Abstract
The importance of differentiating between social concepts when appraising actions (e.g., understanding behavior as critical vs. fault-finding) and its contribution to vulnerability to major depressive disorder (MDD) is unknown. We predicted poor integration of differentiated conceptual knowledge when people with MDD appraise their social actions, contributing to their tendency to grossly overgeneralize self-blame (e.g., “I am unlikable rather than critical”). To test this hypothesis, we used a neuropsychological test measuring social conceptual differentiation and its relationship with emotional biases in a remitted MDD and a control group. During fMRI, guilt- and indignation-evoking sentences were presented. As predicted, conceptual overgeneralization was associated with increased emotional intensity when appraising social actions. Interdependence of conceptual overgeneralization and negative emotional biases was stronger in MDD (reproducible in the subgroup without medication) and was associated with overgeneralized self-blame. This high conceptual–emotional interdependence was associated with functional disconnection between the right superior anterior temporal lobe (ATL) and right dorsolateral prefrontal cortex (PFC) as well as a septal region across groups when experiencing guilt (SPM8). Strong coupling of conceptual information (ATL) with information about the context of actions and emotions (frontal-subcortical regions) is thus associated with appraisal being less dependent on conceptual overgeneralization, thereby protecting against excessive self-blame.
Keywords: Social concepts, Moral emotions, Overgeneralization, Major depression, Vulnerability, Self-blame, Information theory, Differentiation, Redundancy, Emotional biases
The degree of differentiation with which individuals perceive themselves and the external world was at the center of theories on cognitive style, which originated at the beginning of the twentieth century (Hall & Lindzey, 1978; Witkin, Goodenough, & Oltman, 1979). Beck made the seminal observation that patients with major depressive disorder (MDD) failed to differentiate by overgeneralizing information related to self-blame and self-criticism ((Beck, 1963; Beck, Rush, Shaw, & Emery, 1979), e.g., “I spilled a glass of water on my colleague's laptop, this means I am a failure”). One central component of social information necessary for differentiated and thereby precise appraisals is the conceptual meaning of social behavior ((Zahn et al., 2007, Zahn, Moll, Iyengar, et al., 2009), e.g., this means I am “clumsy” rather than “a failure”). A comprehensive understanding of vulnerability to and resilience against MDD, therefore, requires the identification of individual variations in social conceptual differentiation and its interaction with self-blaming emotional biases (e.g., “I am a failure, therefore I hate myself”). To understand this interaction, it is important to assess whether conceptual knowledge is less informative (i.e., more redundant, (Attneave, 1959)) in people with MDD when appraising their behavior, thereby making them more vulnerable to excessive self-blame.
Previous studies of cognitive differentiation and their role in vulnerability to MDD have relied on questionnaire measures of attributional style (Alloy, Abramson, Peterson, & Seligman, 1984) or emotional differentiation (Lane, Quinlan, Schwartz, Walker, & Zeitlin, 1990). Other studies have employed experimental probes of autobiographical memory (Williams et al., 2007), personality traits attributed to oneself (Dalgleish, Hill, Golden, Morant, & Dunn, 2011; Dozois & Dobson, 2001; Goldston, Gara, & Woolfolk, 1992), or visuospatial perception (Kingsland & Greene, 1984). Although these studies confirmed the importance of cognitive overgeneralization in MDD, the underpinning neurocognitive components, especially the role of conceptual differentiation, remain elusive. Overgeneralized forms of self-blaming emotions (e.g., self-hate, exaggerated guilt) remain detectable after remission of symptoms (Ghatavi, Nicolson, MacDonald, Osher, & Levitt, 2002; Green, Moll, Deakin, Hulleman, & Zahn, 2013), confirming their importance as “trait markers” of vulnerability to MDD according to one influential cognitive model (Abramson, Seligman, & Teasdale, 1978). Attribution questionnaire measures of overgeneralization (Abramson et al., 1978; Alloy et al., 1984), however, did not explain increased self-hate in people with remitted MDD (Green, Moll, et al., 2013). It is thus unknown whether there is a link between cognitive overgeneralization and self-blaming emotions.
Recent evidence from fMRI provided new clues to the understanding of individual differences in self-blaming emotions such as guilt. Guilt-evoked activations in the septal region and subgenual cingulate cortex on fMRI were reproducibly shown to vary between healthy individuals (Basile et al., 2011; Zahn, de Oliveira-Souza, Bramati, Garrido, & Moll, 2009; Zahn, Moll, Paiva, et al., 2009). Further, these septal-subgenual cingulate activations were selective for guilt compared with emotions related to blaming others (indignation) while controlling for the degree of negative valence (Green, Lambon Ralph, Moll, Deakin, & Zahn, 2012; Zahn, Moll, Paiva, et al., 2009).
In contrast to the guilt-selectivity of the septal-subgenual region, there were equal levels of fMRI activation in healthy people within the right superior anterior temporal lobe (ATL) irrespective of the context of emotions (guilt, indignation) and agency (self or others (Green, Lambon Ralph, et al., 2012; Zahn, Moll, Paiva, et al., 2009)) or valence (positive, negative (Zahn et al., 2007, Zhan, Moll, Paiva, 2009)). The right superior ATL was previously associated with selective activations for social versus nonsocial concepts (Skipper, Ross, & Olson, 2011; Zahn et al., 2007), and its lesion was associated with selective impairments of social relative to nonsocial concepts (Zahn, Moll, Iyengar, et al., 2009). The importance of the right superior ATL for social concepts was in keeping with a large body of evidence that the ATL is the only brain region representing conceptual knowledge irrespective of modality (e.g., visual, verbal) and context (e.g., the core meaning of stinginess across different behavioral contexts such as giving away time or donating money remains invariant (Lambon Ralph & Patterson, 2008; Lambon Ralph, Sage, Jones, & Mayberry, 2010; Patterson, Nestor, & Rogers, 2007)).
Healthy participants at low risk of MDD showed strong coupling between the ATL and the septal-subgenual cingulate region, whilst feeling guilty during fMRI (Green et al., 2010) as a sign of functional integration of information (Friston et al., 1997). This led to the prediction that people with MDD, even when remitted from symptoms, should show poor functional integration between these regions while feeling guilty, thereby making them more vulnerable to overgeneralization of self-blame. This hypothesis was confirmed in a previous fMRI study, which showed guilt-selective functional disconnection of the right superior ATL (Green, Lambon Ralph, et al., 2012). Individual variations in the degree of differentiation with which social concepts are represented, however, were not assessed so far, so that the exact contribution of the right superior ATL to individual styles of social information processing and self-blaming biases remained elusive.
Here, we used a novel neuropsychological test, the conceptual social knowledge differentiation (CSKD) task, to directly probe access to differentiated conceptual knowledge (i.e., how distinctively participants perceived the meaning of social concepts) when appraising social behavior in different contexts of agency (self vs. other) and emotional valence (positive and negative behaviors). For example, in the negative self-agency condition, participants saw descriptions such as “[participant's name] deceiving [best friend's name] to gain achievements in sports,” and in the negative other-agency condition, the names were reversed. They had to pick the concept that best described one imagined example of this behavior from a selection of 19 concepts. Participants were only allowed to pick more than one concept if this described the behavior equally well. The 19 concepts were carefully selected based on normative data to include few concepts with overlapping meaning that can be applied to several of the social behaviors. This means that if participants chose a higher number of concepts than two (e.g., “deceitful,” “dishonest”) to describe a given social behavior (e.g., “deceiving one's best friend to gain achievements in sports”), they picked concepts that were more likely to be only loosely related (e.g., “selfish”) or unrelated to the social behavior, indicating conceptual overgeneralization (see Figure 1, Methods section, and Supplementary Materials). Participants also rated the degree of pleasantness or unpleasantness (i.e., emotional valence) of imagined examples of these social behaviors. This allowed us to probe whether participants accessed conceptual information in a more differentiated or generalized fashion and whether this information was integrated in a nonredundant or redundant way with other information relevant for appraisal of social behavior.
Figure 1.
