Abstract
The subventricular zone retains its neurogenic capacity throughout life and, as such, is often considered a potential source for endogenous repair in neurodegenerative disorders. Because dopamine is believed to stimulate adult neurogenesis, we looked for possible variations in the dopaminergic innervation of the subventricular zone between cases of Huntington’s chorea and Parkinson’s diseases. Antibodies against tyrosine hydroxylase (TH) and proliferating cell nuclear antigen (PCNA) were used as specific markers of dopaminergic axons and cell proliferating activity, respectively. The immunohistochemical approach was applied to postmortem tissue from 2 Parkinson’s disease cases, 4 Huntington’s disease cases, along with age-matched controls. The immunostaining was revealed with either diaminobenzidine or fluorescent-conjugated secondary antibodies. Optical density measurements were made along the entire dorso-ventral extent of the caudate nucleus. An intense TH+ zone was detected along the ventricular border of the caudate nucleus in Huntington’s disease cases, but not in patients with Parkinson’s disease or age-matched controls. This thin (287±38 μm) paraventricular zone was composed of numerous small and densely packed dopamine axon varicosities and overlapped the deep layers of the subventricular zone. Its immunoreactivity was 47±8% more intense than that of adjacent striatal areas. The dopamine innervation of the subventricular zone is strikingly massive in Huntington’s chorea compared to Parkinson’s disease, a finding that concurs with the marked increase in neurogenesis noted in the subventricular zone of Huntington’s disease patients. This finding suggests that dopamine plays a crucial role in mechanisms designed to compensate for the massive striatal neuronal losses that occur in Huntington’s disease.
Keywords: Stem cells, adult neurogenesis, basal ganglia, Parkinson’s disease, Huntington’s chorea, subventricular zone, neurodegenerative disorders, human striatum
Introduction
The subventricular zone (SVZ) is one of the rare brain areas where new neurons are produced throughout life [1,2]. This germinal zone covers much of the anterior lateral ventricles, including the ventricular surface of the head of caudate nucleus, just beneath the ependymal layer. Under physiological conditions, the SVZ provides neuroblasts that migrate towards the olfactory bulb, where they differentiate into GABAergic and dopaminergic interneurons that integrate themselves into local networks [3-5]. In pathological conditions, such as stroke, demyelinating disorders and neurodegenerative diseases, the rate of adult neurogenesis can be either positively or negatively altered [6-9] and new neurons can migrate towards sites of injury [7,10]. The close proximity of the SVZ with the striatum makes it a potential source of endogenous neurons that could be engaged in brain repair strategies for Huntington (HD) and Parkinson’s diseases (PD), two neurodegenerative disorders that are characterized by opposite motor deficits and by a marked alteration of the striatal microcircuitry. The pathological hallmark of HD is a massive atrophy of the striatum [11,12] largely caused by degeneration of the medium spiny projection neurons. In this hereditary hyperkinetic disorder, a robust progenitor cell proliferation in the SVZ has been reported [7]. The pathological changes in PD most consistently affect dopaminergic neurons of the substantia nigra that innervate the striatum and the adjacent SVZ. This hypokinetic disorder is generally associated with a decrease of precursor cell proliferation, although there exist conflicting results on this issue [8,13-15].
Animal studies have been useful to improve our understanding of the role played by various growth factors and signaling molecules in the control of adult neurogenesis. For example, investigations in rodents have led to the concept that dopamine promotes the production of new neurons in the adult SVZ [8,16,17]. This view is supported by the fact that experimental lesions of the nigrostriatal dopaminergic pathway in an animal model of PD decreases precursor cell proliferation in the SVZ, whereas proliferation is restored completely by selective agonists of D2-like receptors [8]. Altogether, these findings prompted us to compare the status of the dopamine innervation of the SVZ and adjacent striatal parenchyma in patients who have suffered from idiopathic PD and others affected by HD. The results pertaining to HD patients have been presented in an abbreviated form elsewhere [18], but this material has been reanalyzed here in more details and its functional significance underlined in the light of novel findings gathered in PD patients with the very same quantitative immunohistochemical procedures. Hence, the present paper provides the very first direct comparative study of the dopaminergic innervation of the human SVZ in HD and PD, two neurodegenerative disorders that affect basal ganglia microcircuitry and motor behavior quite differently.
