and placebo-controlled trial. BJOG. 2008;115:515–519. doi: 10.1111/j.1471-0528.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 73.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, Khani M. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19:148–151. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- 74.Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97:281–284. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Hosseinzadeh H, Ziaee T, Sadeghi A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine. 2008;15:491–495. doi: 10.1016/j.phymed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 76.Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. Effects of the active constituents of Crocus sativus L. , crocins on recognition and spatial rats’ memory. Behav Brain Res. 2007;183:141–146. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Premkumar K, Thirunavukkarasu C, Abraham SK, Santhiya ST, Ramesh A. Protective effect of saffron (Crocus sativus L. ) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum Exp Toxicol. 2006;25:79–84. doi: 10.1191/0960327106ht589oa. [DOI] [PubMed] [Google Scholar]
- 78.Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Inhibitory effects of aqueous crude extract of Saffron (Crocus sativus L. ) on chemical-induced genotoxicity in mice. Asia Pac J Clin Nutr. 2003;12:474–476. [PubMed] [Google Scholar]
- 79.Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocus sativus Linn. ) on genotoxins-induced oxidative stress in Swiss albino mice. Phytother Res. 2003;17:614–617. doi: 10.1002/ptr.1209. [DOI] [PubMed] [Google Scholar]
- 80.Premkumar K, Abraham SK, Santhiya ST, Gopinath PM, Ramesh A. Inhibition of genotoxicity by saffron (Crocus sativus L. ) in mice. Drug Chem Toxicol. 2001;24:421–428. doi: 10.1081/dct-100106266. [DOI] [PubMed] [Google Scholar]
- 81.Pellegrini N, Serafini M, Salvatore S, Del Rio D, Bianchi M, Brighenti F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol Nutr Food Res. 2006;50:1030–1038. doi: 10.1002/mnfr.200600067. [DOI] [PubMed] [Google Scholar]
- 82.Asai A, Nakano T, Takahashi M, Nagao A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J Agric Food Chem. 2005;53:7302–7306. doi: 10.1021/jf0509355. [DOI] [PubMed] [Google Scholar]
- 83.Umigai N, Murakami K, Ulit MV, Antonio LS, Shirotori M, Morikawa H, Nakano T. The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomedicine. 2011;18:575–578. doi: 10.1016/j.phymed.2010.10.019. [DOI] [PubMed] [Google Scholar]