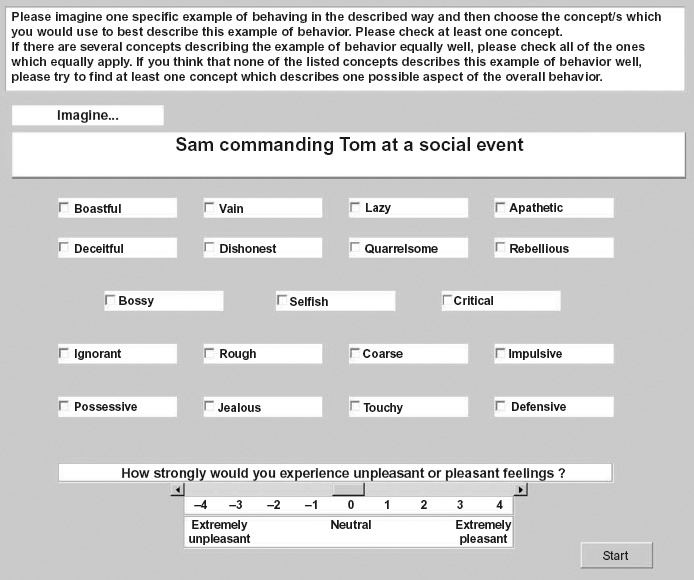
An example screen from the CSKD task is depicted. Beneath the denoted social behavior, participants were presented with 19 negative social concepts in the negative condition (as displayed) and 19 positive social concept labels in the positive condition. Concepts were taken from our normative pre-study. Participants were provided with the instructions as displayed above. To clarify this instruction, participants were reminded by the experimenter that when choosing multiple concepts, “they should all be equally good labels of the described behavior.” Participants were asked to rate to what extent they would experience unpleasant or pleasant feelings (valence) as a result of the social behavior on a bipolar scale (−4 = extremely unpleasant to +4 = extremely pleasant).
This approach was based on information theory, which defines information gain as highest when novel bits of information are nonredundant with existing bits of information (Attneave, 1959). This means that conceptual information gain is highest if it contributes noninterdependently (i.e., nonredundantly) to appraisal of social behavior rather than being interdependent (i.e., redundant) with other information relevant to appraisal (e.g., the context of actions/agency and emotions). Nonredundant conceptual integration was captured as noninterdependence between conceptual differentiation/generalization and perceived emotional intensity on the CSKD task when comparing actions of oneself (self-agency) relative to those of others (other-agency). This was based on the expectation that the degree of negative or positive appraisal of actions is most independent of conceptual generalization if conceptual information is nonredundant with other information relevant to the appraisal. We further examined the association of this neuropsychological measure of conceptual–emotional interdependence on the negative conditions of the CSKD task with separately obtained fMRI measures of ATL coupling during the experience of self-blame (i.e., guilt) and blaming of others (i.e., indignation).
The investigation of participants with remitted MDD allowed us to probe “trait” vulnerability factors (Bhagwagar & Cowen, 2008) that are independent of the depressive state. We chose closely matched individuals with no personal or family history of MDD as a comparison group so that group differences could be interpreted as arising from differences in MDD vulnerability.
First, we hypothesized that conceptual overgeneralization when appraising social actions on the CSKD task is associated with more intense emotions than conceptual differentiation. Second, we predicted that high interdependence of conceptual overgeneralization and negative emotions when appraising one's own versus one's best friend's actions on the CSKD task would be associated with MDD and with higher self-hate scores on the Interpersonal Guilt Questionnaire (IGQ-67, (O'Connor, Berry, Weiss, Bush, & Sampson, 1997)). Third, based on our previous work, we hypothesized this high conceptual–emotional interdependence on the CSKD task to be reflected in reduced ATL coupling with frontal-subcortical circuits, in particular the septal and subgenual cingulate region when blaming oneself relative to blaming others (Green, Lambon Ralph, et al., 2012; Green et al., 2010).
This latter hypothesis was also based on a model (Henson & Gagnepain, 2010), which conceives of strong coupling between brain regions as a sign of reiterative interactions needed because information in one brain region (e.g., conceptual information in the ATL) does not allow for prediction of information in the other brain region (e.g., guilt-specific contextual information in subgenual region) without strong interaction (i.e., information in both regions being nonredundant). Thus, strong interactions between regions reflect the integration of nonredundant information. We, therefore, expected strong coupling with the ATL to be associated with nonredundant integration of conceptual information when appraising social actions. We predicted this neurocognitive architecture to be associated with resilience against self-blaming emotional biases and MDD. This is because appraisal of one's behavior draws upon a richer variety of information when conceptual information contributes in a nonredundant fashion (Attneave, 1959), thereby protecting against overgeneral forms of self-blaming emotions (e.g., self-hate). This prediction was also based on our previous finding that individuals showing stronger ATL-coupling, while experiencing guilt, exhibited lower levels of self-hate and lower MDD risk (Green, Lambon Ralph, et al., 2012).
METHODS
Participants
This study was approved by the South Manchester NHS Research Ethics Committee, and all participants gave informed consent. Participants in the MDD group fulfilled criteria for a past major depressive episode according to Diagnostic and Statistical Manual IV-TR (DSM-IV, American Psychiatric Association, 2000) and for a moderate to severe depressive episode according to the International Classification of Diseases-10 with at least 2 months duration requiring treatment and remission of symptoms for at least 12 months. Exclusion criteria were current axis-I disorders and history of alcohol or substance abuse, or past co-morbid axis-I disorders being the likely primary cause of the depressive syndrome. The healthy control group had no current or past axis-I disorders and no first degree family history of MDD, bipolar disorder, or schizophrenia. Detailed clinical characteristics of the MDD group including medication history are further described in Green, Moll, et al. (2013).
Volunteers were invited for a clinical interview that included psychiatric, medical, and family history and a neurological exam by a board-certified psychiatrist (RZ). Further, a Structured Clinical Interview for DSM-IV-TR Mood Disorders Module A (First, Spitzer, Gibbon, & Williams, 2002) and the International Neuropsychiatric Interview (Lecrubier et al., 1997), which was adapted to allow assessment of lifetime axis-I disorders including substance and alcohol abuse, and a shortened version of the Weissman Family History Screen (Weissman et al., 2000), the Montgomery Asberg Depression Rating Scale (MADRS, (Montogomery & Asberg, 1979)), and the Global Assessment of Functioning (GAF) scale (Axis V, DSM-IV) were employed.
Fifty-seven participants (27 remitted MDD and 30 control participants) received the CSKD task. One control participant did not complete the task and was excluded from the analysis. There were no significant differences between the final groups (N = 29 control and N = 27 remitted MDD) on education (control, 15.7 ± 1.6; remitted MDD, 16.0 ± 2.10; t = .73; p = .47; ± refers to standard deviations throughout the text), gender (control, 22 female/7 male, 22 female/5 male; contingency coefficient = .07; p = .61), or age (control, 22.7 ± 3.5 years; remitted MDD, 25.6 ± 7.5 years; t = 1.85; p = .08). Depression scale and psychosocial function scores in the MDD differed from the control group, but were within the nonclinically relevant range (MADRS: control, .21 ± .6; remitted MDD, 1.33 ± 1.8; Mann–Whitney U = 263, p = .006; GAF scores: control, 89.3 ± 4.6; remitted MDD, 83.3 ± 7.2; Mann–Whitney U = 199.5, p = .001). In total, fMRI data were complete and had good coverage of ventral frontal, frontopolar, and anterior temporal lobes in 46 participants (21 healthy control participants and 25 individuals with remitted MDD, 16 with no current antidepressant medication) and were included in the final fMRI data analysis (Green, Lambon Ralph, et al., 2012).
Conceptual social knowledge differentiation task design
The CSKD task was based on a normative study, full results of which are available on request (please see Supplementary Methods section). In the final CSKD task (available at http://www.translational-cognitive-neuroscience.org/start/test-materials), participants were presented with sentences describing social interactions between themselves and their best friends in which either they (self-agency) or their best friend (other-agency) were the agents of the behavior. The social actions were derived from our normative pre-study as primary social action categories (e.g., “bragging,” “cheating,” “apologizing,” “joking”) and combined with co-occurring sequential context categories (e.g., “work,” “leisure,” “social event”) and were either of negative or positive valence as based on the norms and were generated as an example of one of the social concepts used in the task to prompt participants. For example, in the negative self-agency condition (NEG_S-AG, 30 items), “[participant's name] ignoring [best friend's name] at a social event;” in the negative other-agency condition (NEG_O-AG, 30 items), “[best friend's name] ignoring [participant's name] at a social event;” in the positive self-agency (POS_S-AG) condition, “[participant's name] giving [best friend's name] a gift whilst shopping;” and in the positive other-agency (POS_O-AG) condition, “[best friend's name] giving [participant's name] a gift whilst shopping.” Participants were shown the same stimuli for both the NEG_S-AG and NEG_O-AG condition and the POS_S-AG and POS_O-AG condition, switching only the names of participants to denote the role changes of agent and recipient. The order of administration of positive and negative conditions was randomized across participants. The order of self- and other-agency trials was fully randomized throughout the experiment for each person. Participants were asked to imagine one specific example of the behavior and then to choose the concept which they would use to best describe this example of behavior. Participants were reminded by the experimenter that when choosing multiple concepts, “they should all be equally good labels of the described behavior” (further details, see Figure 1).