Materials and methods
Tissue collection
The post-mortem material used in this study was gathered from the brains of 2 PD patients, 4 patients who suffered from HD, together with 5 age-matched controls (Table 1). Tissue samples were obtained from the human brain bank of the Centre de recherche de l’Institut universitaire en santé mentale de Québec, which required informed consent before donation of tissues. The Ethics Committee at Université Laval approved the brain collecting procedures, as well as the storage and handling of post-mortem human brain material that has been described previously [19].
Table 1.
Clinical data on brain used
| Brain | Sex | Age (yrs) | Post-mortem delay (h) | Cause of death | Duration of the disease (yrs) |
|---|---|---|---|---|---|
| Parkinson’s disease | |||||
| PD1 | F | 68 | 14 | Bronchoaspiration | 19 |
| PD2 | M | 68 | 17 | Septic shock | 11 |
| Huntington’s disease (grade [12]) | |||||
| HD1 (3/4) | F | 43 | 3 | Pneumonia | 15 |
| HD2 (4/4) | M | 51 | 12 | Bronchoaspiration | 17 |
| HD3 (4/4) | M | 56 | 20 | Pneumonia | 21 |
| HD4 (4/4) | F | 52 | 10 | Bronchoaspiration | 20 |
| Controls | |||||
| C1 | M | 43 | 8 | Aortic rupture | n/a |
| C2 | M | 49 | 16 | Multi-traumatism | n/a |
| C3 | M | 55 | 18 | Pulmonary edema | n/a |
| C4 | F | 51 | 6 | Abdominal aortic aneurysm | n/a |
| C5 | F | 52 | 9 | Coronary atherosclerosis | n/a |
In the present study, all PD and HD patients satisfied clinical and neuropathological criteria established for these diseases. The two PD patients were considered at stage 5 on the Hoehn and Yahr scale [20]. The duration of the disease was 19 and 11 years and the daily dose of L-Dopa taken at the time of death was 825 and 500 mg, respectively. One PD patient showed dyskinesias. Lewy bodies and neuronal loss were documented within the substantia nigra of the 2 PD brains. All 4 HD patients had a positive family history for the disease. The striatal atrophy was documented by magnetic resonance imaging at least 10 years before the death of the patients, who all displayed choreic movements and severe cognition impairment at the time of death. The post-mortem neuropathological examination of the brains confirmed the marked volumetric atrophy of the striatum and histological analyses of hematoxylin/eosin-stained sections showed important neuronal losses associated with a compensatory gliosis, as revealed by immunostaining for GFAP and CD68. No signs of neurofibrillary degeneration (Gallyas staining) or any other major neuropathological features were disclosed. They were characterized as either grade 3 or 4 according to the Vonsattel’s scale [12]. The duration of the disease ranged from 15 to 21 years. Individuals used as controls had no clinical or pathological signs of neurological or psychiatric diseases. Their striata did not display neuronal losses nor reactive astrocytosis.