In a pilot study carried out by JZ (Zakrzewski, 2008), 24 native English speakers (10 male) received the CSKD (age mean = 24.3 ± 2.4, years of education mean = 18.4 ± 1.7). This study showed an increase in rated emotional intensity with higher numbers of concepts used to describe a social behavior on a case-by-case basis (group average regression coefficient above .30 in the positive and below −.30 in the negative conditions). A higher number of concepts used indicated that they were perceived as relatively similar in meaning and thereby indicates conceptual overgeneralization (see also Supplemental Results section and Figure 1).
Behavioral data analysis
All data were checked for univariate outliers using standardized scores (outside z = ±2.5 standard deviations from the mean) and bivariate outliers using Mahalanobis distance measure (threshold, c2(2) = 9.21, p = .01) for the remitted MDD group and control group separately using SPSS 15 (www.spss.com). Additional analyses confirmed results after excluding univariate outliers from parametric group comparisons and both univariate and bivariate outliers from parametric correlational analyses.
Data were screened for normal distribution within each group with Kolmogorov–Smirnov tests and non-parametric Mann–Whitney-U tests were performed to investigate between-group differences on non-normally distributed measures. The significance threshold was p = .05, 2-sided.
The task was designed to investigate differences in conceptual differentiation depending on agency-role (self vs. other). We, therefore, computed negative CONCEPT scores as the differences in number of concepts chosen in the negative self-agency (NEG_S-AG) relative to negative other-agency (NEG_O-AG) conditions as a measure of relative indistinctiveness of concepts in one condition relative to the other (i.e., conceptual overgeneralization). Positive CONCEPT scores were computed in the same way. Negative VALENCE scores were computed as the difference in valence ratings between NEG_S-AG and NEG_O-AG conditions. Positive VALENCE scores were computed in the same way. To capture the interdependence of VALENCE and CONCEPT scores, we computed an interaction score VALENCE × CONCEPT by multiplying VALENCE and CONCEPT scores. This VALENCE × CONCEPT score was calculated separately for negative and positive conditions.
Functional MRI methods
The functional MRI methods and group comparisons have been described elsewhere (Green, Lambon Ralph, et al., 2012) and are only briefly summarized here. This study focused on how patterns of ATL-coupling for guilt versus indignation were affected by between-subject differences on the CSKD task.
fMRI paradigm
Participants were presented with written statements describing actions counter to social and moral values described by social concepts (e.g., “stingy,” “tactless”) in which the agent was either the participant (“self-agency” condition, N = 90) or their best friend (“other-agency” condition, N = 90, norms for the stimuli are further described in Zahn, Moll, Paiva, et al. (2009) and a full list of stimuli is available on request). Self- and other-agency conditions used the same social concepts (self-agency: e.g., “[participant's name] does act stingily toward [best friend's name];” other-agency: e.g., “[best friend's name] does act stingily toward [participant's name]”). 50% of trials used negative social concepts (e.g., “does act stingily”) and 50% used negated positive social concepts (e.g., “does not act generously”). In addition, we used a low-level resting-state baseline condition: fixation of visual pattern with no button press (null event, N = 90). Stimuli were presented in an event-related design for a maximum of 5 seconds within which participants had to make a decision by pressing one of two buttons with their right hand whether they would feel “extremely unpleasant” or “mildly unpleasant” from their own perspective.
After the scanning session, participants rated each statement on the degree of unpleasantness (7-step scale) to control for the degree of negative valence and emotional intensity. Further they were required to “choose the feeling that (they) would feel most strongly” from a choice of guilt, contempt/disgust toward self, shame, indignation/anger toward self, indignation/anger toward other, contempt/disgust toward other, none, other feeling. As in our previous studies (Green et al., 2010; Zahn, Moll, Paiva, et al., 2009), guilt and indignation toward other trials for the fMRI analysis were defined on the basis of individual ratings and restricted to agency-role-congruent responses (i.e., guilt in the self-agency condition and indignation toward others in the other-agency condition). Indignation toward others was abbreviated to indignation throughout the manuscript.
Image acquisition
Echo-planar T2∗-weighted images (405 volumes in each of the 3 runs with 5 dummy scans for each run of 13 min 40 sec) were acquired on a Philips 3 Tesla Achieva MRI scanner with an 8 channel coil, 3-mm slice thickness, and ascending continuous acquisition parallel to the anterior to posterior commissural line (between 35 and 40 slices depending on size of the participant's head, repetition time (TR) = 2000 ms, echo time (TE) = 20.5 ms, field of view (FOV) = 220 × 220 × 120 mm3, acquisition matrix = 80 × 80, reconstructed voxel size = 2.29 × 2.29 × 3 mm3, SENSE factor = 2). In addition, three-dimensional T1-weighted magnetization-prepared rapid acquisition gradient echo structural images were obtained (reconstructed voxel size = 1 mm3, 128 slices, TE = 3.9 ms, FOV = 256 × 256 × 128, acquisition matrix = 256 × 164, slice thickness = 1 mm, TR = 9.4 ms).
Image analysis
Functional images were realigned, unwarped, and coregistered to the subject's T1 images. These images were normalized by first normalizing the participant's T1 image to the standard T1-template in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and applying the same transformations to the functional images. A smoothing kernel of FWHM = 6 mm was used.
To investigate functional integration, we used a psychophysiological interaction (PPI) analysis in SPM8 (Friston et al., 1997). PPI requires the extraction of the signal from a seed region (in this case, the right superior ATL) and the creation of the interaction term, which is the multiplication of the psychological variables (the main effects of the conditions) with the physiological variable (the ATL signal time course irrespective of condition). A whole-brain search identifies all voxels in which a significant fraction of variance in signal can be explained by the PPI term. “Physiological” coupling refers to the ATL signal time course predicting activity in another brain area throughout the experiment (independent of psychological condition). In contrast, a PPI effect refers to the slope of the regression effect of the ATL on another brain area changing for one condition (e.g., guilt) relative to another (e.g., indignation). The PPI effect, therefore, indicates a selective modulation of functional integration by psychological condition.
The seed region was a sphere with a radius of 4-mm around the peak coordinate of the ATL activation in the standard BOLD analysis that was common to both the comparisons of guilt versus fixation and indignation versus fixation (x = 58, y = 0, z = −12, (Green, Lambon Ralph, et al., 2012)).
At the single subject level, the physiological variable, the psychological variable, and the PPI terms for guilt versus fixation and indignation versus fixation were entered into a common general linear model.
Here, we investigated between-subject differences on conceptual differentiation (CONCEPT scores) and its interdependence with emotional valence (CONCEPT × VALENCE scores) in the NEG S-AG relative to the NEG O-AG condition of the CSKD task. We modeled CONCEPT × VALENCE and CONCEPT scores as between-subject covariates of interest and modeled VALENCE scores and group (MDD vs. CONTROL) as covariates of no interest. Because CONCEPT × VALENCE scores differed between groups, we present supporting analyses showing its between-subject effects separately in each group. These between-subject covariate analyses were carried out on contrast images for each participant, which compared the PPI effect of guilt relative to indignation on ATL coupling with an uncorrected voxel-level threshold of p = .005 and extent threshold of 4 voxels. Results were inclusively masked with the same between-subject covariate effects on guilt versus fixation (mask at uncorrected voxel-level threshold p = .005). Thereby, we ensured that reported PPI effects were due to positive effects in the guilt condition rather than due to negative effects in the subtracted indignation condition. Only areas are reported that survived additional voxel- or cluster-level family-wise-error (FWE)-corrected thresholds of p = .05 across a priori region of interests (ROIs) (small volume correction) or the whole brain. A gray matter mask based on brains of all participants was used as an inclusive mask in all analyses (Green, Lambon Ralph, et al., 2012).
ROI definition
All regions surviving our uncorrected voxel-level threshold (minimum cluster size of 4 voxels) that did not survive a whole brain FWE-corrected threshold of p = .05 were further examined using FWE-correction over bilateral a priori ROIs in two tiers, as in Green et al. (2010). Tier 1 regions were regions that we had no specific hypothesis about, but which have been previously associated with moral and social cognition (Moll, de Oliveira-Souza, & Zahn, 2008) including posterior superior temporal sulcus/temporo-parietal junction, ventromedial prefrontal cortex (PFC), dorsolateral PFC, dorsomedial PFC, insula, amygdala, basal ganglia, hypothalamus, ventral tegmental area, anterior temporal lobes, and additional areas highlighted in cortico-limbic network models of MDD (Seminowicz et al., 2004): medial temporal lobes and frontopolar cortex (BA 10, (Green, Lambon Ralph, et al., 2012) for further details).
Activations that did not survive FWE-correction over these ROIs were then subjected to FWE-correction over tier 2 ROIs. Tier 2 ROIs were constructed as spheres with 6 mm radius around center coordinates that have been consistently identified for guilt (SCSR ROI center coordinate: x = −4, y = 23, z = −5) and indignation/anger (lateral orbitofrontal cortex ROI: x = 41, y = 33, z = −2), as well as the left hemispheric homologue of the previously identified superior ATL region (x = −57, y = −3, z = −6) and were taken from a previous independent study (further described in Green et al. (2010)).
RESULTS
Conceptual social knowledge differentiation task results
Reliability
Split-half reliability of the CSKD task was computed using the Spearman–Brown formula (Wilson, 2010) after randomly splitting items in each condition into parallel forms based on alphabetical order of stimuli. Reliability of measures in the different conditions (SA_POS, OA_POS, SA_NEG, OA_NEG) was very high (range of reliability coefficients: valence, .88–.89; number of concepts chosen, .95–.98).
Trial-by-trial-associations of conceptual and emotional measures
To investigate the relationship between conceptual generalization and emotional intensity when appraising social actions, we computed Spearman rank correlations between the number of concepts used to describe social behavior and the valence ratings in each condition (POS_S-AG, POS_O-AG, NEG_S-AG, NEG_O-AG) in each participant on a trial-by-trial basis. Correlation coefficients were significantly higher than zero in the positive conditions (POS_S-AG: mean = .21 ± .25, t[54] = 6.1, p < .0000001; POS_O-AG: mean = 21 ± .26, t[55] = 6.3, p < .0000001) and significantly below zero in the negative conditions (NEG_S-AG: mean = −.39 ± .20, t[50] = −14.4, p < .0000001; NEG_O-AG: mean = −.45 ± .20, t[49] = −15.8, p < .0000001) with no difference between groups (t < .44, p > .65 in all comparisons). These results show that conceptual generalization was associated with increased emotional intensity of appraised actions (increased unpleasantness in the negative and increased pleasantness in the positive conditions) across groups.
Differences between negative self-agency and other-agency conditions
Figure 2 shows the relationship of differences between NEG_S-AG and NEG_O-AG conditions on number of concepts chosen (CONCEPT scores) as a measure of relative indistinctiveness of concepts in one condition relative to the other (i.e., conceptual overgeneralization) and valence differences between conditions (VALENCE scores). Figure 3 gives an example of one MDD participant with conceptual overgeneralization for NEG_S-AG relative to the NEG_O-AG condition. CONCEPT and VALENCE scores were negatively associated such that people felt more unpleasantly about their own negative actions relative to their best friend's negative actions, when conceptually overgeneralizing while appraising their own relative to their best friend's actions (see legend to Figure 2). This interdependence of CONCEPT and VALENCE scores was robust in the MDD group, but not observed in the control group (see Figure 2).
Figure 2.
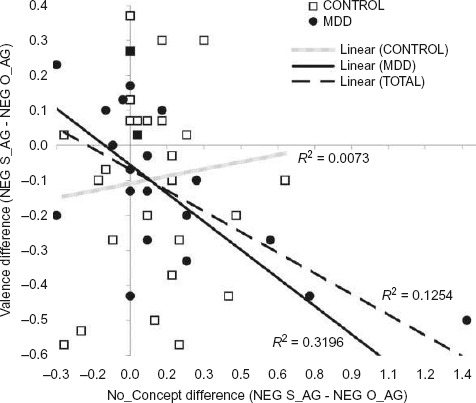
Displayed are individual values of VALENCE scores = difference of mean valence (NEG_SA – NEG_OA) and CONCEPT scores = difference of mean number of concepts (NEG_SA – NEG_OA) for N = 56 participants (29 control, 27 MDD). A univariate AN(C)OVA (corrected model: R2 = .26, F = 6.2, p = .001) showed a main effect of VALENCE on CONCEPT (F = 6.5, p = .01) and an interaction of group by VALENCE (F = 9.2, p = .004) with no main effect of group (F = .09, p = .76). The interaction was due to people with MDD showing a higher interdependence of CONCEPT and VALENCE scores in the expected direction (MDD: B = −.60, t = −4.2, p < .0001; Control: B = .05, t = .33, p = .75) such that self-agency-selective conceptual overgeneralization (i.e., high CONCEPT scores) was associated with self-agency-selective negative valence biases (i.e., low VALENCE scores).
Figure 3.
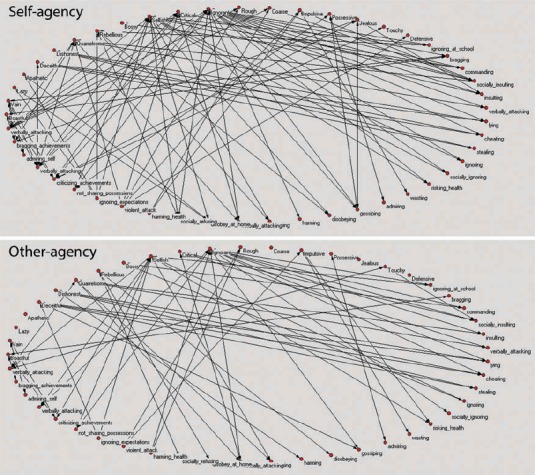
Displayed are connections between the 19 negative concepts and 30 social behaviors in the self-agency and other-agency condition made by one participant with remitted MDD (using PAJEK software for network analysis, (de Nooy, Mrvar, & Batgelj, 2005)). As can be seen, the participant used more concepts as descriptive of her own (92) relative to her best friend's behavior (56) indicating conceptual overgeneralization for negative self- relative to the other-agency condition.
To capture the observed interdependence of VALENCE and CONCEPT scores, we computed a VALENCE × CONCEPT interaction score by multiplying VALENCE and CONCEPT scores and showed that the MDD group had more negative interaction scores, indicating higher interdependence of VALENCE and CONCEPT scores (MDD: −.11 ± .26; control: −.004 ± .075; t = 2.0, p = .05; Mann–Whitney-U test = 263.5, p = .04). This result was reproduced when only including the 17 participants with MDD not taking antidepressant medication (t = 2.2, p = .03). There were no group differences on VALENCE (MDD: −.15 ± .38; control: −.095 ± .33; t = .5, p = .60) or CONCEPT scores (MDD: .17 ± .40; control: .099 ± .20; t = .8, p = .44).
In a linear regression model (R2 = .46), higher CONCEPT × VALENCE interdependence scores predicted higher IGQ-Self-Hate (β = −.32, p = .004), and this effect was adjusted for overall higher IGQ-Self-Hate scores in the remitted MDD group (β = .52, p = .000008).
Differences between positive self- and other-agency conditions
When computing differences between POS-S-AG and POS-O-AG conditions for VALENCE and CONCEPT scores and their interdependence, we found no significant differences between groups on either VALENCE × CONCEPT (MDD: 1.33 ± 2.8; control:.47 ± 1.5; t = −1.43, p = .16; Mann–Whitney-U test = 326.5, p = .29), VALENCE (MDD: .09 ± .40; control: −.05 ± .31; t = −1.47, p = .15) or CONCEPT scores (MDD: 59.9 ± 25.0; control: 59.6 ± 20.9; t = −.06, p = .95).
Imaging results
Across-group effects of variations in conceptual overgeneralization and its interaction with emotional biases
In this analysis, we investigated between-subject differences in conceptual generalization and its interaction with emotional appraisal whilst controlling for group differences (reported in Green, Lambon Ralph, et al. (2012)) and for differences in VALENCE scores (Table 1, Figure 4). Individuals who used social concepts in a more differentiated way (i.e., used fewer concepts as indistinctive) when evaluating their own relative to their best friend's negative behavior (i.e., had more negative CONCEPT [NEG_S-AG – NEG_O-AG] scores) exhibited a stronger transhemispheric coupling of the right ATL with the left middle ATL and left parahippocampal gyrus for guilt relative to indignation (Table 1, Figure 4). Individuals who overgeneralized conceptually when appraising their own relative to their best friend's behavior (i.e., had more positive CONCEPT [NEG_S-AG – NEG_O-AG] scores) showed hypothalamic/thalamic and right anterior dorsolateral PFC areas of increased coupling for guilt versus indignation.