Immunohistochemistry
Antibodies raised against tyrosine hydroxylase (TH) and proliferating cell nuclear antigen (PCNA) were used as specific markers of dopamine and cell proliferating activity, respectively. Free-floating brain sections were incubated with the primary antibody against TH (mouse monoclonal IgG 1:500, ImmunoStar, Hudson, WI) and PCNA (rabbit polyclonal IgG 1:50, Santa Cruz Biotechnology, Santa Cruz, CA). Biotinylated horse anti-mouse IgG (Vector Laboratories, Burlingame, CA) was used as secondary antibody for TH immunolabeling. For optical density measurements, TH immunostaining was achieved by incubating sections with 2% avidin-biotin complex (ABC standard kit; Vector Laboratories) followed by diaminobenzidine (DAB, 0.05%), to which 0.005% H2O2 was added (see 19 for details). TH and PCNA immunofluorescent staining was revealed with Alexa 488-conjugated streptavidin antibody (1:200, Invitrogen, Carlsbad, CA) and Alexa 568-conjugated goat anti-rabbit secondary antibody (1:200, Invitrogen), respectively. Once dried, sections were treated with an autofluorescence eliminator reagent (Millipore, Billerica, MA). In addition, some sections adjacent to those used for TH/PCNA visualization were stained with an antibody raised against the serotonin (5-hydro- xytryptamine) transporter (SERT) to compare the patterns of dopamine and serotonin innervations of the striatum in PD and HD cases. These sections were incubated overnight with a SERT antibody (1:1000, goat polyclonal antibody; Santa Cruz Biotechnology) and immunoreactivity was revealed using DAB as the chromogen.
Material analysis
TH-immunostained sections from PD, HD and control brains were carefully examined using a Leica Leitz DM RB light microscope (Leica, ON, Canada). Optical density measurements of TH immunoreactivity were performed on transverse HD brain sections taken at three anteroposterior levels of the striatum (pre-commissural, commissural and post-commissural) with the anterior commissure as a mid-landmark. On each section, 6 photomicrographs were taken at 2 mm interval along the dorsoventral axis of the caudate nucleus, and at 4 mm from the ependymal layer along the mediolateral axis, using a 10X/0.30 objective. Six other photomicrographs equally spaced along the dorsoventral axis were taken at 150 μm from the ependymal layer, upon the intense TH-immuno-positive (+) zone of the caudate nucleus. From these photomicrographs, luminance information was collected using the public domain “Image J” processing software from NIH (v. 1.43r). For double-immunostained sections, fluorescence signals were imaged with a confocal laser-scanning microscope LSM 700 (Zeiss, Oberkochen, Germany). The emission signals of Alexa 488 (TH) and Alexa 568 (PCNA) were assigned the green and red colors, respectively. Statistical significance of optical density measurements was assessed by using two-tailed unpaired t-test.
Results
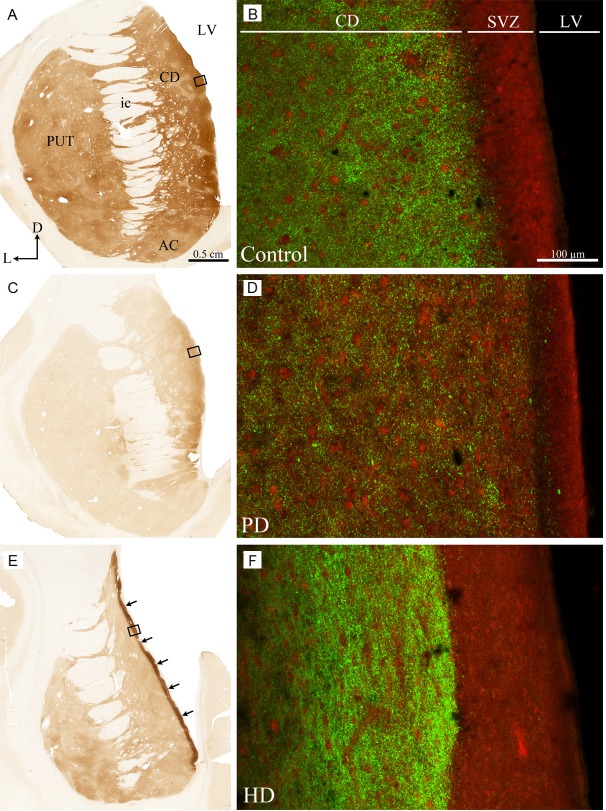
Examination of TH-immunostained sections through the human striatum revealed the presence of a thin but very intense TH+ zone lying along the inner surface of the ventricular lining of the caudate nucleus in HD brains but not in PD or control brains (Figure 1). In the pre-commissural portion of the striatum, this TH+ zone covered the entire dorsoventral extent of the ventricular border of the caudate nucleus (Figure 1E), whereas it was restricted to its dorsal two-thirds at commissural and post-commissural levels. This TH+ paraventricular zone, whose thickness ranged from 150 to 400 μm, with a mean value of 287±38 μm, was composed of many small and closely packed TH+ axon varicosities among which some thin and varicose immunoreactive fibers were scattered. In sections immunostained for both TH and PCNA, the intense TH+ zone was found to lie immediately beneath the SVZ, with some overlap with the deeper layers of the SVZ. Several varicose TH+ fibers emerging from this zone were seen to penetrate the more superficial layers of the SVZ, where they arborize in a rather diffuse manner. The SVZ itself was found to be 2 to 3 times thicker in HD cases than in controls (Figure 1B, 1F). In PD, the thickness of the SVZ appears slightly thinner than in controls (Figure 1B, 1D). As expected, TH immunoreactivity was significantly lower in the striatum of PD brains than in that of controls and lower in the putamen compared with the caudate nucleus, whose immunoreactivity displayed a marked mediolateral decreasing gradient (Figure 1C). The TH immunoreactivity of the paraventricular zone located beneath the SVZ was weaker in PD than in controls and HD brains.
Figure 1.
Transverse sections taken through the pre-commissural striatum of a control (A, B), Parkinson’s disease (C, D) and Huntington’s disease (E, F) brains immunostained for TH (A, C, E) and PCNA/TH (B, D, F). Arrows in E point to the intense TH+ zone lying along the ventricular border of the caudate nucleus in HD patients. This zone is composed of many small and closely packed TH+ axon varicosities among which some thin and varicose immunoreactive fibers are scattered. Rectangles depicted on the left panels indicate precisely the location of confocal photomicrographs shown in B, D and F. PCNA immunofluorescence appears red whereas TH immunolabeling is green. AC, nucleus accumbens; CD, caudate nucleus; D, dorsal; ic, internal capsule; L, lateral; LV, lateral ventricle; PUT, putamen; SVZ, subventricular zone.
Optical density measurements revealed that TH immunostaining was significantly more intense in the paraventricular zone compared to the remaining sectors of the caudate nucleus in all 4 HD brains examined. Despite some inter-individual variations in the mean optical density values, the TH immunostaining in the paraventricular zone was 36% to 55% (mean value of 47±8%) higher than that of adjoining striatal areas. This increase appears to be the result of an augmentation of the number of TH+ axon terminals rather than an increase in TH immunostaining intensity of single TH+ axon terminals.
In sections immunostained for SERT, numerous labeled axons were present in and around the paraventricular zone [18]. Long, thin and varicose SERT+ axons coursed among the myelinated fibers that separate the SVZ from the striatal parenchyma. Some of these SERT+ axons emitted collaterals that penetrated the SVZ as well as the ependymal layer to form a dense plexus directly within the cerebral ventricle. This pattern of distribution of serotonin axons, whose presence has been documented previously in both animals and human [21,22], was identical in HD cases and age-matched controls as well as in PD case where SERT+ striatal axons appear to be preserved. There was no dense region of SERT+ axon varicosities in the vicinity of the SVZ equivalent to the dense TH+ paraventricular zone in any of the 4 HD brains examined.
Discussion
This study has revealed the existence of an intense TH+ zone lining the ventricular border of the caudate nucleus in patients with HD but not in PD or age-matched controls. Giving that TH is the rate limiting enzyme in dopamine synthesis, the presence of a multitude of densely packed TH+ axon terminals suggests the existence in that specific locus of an intense dopamine activity, which might significantly contributes to the robust progenitor cell proliferation that has been previously reported in the SVZ of HD [7]. This increase in the degree of cell proliferation leads to a marked augmentation of the size of the SVZ zone itself, which contrasts strikingly with the severe cell loss and atrophy that occurs in the adjoining portion of the striatum in this neurodegenerative disease. Interestingly, no similar intense SERT+ zone has been observed in the paraventricular region of the striatum in HD brains, nor in that of PD brains, suggesting that serotonin does not play a major role in the increase in size and activity of the SVZ noted in HD brains, nor in the relative reduction of this zone in PD. The absence of a SERT+ paraventricular zone also demonstrates that the atrophy of the caudate nucleus in HD is not sufficient to explain the increased density of TH+ axons reported here.