TABLE 1.
Individual differences on ATL-PPI effects for guilt versus indignation irrespective of group
| Between-subject covariate of interest | Interpretation | Hemisphere | Anatomical description | Brodmann area |
MNI coordinates |
t-value | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Positive effect of interaction score (VALENCE × CONCEPT) | Increased guilt-selective coupling in individuals with relative noninterdependence of conceptual generalization and valence biases | R | Anterior dorsolateral prefrontal cortex | 46 | 44 | 36 | 8 | 4.59∗ |
| R | Septal part of nucleus accumbens | – | 8 | 6 | −4 | 3.34∗∗ | ||
| Negative effect of interaction score (VALENCE × CONCEPT) | Increased guilt-selective coupling in individuals with high interdependence of conceptual generalization and valence biases | L | Middle anterior temporal cortex | 21 | −58 | −4 | −2 | 4.31∗ |
| L | Superior anterior temporal cortex | 22 | −58 | 0 | −6 | 3.36∗∗ | ||
| Positive effect of conceptual score (S_AG – O_AG) | Increased guilt-selective coupling in S_AG-selective conceptual overgeneralizers | R | Thalamus/hypothalamus | – | 8 | −6 | 2 | 3.39∗ |
| R | Anterior dorsolateral prefrontal cortex | 46 | 42 | 38 | 10 | 3.43∗ | ||
| Negative effect of conceptual score (S_AG – O_AG) | Increased guilt-selective coupling in S_AG-selective conceptual overdifferentiators | L | Middle anterior temporal cortex | 21 | −58 | −4 | −22 | 4.96∗ |
| L | Parahippocampal gyrus | 35 | −18 | −8 | −28 | 4.54∗ | ||
Notes: Only regions surviving inclusive masking with guilt versus null are reported. No other regions survived an uncorrected threshold of p = .005 (extent threshold of 4 voxels) and a voxel- or cluster-level p = .05 over the whole brain or our a priori ROIs. ∗Survived voxel-wise FWE-correction over tier 1 ROI; ∗∗Survived voxel-wise FWE-correction over tier 2 ROI. S_AG = self-agency condition, O_AG = other-agency condition, between-subject covariates in all models were group (MDD/control), the main effects of conceptual (CONCEPT) and valence (VALENCE) difference scores (S_AG – O_AG), and their interaction score (VALENCE × CONCEPT).
Figure 4.
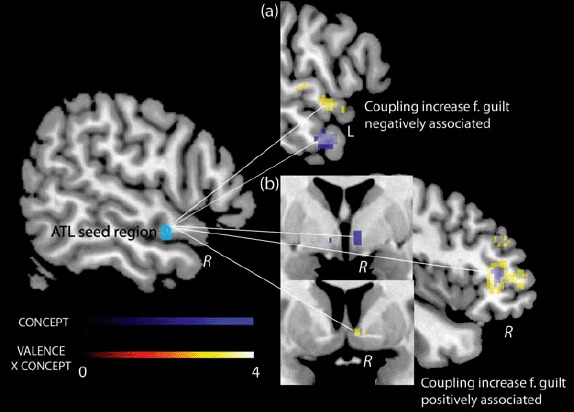
Regions showing increased coupling with the right superior ATL during the experience of guilt relative to indignation in individuals with lower (a) or higher (b) CONCEPT [NEG_S-AG – NEG_O-AG] (blue scale) or VALENCE [NEG_S-AG – NEG_O-AG] × CONCEPT [NEG_S-AG – NEG_O-AG] interaction (hot scale) scores irrespective of VALENCE scores and group. NEG_S-AG-selective conceptual differentiation (negative CONCEPT scores) was associated with left middle ATL increases in coupling for guilt (a) while high interdependence of conceptual overgeneralization and negative valence (more negative interaction scores) were associated with left superior ATL coupling increases for guilt (a). Higher coupling in right dorsolateral PFC and the septal part of the nucleus accumbens were associated with relative noninterdependence of conceptual overgeneralization and negative valence (i.e., more positive CONCEPT × VALENCE scores). Higher coupling in the same right dorsolateral PFC area and in the hypothalamus were also associated with higher NEG_S-AG-selective conceptual overgeneralization (i.e., higher CONCEPT scores). Cropped whole brain images were displayed at an uncorrected threshold of p = .005 (extent threshold of 4 voxels). All depicted regions survived FWE-correction over a priori ROIs at p = .05 in separate analyses.
The same right anterior dorsolateral PFC area together with the part of the nucleus accumbens which belongs to the septum (septal part of the nucleus accumbens, (Nieuwenhuys, Voogd, & Van Huijzen, 1978)) exhibited increased coupling for guilt versus indignation in individuals with noninterdependence of conceptual overgeneralization and emotional appraisal biases (i.e., those with less negative CONCEPT [NEG_S-AG-NEG_O-AG] × VALENCE [NEG_S-AG-NEG_O-AG] interaction scores). In contrast, individuals with high interdependence of conceptual overgeneralization and emotional valence (i.e., those with more negative CONCEPT [NEG_S-AG-NEG_O-AG] × VALENCE [NEG_S-AG-NEG_O-AG] interaction scores) showed higher coupling between the right superior ATL and its left hemispheric homologue as well as a left middle ATL region.
Within-group effects of variations in interaction of conceptual overgeneralization and emotional biases
Because average CONCEPT × VALENCE interaction scores when appraising negative behavior were higher in the CONTROL than in the MDD group, we also investigated their effect on right superior ATL coupling in each group separately. Within the MDD group, individual variation on CONCEPT × VALENCE scores (i.e., interdependence of conceptual overgeneralization and emotional appraisal biases) was not associated with individual differences in coupling for guilt versus indignation. In contrast, within the CONTROL group, individuals with lower interdependence of conceptual overgeneralization and emotional appraisal biases showed higher coupling for guilt versus indignation within a network of subgenual and dorsal anterior cingulate, right dorsolateral PFC, right medial and left lateral ventral frontopolar cortices, left temporoparieto-occipital junction, and hypothalamus (Table 2, Figure 5(b)). Within the subgenual cingulate region resulting from this analysis, regression coefficients for the MDD group showed that a lack of effect may be due to an overall lack of coupling increase for guilt relative to indignation in the MDD group (Figure 5(a)) as reported previously (Green, Lambon Ralph, et al., 2012).
TABLE 2.
VALENCE × CONCEPT interaction score effects on ATL-PPI within each group
| Between-subject covariate of interest | Hemisphere | Anatomical description | Brodmann area |
MNI Coordinates |
t-value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Positive effect of VALENCE × CONCEPT interaction score on ATL-PPI for guilt versus indignation in the control group | R | Medial ventral frontopolar cortex | 10 | 10 | 56 | 0 | 6.49c |
| R | Anterior subgenual cingulate cortex | 24 | 8 | 30 | −2 | 6.47c | |
| L | Lateral ventral frontopolar cortex | 10 | −38 | 56 | −2 | 5.92∗ | |
| R | Anterior dorsolateral prefrontal cortex | 46 | 40 | 40 | 16 | 5.92c | |
| L and R | Dorsal anterior cingulate cortex | 24 | −2 | −2 | 32 | 6.14∗ | |
| L | Hypothalamus | – | −14 | −8 | −10 | 5.18∗ | |
| L | Parieto-temporo-occipital junction | 40 | −48 | −42 | 36 | 7.07c | |
Notes: Only regions surviving inclusive masking with guilt versus null are reported. No other regions survived an uncorrected threshold of p = .005 (extent threshold of 4 voxels) and a voxel- or cluster-level (=c) p = .05 over the whole brain or our a priori ROIs. ∗Survived voxel-wise FWE-correction over tier 1 ROI, There was no significant positive effect of the interaction score within the MDD group. Effects of conceptual scores were not examined separately for each group, because there was no difference between groups on this measure.
Figure 5.
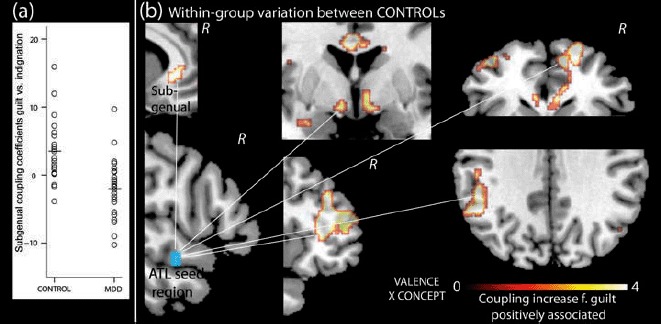
(a) Displays right superior ATL coupling coefficients from the subgenual cingulate peak voxel of the positive effect of CONCEPT × VALENCE interaction scores on guilt versus indignation within the control group. Within this voxel, the control group showed higher ATL coupling for guilt versus indignation compared with the MDD group (CONTROL mean = 3.5 ± 4.7, MDD mean = −1.9 ± 4.4, t[43] = 4.02, p < .0001). (b) Depicts the subgenual, hypothalamic, right anterior dorsolateral PFC, medial and lateral frontopolar, and left temporo-parietal regions showing increased coupling for guilt versus indignation in control individuals with relative noninterdependence of conceptual overgeneralization and negative valence when appraising social behaviors (i.e., more positive CONCEPT × VALENCE interaction scores).
DISCUSSION
Our first hypothesis was confirmed; when appraising one's own or others social actions, conceptual generalization was associated with higher emotional intensity. This association was consistent across MDD and control groups as well as conditions (POS_S-AG, NEG_S-AG, POS_O-AG, NEG_O-AG). The second hypothesis was also confirmed; redundant integration of conceptually differentiated information regarding one's own relative to others’ negative actions was associated with self-hate on the IGQ-67 and MDD. This was demonstrated by showing higher interdependence of conceptual overgeneralization and negative emotional biases in the MDD group. This was reproducible in the MDD subgroup not taking medication, thereby ruling out abnormal performance due to effects of antidepressants. Individuals with higher interdependence of conceptual overgeneralization and negative emotional biases also showed stronger self-hate, even when correcting for effects of group.
Confirming our third hypothesis, we found that individuals displaying nonredundant integration of conceptual information when appraising negative actions showed increased coupling of the right superior ATL with frontal-subcortical circuits, for guilt relative to indignation, irrespective of group. Interestingly, this included an anterior right dorsolateral PFC area, previously associated with cognitive reappraisal of emotions (Ochsner & Gross, 2005; Wiech et al., 2006). As predicted, this analysis yielded ATL-septal area coupling. However, this did not include the expected subgenual cingulate region across groups, which only emerged when focusing the analysis on between-subject differences in the control group. In addition to the subgenual cingulate/septal area, this analysis revealed frontopolar and hypothalamic areas of ATL coupling previously shown to be strong in people at low risk of MDD (Green, Lambon Ralph, et al., 2012). This result demonstrated that control individuals with high ATL coupling in these areas when feeling guilty display nonredundant conceptual–emotional integration.
We were able to show that perceived emotional intensity and conceptual generalization are interdependent on a trial-by-trial basis. We further validated the number of concepts chosen as a measure of conceptual generalization by showing its high association with a more complex measure that takes normative data on the associative strength (i.e., dominance, (Ashcraft, 1978)) between a given concept and a given social behavior into account (Supplementary Results). This dominance-weighted measure showed that participants choosing a high number of concepts to describe social behavior did so, because they selected concepts that were not highly associated with the given behavior in a normative sample (e.g., choosing the concept “possessive” among others to describe one's act of gossiping about one's best friend, Figure 3) as a sign of conceptual overgeneralization. This demonstrated that the concepts provided in the task were unrelated enough to one another to prevent participants from describing multiple distinct aspects equally related to a given social behavior by using multiple concepts. Providing a selection of distinct but equally applicable concepts would have rendered the number of concepts chosen unusable as an index of conceptual generalization.
This confirms the hypothesis that conceptual differentiation plays a role in the experience of emotions. However, the task did not allow us to determine whether conceptual generalization caused increased perception of emotional intensity or whether access to conceptual information was reduced when participants faced more emotionally intense stimuli. The degree of interdependence between conceptual overgeneralization and emotional appraisal was found to be stronger in MDD and in individuals with overgeneralized self-blaming emotions as measured on the IGQ-67 self-hate scale. Since we controlled for the overall degree of conceptual generalization/differentiation and emotional intensity, which did not differ between groups and were not associated with self-hate, this result points to other complementary cognitive components of appraisal that must influence the relationship between conceptual generalization and perceived emotional intensity of social behavior. Although, the CSKD task did not identify those other cognitive components directly, we were able to identify their neural correlates by correlating between-subject differences on conceptual generalization-dependence of emotional intensity with fMRI measures of functional integration with the right superior ATL.
Individuals showing noninterdependence between conceptual overgeneralization and intensity of negative emotions (i.e., nonredundant integration of conceptual information) exhibited stronger functional coupling of the right ATL and the ipsilateral anterior dorsolateral PFC, as well as the septal part of the nucleus accumbens for guilt (self-blaming emotion) relative to indignation (other-blaming emotion). By inclusively masking with guilt versus fixation, we ensured that these effects arose as relative increases in coupling for guilt compared with both control conditions. This sheds new light on the evidence that dysfunction of the dorsolateral PFC contributes to symptoms of major depression (Price & Drevets, 2010; Siegle, Thompson, Carter, Steinhauer, & Thase, 2007) and that its lesion was associated with higher rates of depression in patients with penetrating head injuries (Koenigs et al., 2008). While the lack of dorsolateral PFC function in MDD is usually seen as due to a lack of regulating negative emotions (Price & Drevets, 2010; Siegle et al., 2007), our findings point to a more specific role in protecting against self-blaming biases.
Similar right anterior dorsolateral PFC areas have been shown to be activated for reappraisal (i.e., changing the meaning (Ochsner & Gross, 2005)) of emotional stimuli and thereby decreasing their perceived intensity (Ochsner & Gross, 2005; Wiech et al., 2006). While there is general agreement on the importance of the right anterior dorsolateral PFC for reappraisal of emotions, for moral judgments (Greene, Nystrom, Engell, Darley, & Cohen, 2004), and for social-executive functions including empathic perspective taking (Eslinger, Moore, Anderson, & Grossman, 2011), there are different views on the exact contribution of the dorsolateral PFC to socio-moral functioning (Moll & de Oliveira-Souza, 2007). The cognitive control model predicts that the dorsolateral PFC modulates information stored elsewhere in the brain (Miller & Cohen, 2001), whereas representational models of dorsolateral PFC function predict sequential event and action context information to be stored in this area (Moll, Zahn, de Oliveira-Souza, Krueger, & Grafman, 2005; Wood & Grafman, 2003). Our results would be compatible with both theoretical perspectives.
Our finding of increased ATL coupling with the septal part of the nucleus accumbens when feeling guilty relative to indignation in individuals showing adaptive integration of conceptual information was predicted from our previous work on guilt-selective functional disconnections of these regions in the remitted MDD group (Green, Lambon Ralph, et al., 2012) and strong coupling in people at low risk of MDD (Green et al., 2010). The current results confirm the hypothesis that the degree of coupling when experiencing guilt is associated with the degree of nonredundancy with which conceptual information is integrated into the experience of emotions. Previous studies have shown that the septal region is selectively activated when donating to charity relative to receiving monetary reward (Moll et al., 2006) and when cooperating in trust games (Krueger et al., 2007). The septal region was also more active in guilt-prone people (Green, Lambon Ralph, et al., 2012; Zahn, Moll, Paiva, et al., 2009) when feeling guilt compared with indignation. Moreover, neurodegeneration in this region was shown to lead to a loss of experimentally probed guilt and pity while controlling for other negative emotions such as embarrassment, disgust, and anger (Moll et al., 2011). This evidence is in keeping with the prediction that the septal region hosts representations of key ingredients for affiliative emotions such as guilt and pity (Moll et al., 2008). The current study shows that individuals differ with respect to how they integrate conceptual knowledge within the ATL with emotional information in the septal region and that this affects their resilience toward MDD.
Unexpectedly, however, ATL coupling with the adjacent subgenual cingulate region, reproducibly shown to be activated selectively for guilt (Basile et al., 2011; Green, Lambon Ralph, et al., 2012; Morey et al., 2012; Zahn, de Oliveira-Souza, et al., 2009, Zahn, Moll, Paiva, 2009), did not differ between participants with redundant and nonredundant integration of conceptual information when appraising negative emotions across both groups. The subgenual cingulate region emerged, however, together with a network of hypothalamic and frontopolar regions previously shown to display guilt-selective functional disconnection in MDD (Green, Lambon Ralph, et al., 2012), when repeating the analysis in the control group only. A further analysis showed that there were relatively few individuals in the MDD group who exhibited higher subgenual cingulate coupling in the guilt relative to the indignation condition at all. This “floor” effect in the MDD group may explain why analyses of between-subject differences across groups in this area did not yield significant results. This is in keeping with the evidence that the subgenual cingulate plays a key role in the pathophysiology of MDD (Drevets, Ongur, & Price, 1998; Ressler & Mayberg, 2007) and that its stimulation leads to remission of depressive symptoms (Mayberg et al., 2005).