As in rodents, the adult SVZ in human is composed of three distinct types of cells (i.e. A, B and C) that are differentially distributed in the various layers [7]. Among these three cell types, type C cells are of particular interest because they appear to be preferentially targeted by the dopamine axons and their proliferative capacity is reportedly regulated by this neurotransmitter through D2-like receptors [8]. Type C cells, also known as transit-amplifying progenitor cells, are located in the deeper part of the SVZ, close to the myelin layer [7], a portion of the SVZ that overlapped the intense TH+ paraventricular zone identified in the present study. In adult rodents, dopamine appears to stimulate the release of epidermal growth factor, which in turn causes C cells to become activated B (glial) cells by acting upon epidermal growth factor receptors that are coexpressed with D2/D3 receptors on C cells [23]. Dopamine can act either through direct synaptic contact via the varicose dopamine axons that invade the more superficial layers of the human SVZ or through indirect, volumic transmission, both modes of dopamine release having been well documented at the striatal level [24,25].
Degeneration of the dopamine nigrostriatal pathway in PD has been associated with a reduction in progenitor cell proliferation [8,23,26]. In accordance with these findings, the SVZ of the PD patients examined in the present study was slightly thinner than that of controls. However, whether this relatively minor thinning of the SVZ in PD is associated with a reduction in neurogenesis is unclear since, in contrast to the results of Hoglinger and collaborators [8], van den Berge and coworkers found no difference in the number of proliferating cells in the SVZ of PD brains compared to that of controls [15]. The discrepancy between these two sets of data might simply reflect methodological differences [13] or variability in pharmacotherapy administered to PD patients, including L-Dopa that is known to positively regulate neurogenesis [26]. In contrast to PD, a clear numerical increase in the three types of SVZ cells occurs in HD brains, but much of the cell proliferation is reportedly due to a massive transformation of C cells into B cells [27]. The mechanism responsible for such a major change is unknown but, based on the intense dopamine activity that occurs in this specific portion of the human striatum, as evidenced by the intense TH+ paraventricular zone disclosed in HD brains, we hypothesise that dopamine is a key factor in this remarkable SVZ transformation. The reason for such a prominent increase in the degree of SVZ cell proliferation in HD brains is unknown, but the increase in the production of B cells could be related to the robust gliogenesis that occurs in the striatal tissue in such a pathological condition [28]. It could also represent an attempt to produce new neurons to compensate for the massive losses of striatal projection neurons that characterize this neurodegenerative disease. If this is the case, the newly generated neurons obviously do not succeed in coping with the powerful neurodegenerative mechanisms at plays in this devastating disorder.
Conclusion
The present study has revealed the existence of a thin but intense dopamine zone lying immediately beneath the ventricular surface of the caudate nucleus in HD patients, but not in PD and age-matched controls. The synaptic or volumic release of dopamine by the multitude of small and densely packed dopamine axon varicosities that compose this paraventricular zone, which overlap the deep layers of the SVZ, might play a crucial role in the striking progenitor cell proliferation noted in the SVZ of HD patients. Our findings suggest that the dopamine innervation of the SVZ is a key element in intrinsic cellular mechanisms designed to compensate for the massive striatal neuronal losses that occur in HD.
Acknowledgements
This research was supported by grants from the Canadian Institutes of Health Research (CIHR, MOP-115008 to M.P.). The authors express their gratitude to Dr. Peter V. Gould for the neuropathological analysis of the brains and to Mrs. Doris Côté and Caroline Côté for expert technical assistance.