Although our main analysis focused on the interdependence of conceptual information and emotions, we also explored neural correlates of individual differences in conceptual differentiation of social actions irrespective of its influence on emotions. Interestingly, individuals who conceptually differentiated to a higher degree in the NEG_S-AG than in the NEG_O-AG condition exhibited stronger inter-hemispheric ATL coupling for guilt relative to indignation. Higher inter-hemispheric ATL coupling, however, was also displayed by individuals who showed redundant integration of conceptual information when making emotional appraisals. Thus, inter-hemispheric ATL coupling appears to be employed by individuals who are well able to differentiate conceptually when appraising their negative actions, but who may over-rely on their conceptual system when making emotional appraisals and may, therefore, be more vulnerable to self-negative biases when their conceptual system fails to protect against overgeneralization. Integration of conceptual information across hemispheres is compatible with the view that conceptual knowledge is represented bilaterally (Ralph, Pobric, & Jefferies, 2009), thereby allowing for compensation of unilateral ATL lesions through contralateral take-over of function (Ralph, Ehsan, Baker, & Rogers, 2012) (Warren, Crinion, Ralph, & Wise, 2009). Bilateral organization of social conceptual information would also explain why some studies found selective activations for social stimuli in the left (Ross & Olson, 2010), some in the right (Skipper et al., 2011; Zahn et al., 2007), and others in bilateral ATLs (Simmons, Reddish, Bellgowan, & Martin, 2010). Further studies are needed to confirm a role of inter-hemispheric ATL coupling in enhancing conceptual differentiation capacity and to determine the unique contributions of each hemisphere.
Previous studies assessing the redundancy of associations between social concepts across different aspects of the self (e.g., work, relationship) used card sorting tasks in remitted MDD and found either reduced redundancy for positive (Dalgleish et al., 2011) or increased redundancy for negative information (Dozois & Dobson, 2001). This is concordant with our finding of changes in the cognitive architecture of social knowledge in MDD persisting into the remitted stage. Redundancy was previously defined as the repeated use of a given social concept or phrase to describe several aspects of one's self (Dozois & Dobson, 2001) or periods of one's past self (Dalgleish et al., 2011). Thus, redundancy referred to the architecture of associations between social concepts and autobiographical or situational context. The finding of increased redundancy of associations for negative social information (Dozois & Dobson, 2001), despite not specifically assessing the contribution of conceptual differentiation (i.e., whether people accessed knowledge of differentiated aspects of the meaning of their behavior), concurs with our result of higher redundancy between conceptual and other aspects of information relevant to emotional appraisal in remitted MDD.
On a more cautionary note, this study did not determine to what degree redundant social conceptual integration is a scar effect (Lewisohn, Steinmetz, Larson, & Franklin, 1981; Wichers, Geschwind, van Os, & Peeters, 2010) of a previous depressive episode versus the correlate of a primary vulnerability to MDD. Regarding confounding factors that may have affected task performance, one needs to consider executive demands of the task, ability, and motivation to follow the instructions, as well as the general level of conceptual differentiation, which may be influenced by educational factors. All these factors were carefully controlled by focusing the analysis on the comparison of carefully matched task conditions of self- and other-agency. Thus, all these confounding factors were equal for the compared conditions and can therefore not explain individual differences in conceptual differentiation depending on agency.
CONCLUSIONS
Taken together, our results show that conceptual overgeneralization is associated with increased emotional intensity when appraising social actions. Interdependence of conceptual overgeneralization and negative emotional biases was higher in individuals with overgeneralized forms of self-blame (i.e., self-hate) and vulnerability to MDD. Further, high interdependence of conceptual overgeneralization and negative emotional biases indicating redundant conceptual integration was associated with guilt-selective decoupling between the right superior ATL and right dorsolateral PFC as well as the septal part of the nucleus accumbens across MDD and control groups.
In addition, those areas of ATL coupling previously associated with low MDD vulnerability, including subgenual cingulate, frontopolar, and hypothalamic areas, were associated with relative noninterdependence of conceptual overgeneralization and emotional biases in the control group, indicating nonredundant integration of conceptual information. This effect did not survive across both groups, possibly because of “floor” effects of coupling within those areas in the MDD group. We speculate that ATL-fronto-subcortical coupling is the correlate of nonredundant integration of complementary conceptual knowledge (i.e., emotional and action-context-independent) in the ATL with representations of the context of action sequences and associated emotional qualities in frontal-subcortical networks (Zahn, Moll, Paiva, et al., 2009). This hypothesis needs to be further tested with direct measures capturing the degree of differentiation of action and emotional context and their association with specific frontal and subcortical brain regions.
Supplementary material
Supplementary is available via the “Supplementary” tab on the article's online page (http://dx.doi.org/doi=10.1080/17470919.2013.810171).
REFERENCES
- Abramson L. Y., Seligman M. E., Teasdale J. D. Learned helplessness in humans: Critique and reformulation. Journal of Abnormal Psychology. 1978;87(1):49–74. [PubMed] [Google Scholar]
- Alloy L. B., Abramson L. Y., Peterson C., Seligman M. E. P. Attributional style and the generality of learned helplessness. Journal of Personality and Social Psychology. 1984;46(3):681–687. doi: 10.1037//0022-3514.46.3.681. [DOI] [PubMed] [Google Scholar]
- Ashcraft M. H. Property norms for typical and atypical items from 17 categories: A description and discussion. Memory & Cognition. 1978;6:227–232. [Google Scholar]
- Attneave F. Applications of information theory to psychology. New York, NY: Henry Holt; 1959. [Google Scholar]
- Basile B., Mancini F., Macaluso E., Caltagirone C., Frackowiak R. S. J., Bozzali M. Deontological and altruistic guilt: Evidence for distinct neurobiological substrates. Human Brain Mapping. 2011;32(2):229–239. doi: 10.1002/hbm.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. T. Thinking and depression 1. Idiosyncratic content and cognitive distortions. Archives of General Psychiatry. 1963;9:324–333. doi: 10.1001/archpsyc.1963.01720160014002. [DOI] [PubMed] [Google Scholar]
- Beck A. T., Rush A. J., Shaw B. F., Emery G. Cognitive therapy of depression. New York, NY: Guilford Press; 1979. [Google Scholar]
- Bhagwagar Z., Cowen P. J. “It's not over when it's over”: Persistent neurobiological abnormalities in recovered depressed patients. Psychological Medicine. 2008;38(3):307–313. doi: 10.1017/s0033291707001250. [DOI] [PubMed] [Google Scholar]
- Dalgleish T., Hill E, Golden A. M. J., Morant N., Dunn B. D. The structure of past and future lives in depression. Journal of Abnormal Psychology. 2011;120(1):1–15. doi: 10.1037/a0020797. [DOI] [PubMed] [Google Scholar]
- de Nooy W., Mrvar A., Batgelj V. Exploratory social network analysis with pajek. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Dozois D. J. A., Dobson K. S. A longitudinal investigation of information processing and cognitive organization in clinical depression: Stability of schematic interconnectedness. Journal of Consulting and Clinical Psychology. 2001;69(6):914–925. [PubMed] [Google Scholar]
- Drevets W. C., Ongur D., Price J. L. Neuroimaging abnormalities in the subgenual prefrontal cortex: Implications for the pathophysiology of familial mood disorders. Molecular Psychiatry. 1998;3(3):220–226. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- DSM-IV (American Psychiatric Association) Diagnostic and statistical manual of mental disorders. 4th ed. – text revision. Arlington, VA: American Psychiatric Association; 2000. (DSMIV-TR)) [Google Scholar]
- Eslinger P. J., Moore P., Anderson C., Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23(1):74–82. doi: 10.1176/appi.neuropsych.23.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. W. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Friston K. J., Buechel C., Fink G. R., Morris J., Rolls E., Dolan R. J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Ghatavi K., Nicolson R., MacDonald C., Osher S., Levitt A. Defining guilt in depression: A comparison of subjects with major depression, chronic medical illness and healthy controls. Journal of Affective Disorders. 2002;68(2–3):307–315. doi: 10.1016/s0165-0327(01)00335-4. [DOI] [PubMed] [Google Scholar]
- Goldston R. B., Gara M. A., Woolfolk R. L. Emotion differentiation – A correlate of symptom severity in major depression. Journal of Nervous and Mental Disease. 1992;180(11):712–718. [PubMed] [Google Scholar]
- Green S., Lambon Ralph M. A., Moll J., Deakin W. F., Zahn R. Guilt-selective functional disconnection of anterior temporal and subgenual cortices in major depressive disorder. Archives of General Psychiatry. 2012;69(10):1014–1021. doi: 10.1001/archgenpsychiatry.2012.135. [DOI] [PubMed] [Google Scholar]
- Green S., Lambon Ralph M. A., Moll J., Stamatakis E. A., Grafman J., Zahn R. Selective functional integration between anterior temporal and distinct fronto-mesolimbic regions during guilt and indignation. NeuroImage. 2010;52(4):1720–1726. doi: 10.1016/j.neuroimage.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Moll J., Deakin J. F., Hulleman J., Zahn R. Proneness to decreased negative emotions in major depressive disorder when blaming others rather than oneself. Psychopathology. 2013;46(1):34–44. doi: 10.1159/000338632. [DOI] [PubMed] [Google Scholar]
- Greene J. D., Nystrom L. E., Engell A. D., Darley J. M., Cohen J. D. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44(2):389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Hall C. S., Lindzey G. Lewin's field theory. Theories of personality. 3rd ed. New York, NY: Wiley; 1978. pp. 383–437. [Google Scholar]
- Henson R. N., Gagnepain P. Predictive, interactive multiple memory systems. Hippocampus. 2010;20(11):1315–1326. doi: 10.1002/hipo.20857. [DOI] [PubMed] [Google Scholar]
- Kingsland R. C., Greene L. R. Psychological differentiation and clinical depression. Cognitive Therapy and Research. 1984;8(6):599–605. [Google Scholar]
- Koenigs M., Huey E. D., Calamia M., Raymont V., Tranel D., Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. Journal of Neuroscience. 2008;28(47):12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F., McCabe K., Moll J., Kriegeskorte N., Zahn R., Strenziok M., Grafman J. Neural correlates of trust. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):20084–20089. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph M. A., Patterson K. Generalization and differentiation in semantic memory: Insights from semantic dementia. Annals of the New York Academy of Sciences. 2008;1124:61–76. doi: 10.1196/annals.1440.006. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M. A., Sage K., Jones R. W., Mayberry E. J. Coherent concepts are computed in the anterior temporal lobes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(6):2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R. D., Quinlan D. M., Schwartz G. E., Walker P. A., Zeitlin S. B. The levels of emotional awareness scale – A cognitive-developmental measure of emotion. Journal of Personality Assessment. 1990;55(1–2):124–134. doi: 10.1080/00223891.1990.9674052. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D. V., Weiller E., Amorim P., Bonora I., Sheehan K. H., Dunbar G. C. The mini international neuropsychiatric interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry. 1997;12(5):224–231. [Google Scholar]
- Lewisohn P. M., Steinmetz J. L., Larson D. W., Franklin J. Depression-related cognitions: Antecedent or consequence? Journal of Abnormal Psychology. 1981;90(3):213–219. doi: 10.1037//0021-843x.90.3.213. [DOI] [PubMed] [Google Scholar]
- Mayberg H. S., Lozano A. M., Voon V., McNeely H. E., Seminowicz D., Hamani C., Kennedy S. H. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Miller E. K., Cohen J. D. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moll J., de Oliveira-Souza R. Response to Greene: Moral sentiments and reason: Friends or foes? Trends in Cognitive Sciences. 2007;11(8):323–324. doi: 10.1016/j.tics.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Moll J., de Oliveira-Souza R., Zahn R. The neural basis of moral cognition – Sentiments, concepts, and values. Year in Cognitive Neuroscience. 2008;2008(1124):161–180. doi: 10.1196/annals.1440.005. [DOI] [PubMed] [Google Scholar]
- Moll J., Krueger F., Zahn R., Pardini M., de Oliveira-Souza R., Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(42):15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Zahn R., de Oliveira-Souza R., Bramati I. E., Krueger F., Tura B., Grafman J. Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. NeuroImage. 2011;54(2):1735–1742. doi: 10.1016/j.neuroimage.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Zahn R., de Oliveira-Souza R., Krueger F., Grafman J. The neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Montogomery S. A., Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morey R. A., McCarthy G., Selgrade E. S., Seth S., Nasser J. D., LaBar K. S. Neural systems for guilt from actions affecting self versus others. NeuroImage. 2012;60(1):683–692. doi: 10.1016/j.neuroimage.2011.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R., Voogd J., Van Huijzen C. The human central nervous system. Berlin: Springer; 1978. [Google Scholar]
- Ochsner K. N., Gross J. J. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- O'Connor L. E., Berry J. W., Weiss J., Bush M., Sampson H. Interpersonal guilt: The development of a new measure. Journal of Clinical Psychology. 1997;53(1):73–89. doi: 10.1002/(sici)1097-4679(199701)53:1<73::aid-jclp10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Patterson K., Nestor P. J., Rogers T. T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Price J. L., Drevets W. C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph M. A. L., Ehsan S., Baker G. A., Rogers T. T. Semantic memory is impaired in patients with unilateral anterior temporal lobe resection for temporal lobe epilepsy. Brain. 2012;135:242–258. doi: 10.1093/brain/awr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph M. A. L., Pobric G., Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: Convergent evidence from rTMS. Cerebral Cortex. 2009;19(4):832–838. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Ressler K. J., Mayberg H. S. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nature Neuroscience. 2007;10(9):1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. A., Olson I. R. Social cognition and the anterior temporal lobes. NeuroImage. 2010;49(4):3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D. A., Mayberg H. S., McIntosh A. R., Goldapple K., Kennedy S., Segal Z., Rafi-Tari S. Limbic-frontal circuitry in major depression: A path modeling metanalysis. NeuroImage. 2004;22(1):409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Siegle G. J., Thompson W., Carter C. S., Steinhauer S. R., Thase M. E. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biological Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Simmons W. K., Reddish M., Bellgowan P. S. F., Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cerebral Cortex. 2010;20(4):813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper L. M., Ross L. A., Olson I. R. Sensory and semantic category subdivisions within the anterior temporal lobes. Neuropsychologia. 2011;49(12):3419–3429. doi: 10.1016/j.neuropsychologia.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. E., Crinion J. T., Ralph M. A. L., Wise R. J. S. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–3442. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M. M., Wickramaratne P., Adams P., Wolk S., Verdeli H., Olfson M. Brief screening for family psychiatric history – The family history screen. Archives of General Psychiatry. 2000;57(7):675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- Wichers M., Geschwind N, van Os J., Peeters F. Scars in depression: Is a conceptual shift necessary to solve the puzzle? Psychological Medicine. 2010;40(3):359–365. doi: 10.1017/s0033291709990420. [DOI] [PubMed] [Google Scholar]
- Wiech K., Kalisch R., Weiskopf N., Pleger B., Stephan K. E., Dolan R. J. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. Journal of Neuroscience. 2006;26(44):11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M. G., Barnhofer T., Crane C., Hermans D., Raes F., Watkins E., Dalgleish T. Autobiographical memory specificity and emotional disorder. Psychological Bulletin. 2007;133(1):122–148. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. Constructing measures. New York, NY: Routledge; 2010. [Google Scholar]
- Witkin H. A., Goodenough D. R., Oltman P. K. Psychological differentiation: Current status. Journal of Personality and Social Psychology. 1979;37(7):1127–1145. doi: 10.1037//0022-3514.32.4.730. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Grafman J. Human prefrontal cortex: Processing and representational perspectives. Nature Reviews Neuroscience. 2003;4(2):139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Zahn R., de Oliveira-Souza R., Bramati I., Garrido G., Moll J. Subgenual cingulate activity reflects individual differences in empathic concern. Neuroscience Letters. 2009;457(2):107–110. doi: 10.1016/j.neulet.2009.03.090. [DOI] [PubMed] [Google Scholar]
- Zahn R., Moll J., Iyengar V., Huey E. D., Tierney M., Krueger F., Grafman J. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132:604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R., Moll J., Krueger F., Huey E. D., Garrido G., Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences. 2007;104(15):6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R., Moll J., Paiva M., Garrido G., Kruger F., Huey E. D., Grafman J. The neural basis of human social values: Evidence from fMRI. Cerebral Cortex. 2009;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewski J. Differentiation of conceptual social knowledge and the relationship with negative self-evaluations: Individual differences and implications for vulnerability to major depression. Manchester: University of Manchester; 2008. [Google Scholar]