References
- 1.Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 3.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 5.Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 7.Curtis MA, Eriksson PS, Faull RL. Progenitor cells and adult neurogenesis in neurodegenerative diseases and injuries of the basal ganglia. Clin Exp Pharmacol Physiol. 2007;34:528–532. doi: 10.1111/j.1440-1681.2007.04609.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 9.Marti-Fabregas J, Romaguera-Ros M, Gomez-Pinedo U, Martinez-Ramirez S, Jimenez-Xarrie E, Marin R, Marti-Vilalta JL, Garcia-Verdugo JM. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357–365. [Google Scholar]
- 10.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 12.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Hoglinger GU, Barker RA, Hagg T, Arias-Carrion O, Caldwell MA, Hirsch EC. Quantitative evaluation of the human subventricular zone. Brain. 2012 Aug;135:e221, 1–4. doi: 10.1093/brain/aws087. author reply e222, 1-6. [DOI] [PubMed] [Google Scholar]
- 14.Marxreiter F, Regensburger M, Winkler J. Adult neurogenesis in Parkinson’s disease. Cell Mol Life Sci. 2013;70:459–473. doi: 10.1007/s00018-012-1062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Berge SA, van Strien ME, Korecka JA, Dijkstra AA, Sluijs JA, Kooijman L, Eggers R, De Filippis L, Vescovi AL, Verhaagen J, van de Berg WD, Hol EM. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain. 2011 Nov;134:3249–63. doi: 10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- 16.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Kampen JM, Hagg T, Robertson HA. Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D3 receptor stimulation. Eur J Neurosci. 2004;19:2377–2387. doi: 10.1111/j.0953-816X.2004.03342.x. [DOI] [PubMed] [Google Scholar]
- 18.Bédard C, Wallman MJ, Pourcher E, Parent A, Parent M. Intense dopamine innervation of the subventricular zone in Huntington’s disease. Neuroreport. 2010;21:1074–1079. doi: 10.1097/WNR.0b013e32834052a6. [DOI] [PubMed] [Google Scholar]
- 19.Huot P, Lévesque M, Parent A. The fate of striatal dopaminergic neurons in Parkinson’s disease and Huntington’s chorea. Brain. 2007;130:222–232. doi: 10.1093/brain/awl332. [DOI] [PubMed] [Google Scholar]
- 20.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 21.Richards JG, Lorez HP, Colombo VE, Guggenheim R, Kiss D, Wu JY. Demonstration of supra-ependymal 5-HT nerve fibres in human brain and their immunohistochemical identification in rat brain. J Physiol (Paris) 1981;77:219–224. [PubMed] [Google Scholar]
- 22.Richards JG, Lorez HP, Tranzer JP. Indolealkylamine nerve terminals in cerebral ventricles: identification by electron microscopy and fluorescence histochemistry. Brain Res. 1973;57:277–288. doi: 10.1016/0006-8993(73)90136-4. [DOI] [PubMed] [Google Scholar]
- 23.O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A. 2009;106:8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Descarries L, Watkins KC, Garcia S, Bosler O, Doucet G. Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: a quantitative autoradiographic and immunocytochemical analysis. J Comp Neurol. 1996;375:167–186. doi: 10.1002/(SICI)1096-9861(19961111)375:2<167::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.O’Sullivan SS, Johnson M, Williams DR, Revesz T, Holton JL, Lees AJ, Perry EK. The effect of drug treatment on neurogenesis in Parkinson’s disease. Mov Disord. 2011;26:45–50. doi: 10.1002/mds.23340. [DOI] [PubMed] [Google Scholar]
- 27.Curtis MA, Waldvogel HJ, Synek B, Faull RL. A histochemical and immunohistochemical analysis of the subependymal layer in the normal and Huntington’s disease brain. J Chem Neuroanat. 2005;30:55–66. doi: 10.1016/j.jchemneu.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Benraiss A, Toner MJ, Xu Q, Bruel-Jungerman E, Rogers EH, Wang F, Economides AN, Davidson BL, Kageyama R, Nedergaard M, Goldman SA. Sustained Mobilization of Endogenous Neural Progenitors Delays Disease Progression in a Transgenic Model of Huntington’s Disease. Cell Stem Cell. 2013;12:787–799. doi: 10.1016/j.stem.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]